Abstract
Several brain areas in the diencephalon are involved in the activation and expression of sexual behavior, including in quail, the medial preoptic nucleus (POM). However, the ontogeny of these diencephalic brain nuclei has not to this date been examined in detail. We investigated the ontogeny of POM and other steroid-sensitive brain regions by injecting quail eggs with 5-bromo-2-deoxyuridine (BrdU) at various stages between E3 and E16 and killing animals at postnatal (PN) days 3 or 56. In the POM, large numbers of BrdU-positive cells were observed in subjects injected from E3 to E10, the numbers of these cells was intermediate in birds injected on E12, and most cells were post-mitotic in both sexes on E14-E16. Injections on E3-E4 labeled large numbers of Hu positive cells in POM. In contrast, injections performed at a later stage labeled cells that do not express aromatase nor neuronal markers such as Hu or NeuN in the POM and other steroid-sensitive nuclei and thus do not have a neuronal phenotype in these locations contrary to what is observed in the telencephalon and cerebellum. No evidence could also be collected to demonstrate that these cells have a glial nature. Converging data, including the facts that these cells divide in the brain mantle and express PCNA, a cell cycling marker, indicate that cells labeled by BrdU during the second half of embryonic life are slow cycling progenitors born and residing in the brain mantle. Future research should now identify their functional significance.
Keywords: preoptic area, sex steroids, neurogenesis, neural progenitors, glial cell, sexual behavior
Introduction
The discovery by Nottebohm and colleagues of a previously unsuspected degree of seasonal plasticity in the brain of songbirds (Nottebohm, 1981, 2004) played a key role in renewing investigations on the potential existence of neurogenesis in the adult brain (Kemperman, 2006; Gage et al., 2007). Birds, in particular songbird species such as the canary (Serinus canaria) and zebra finch (Poephila guttata), have now been used extensively in studies of adult neurogenesis (Brenowitz, 2008, Nottebohm, 2004, 2008). The focus of these studies has been almost exclusively on neuronal proliferation at the wall of the lateral ventricle and their migration and integration into functional telencephalic circuits involved in the control of song production. It is now recognized that the extensive neurogenesis observed in the post-natal or even adult telencephalon is not specific to songbirds and occurs in avian species belonging to other orders such as quail (Nikolakopoulou et al., 2006; Balthazart et al., 2010) or doves (Cao et al., 2002; Ling et al., 1997).
This interest for adult telencephalic neurogenesis in birds has to a large extent shifted attention away from the mechanisms by which the avian brain develops. Substantial interest has been devoted to the relative growth of the telencephalon in different avian groups (Charvet and Striedter, 2008, 2009a,b; Striedter and Charvet, 2008) but the ontogeny of the diencephalic brain nuclei that control reproductive behavior has not to this date been analyzed in much detail.
Birds have been broadly used for investigating the endocrine controls of sexual behavior. The Japanese quail (Coturnix japonica) in particular has been widely used for dissecting hormonal mechanisms mediating the activation and sexual differentiation of behavior (Adkins, 1978; Adkins-Regan, 1983; Ball and Balthazart, 2011; Balthazart and Ball, 1998). In male quail, copulatory behavior disappears following castration but is restored within a few days by a treatment with exogenous testosterone (Adkins, 1978; Balthazart et al., 1983).
The neural sites mediating the activation of male sexual behavior have been extensively characterized in quail. Lesion studies and experiments based on the stereotaxic implantation of steroids clearly demonstrated that testosterone action in the medial preoptic nucleus (POM) is necessary and sufficient to activate both appetitive and consummatory aspects of this behavior in a castrated male presented with a sexually receptive female (Balthazart and Ball, 2007; Panzica et al., 1996). The effects of testosterone on a wide range of behaviors are mediated through its aromatization in the POM (Balthazart et al., 2004; Balthazart and Ball, 2007). These behavioral effects of testosterone are correlated with a number of testosterone-induced neuroanatomical, neuroendocrine and neurochemical changes (increase in POM volume and in the size of its neurons, increase of aromatase transcription and of the density of vasotocinergic inputs, changes in dopamine release; see for reviews: Balthazart et al., 2004; Balthazart and Ball, 2007; Balthazart et al., 2009; Panzica et al., 1996).
Copulatory behavior (Adkins, 1975; Balthazart et al., 1983) and many of the testosterone-sensitive features of the preoptic area are sexually differentiated in adult quail (e.g., POM volume: Viglietti-Panzica et al., 1986; size of its neurons: Panzica et al., 1987; aromatase activity: Schumacher and Balthazart, 1986; vasotocinergic innervation: Panzica et al., 1998), but how and when they differentiate remains poorly understood (see Balthazart et al., 1996 for discussion). It is however established that the differentiated behavioral response to testosterone is organized by ovarian estrogens during a critical period of the embryonic life ending on day 12 of incubation (Adkins, 1978; Balthazart and Adkins-Regan, 2002; Schumacher et al., 1989).
It should also be noted that even if testosterone action is necessary and suffIcient to activate male sexual behavior, this activation involves multiple other brain areas directly or indirectly connected to POM such as the bed nucleus of the stria terminalis, the periaqueductal central gray or the ventromedial nucleus of the hypothalamus (Balthazart and Ball, 2007; Ball and Balthazart, 2011).
Despite extensive research into the factors required for expression of sexual behaviors in adulthood, the development during ontogeny of the diencephalic regions underlying these behaviors has not yet been investigated (see Balthazart et al., 1996 for discussion). More generally, there is a lack of understanding in quail and other avian species of the mechanisms underlying brain development and in particular ontogeny of the brain regions such as the preoptic area and hypothalamus that control motivated behaviors. The overall goal of the present research was therefore to produce a general description of the ontogeny of this brain region in an avian species largely used in behavioral studies. In particular, we were interested in determining when cell (neuronal) proliferation takes place in various nuclei that control behavior, what is the birth date of preoptic neurons and whether any of the processes underlying the development of these nuclei is sexually differentiated.
To this end, quail eggs were injected with 5-bromo-2-deoxyuridine (BrdU) at different embryonic (E) stages between E3 and E16 and BrdU-labeled cells were quantified after hatching (at postnatal day 3, PN3) or in adulthood (PN56). We show here that neurons are already post-mitotic in the quail POM on E6 and are thus probably born before the establishment of sexually differentiated secretions of sex steroids by the gonads. An active neurogenesis persists however at later ages in other brain areas such as the telencephalon and cerebellum. In the POM, decreasing numbers of BrdU-positive cells are produced between E6 and E14 but analysis by double-label immunocytochemistry of their cellular phenotype indicates that these cells are slow cycling progenitors that express PCNA, a cell cycling marker. Their functional significance is unknown at this date but they may contribute to the plasticity of the POM observed later in life.
Material and Methods
Experimental animals
All experiments were carried out on Japanese quail (Coturnix japonica) hatched from eggs incubated in the laboratory and obtained from a local breeder in Belgium. All experimental procedures were in agreement with the relevant Belgian laws on the “Protection and Welfare of Animals” and on the “Protection of Experimental Animals” and the International Guiding Principles for Biomedical Research involving Animals published by the Council for International Organizations of Medical Sciences. The protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Throughout their life in the laboratory, birds were maintained on a photoperiod simulating long summer days (16L:8D) and had access to food and water ad libitum. During the first three weeks of life, chicks were kept in brooders at 30°C in heterosexual groups. Temperature was then progressively lowered to room temperature and around 5 weeks of age, all birds were transferred to individual cages.
Eggs were incubated, pointed end down, at 39°C, 50–60% relative humidity in a forced-air incubator on trays that were rotated by 90° twice a day. On embryonic day 15 (E15; the day when eggs were set in the incubator was counted as E0), eggs were transferred for hatching in separate wire mesh boxes that were placed at the bottom of the incubator and no longer rotated until hatching.
Brain histogenesis and BrdU labeling during the critical period of sexual differentiation
Eggs were injected with the thymidine analog 5-bromo-2’-deoxyuridine (BrdU, Fluka 16880; Sigma Aldricht, Seelze, Germany) to label cells during the S phase of cell cycle preceding mitosis (Miller and Nowakowski, 1988). In a first series, a total of 200 eggs were injected at E8, E10, E12, E14 or E16 (40 eggs/ group). To confirm the exact developmental stages, a few embryos from each group were killed and staged according to Hamburger and Hamilton before injection of BrdU to the remaining embryos (Hamburger and Hamilton, 1951). The age at which BrdU was injected had no detectable impact on the hatching success of the eggs. The percentage of eggs hatching in each experimental group ranged between 45.0 and 57.5 % (E8: 23/40; E10: 21/40; E12: 22/40; E14: 20/40; E16: 18/40), which is typical for our incubation and breeding conditions. These hatching rates suggest that, at the dose used here, BrdU has no major toxic effect on embryonic development; otherwise a differential impact would be expected as a function of the developmental stages.
For injections, eggs were cleaned with alcohol, the shell at the small end of the egg (opposite to the air chamber) was pierced with a needle previously sterilized in a flame, and 200 µg of BrdU dissolved in 20 µl sterile 0.9% NaCl was injected with an automatic pipette fitted with autoclaved thin tips inserted approximately 5 mm into the albumen. The hole in the shell was sealed with melted paraffin and immediately returned to the incubator until hatching.
Birds were allowed to survive until post-natal day 56 (PN56), after they reached sexual maturity. The body mass of each bird was recorded once a week to confirm the normal growth of the experimental subjects.
At PN56, all birds were killed by decapitation and their brain was quickly removed from the skull. Brains were placed in the aldehyde fixative acrolein (5% in Phosphate-buffer 0.01 M-saline 0.9% [PBS], pH 7.2) for 2.5 hours, washed twice in PBS (30 min) and cryoprotected in 30% sucrose for 24 h at 4°C. The brains were then frozen on dry ice and stored at −80 °C until used. A total of 86 brains were successfully collected that were more or less evenly distributed between males (M) and females (F) and between the 5 experimental groups (E8: 6F, 10M; E10: 10F, 8M; E12: 8F, 11M; E14: 8F, 10M; E16: 10F, 5M).
In order to produce additional brains that could be used to study the phenotype of cells that had experienced their last DNA replication on the last day of the period of sexual differentiation, an additional small batch of eggs was later injected with BrdU on E12 as explained before. This produced another group of 8 males and 8 females that were killed at PN56. Like previously explained, brains were immediately collected, frozen, and stored at −80°C until analyzed.
BrdU labeling before E8
Because BrdU injections between E8 and E16 failed to label neurons in the preoptic area and hypothalamus, it was speculated that all neurons in these brain areas become post-mitotic at an early stage of development. To test this idea, BrdU was injected as described above to batches of Japanese quail eggs at embryonic days 3, 4, 5, or 6 (E3 to E6). Chicks were then killed by decapitation on post-natal day 3 (PN3), brains were quickly removed from the skull, fixed with acrolein and processed as described for experiment 1.
Brain were collected here soon after hatching rather than in adulthood to optimize the detection of neurons labeled by BrdU since it is known that a substantial fraction of the neurons formed during early ontogeny die during the brain maturation. The purpose of this experiment was no longer to detect the potential origin of a sexually differentiated neuronal population in the adult preoptic area but, given the negative results collected previously, to determine the birth date of neurons populating this brain region. This experiment was additionally designed to investigate the ontogeny of aromatase-expressing neurons and therefore to optimize their detection in these young birds (see Bardet et al., 2010), all chicks were injected in the pectoral muscle on PN1 and again on PN2 with 1 mg testosterone propionate (TP; Fluka 86541, Buchs, Switzerland) dissolved in 50 µl sesame oil. The sex of the subjects was determined post mortem by visual inspection of the gonads with the help of a binocular microscope. BrdU injections at these early ages do not affect the hatching success of the chicks. Over several successive experiments performed in the lab with this procedure, no systematic variation in hatching success has been detected (E3: 30/55, 54.6%; E4: 26/50, 32.0%; E533/60, 55.0%; Saline injection: 11/20, 55%).
BrdU labeling in adult quail
To study the potential presence of cell proliferation in the adult brain, we injected BrdU (50mg/kg) every two hours for ten hours in 6 males that were 12 weeks of age. Four subjects were then killed 24h hours later and two subjects were killed 48h later. The brains were fixed with acrolein, rinsed with PBS followed by sucrose, and immediately frozen as described above. Tissue was later sectioned and stained by immunocytochemistry for BrdU (see next section).
BrdU immunocytochemistry
The forebrain of each subject (from the level of the tractus septopallio-mesencephalicus (TSM) to the caudal end of the telencephalon was cut in the coronal plane with a cryostat (Leica, Groot-Bijgaarden, Belgium) at −18°C in 4 series of 30 μm thick sections (3 series only for birds killed at PN3) that were collected in phosphate buffer (PB; 0.1 M, pH7.4). One series of free floating sections for all brains (120 μm between two successive sections in adults, 90 µm in PN3 birds) was stained by immunocytochemistry for BrdU with a rat polyclonal antibody. Aldehydes were first deactivated by a 15 min incubation in 0.1% sodium borohydride in phosphate buffer-saline (PBS) and endogenous peroxidases were then inactivated by a 20 min incubation in 0.6% H2O2 in PBS. Sections were rinsed 3 times with PBS between each step. Sections were treated with 2N HCL for 20 min at 37°C to denature DNA, neutralized in 0.1 M sodium borate buffer at pH 8.5 for 10 min, and rinsed 5 min in PBST (PBS with 0.1% Triton X-100). The non-specific sites were blocked during 30 min in 10% horse serum in PBST and sections were incubated overnight at 4 °C with a monoclonal rat anti-BrdU antibody (AbD Serotec, Dusseldorf, Germany; OBT-0030, 1/2000 in PBST). After 3 washes in PBST, sections were incubated in a biotinylated donkey anti-rat antibody (Jackson Immunoresearch Inc, West grove, PA, USA; 712-065-150, 1/2000 in PBST) for 2 hours and afterwards washed 3 times in PBST. The sections were placed in an avidin-biotin peroxidase solution (ABC Elite kit, diluted 1/50 A and 1/50 B in PBST; Vector laboratories, Burlingame, CA) for 90 min, followed by two consecutive rinses in PBST and one in PBS. The peroxidase activity was finally revealed with 3,3’–diaminobenzidine tetrahydrochloride (DAB, 0.04% in PBS) serving as the chromogen and 0.012% H2O2 for 10 min. After repeated washes in PBS, sections were mounted on microscope slides in a gelatin-based medium and covered with a gelatin phenol aqueous medium.
Phenotypic characterization of BrdU labeled cells
Series of sections coming from the first or second batch of eggs were also double-labeled for BrdU and for various markers of specific neuronal or glial cell lines in order to identify the nature of the cells that had been labeled by BrdU.
One series of sections from one adult female and one adult male in each injection group from the first batch (BrdU injections on E8, E10, E12, E14, E16) was stained for BrdU as previously described and subsequently double stained for aromatase. At the end of the first staining sequence (after BrdU was revealed with DAB), the free-floating sections were washed 3 times in PBS and incubated overnight with a rabbit anti-quail aromatase antibody (1/3000, gift of Dr. N. Harada; see Carere et al., 2007 for antibody preparation and validation).
Another series from one adult female and one adult male in each injection group (E8, E10, E12, E14, E16) was similarly stained for BrdU followed by an overnight incubation with a mouse antibody raised against the Human neuronal protein HuC-HuD (Hu 1/1000, Molecular Probes, A-21271; Invitrogen Molecular Probes, Eugene, Oregon, USA). Sections from embryos injected with BrdU between E3 and E6 (n=4 males at each age except for E3 where n=2 males) were also double labeled for BrdU and the neuronal marker Hu as described above.
Additional characterization subsequently focused on the cell populations labeled by BrdU on E12 with brains collected on PN56. Representative sections from these birds were first stained for BrdU as described above. At the end of the first staining sequence (after BrdU was revealed with DAB), the free-floating sections were washed 3 times in PBS and incubated overnight with one of the following antibodies raised against the pan-neuronal marker HuC-HuD (Monoclonal mouse antibody Hu 1/1000, Molecular Probes, A-21271; staining in 4 birds of each sex), the marker of mature neurons NeuN (monoclonal Mouse antibody against Neuronal Nuclei, Chemicon millipore, Temecula, CA, USA; Clone A60/ Millipore MAB377; 1/500, one male and one female), or the marker of glial cells GFAP (polyclonal rabbit antibody against Glial Fibrillary Acid Protein, 1/500, Dako, Z 0334, Glostrup, Denmark; two males and one female).
Binding of these antibodies was identified with a biotinylated goat anti-rabbit antibody (1/400, Dako E-0432 for aromatase and GFAP) or a biotinylated goat anti-mouse antibody (1/400, Dako, E-0433 for Hu and NeuN). Staining was finally revealed with the avidin/biotin system and the peroxidase substrate Vector SG kit providing a blue label for stained cells (Vector Laboratories PK6100, SK4700). Sections were mounted on slides, dried and covered with Eukitt Mounting Medium (Electron Microscopy Sciences 15320; Sigma Aldricht, Seelze, Germany).
Double-labeling BrdU and PCNA
To determine whether BrdU-ir cells born on E12 could be cycling and dividing in the adult brain, we labeled sections through adult brains (three males and three females killed on PN56 after an injection of BrdU at E12) by fluorescent double immunohistochemistry for BrdU and the Proliferating Cell Nuclear Antigen (PCNA), a marker of cell proliferation. The first step of the protocol that was labeling BrdU is the same as described above except that sections were incubated overnight with the primary antibody diluted 1/200. This increased concentration of primary antibody was used to compensate for the fact that no amplification of signal was used in fluorescence microscopy whereas an amplification step (ABC detection system) was present in the staining procedure with DAB as a chromogen. After 3 washes in PBST, sections were incubated with a goat anti-rat coupled with FITC (Jackson Immunoresearch, 1/200 in PBST) for two hours and washed 3 times in PBST. The sections were rinsed and the non-specific sites were blocked for 30 min in 5% goat serum in PBST. The sections were then incubated overnight at 4°C with the monoclonal mouse NCL-L-PCNA antibody (Novocastra Leica, Newcastle, UK; Clone PC10, 1/500). After 3 washes in PBST, sections were incubated with a goat anti-mouse coupled with horse radish peroxidase (HRP; Dako, 1/500 in PBST) for two hours. An amplification was performed for an hour with the tyramide cy3 system (Invitrogen, 1/25 in amplification buffer/0.0015% H2O2 protected from light from this step until the end). After repeated washes in PBS, sections were mounted on microscope slides in and covered with Vectashield Hard Set Mounting Medium with DAPI (Vector Laboratories).
Antibody characterizations
All primary antibodies used here are listed in the JCN antibody Database V.4 and have therefore been previously tested for specificity in immunohistochemical procedures (see http://onlinelibrary.wiley.com/journal/10.1002/%28ISSN%291096-9861/homepage/jcn_antibody_database.htm). We are presenting below additional information supporting the notion that these antibodies specifically recognize their target protein in quail brain tissue. Key aspects of these characteristics are summarized in table 1.
Table 1.
List of primary antibodies used and their key characteristics.
| Antigen Host Species |
Immunogen | Manufacturer | Dilution used |
|---|---|---|---|
| BrdU Rat monoclonal |
5-Bromo-2-deoxyuridine attached to a single stranded DNA | AbD Serotec (Dusseldorf, Germany) #OBT-0030 |
1: 2,000 |
| Cytochrome P450 Aromatase Rabbit polyclonal |
Recombinant protein E.Coli corresponding to amino acids 249–448 of the quail aromatase sequence | N. Harada, Toyoake Japan Serum QR 2/5 (see Harada 1988b; Foidart et al. 1995) |
1: 3,000 |
| HuC-HuD Mouse monoclonal |
Human HuD peptide (QAQRFRLDNLLN-C)-Keyhole Limpet Hemocyanin (KLH) conjugate | Molecular Probes (Eugene, OR), clone 16A11, No. A-21271 |
1: 1,000 |
| NeuN Mouse monoclonal |
Purified cell nuclei from mouse brain | Chemicon (Temecula, CA), clone A60, No. MAB377 |
1: 500 |
| GFAP Rabbit Polyclonal |
GFAP isolated from cow spinal cord (50 KDa intracytoplasmic filamentous protein) |
DAKO (Glostrup, Denmark), No. Z0334 |
1: 500 |
| PCNA Mouse monoclonal |
36 KDa subunit of the DNA polymerase | Novocastra Leica (Newcastle, UK) Clone PC10 |
1: 200 |
The halogenated nucleotide 5-bromo-2’-deoxyuridine (BrdU) is a thymidine analog that is incorporated into newly synthesized DNA strands during the S-phase of the cell cycle. The anti-BrdU antibody used here (AbD Serotec, OBT-0030) is a rat monoclonal IgG2a antibody that has been broadly used in a variety of species (e.g., mouse: Encinas et al., 2006; rat: Sadgrove et al., 2006; canary: Balthazart et al., 2008; quail: Nikolakopoulou et al., 2006). OBT-0030 recognizes BrdU incorporated into single stranded DNA, attached to a protein carrier and free BrdU. OBT-0030 does not cross react with thymidine but does react weakly with chlorodeoxyuridine (AbD Serotec Specifications). In quail it never stained any structure in subjects that had not been injected with BrdU thus demonstrating that it does not cross-react with endogenous thymidine or any other endogenous component in the quail brain.
The anti-aromatase antibody was prepared by Dr. N. Harada (Fujita Health University, Toyoake, Japan, Antiserum ARO-QR2/05) against a recombinant protein produced in Escherichia coli and corresponding to a 230 amino acid sequence from the quail aromatase gene cloned, sequenced, and described in Harada et al. (Harada et al., 1992). This sequence corresponds to amino acids 219 to 448 in the sequence of the human aromatase (Harada, 1988a) and includes the catalytic site of the enzyme (See Balthazart et al., 2003 for comparison of this sequence with aromatase in other species). The full procedure used to prepare this antibody and validate its specificity has been described (Carere et al., 2007; Foidart et al., 1995). The antibody used here (QR2/05) is part of a set of 7 new antisera (QR1/05 to QR7/05; seven rabbits were injected in parallel with the same antigen) that all identify exactly the same cell populations when used in parallel on adjacent sections (Balthazart J., unpublished data) as a previous batch of antibody prepared in the same way (QR1; see Foidart et al., 1995) which itself identified the same populations as an earlier antibody raised against human placental aromatase (HP1; see Balthazart et al., 1990a; Balthazart et al., 1990b). The specificity of antibodies HP1 and QR1 were confirmed by Ouchterlony double diffusion tests (single precipitation line) and Western blotting (single band at the expected mass of 51 kDa) (Harada, 1988b; Balthazart et al., 1990a; Foidart et al., 1995). When these antibodies are preadsorbed with their respective antigen or with recombinant aromatase sequences from other species (human or mouse), immunostaining is completely abolished (Balthazart et al., 1990a; Foidart et al., 1995). Furthermore, incubation of quail tissue extract with antibody HP1 abolished in a dose-dependent manner aromatase activity measured in these extract by an in vitro product formation assay, which indicates that this antibody actually recognizes the active enzymatic protein (Balthazart et al., 1990a). This specificity is further supported by the identical distribution of the immunoreactive protein as detected by these antibodies (HP1, QR1 and QR2/05) and the distribution of the corresponding messenger RNA as detected by isotopic or non-isotopic in situ hybridization histochemistry procedures (Aste et al., 1998; Voigt et al., 2007).
The pan-neuronal marker HuC-HuD is a RNA-binding protein that is specifically present in post-mitotic neurons both in mammals (Marusich et al., 1994) and birds (Barami et al., 1995; Cao et al., 2002; Wakamatsu and Weston, 1997). It was visualized here with a mouse monoclonal anti HuC-HuD antibody (Molecular Probes, A-21271, originally produced by M. Marusich in the University of Oregon Monoclonal Antibody Facility as mAb 16A11. The antibody was generated against human HuD peptide (QAQRFRLDNLLN-C)-Keyhole Limpet Hemocyanin (KLH) conjugate. The antibody recognizes 39-, 43-, and 49-kDa bands on Western blots of canary brain, similar to what is observed in human tissue. It stains neurons in avian tissue (see for example Barami et al., 1995; Bhattacharyya and Bronner-Fraser, 2008; Wakamatsu and Weston, 1997) and in quail specifically (Nikolakopoulou et al., 2006). Using the same antibody combined with tritiated thymidine labeling of new neurons in the adult songbird brain, Barami and collaborators showed that Hu is not expressed by premitotic precursor cells but appears within hours in their neuronal progenitors even before they embark on parenchymal migration (Barami et al., 1995). Hu is then expressed by cells that have a neuronal morphology and double stain for two other neuronal markers but not in glia both in vivo and in vitro. This conclusion was confirmed by co-localization studies with a variety of other neuronal or glial markers and by ultrastructural analysis. Hu can thus be considered as a pan neuronal marker. In chicks, antibody 16A11 is specific for HuC/D, an antigen specifically expressed by cells undergoing neuronal differentiation (Wakamatsu and Weston, 1997).
The mouse monoclonal NEUronal Nuclei (NeuN) antibody (Chemicon; clone A60) specifically recognizes the DNA-binding, neuron-specific protein, NeuN, which is present in most neuronal cell types of the central and peripheral nervous system of all vertebrates tested so far. The immunohistochemically detectable NeuN protein first appears at developmental time points that correspond to the withdrawal of the neuron from the cell cycle and/or with the initiation of its terminal differentiation (Mullen et al., 1992). The NeuN antibody used here recognizes 2–3 bands in the 46–48 kDa range on Western Blots (manufacturer’s datasheet; Mullen et al., 1992; Lind et al., 2005). The antibody was shown to label neurons specifically in different species including mammals (Goodman et al., 2010), birds (Newman et al., 2010; Vellema et al., 2010) and quail (Stamatakis et al., 2004) specifically. This NeuN antibody was also shown to colocalize with Hu-immunoreactive neurons in a variety of avian and mammalian species (see Velleman et al., 2010). The NeuN protein is present in the nuclei, perikarya and some proximal processes of most neurons in both fetal and adult brains but, some neuronal types are not expressing this protein at any point during development, for example the Purkinje cells or the inferior olivary and dentate nucleus neurons (Mullen et al., 1992; Wolf et al., 1996). Accordingly, no NeuN positive cells can be detected in the structures in the species mentioned above and in particular in the Purkinje cells of song sparrows (Newman et al., 2010) and quail (Stamatakis et al., 2004).
GFAP is an intracytoplasmic filamentous protein that constitutes a portion of the cytoskeleton in astrocytes (Eng et al., 2000) and has proved to be a specific marker for cells of astrocytic origin. The GFAP antibody used here is a polyclonal rabbit antibody (Dako, Z0334) that has been used in multiple studies to identify the cells with an astrocytic phenotype in the central nervous system under normal and pathological conditions, at various ages in different mammalian species (Borregon et al., 1993; Boya and Calvo, 1993; Eng et al., 2000; Reske-Nielsen et al., 1987). The distribution of GFAP-immunopositive structures was described within the central nervous system of the Japanese quail with the antibody used here (Cameron-Curry et al., 1991) and this study showed that GFAP represents a good marker for at least part of the astroglial population in quail (Cameron-Curry et al., 1991) and in other birds (Vellema et al., 2010). The staining pattern observed here with this antibody is similar to previous report in birds and mammals.
Finally, the protein Proliferating Cell Nuclear Antigen (PCNA) is found in the cell nucleus and is a cofactor of DNA polymerase delta. The antibody against PCNA recognizes a 36-kDa subunit of the DNA polymerase that is expressed selectively in proliferating cells. PCNA has been shown to be highly conserved among eukaryotes and was identified in several cell types of human, mouse, hamster and avian origin (Bravo et al., 1987). The antibody labels cells in the pattern one expects from previous studies in a variety of avian species including quail, parakeets and zebrafinch (Charvet and Striedter, 2008; 2009a). In quail specifically, the pattern of anti-PCNA staining coincides with the labeling observed after BrdU injections with short survival times which confirms that the PCNA antibody recognizes its intended antigen (Striedter and Charvet, 2008).
The specificity of all secondary antibodies used in the present studies was additionally confirmed by staining control sections with the procedures described above but with primary antibodies replaced by equivalent volumes of buffer. No signal was ever obtained in these conditions.
Data analysis
The number of BrdU-ir cells present in various brain regions was quantified by one of the authors (SMB) who was blind to the experimental conditions (sex of the birds, age of embryonic BrdU administration) using computer-assisted image analysis. Images were acquired with a video camera attached to an Olympus (Aartselaar, Belgium) BH-2 microscope (20 × objective) connected to a Macintosh computer running the image analysis software Image J 1.36b (NIH, USA; see http://www.rsb.info.nih.gov/ij/).
Digital images were made binary and the manual threshold function was used to adjust contrast individually for each section in order to optimize the discrimination between labeled material and background. Unless otherwise mentioned, the computer then automatically counted the number of labeled objects in the entire digitized camera field (536 × 360 µm or 0.193 mm2; field always placed in landscape position). Exclusion thresholds were set at 20 (low threshold) and 200 (high threshold) pixels to remove from the counts dark objects that did not correspond to the size of a cell nucleus.
BrdU incorporation was originally quantified in 8 different brain areas. Additionally, in a second step, the number of BrdU-immunoreactive (-ir) cells was counted in the preoptic area for the E12 group at 4 rostro-caudal levels defined in a standardized manner based on anatomical landmarks (e.g., edge of ventricles or prominent fiber tracts). In each case, quantification was performed in one single camera field that was placed in a standardized manner (described below) in the brain area of interest. All these areas are schematically illustrated in Figure 1.
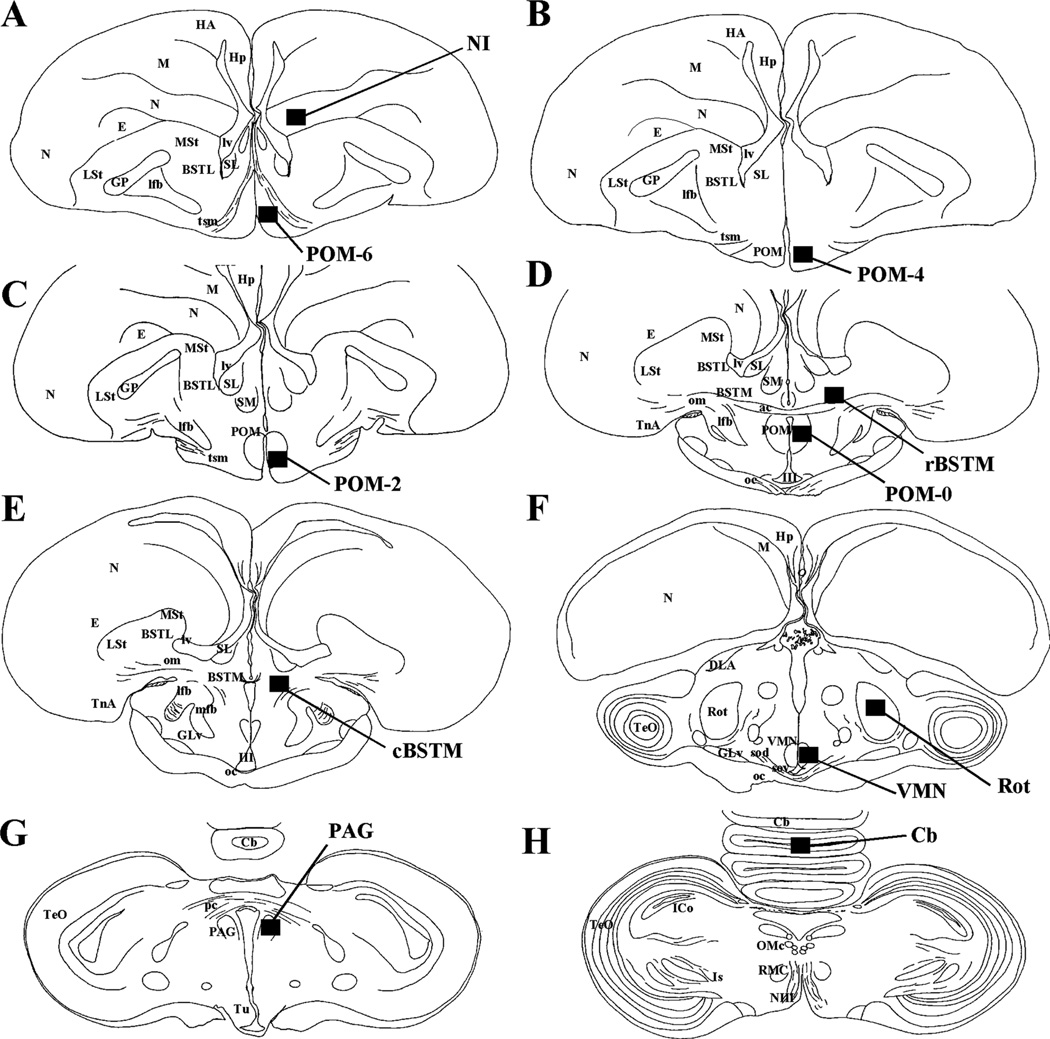
Figure 1.
Schematic drawings of coronal sections through the adult quail brain illustrating the areas where BrdU expression was quantified (dark rectangular areas). Sections are organized in a rostral to caudal order (A to H) and are not always separated by the same interval (see text for detail).
Abbreviations: ac, anterior commissure; BSTL, lateral Bed nucleus of the Stria Terminalis; BSTM, medial Bed nucleus of the Stria Terminalis; Cb, Cerebellum; cBSTM, medial Bed nucleus of the Stria Terminalis, caudal part; DLA, Dorsolateral Anterior nucleus of the Thalamus; E, Entopallium; GLv, Ventral Geniculate nucleus; GP, Globus Pallidus; HA, Hyperpallium Apicale; Hp, Hippocampus; ICo, Intercollicular nucleus of the mesencephalon; Is, Isthmic region; lfb, lateral forebrain bundle; LSt, Lateral Striatum; lv, lateral ventricle; M, Mesopallium; mfb, medial forebrain bundle; MSt, Medial Striatum; N, Nidopallium; NI, Nidopallium Intermedium; NIII, oculomotor nerve; oc, optic chiasma; om, tractus occipitomesencephalicus; OMc, Oculomotor nucleus complex; PAG, Periaqueductal Gray; pc, posterior commissure; POM, Medial Preoptic nucleus; rBSTM, medial Bed nucleus of the Stria Terminalis, rostral part; RMC, Red nucleus, magnocellular part; Rot, nucleus Rotundus; sod, dorsal supraoptic decussation; sov, ventral supraoptic decussation; TeO, Optic Tectum; TnA, nucleus Taeniae of the Amygdala; tsm, tractus septopallio-mesencephalicus; Tu, Hypothalamic Tuberal region; VMN, Hypothalamic Ventromedial nucleus; III, third ventricle.
The number of BrdU-ir cells was counted on one side of the brain (left or right) that was selected in an arbitrary (random) manner (information about lateralization of sections was lost during the free-floating staining procedure). Brain structures were identified based on the atlas of the quail or chicken brain (Baylé et al., 1974; Kuenzel and Masson, 1988; Puelles et al., 2007) and we used the nomenclature adopted by the avian brain forum (Reiner et al., 2004).
BrdU-ir cells were quantified in following areas of the adult brains:
Medial Preoptic Nucleus (POM)
BrdU-ir cells were initially counted for all subjects in the medial preoptic area (mPOA) at its most caudal level where the anterior commissure (ac) reaches its largest extension and crosses the midline. This level corresponds to plate A 8.2 in Kuenzel and Masson (Kuenzel and Masson, 1988) or plate 14 in Puelles et al. (Puelles et al., 2007). The camera field was aligned with the lateral edge of the third ventricle and the ventral edge of the anterior commissure and moved half a field (180 μm) ventrally along the edge of the third ventricle. Quantification was performed at this location that corresponds to the caudal end of the sexually dimorphic medial preoptic nucleus (POM; see Balthazart and Ball, 2007; Panzica et al., 1996) and is called POM-0 in the following.
In a second step, counting was additionally performed at two or three more rostral levels to investigate the distribution of BrdU-ir cells throughout the whole rostro-caudal extension of the POM. These levels are located 240 μm (called POM-2 since one section every 120 µm was present in each series), 480 μm (POM-4) and 720 μm (POM-6) more rostrally. The POM-2 level corresponds to the most rostral appearance of the anterior commissure fibers at the lateral edge of the preoptic area. The counting field was placed along the third ventricle and aligned with the floor of the brain and then moved half a field dorsally (180 μm). The third level (POM-4) was located at the level where the caudal end of the tractus septopallio-mesencephalicus (tsm) is visible in lateral position just ventral to the lateral forebrain bundle (lfb). Here also the counting area was aligned with the floor of the brain and the inter-hemispherical fissure before being moved half a field dorsally (180 μm). Finally at the most rostral level in the mPOA (POM-6) where the tsm forms an inverted V-shape, the counting field was similarly located in a position adjacent to the hemispherical fissure and 180 µm (half a field) dorsal to the floor of the brain.
Bed nucleus of the stria terminalis, medial part (BSTM)
BrdU-ir cells were counted in two different parts of the BSTM. In the rostral BSTM (rBSTM) quantification was performed in an area just dorsal the anterior commissure located in the same brain section as POM-0. The camera field was originally aligned with the dorsal edge of the anterior commissure and the inter-hemispherical fissure. The field was then moved one field (536 μm) laterally along the edge of the anterior commissure and quantification was carried out at this location.
BrdU-ir cells in the caudal BSTM (cBSTM) were counted in the next section immediately caudal (120 µm) to the section containing POM-0. The counting field was placed along the edge of the third ventricle at the level of the commissura pallii and moved half a field laterally to cover the top of one side of the BSTM characteristic V-shape.
Ventromedial Hypothalamic Nucleus (VMN)
BrdU-ir cells were quantified in a section of the brain that corresponds in the chicken brain atlas with plate A 6.4 (VMN, Kuenzel and Masson, 1988) or plate 21 (VMH, Puelles et al., 2007) in the hypothalamus. The field was originally positioned in the angle formed by the third ventricle and the dorsal edge of the dorsal supraoptic decussatio. It was then moved one field (360 μm) dorsally along the ventricle and quantification was performed at this point.
Nucleus Rotundus (Rot)
BrdU-ir cells were counted in the nucleus Rotundus of the thalamus in the same section where counting was performed in VMN. The camera field was placed in the center of the circular area so that it was entirely contained in the nucleus.
Periaqueductal Gray (PAG)
BrdU-ir cells were quantified in the section of the PAG where the posterior commissure reaches its largest extension. This rostro-caudal level corresponds to plate A 4.8 in the chicken brain atlas of Kuenzel and Masson (1988) or plate 27 in Puelles et al. (2007). The counting field was placed at the edge of the third ventricle just ventral to the posterior commissure.
Cerebellum (Cb)
A section of the cerebellum was chosen that corresponds approximately to plate A 3.6 or plate 32 in the two chicken brain atlas (respectively, Kuenzel and Masson, 1988 and Puelles et al., 2007). The camera field was placed so that it was entirely located in a lobule of the cerebellar vermis within the granule cell layer with the white matter located close to the center of the image, leaving the molecular layer outside the field. Only half of the field in the dorso-ventral axis (536 × 180 µm) was quantified: it was positioned entirely in the granule cell layer between the white matter and the molecular layer.
Nidopallium Intermedium (NI)
BrdU-ir cells were quantified in a section of the nidopallium corresponding approximately with plate A 9.0 or plate 12 in the two chicken brain atlas (respectively, Kuenzel and Masson, 1988 and Puelles et al., 2007). The camera field was first placed at the edge of the lateral ventricle near the level of the fimbria of the hippocampus that is slightly ventral to the point where the ventricles are closest to the midline of the brain. The field was then moved half a field laterally to be in the central region of the NI, and quantification was done at this point.
BrdU-ir cells in quail that had been injected with BrdU as adults were quantified in six males at three rostro-caudal levels of the POM (POM-0, POM-2 and POM-4) as described above. Sections from one male and one female stained by double-label immunohistochemical procedures for BrdU and PCNA were photographed with a video camera attached to a Zeiss microsope (20 × objective) connected to a computer running the image analysis software FluoUp. Cells were counted in these photomicrographs in a 449 × 335 µm or 0.150 mm2 field of view at each of the 3 rostro-caudal levels of the POM (Figure 1). The BrdU-PCNA co-localization was confirmed in subset of the cells by confocal microscope with an Olympus Fluoview FV1000 confocal system equipped with an Olympus IX81 inverted microscope (Olympus). In both cases (adult BrdU alone and BrdU + PCNA), cells were counted on both the right and the left side at each level of the POM. The means of left and right counts are presented in the results.
Statistical analysis
Numbers of BrdU-ir cells in each area of the adult brains were analyzed two-way analyses of variance (ANOVA), with the sex of the birds and the age of BrdU administration as factors. Other data were analyzed as appropriate by one-way ANOVA or bilateral student t tests. ANOVAs were followed when appropriate by Fisher’s protected least significant difference (PLSD) post-hoc tests. A few selected comparisons were also performed by bilateral student t-tests. Analyses were performed with the SuperAnova software for Macintosh OS9 (Abacus Concepts Inc, Berkeley CA) or Prism 5 software for Mac OS X (GraphPad Software Inc, La Jolla CA).
No correction for double counting was carried out since the size of counted objects (BrdU-ir nuclei, about 3–4 µm) is small relative to the section thickness (30 µm in adults). The Abercrombie correction indicates that in these conditions numbers of positive objects should be inflated by about 10% (N=n *(30/(30+3)) with N= corrected number and n= observed number; (Abercrombie, 1946; Guillery and Herrup, 1997)). Since nuclei size is essentially similar in both sexes, this systematic error has no impact on the conclusions presented in this study. The absolute numbers of BrdU-ir cells is not the focus of the present work, we are only interested in relative differences.
All results are expressed as means ± standard error of the mean (S.E.M.) of the total numbers of BrdU-ir cells in the counting areas. Differences between groups were considered significant for p≤0.05.
Figure preparation
Digital photomicrographs were obtained on a Leica DRMB microscope equipped with a DFC480 Leica digital camera or with a Scion Corporation camera (Model CFW-1612C) attached to an Olympus BH-2 microscope. Fluorescent sections (BrdU and PCNA staining) were photographed with an Olympus Fluoview FV1000 confocal system equipped with an Olympus IX81 inverted microscope (Olympus). Images were processed to homogenize contrast and brightness within a single plate with Adobe Photoshop 7.0.1 or Adobe Photoshop Elements 6. Drawings were created with Canvas 8 or 9 and assembled in Adobe Photoshop.
Results
Brain histogenesis between E8 and E16
BrdU-immunoreactive cells at PN56 after in ovo BrdU injection between E8 and E16
Cells exhibiting BrdU immunoreactivity in all brain areas of adult birds were characterized by a dense nuclear staining with no staining in the cytoplasm so that the outline of the whole cell was not discernable. Examples of photomicrographs of these BrdU-immunoreactive (ir) cells in the POM of male birds injected at each of the 5 embryonic ages are presented in figure 2.
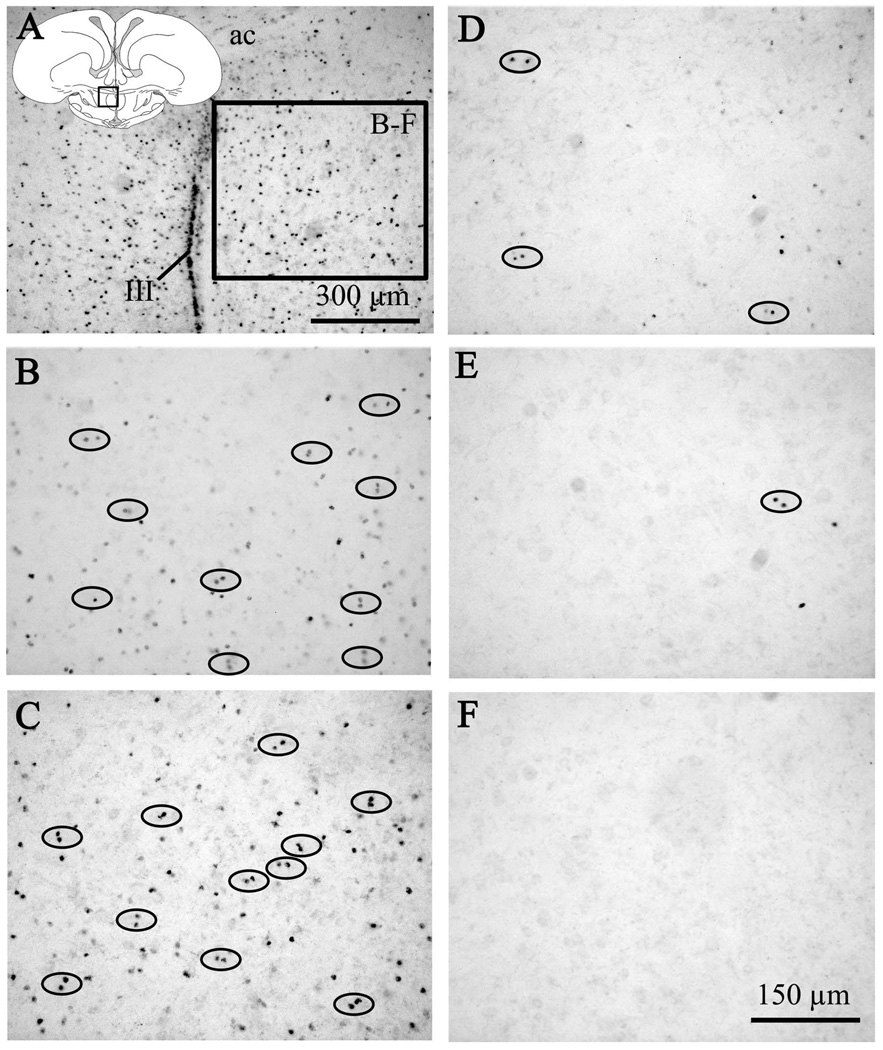
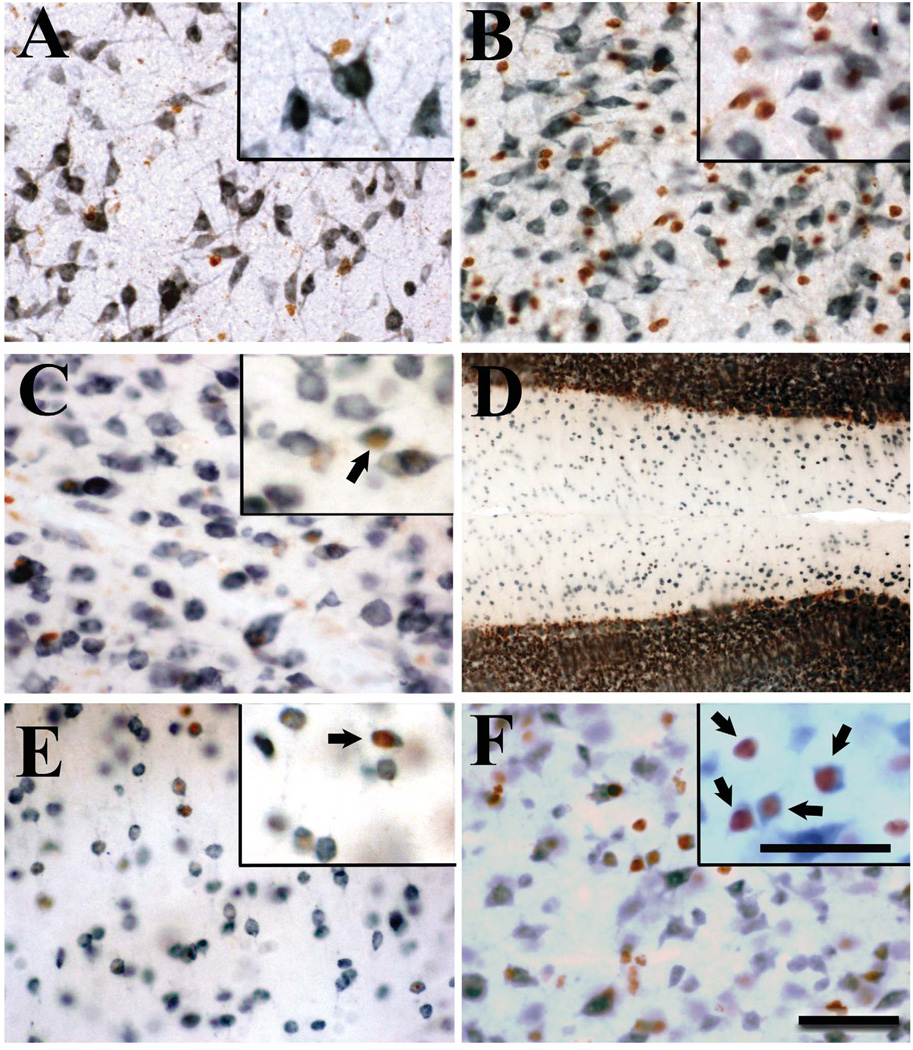
Figure 2.
Photomicrographs from the preoptic region of adult male quail illustrating the differential presence of BrdU-ir cells in birds that had been injected with BrdU on embryonic days 8 (B), 10 (C), 12 (D), 14 (E) or 16 (F). A. Photomicrograph at low magnification of the preoptic area of an adult male quail previously injected with BrdU on E8. The schematic drawing of the quail brain coronal section indicates the area that was photographed. The box labeled “B-F” in panel A indicates the position of the fields shown at higher magnification in the other five panels. B–F. Photomicrographs at higher magnification of BrdU-ir cells in the preoptic area of males injected with BrdU on embryonic days 8 to 16. Note that the large number of positive cells observed in the E8-E10 birds markedly decreased in birds injected on E12 to disappear almost completely in the E14-16 birds. Ovals highlight pairs of BrdU cells (two directly apposed cell nuclei) in the brain parenchyma. The scale bar in F applies to panels B to F. ac: anterior commissure, III: third ventricle.
Following BrdU injections at E8 or E10, large numbers BrdU-ir cells were detected in adulthood in the ventricle walls, but also in the mantle of all brain regions along the rostro-caudal axis between (data not shown). In most brain areas, the number of these immunoreactive cells was intermediate in the E12 groups and extremely low in birds injected on E14 or E16.
Systematic counts indicated that the largest numbers of BrdU-ir cells were observed in the nidopallium and cerebellum of males and females injected on E8-E10 while the lowest numbers were detected in the BSTM (rostral and caudal), thus indicating the presence of differential rates of cell proliferation or survival in different parts of the brain. In birds injected with BrdU on E14 or E16 very few BrdU-ir cells were detected in adulthood except in the cerebellum of E14 birds in agreement with the previously described delayed cellular proliferation in this structure during the late embryonic and early postnatal stages (Stamatakis et al., 2004).
Interestingly, many of the BrdU-ir cells were located in pairs or “doublets”. This is illustrated for the POM in figure 2B–E (doublets surrounded by ovals) but the same cellular organization was found in other brain areas (not shown). Given the larger number of total BrdU-ir cells in birds injected on E8 or E10, the doublets were also more frequent in absolute numbers in birds injected at these ages but they were still clearly present in the E12 and E14 groups.
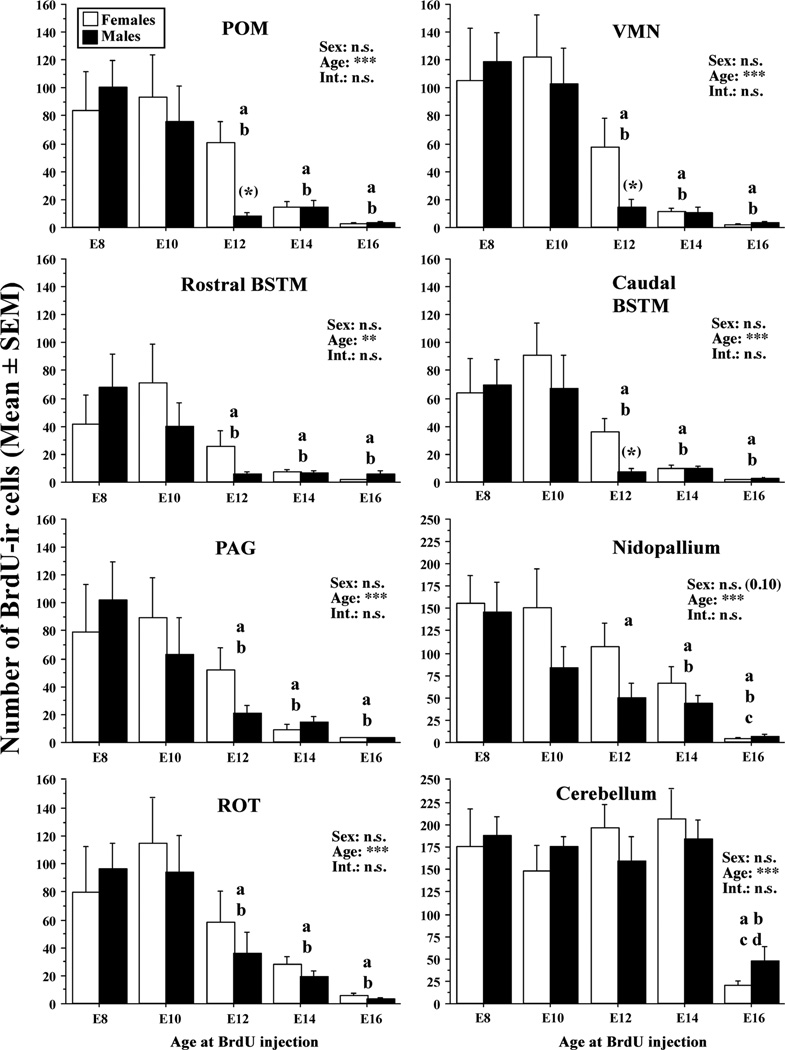
Systematic counts of these BrdU-ir cells were performed in 8 brain regions in adult males and females and these data are summarized in figure 3. Data were analyzed region by region by two-way ANOVAs with the sex of the bird and age at injection as independent factors. Results of these statistical analyses are also indicated in figure 3.
Figure 3.
Quantification of BrdU-ir cells surviving in the adult brain following injections of BrdU at embryonic (E) days 8, 10, 12, 14 or 16 in 8 brain areas illustrated in figure 1 namely the medial preoptic nucleus (POM), hypothalamic ventromedial nucleus (VMN), rostral and caudal parts of the bed nucleus of the stria terminalis (BSTM), periaqueductal gray (PAG), nidopallium, nucleus rotondus (ROT) and cerebellum. Data for each brain area were analyzed by a two-way ANOVA with the sex of the birds and the age at BrdU injection as independent factor (Age). Results of these analysis are summarized in each panel as follows: ***= p<0.001, **= p<0.01, n.s.=not significant (P>0.05), Int: interaction sex by age. Fisher PLSD post hoc tests were used to compare results at different ages and significant differences are indicated by letter above the columns (a=p<0.05 vs. E8, b=p<0.05 vs. E10, c=p<0.05 vs. E12, d=p<0.05 vs. E14). The results of planned t-tests comparing the numbers of BrdU-ir cells in males and females of the E12 groups are indicated in parentheses above the corresponding columns (*= p<0.05). Data are represented by their means ± standard errors of mean (SEM).
A significant effect of the age at BrdU injection on the number of BrdU-ir cells counted in adulthood at PN56 was detected in all areas considered (p<0.0001 in all cases except in the rostral BSTM where p=0.0014). Fisher post-hoc tests showed that in most brain regions the numbers of BrdU-ir cells were lower in the E12, E14 and E16 groups than in E8 and E10 groups (see symbols at the top of corresponding bars in Fig. 3). The cerebellum made an exception to this rule in that the decrease in numbers of BrdU-ir cells was only observed in the E16 group as compared to all others.
No significant overall effect of sex and no interaction of sex with age of injection were detected in the 8 brain regions that had been investigated in detail. However, a qualitative inspection of results in figure 3 indicated that in several brain regions a larger number of BrdU-ir cells was observed in females than in males in the E12 group. This difference was especially prominent in brain areas that are known to be targets of sex steroids such as the POM, BSTM and VMN. Planned t-tests were therefore performed to compare the numbers of BrdU-ir cells in males and females of the E12 groups specifically. These tests suggested the existence of significant sex differences at E12 in the POM (t14= 3.112, p= 0.0076), caudal BSTM (t14= 3.288, p= 0.0054) and VMN (t13= 2.354, p= 0.0650). Differences between E12 males and females in the 5 other nuclei were not significant (p≥ 0.05). Photomicrographs in figure 4A–B illustrate this larger number of BrdU-ir cells in the POM of E12 females as compared to males injected at the same embryonic age.
Figure 4.
Sex difference in the number of BrdU-ir cells present in the medial preoptic nucleus (POM) of adult quail injected with BrdU on embryonic day 12 (E12). A–B. high magnification photomicrographs of the caudal POM of female (A) and male (B) quail injected with BrdU at E12. C. Quantification of BrdU-ir cells at four rostro-caudal levels in the POM of male and female quail injected with BrdU on E12. POM-0 represents the section located at the level of the anterior commissure and POM-2, -4 and -6 correspond to sections located respectively 240, 480 and 720 µm more rostrally. *= p<0.05 compared to females at the same rostro-caudal level. Scale bar in B is equal to 300 µm and also applies to A.
Distribution of BrdU-ir cells in the POM
To obtain more information about the potential sex difference in preoptic BrdU-ir cells born at E12 and its anatomical extension, these cells were counted at 4 different rostro-caudal levels in the POM (6 females and 10 males except at the most rostral level where N= 5 females and 7 males), a key nucleus in the control of male sexual behavior (Fig. 4C). These counts were first analyzed by a two way ANOVA in which the 4 missing data at level POM-6 were replaced by the average of male and female data at this level (thus decreasing Type I error, i.e. the risk of identifying a sex difference that would not exist). This analysis identified the presence of a significance sex difference (F1,14=6.24, p= 0.0255) and of a significant interaction between the sex of the birds and the position in the rostro-caudal axis (F3,42= 4.54, p= 0.0076). There was no overall effect of position (F3,42= 1.61, p=0.2012). A similar analysis focusing on the three most caudal levels only and thus including no replacement data produced similar conclusions (Sex: F1,14= 7.98, p= 0.0135; Position: F2,28= 1.99, p= 0.1545; Interaction: F2.28=5.07, p= 0.0131). T tests performed to identify the origin of the interaction indicated the presence of a significant sex difference (E12 females>E12 males) at the two most caudal levels of the POM (POM-0: t14=2.991, p= 0.0097 and POM-2: t14=3.109 p=0.0077; see Fig. 4C). These differences remain significant even after a Bonferroni correction is applied to take into account the fact that multiple tests (n=4) were performed here (p < 0.0388 and p<0.0308 for POM-0 and POM-2 respectively). At more rostral levels (POM-4 and POM-6) a numerical difference in the same direction was also observed but this difference did not reach statistical significance (POM-4: t14= 1.627, p= 0.1252 and POM-6: t10= 1.328, p=0.2137).
Phenotypic characterization of BrdU-ir cells
We then wondered what is the nature of the BrdU-ir cells found in adult brains after a single injection of BrdU between embryonic days 8 and 16, focusing on cells born during the critical period of sexual differentiation (E8-E12) and particularly on the last batch of cells born on E12, i.e. on the days that marks the end of the sexual differentiation of male-typical copulatory behavior.
Cells born between E8 and E16 do not express aromatase
Because we were particularly interested in the development of the aromatase-expressing cells and their implication in the control of sexual behavior, we first double-labeled one series of sections for BrdU and aromatase in one male and one female injected with BrdU on E12 Somewhat unexpectedly not a single aromatase-immunoreactive (ARO-ir) cell had a BrdU-positive nucleus in all these sections. These results are illustrated in figure 5 for the three main brain areas that express aromatase (POM, BSTM and VMN) in female sections.
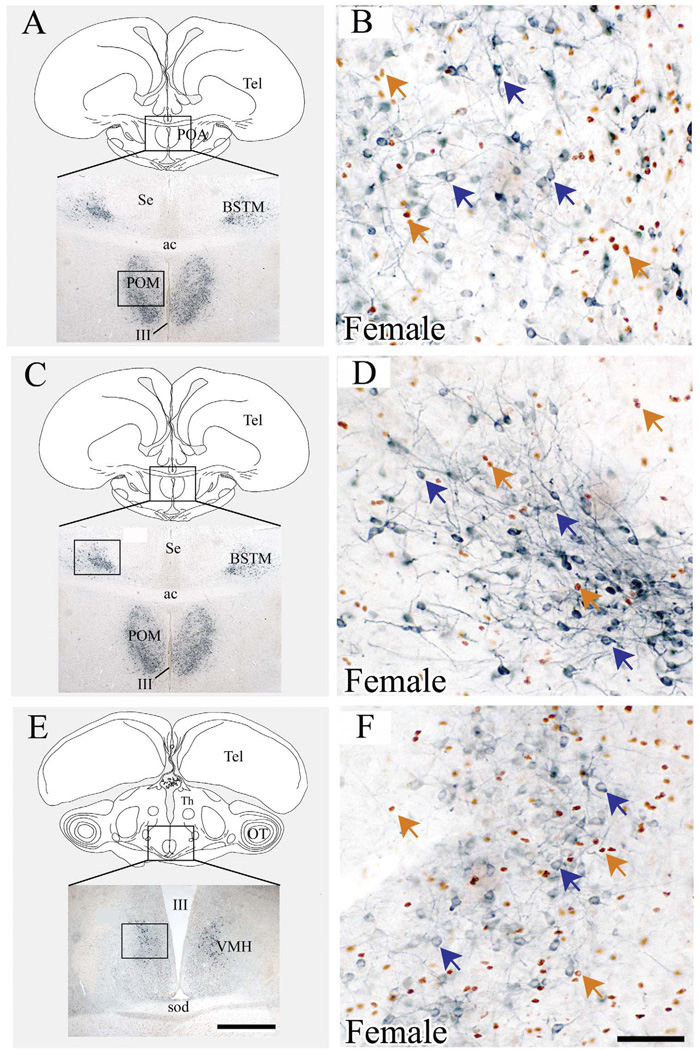
Figure 5.
BrdU-ir cells born on E12 do not express aromatase in the adult brain quail. This figure presents photomicrographs of coronal sections through the POM (A–B), BSTM (C–D) and VMN (E–F) that were double-labeled by immunohistochemistry for BrdU (brown nuclear label) and aromatase (blue cytoplasmic label). A, C and E present a schematic drawing of a coronal section in the quail brain indicating the position of the nucleus of interest (top) and a view at low magnification of a double-labeled section at the level of this nucleus (bottom). A box in the drawing indicates the area illustrated in the photomicrograph. Panels B, D and F present high magnification photomicrographs of double-labeled sections respectively through the POM, BSTM and VMN in the brain of an adult female injected with BrdU on E12. Brown arrows point to BrdU-ir nuclei, blue arrows to ARO-ir perikarya.
Scale bars = 600 µm in E (applies to A, C, E); 75 µm in F (applies to B, D, F). Abbreviations: ac, anterior commissure; BSTM, Bed nucleus of the Stria Terminalis, medial part; III, third ventricle; POA, Preoptic area; POM, Medial preoptic nucleus; Se, Septum; sod, dorsal supraoptic decussation; OT, Optic Tectum; Tel, Telencephalon; Th, Thalamus; VMN, Ventromedial nucleus of the hypothalamus.
Although a large number of BrdU-ir and of ARO-ir cells were visible in these sections attesting that the immunocytochemical staining was working properly, the two labels were never found in the same cells. It should also be noted that the nuclei of BrdU-ir cells (brown arrows in Fig. 5, panels B, D and F) were systematically and markedly smaller than the nuclei of ARO-ir cells that appear unstained in the center of the neuronal perikarya labeled in blue (blue arrows). This clear size difference further indicates that BrdU-ir nuclei belong to a different cell type.
We then wondered whether aromatase-expressing cells were born earlier (during the sensitive period of sexual differentiation between E8 and E12) or later than E12 and to test this idea, one series of sections from one male and one female injected with BrdU at each of the experimental ages (E8 to E16) was double-labeled for BrdU and aromatase. Similar what had been observed in birds injected with BrdU on E12, no double-labeled cell could be detected in either sex throughout the entire age range that was investigated. Large numbers of cells positive for either BrdU or aromatase were again detected at all other embryonic ages investigated except that the number of BrdU-ir nuclei was much smaller in birds injected with BrdU on E14 or E16 (data not shown).
Double-labeling for neuronal markers
We then wondered whether, more generally, these BrdU-ir cells were neurons. To address this question, we first double-labeled one series of sections from one male and one female at each age of BrdU injection (E8-E16) for BrdU and the pan-neuronal marker HuC-HuD. As expected a very broad distribution of Hu-ir cells was observed throughout the brain including the nuclei containing a high density of ARO-ir cells (POM, BST, VMN). However, in the three steroid-sensitive nuclei containing dense populations of ARO-ir neurons, absolutely no colocalization was observed between Hu and BrdU immunoreactivities indicating that these cells born between E8 and E16 are not neurons. This lack of colocalization Hu/BrdU is illustrated for the POM in a male and a female that had been injected with BrdU on E12 (Fig. 6A–B) and in the VMN in a female that had been injected with BrdU on E8 (Fig. 6C). Note that in these pictures also the BrdU-ir nuclei are substantially smaller than the unstained nuclei of the Hu-ir cells as previously mentioned for the sections double-labeled for aromatase and BrdU.
Figure 6.
Photomicrographs of sections from birds injected with BrdU on E8 (B), E10 (E) or E12 (A, C, D, F) and killed on PN56 that were double-labeled for BrdU and for the neuronal markers Hu (A-E) or NeuN (F). No colocalization between BrdU and Hu is observed in the POM (A) and VMN (B). A limited amount of colocalization of BrdU and Hu was detected in n the nidopallium (C) and the molecular layer of the cerebellum (D, E). Similar results are obtained for the colocalization of BrdU and NeuN and illustrated here for the nidopallium (substantial colocalization in F). No obvious sex difference in the degree or absence of colocalization could be detected. Sections A, C and D are from male brains, sections B, E and F come from female brains. Magnification bar = 200 µm in D, 100 µm in B and 50 µm in A, C, E and F. In the higher magnification inserts, the magnification bar shown in F represents 20 µm in A, C, E and F and 40 µm in B and arrows point to double-labeled cells.
In contrast to what was observed in steroid-sensitive nuclei, some colocalization between Hu and BrdU was observed in other brain areas such as the nidopallium (infrequently; Fig. 6D) and the cerebellum (Fig. 6E–F). In this latter structure, colocalization was clearly visible and abundant for cells scattered in the molecular layer where most BrdU positive cells were also Hu-ir (Fig. 6F). Density of cells in the granular layer was too high to allow precise detection of colocalizations (Fig. 6E). These data thus indicate that the double-label immunocytochemical technique we used is able to detect BrdU-Hu colocalizations but that all cells born in POM, BSTM and VMN on between E8 and E16 are not neurons. This notion is also supported by currently ongoing experiments indicating that, if BrdU is injected earlier during incubation (E3-E5), a very large number of BrdU-ir cells are then Hu positive in these nuclei. These data indicated that cells born between E8 and E16 in the steroid-sensitive nuclei of the quail preoptic area and hypothalamus are not neurons, in contrast to more dorsal areas such as the nidopallium or cerebellum where many BrdU positive cells have a neuronal phenotype.
We further tested this conclusion by staining a series of sections coming from three males and three females that had been injected with BrdU on E12 by double immunohistochemistry for BrdU and for NeuN, a neuron-specific nuclear protein in vertebrates (Mullen et al., 1992). Overall, the number of NeuN-ir neurons that were present in these brains was smaller than the number of Hu-ir neurons thus confirming that these two proteins are not expressed in the same cell populations. It is indeed established that NeuN only labels mature neurons whereas Hu is expressed in cells as soon as they begin their neuronal differentiation, even before they have migrated to their final destination (Magavi and Macklis, 2008). As observed with Hu, cells immunoreactive for both NeuN and BrdU were found in the nidopallium (Fig. 8G) but no colocalization between these markers was present in the steroid-sensitive regions that express aromatase such as the POM, BSTM and VMN (see Fig. 6H for BSTM).
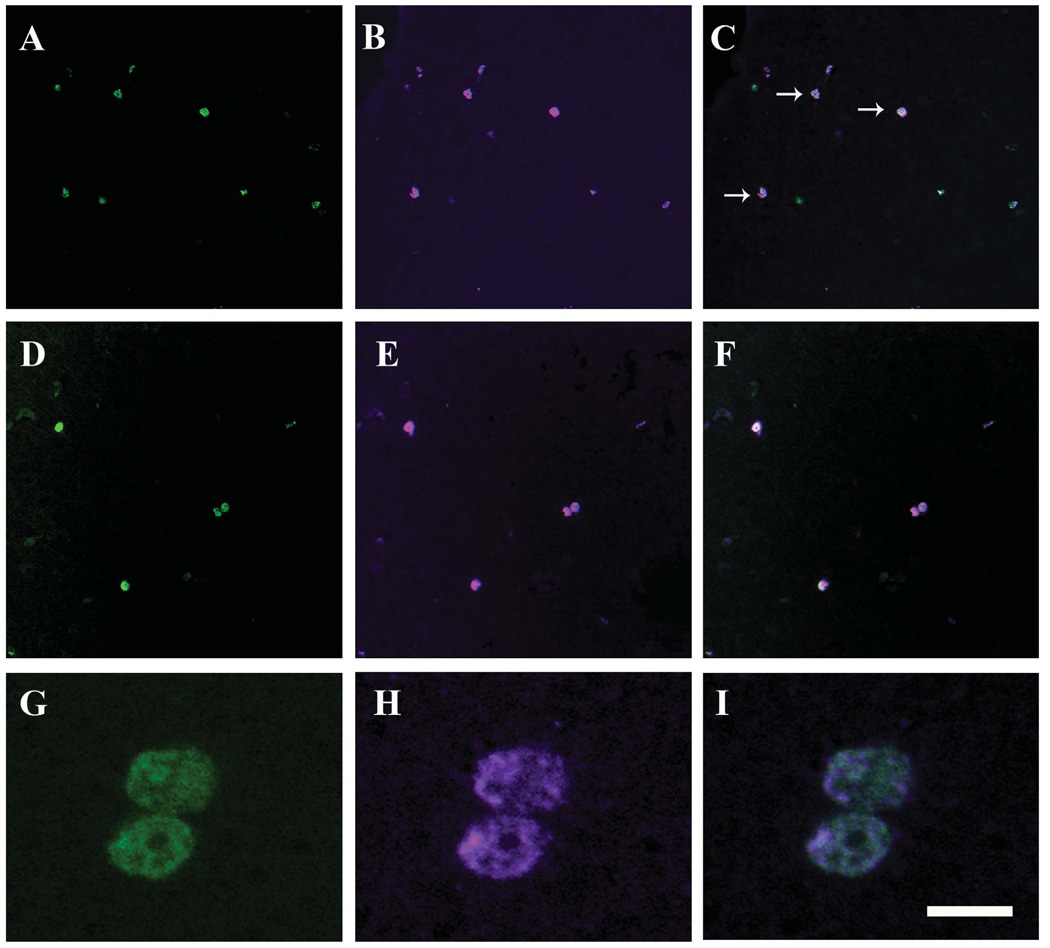
Figure 8.
Photomicrographs of sections through the POM stained by immunofluorescence from bird injected with BrdU at E12 and killed at PN56 that were double-labeled for BrdU (green; panels A,D and G) and the proliferation marker, PCNA (magenta; panels B, E and H). Panels C,F and I present the overlap of both signals. Panels A-C illustrate three BrdU-ir nuclei that are clearly co-localized with PCNA-ir nuclei (arrows), and a few BrdU-ir cells that do not express PCNA. In panel D-F, all BrdU-ir cells are co-localized with PCNA. Panels G-I illustrate at higher magnification a doublet labeled by both BrdU and PCNA. All sections are from a male brain. Magnification bar = 40 µm in A-F, 5 µm in G–I.
Double-labeling for glial and other markers
We next tested whether BrdU cells populating the steroid-sensitive areas of the preoptic area and hypothalamus had a glial phenotype. We therefore stained by double-label immunohistochemistry for BrdU and the glial fibrillary acidic protein (GFAP) sections from three males and three females that had been injected with BrdU on E12 and killed on PN56. GFAP is an intermediate filament protein that is specifically expressed in astrocytes in the central nervous system. In most locations within the brain mantle, GFPA-ir cells appeared as multipolar astrocytes of variable sizes displaying an irregular cell body. Along the ventricle, the GFAP positive cells had the morphology of radial glial cells with a nucleus closed to the ventricle associated with long process perpendicular to the ventricle wall (Fig. 7A–C, E). The density of GFAP-ir cells was extremely variable from one brain area to another and the degree of colocalization with BrdU also varied quite significantly. In the preoptic area hypothalamus, few GFAP-ir cells were present in rostral areas (preoptic area) but their density increased progressively in a rostral to caudal direction.
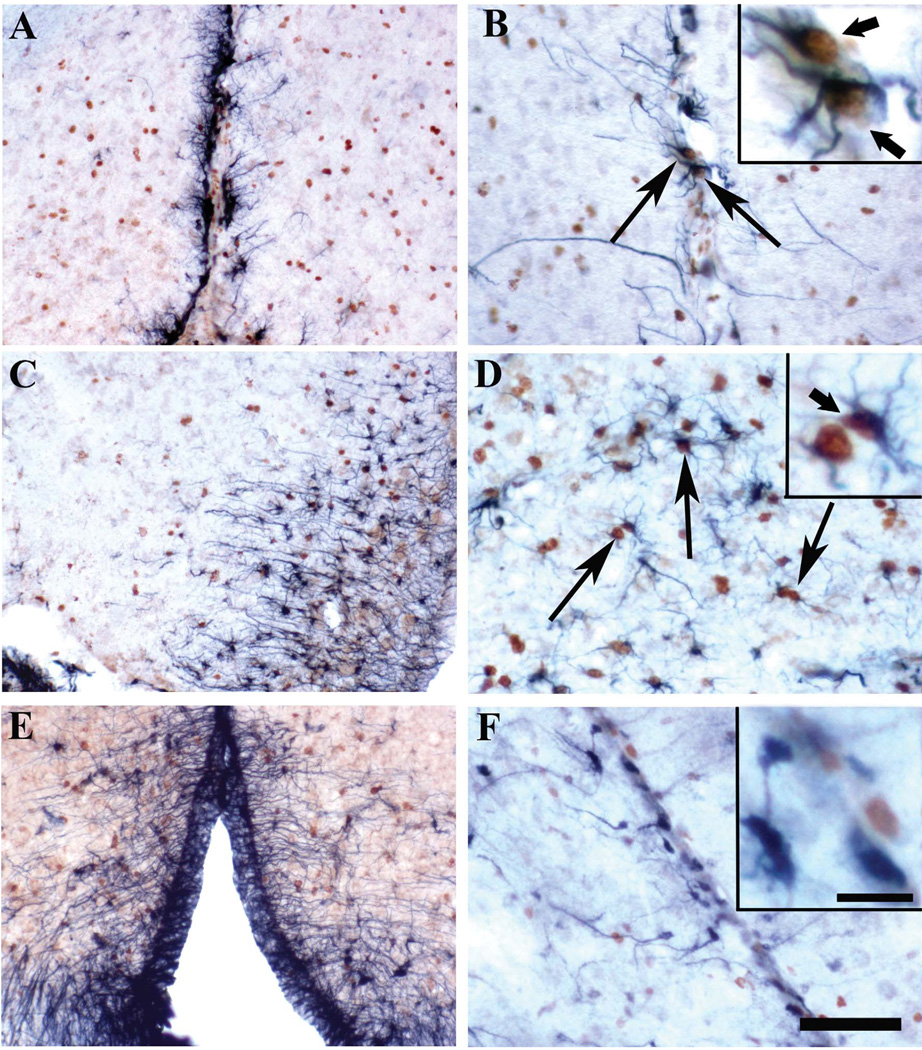
Figure 7.
Photomicrographs of sections from birds injected with BrdU on E12 and killed on PN56 that were double-labeled for BrdU and for the glial markers GFAP (Glial fibrillary acidic protein, A-D), Vimentin (E) or BLBP (Brain Lipid Binding Protein; F). A. Numerous GFAP positive cells and fibers are lining the border of the 3rd ventricle but few of these cells are present in the mantle of the preoptic area where BrdU-ir cells are located. B. At higher magnification, BrdU-ir nuclei can be detected in some of the GFAP-positive cells lining the wall of the 3rd ventricle (arrows). C. GFAP-ir cells extend to the proximal parts of the brain mantle at the level of the VMN where they coexist with BrdU-ir nuclei. D. At higher magnification, BrdU-ir nuclei can be observed in some GFAP-ir cells (arrows). E. As observed with GFAP, Vimentin-ir cells and fibers extend from the third ventricle into the brain mantle at the level of VMN. F. In the quail brain, BLBP-ir cells are observed only at the level of the lateral ventricle and they do not contain BrdU-ir nuclei. Sections B and F are from male brains, other sections come from female brains. Magnification bar = 100 µm in A, C and E, 50 µm in B, D and F. The magnification bar for all higher magnification inserts is shown in panel F and represents 10 µm. In the inserts arrows point to double-labeled cells.
In the POM, most GFAP positive cells were radial cells located along the ventricle wall and the high density of staining prevented us from assessing whether their nucleus was immunoreactive for BrdU (Fig. 7A). A very small number of positive astrocytes was additionally present in the mantle of the preoptic area and most of these cells were not BrdU-ir. Conversely most BrdU-ir cells were not double-labeled with GFAP (Fig. 7B). A similar situation was observed in the BSTM: very few GFAP-ir cells were present in this nucleus and none of them was labeled with BrdU. In contrast, the VMN contained a substantial number of GFAP-ir cells and many of them were BrdU-positive (Fig. 7C–D). In the median eminence of the hypothalamus, we also observed numerous GFAP-ir cells that were quite often labeled by BrdU (over 50%); some of the double-labeled cells were found in doublets.
The lateral ventricle is the only neurogenic zone of the adult brain in birds and progenitors are known to be radial glial cells in both birds and mammals (Alvarez-Buylla et al., 1994; Alvarez-Buylla and Nottebohm, 1988; Goldman and Nottebohm, 1983). Accordingly, we observed a large number of GFAP-positive cells along the lateral ventricles and also along the third ventricle. Within the telencephalon, we did not observe any GFAP positive cells in the nidopallium, but numerous GFAP-ir cells with an astrocytic morphology were present in the hippocampus, area parahippocampalis and hyperpallium apicale and a small fraction of these cells were double-labeled with BrdU. Some of these double-labeled cells were found in doublets.
A similar pattern of staining was obtained with an antibody directed against vimentin. Large numbers of immunoreactive were detected along the lateral ventricles and the third ventricle (see Fig 7E). In these areas, the vimentin-ir cells were actually more numerous than the cells expressing GFAP. Numerous vimentin-ir cells were also present in the hippocampus, area parahippocampalis and hyperpallium apicale as also observed with GFAP. In contrast to the lack of expression of GFAP in the nidopallium, we observed fibers (but no perikarya) expressing vimentin in this region. Very few vimentin-positive cells were detected in the POM. However, as observed with GFAP, an increasing number of vimentin-ir cells were observed along the rostral to caudal axis of the hypothalamus; a small fraction of the vimentin-ir cells were double-labeled with BrdU.
The marker BLBP (Brain Lipid Binding Protein), which is known as a marker of neuronal progenitor cells, was shown previously to also label glial cells in the canary brain (Rousselot et al., 1997). In the quail brain, a more restricted pattern of BLBP expression was detected as compared to the canary brain. Labeled cells in quail were only detected in the hippocampus and along the walls of the lateral ventricles (Fig. 7F). BLBP also labeled the Bergmann glia in the cerebellum and a population of cells migrating in the telencephalon. These results confirm the nature of the neuronal progenitor cells present along the lateral ventricle. However, we did not observe any BLBP-ir cells in other regions such as the nidopallium and the preoptic area-hypothalamus. This label therefore provided no information on the nature of BrdU-ir cells located in the brain mantle both in the hypothalamus and in large parts of the telencephalon.
In summary, these data strongly suggest that, with rare exceptions, BrdU-ir cells are not glial cells in the POM and BSTM since no colocalization was detected with markers such as GFAP, vimentin or BLBP. A fraction of these BrdU-ir cells are GFAP-positive in other brain regions such as the VMN and other caudal hypothalamic regions, and to a lesser extend in parts of the telencephalon (hippocampus, hyperpallium apicale) but not in the nidopallium. However, considering that the glial markers used here obviously label only a fraction of the astrocytes in the quail brain (astrocytes must be present everywhere but react with GFAP and vimentin only in specific areas), it is difficult to assess the exact percentage of BrdU-ir cells born during the period of sexual differentiation that have a glial nature. Furthermore, it is known in mammals that glial cells, such as astrocytes, do not express throughout their life time all their specific markers such as GFAP (Gotz et al., 2002). At any time point, the brain therefore contains glial cells that are not specifically identified and as a consequence the percentage of BrdU-ir cells that have a glial nature is difficult if not impossible to determine.
In addition, very few BrdU-positive cells were organized in a linear fashion in the adult brain as could be expected by cells lining a blood vessel. This arrangement was observed in embryonic brains that had been injected with BrdU on E12 and killed on the next day (Bardet S.M., Mouriec K. and Balthazart, J, unpublished data) but was no longer present in adult brains presumably because endothelial cells do not become post-mitotic and continue to divide though the entire life of the animals thus progressively diluting the BrdU marker.
POM cells born on E12 are slow cycling progenitors
Since cells located in POM that had incorporated BrdU on E12 were apparently not neurons nor glial or endothelial cells, we came by exclusion to the conclusion that these cells were non-differentiated progenitors still capable of dividing but cycling at a very slow pace. This conclusion is supported by various arguments and additional data collected in adult birds.
It was first noted that a very large proportion of BrdU-ir cells were found in pairs (doublets) in the mantle of the brain. This observation suggests that these cells had divided at this location in contrast to neurons that are all supposedly born at the level of the ventricle wall and then migrate to their final location in the brain mantle. To document this feature of POM cells, we quantified the total number of BrdU-ir cells, the number of single BrdU positive cells and the number of BrdU-ir doublets in the POM of 6 adult (PN56) males and females that had been injected with BrdU on E12. This confirmed that the majority of BrdU-ir cells are present in doublets (females: 79.6±1.54%, males: 77.3±1.40 %, means ± SEM). Division of these cells are, however, not frequent since they had incorporated BrdU on E12 and retained the label more than 8 weeks later (at PN56). If they had divided multiple times, the BrdU label would be diluted and presumably undetectable.
To provide additional evidence for the idea that cells labeled by BrdU during embryonic life are actually slow cycling progenitors, sections coming from subjects injected with BrdU on E12 and killed on PN56 (four males and two females) were double-labeled by immunocytochemistry for BrdU and for the proliferation marker (PCNA). Although this staining concerns the intersection of two relatively rare events, we could identify a small but reproducible number of cells labeled with BrdU on E12 that were actually in the process of replicating their DNA and preparing for cell division in adulthood. As could be expected based on the idea of slow cycling, we detected more BrdU-ir cells (labeled on E12) than PCNA-ir cells. Small numbers of PCNA-ir cells were however present in various brain areas including the POM, hypothalamus and telencephalon (see Fig. 8 B, E, H for examples in POM).
Systematic counts of these cells (average number of cells in one microscopic field on the left and right side of POM at three rostro-caudal levels [POM-0, POM-2, POM-4] in one male and one female) indicated the presence of 5.75 ± 1.41 PCNA-ir cells per field covering a large fraction of the POM (mean ± SEM). A small percentage of PCNA-ir cells did not contain immunoreactive BrdU but the vast majority of PCNA-ir cells (72.46±12.5 %) were double labeled as illustrated in Figure 8.
Finally, in order to further confirm the presence of these progenitor cells in the POM mantle, we injected six adult male quail with 50 mg/kg of BrdU every two hours for 10 hours (total of 5 injections) and killed them after 24h (four birds) and 48h (two birds). Sections through these brains were then stained by immunocytochemistry for BrdU.
Classically, the lateral ventricle is the only region of the adult avian brain capable of neurogenesis. As expected, we observed at this level a large number of BrdU-ir cells (Fig. 9A) that had thus replicated their DNA soon after the BrdU injections. Moreover, these cells often appeared in doublets indicating that they had recently divided. Numerous BrdU-ir cells were also observed in the cerebellum as expected, namely in the molecular layer. More surprisingly but in agreement with the presence of slow cycling progenitor cells, we also observed BrdU-ir cells in the mantle of other brain regions such as the POM (Fig. 9B).Systematic counts of these cells at different rostro-caudal levels in POM revealed a more or less even distribution of this sparse cell population that had incorporated BrdU (4.6±1.4 BrdU-ir cells in POM at the level of the anterior commissure, 4.3±1.8 cells in POM-2 and 4.1±1.5 cells at POM-4; means ± SEM). A spot check on two birds killed 48 hours after the BrdU injections suggested that these numbers were stable (3.5±1.0 in the POM, 2.5±1.0 in the POM-2 and 2.0±2.0 in the POM-4; means ± SEM) in agreement with the notion that these progenitors are only cycling at a very low pace.
Figure 9.
Photomicrographs of BrdU-ir nuclei in the brain of 12-week old adult male quail that were injected with BrdU and killed 24h later A. Numerous BrdU-positive cells observed in the lateral ventricle. B. A few BrdU-ir nuclei detected in the POM (arrows point to three doublets). The insert in B shows a BrdU-ir doublet at higher magnification. III= third ventricle, VL= lateral ventricle. Magnification bar = 200 µm in A, and B, 25 µm in the insert of panel B.
Surprisingly, very few BrdU-ir cells were observed along the wall of the third ventricle at the level of the POM (Fig. 9B) again suggesting that the BrdU-ir cells observed in POM had incorporated the tracer and divided in the mantle of the brain rather than along the ventricle. This cellular proliferation in the brain mantle was not limited to the POM. BrdU-ir cells were also detected throughout the hypothalamus including in the VMN and infundibulum and in large fractions of the telencephalon, especially in its dorsal subdivisions including the hippocampus. In all these locations, labeled cells were for the most part present in doublets, again in agreement with the idea that cells divide in the adult brain mantle, in particular in the POM and hypothalamus. In general, the neuroanatomical pattern of BrdU incorporation after injection of the tracer in adults was essentially similar to the pattern of PCNA expression observed.
Earlier BrdU injection label preoptic neuronal cells
Experiments described above indicated that the cells born in the quail preoptic area between E8 and E16 do not express neuronal markers and are in all probability slow cycling progenitor cells. This obviously raised the question of when neurons populating this brain region are born. The most obvious answer to this question was that these neurons are all born before E8 and to investigate this question, we double labeled for BrdU and Hu section from male birds that had been injected with BrdU on E3, 4, 5 or 6 and killed on PN3.
In birds injected on E6, although, we found double-labeled cells (BrdU-ir + Hu-ir) in the telencephalon (data not shown), we did not find any double-labeled cells in steroid-sensitive regions including the preoptic area (Fig 10A). This finding is in contrast to what was observed in birds injected with BrdU on E3 to E5, in which we found large numbers of double-labeled cells in the POM, VMN and BST (see Fig 10B–D for representative photomicrographs in POM). The number of double-labeled cells was very high on E3, markedly decreased on E4 and very low on E5. No Hu-ir cell labeled with BrdU could be detected in birds injected on E6.
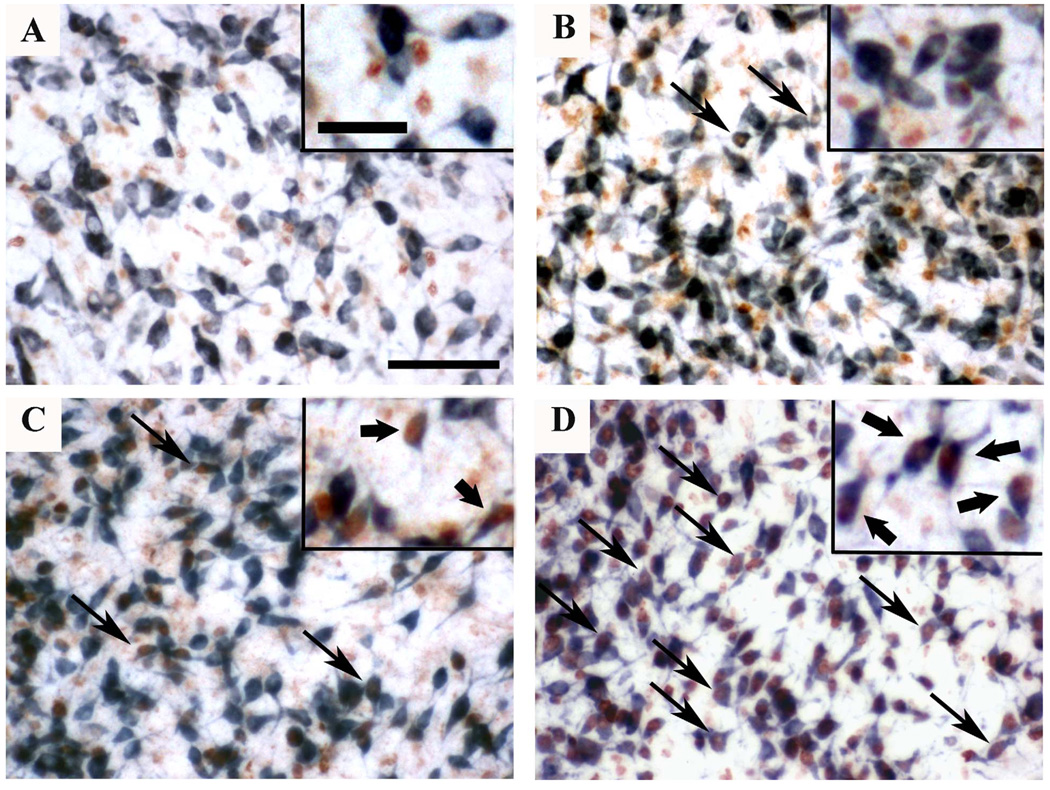
Figure 10.
Photomicrographs of the POM from male birds injected with BrdU on E6 (A), E5 (B), E4 (C) or E3 (D) and killed on PN3 after one injection of testosterone propionate on PN1 and PN2. Sections were double-labeled for BrdU (brown) and for the pan-neuronal marker Hu (blue). No colocalization between BrdU and Hu was observed in the POM when BrdU was injected at E6 (A). A limited amount of colocalization of BrdU and Hu was detected in the POM of birds injected at E5 (B) and E4 (C). In animals injected at E3, most of the cells expressing Hu contain BrdU (D). Magnification bar = 50 µm in the panels and 25 µm in the inserts. Black arrows indicate cells immunoreactive for both BrdU and Hu in the main panels and in the inserts.
These double-labeled cells were counted in the POM of each bird at the level of the anterior commissure. One section was selected for each subject and cells were counted in one microscope field at 40X (0.049 mm2) on both sides of the brain with the help of a camera lucida (drawing made on paper with different symbols for single and double labeled cells). As indicated in Table 2, most BrdU-positive cells in birds injected at E3 had a neuronal phenotype and conversely about 40 % of the neurons present immediately after hatching had been labeled by the BrdU injection on E3. These numbers decreased drastically if the BrdU injection was delayed by one day (E4) and in birds injected on E5 only very few neurons were labeled by BdrU. No double-labeled neurons could be detected following BrdU injections on E6. These findings thus indicate that neuronal proliferation is active on days E3 and E4 in the quail embryos but ceases completely between E5 and E6.
Table 2.
Percentage of colocalization between the neuronal marker Hu and the marker of DNA replication BrdU in male quail injected with BrdU between E3 and E6
| Age at Brdu Injection |
% Hu cells containing BrdU |
% BrdU cells containing Hu |
|---|---|---|
| E3 | 40.7 ± 8.6 | 95,2 ± 1.3 |
| E4 | 5.3 ± 1.8 | 18.2 ± 7.4 |
| E5 | 0.4 ±0.2 | 2.1 ± 1.4 |
| E6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Discussion
This study analyzed in a parametric manner, comparatively in males and females, the histogenesis of the quail brain during the sensitive period of sexual differentiation with a specific attention to steroid-sensitive structures, including the POM that plays a key role in the control of male sexual behavior (Balthazart and Ball, 2007). It is firmly established that in female quail embryos, ovarian estrogens irreversibly demasculinize sexual behavior during a period that extends between the beginning of ovarian steroidogenic activity around days E5-E6 (Guichard et al., 1980; Ottinger et al., 2001; Scheib et al., 1985; Schumacher et al., 1988) and E12, the day that marks the end of the sensitive period (Adkins, 1979; Balthazart et al., 1992; Schumacher et al., 1989). The nature of the cellular and neurochemical estrogen-dependent events that mediate this differentiation of male-typical behavior remains unknown to this date and the present study was therefore carried out in part to research whether a differential histogenesis takes place during this critical period of development and more generally to describe the ontogeny of this functionally important brain region. Following BrdU injections at E8 and E10, large numbers of BrdU-ir cells were detected throughout the brain in adult birds. In most brain areas studied, the number of these cells was intermediate when BrdU was injected at E12 and very low when the injections were done on E14 and E16. For all ages of injection and brain areas investigated, a similar number of BrdU-ir cells was observed in adult males and females with the possible exception that in three steroid-sensitive nuclei, the POM, BSTM and VMN, a numerically larger number of BrdU-ir cells was observed in adult females than in adult males but only in birds that had been injected with BrdU on E12. In POM, this sex difference appeared to be specifically located in the caudal part of the nucleus. Most interestingly, we showed that the BrdU-ir cells which are born at E12 and persist in the adult brain, do not stain for the neuronal nor glial markers that were tested. Data rather indicate that these cells are progenitor cells that retain the capacity until adulthood to divide in the brain mantle but do so at a very slow rate so that many of them still retain at PN56 the BrdU they had incorporated at E12. In contrast, all neurons of the preoptic area appear to be post-mitotic around E5. These observations raise a number of important questions that are discussed in sequence below.
Histogenesis in quail and in previous studies on birds and mammals
One remarkable finding of this study is the overall decrease in cell proliferation that was observed between E10 and E14 in most brain regions that were investigated with the exception of the molecular layer of the cerebellum (and to a lower extent the nidopallium) where this decrease was delayed until after day E14. We had originally anticipated that the embryonic period selected here for study (E8 to E16) would overlap with the end of the neurogenesis in quail at least in the preoptic area so that we could potentially identify, as done in rats previously (Jacobson and Gorski, 1980), a sex difference in the age at which neurons become post-mitotic in the quail preoptic area. To our surprise, we found that, in most brain areas of the adult brain (except in the nidopallium and cerebellum), all cells labeled by BrdU injected between E8 and E16 were not double-labeled with the pan-neuronal marker Hu nor with the marker of mature neurons NeuN, suggesting that these cells are not neurons (see also below for additional discussion on the nature of these cells).
Two interpretations potentially exist for this observation. Either neurons are already post-mitotic by day E8 in most brain areas which explains why new neurons could no longer incorporate BrdU during the S phase of their cycle after E8 or alternatively neurons, contrary to the BrdU-labeled cells identified here, continue to cycle rapidly for some duration after the embryonic period when injections took place so that they dilute the BrdU they had incorporated. Available results strongly favor the first of these interpretations. Most of the neurogenesis is indeed supposed to be completed before hatching in precocial birds such as Japanese quail (Stamatakis et al., 2004; Tsai et al., 1981a; Yurkewicz et al., 1981) and even in altricial birds such as canaries, neurons outside the telencephalon appear to be post-mitotic by embryonic day 12 (Alvarez-Buylla et al., 1994). This interpretation is also supported by the observation that many BrdU-ir neurons (positive for Hu) are detected if quail embryos are injected with BrdU on E3 and 4 but not any more if the tracer is injected on E6. This suggests that indeed neurogenesis in the preoptic area and hypothalamus is completed as early as E5.
Furthermore, if the lack of BrdU in neuronal cells was related to a dilution of the label through additional cell divisions, one should then postulate that these cells remained mitotic for a large number of cycles since BrdU incorporated in a cell remains detectable for only a few divisions. This appears unlikely based on the fact that neurogenesis ends early in the development in birds. It should also be pointed out that, even if some neurogenesis persist throughout life in the telencephalon of adult birds (Alvarez-Buylla et al., 1990; Alvarez-Buylla and Nottebohm, 1988; Goldman and Nottebohm, 1983), the bulk of neurons production takes place in this structure during the early stages of embryonic development (before E7-9 depending on specific location in chicks: Tsai et al., 1981a, before E12 in canaries: Alvarez-Buylla et al., 1994).
Note also that previous work showed that BrdU and thymidine are not rapidly metabolized in young avian embryos and cannot be cleared from the egg by the maternal circulatory system, as it is the case in mammals. The BrdU (or thymidine) injected in one egg thus remains available for incorporation during at least a few days if not for the entire incubation period, depending on the dose that has been injected (LaVail and Cowan, 1971; Tsai et al., 1981a; Yurkewicz et al., 1981). The duration of labeling can be decreased to less than 24 hours by decreasing the amount of BrdU injected (Striedter and Keefer, 2000) but it is clear that given the relatively large amount of BrdU that we injected here in comparison with the pulse-labeling studies described by Striedter and Keefer (200 µg in a quail egg compared to 14–15 µg in a chicken egg) we labeled cells in their S phase during a relatively long period (two days at least) making it impossible that a period of active neurogenesis could have taken place and remained undetected between two of the injections times that were separated by two days. All data thus concur to indicate that neurons in most brain areas in quail (with the exception of the cerebellum, the nidopallium, and probably also other telencephalic regions) are born and post-mitotic before E6. The BrdU-labeled elements observed here that are produced in decreasing numbers between E8 and E14 have therefore clearly of a non-neuronal cell type.
Changes in the numbers of BrdU-ir cells as a function of the injection age were in most brain regions similar in males and females with the notable exception of the three steroid-sensitive regions (POM, BSTM and VMN) where the decrease with age of these numbers seemed to occur more rapidly in males than in females. Planed t test suggested the presence of significant difference between males and females in adult birds that had been injected with BrdU on E12. These tests were, however, performed after overall ANOVAs had failed to identify significant interactions between the sex of the birds and the age at BrdU injection and they were selected ex post facto based on the qualitative inspection of the results, not based on specific pre-existing hypotheses. These sex differences should thus be confirmed by independent experiments before being accepted a truly significant.
Cellular phenotype of BrdU-ir cells born during the period of brain sexual differentiation
As documented in the result section, most of the cells that were labeled here by BrdU between E8 and E16 were not neurons. Two groups of arguments support this conclusion. Firstly, BrdU-ir cells did not colocalize with aromatase (which labels respectively 24 and 41 % of the total number of cells in the medial and lateral POM: Aste et al., 1994) in all aromatase-expressing brain regions but most importantly BrdU immunoreactivity was not colocalized with the pan-neuronal marker Hu nor with the marker of mature neurons NeuN. This conclusion, however, does not apply to the cerebellum that was known before to generate neurons much later in life (Stamatakis et al., 2004) nor to the nidopallium that is the site, like most telencephalic areas, of an active neurogenesis that lasts, contrary to what is observed in mammals, during the entire life in songbirds (Alvarez-Buylla and Nottebohm, 1988; Goldman and Nottebohm, 1983) and in other avian species (Balthazart et al., 2010; Ling et al., 1997). In these two locations, cells double-labeled for BrdU and a neuronal marker were indeed observed therefore confirming that the lack of double-label in the preoptic, hypothalamic and mesencephalic areas is not related to a technical failure in the double-labeling procedure. Furthermore, the same technique double-labels a large number of neurons if BrdU is injected at an earlier age, i.e. on day 3 or 4 of incubation.
Secondly, while it is broadly admitted that in birds like in mammals, most neurons originate from the ventricle wall where precursors of glial nature divide asymmetrically to form a radial glial cell and a young neuron that will subsequently migrate to its final destination during the next 2–3 weeks (Alvarez-Buylla et al., 2002; Ling et al., 1997; Tsai et al., 1981a; b), most BrdU-labeled cells observed here were born in the mantle of the brain (in the tel-, di- and mes-encephalon) at a fairly long distance of the ventricle. Indeed, BrdU labeled cells were already visible in the entire brain parenchyma 3 and 24 hours after the BrdU injection on E12 (Bardet S., Mouriec K. and Balthazart, J., unpublished data) and there was no detectable gradient of labeled cells from the medial periventricular areas to the most lateral aspects of the brain that would be suggestive of a migration of new cells. Most of these labeled cells were also present in doublets at 24 hour post BrdU injection suggesting that they indeed divided in the mantle of the brain rather than at the ventricle walls. If these cells were (migrating) neurons, one could also expect some of them to have a very different morphology with an elongated nucleus associated with a bipolar cell body whereas all BrdU-ir nuclei displayed essentially a round shape. It is thus unlikely that these cells are neurons that migrated from the ventricle because there is no mechanism identified so far that would allow that these cells migrate together in pairs at such a high speed to reach the same final destination. Moreover, double-labeling with glial markers such as GFAP and vimentin failed to identify BrdU-ir cells adjacent to radial glial processes that constitute critical migration guides for the radial migration of neurons. These arguments thus concur to indicate that cells incorporating BrdU on E12 are not neurons and data from the literature as well as more recent work in our laboratory indeed indicates that in the brain stem and hypothalamus (but not in the telencephalon), neurons become postmitotic before E6 in quail (see also before).
We wondered in a second step whether these cells could be glial in nature, which would be in agreement with previous data indicating that glial cells are born later than neurons in the avian (Tsai et al., 1981a) and mammalian brain (Freeman and Doherty, 2006). Testing this idea is technically challenging in birds because many antibodies available to identify glial cells in mammals either do not label any cell in birds or only label subpopulations of glial cells in specific brain regions. For example, in our hands antibodies to the (mostly) astroglial protein S100 or to CD11, a marker of microglia, did not label any cell specifically in the areas of interest in the quail brain. Similarly, Castagna and collaborators reported that immunoreactivity for the S100 protein is essentially absent in the hypothalamus with the exception of the paraventricular nucleus and of some tanycytes bordering the third ventricle (Castagna et al., 2003).
GFAP, a very broadly used marker of astrocytes (see methods), labeled in the quail brain a fair number of radial glial cells along the ventricles, but few GFAP-ir cells were found in the mantle of the POM, although they were more numerous in the VMN. In POM, the few GFAP-ir cells rarely had a BrdU-positive nucleus whereas in the VMN a moderate degree of colocalization could be identified. We did not pursue these analyses because the number of GFAP-positive cells in the parenchyma of the brain is obviously an under-representation of the astrocytes that must be present (see also Cameron-Curry et al., 1991). For some unknown reason, GFAP only labels a fraction of the astrocytic population in quail and therefore cannot be used to assess in a reliable manner their colocalization with BrdU. It must be noted that a similar phenotypical diversity of glial cells has been reported in mammals as a function of the brain region, developmental stage, physiological status and function (Hartfuss et al., 2001; von Bohlen Und Halbach, 2011; Zhang and Barres, 2010). Even if GFAP is considered to be a universal astrocyte marker, it is heterogeneously expressed in different types of astrocytes. For example, GFAP is highly expressed in fibrous astrocytes but not protoplasmic astrocytes (Cahoy et al., 2008). Furthermore, transgenic mice expressing a green fluorescent protein (GFP) under the control of a GFAP promoter display a much larger number of GFP-positive than GFAP-positive cells therefore indicating that GFAP is not stably expressed in all glial cells (Liu et al., 2010). In light of this extensive research demonstrating heterogeneity of the glial lineage in mammals and birds, it appears impossible to completely exclude a glial nature for some BrdU-positive cells.
Despite several attempts we could not identify another glial marker that would stain substantial numbers of cells in the parenchyma of the diencephalon in general and in POM in particular. For example antibodies against vimentin that label in zebra finches (Taeniopygia guttata) reactive astrocytes induced by experimental lesions (Saldanha et al., 2005; Wynne et al., 2008) labeled the present experiment large numbers of (non-BrdU) radial glial cells at the ventricle wall but very few cells in the brain mantle. Available evidence thus suggests that most BrdU-ir cells are not differentiated glial cells. This negative conclusion is, however, not as firmly established as their non-neuronal nature due to limitations concerning antibodies against glial markers in birds so that different glial populations are not recognized in a homogeneous manner by specific antibodies.
Based on morphological criteria (absence of alignment along blood vessels), these BrdU-ir cells also do not appear to be endothelial cells at least in adulthood. Endothelial cells represent at best a minor part of the BrdU-positive cells, except in embryos immediately after injection where lines of positive nuclei are detected along blood vessels (unpublished data). Afterwards endothelial cells continue to cycle and dilute BrdU so that they are no longer detected by immunohistochemistry.
BrdU-ir cells born during the period of brain sexual differentiation in quail are progenitor cells persisting in the adult brain
These negative results lead by exclusion to the notion that the BrdU-ir cells in the adult brain should be progenitor cells that have no differentiated phenotype and cycle very slowly in the mantle of the brain. This conclusion could not be directly supported by double-label studies with specific markers of progenitors because antibodies raised against markers of these cells that we tested (e.g., Sox 2 and nestin, CD133) produced no immunoreactive signal in quail tissue. This conclusion is nevertheless supported by various arguments.
First, the observation of frequent doublets of BrdU-ir cells in the experimental brains strongly suggests that, at the relatively late embryonic ages that were investigated, most cells that accumulated BrdU were actually dividing and thus cycling in the mantle of the brain. When counted in the adult brains, over 80% of the cells that had been labeled by BrdU in the late phases of embryonic development were present in pairs suggesting divisions within the brain mantle. These cells divisions had however to be rare for each cell otherwise their BrdU label would have become undetectable (Doetsch et al., 1999; Seri et al., 2001). Accordingly there was no indication of multiple divisions of these cells as could have been suggested by the presence of groups of 4 or 8 adjacent cells.
Most importantly, we demonstrated by double-label immunocytochemical staining that some of the BrdU-ir cells were positive for the proliferating cell nuclear antigen PCNA in adulthood, a marker of the G1 and S phases of the cell cycle (Kelman, 1997). This PCNA expression suggests that some of the cells labeled by BrdU during embryonic life are cycling at low frequency. The small number of these cells indicates however that they are cycling at a slow rate. This was confirmed by injecting BrdU in adult birds and killing subjects 24h later. This experiment indeed demonstrated the presence of a small number of cells that replicate their DNA and thus proliferate in non-neurogenic zones such as the mantle of the hypothalamus and preoptic area in adult quail.
Nature and function of progenitor cells in the adult preoptic area
The data presented in this paper demonstrate the presence of progenitor cells born on E12 that are still cycling at a low pace in regions of the adult quail brain that are not classically considered as neurogenic. It is broadly accepted since the pioneering studies of Fernando Nottebohm, Steve Goldman and Arturo Alvarez-Buylla that an active neurogenesis takes place in the avian telencephalon at the level of the walls of the lateral ventricle (Alvarez-Buylla et al., 1990; Alvarez-Buylla et al., 1994; Goldman and Nottebohm, 1983). This brain region produces throughout the life of adult birds large numbers of new neurons that are incorporated in functional circuits throughout the dorsal telencephalon and in particular in the song control nucleus HVC of songbirds, but this neurogenesis is classically considered to be missing in the diencephalon, at least in physiological conditions. New neurons were, however, shown to appear in or around the VMN of female ring doves following lesions of this nucleus (Cao et al., 2002; Chen et al., 2006; Ling et al., 1997).
The present demonstration of progenitor cells in the diencephalon fits well with a few recent studies in mammals demonstrating the existence of a low level of cell proliferation and neurogenesis in several brain regions outside of the classical neurogenic areas represented by the subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampal formation (Kemperman, 2006). A low level of neurogenesis has indeed been observed during the last decade in the mammalian hypothalamus (Kokoeva et al., 2007; Migaud et al., 2010; Perez-Martin et al., 2010; Xu et al., 2005; Yuan and Arias-Carrion, 2011) as well as in the mantle of the neocortex and striatum (Dayer et al., 2005) thus confirming the existence (persistence) of progenitor cells in the parenchyma of the brain.
The BrdU-ir nuclei detected here in the quail hypothalamus and preoptic area that belong to mitotically active cells are reminiscent of data recently collected in mammals and primates (Kokoeva et al., 2007; Kornack and Rakic, 2001; Migaud et al., 2010). Several studies have additionally shown that cells labeled in adulthood by BrdU in non-traditional germinal zones of the mammalian brain express neuronal markers (Gould et al., 1999; Perez-Martin et al., 2010). However this is not always the case (Kornack and Rakic, 2001).
The present study identified a cellular proliferation that ends around E12-E14 in the quail preoptic area. This proliferation no longer concerns neuronal cells since neurons become postmitotic at an earlier age (before E5-E6) in this brain region. Between E8 and E12 cell divisions in the preoptic area thus produce new cells with a non differentiated phenotype constituting progenitors embedded in the brain parenchyma that will continue cycling at a very slow pace during post natal development. This observation raises multiple questions concerning namely the final phenotype of these cells born relatively late during the embryonic period, their possible functional significance and the mechanisms that will control their fate.
The fact that this population of cells is born during the end of the period of sexual differentiation of the brain might suggest an implication in the establishment of sexually differentiated brain and behavioral processes. Adult neurogenesis has been show in several cases to be regulated by neurotrophins such as IGF-1 (in the hypothalamus: Perez-Martin et al., 2010) or BDNF (striatum and hypothalamus: Pencea et al., 2001) or by sex steroids (hippocampus: Hajszan et al., 2007; Pawluski et al., 2009; subventrivcular zone and olfactory bulb: Paez-Gonzalez et al., 2011; Veyrac and Bakker, 2011). One could thus speculate that steroids, which are known to modulate activity of some of these neurotrophins (e.g., for BDNF see: Rasika et al., 1999) control the divisions and differentiation of these progenitors in adulthood. The function of these progenitors, however, remains questionable and should be analyzed in future work, first by trying to identify their cellular fate during post-natal life.
Acknowledgments
We thank Jared Woods for help with tissue sectioning and immunohistochemistry. This work was supported by grants from the National Institutes of Mental Health (MH50388), the Belgian FRFC (2.4537.09) and the University of Liège (Fonds Spéciaux 2009) to JB. SMB and KM were supported by postdoctoral incoming fellowships granted by the University of Liège.
References
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Hormonal basis of sexual differentiation in the Japanese quail. J Comp Physiol Psychol. 1975;89:61–71. doi: 10.1037/h0076406. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Sex steroids and the differentiation of avian reproductive behavior. Amer Zool. 1978;18:501–509. [Google Scholar]
- Adkins EK. Effect of embryonic treatment with estradiol or testosterone on sexual differentiation of the quail brain. Neuroendocrinol. 1979;29:178–185. doi: 10.1159/000122920. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Sex steroids and the differentiation and activation of avian reproductive behaviour. In: Balthazart J, Gilles R, editors. Hormones and behaviour in higher vertebrates. 0 ed. Berlin: Springer-Verlag; 1983. pp. 219–228. [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling CY, Yu WS. Contribution of neurons born during embryonic, juvenile, and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song-control nuclei. J Comp Neurol. 1994;347(2):233–248. doi: 10.1002/cne.903470207. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57(6):751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Aste N, Panzica GC, Aimar P, Viglietti-Panzica C, Harada N, Foidart A, Balthazart J. Morphometric studies demonstrate that aromatase-immunoreactive cells are the main target of androgens and estrogens in the quail medial preoptic nucleus. Exp Brain Res. 1994;101:241–252. doi: 10.1007/BF00228744. [DOI] [PubMed] [Google Scholar]
- Aste N, Panzica GC, Viglietti-Panzica C, Harada N, Balthazart J. Distribution and effects of testosterone on aromatase mRNA in the quail forebrain: A non-radioactive in situ hybridization study. J Chem Neuroanat. 1998;14:103–115. doi: 10.1016/s0891-0618(97)10023-0. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2011;51(4):310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. pp. 223–301. [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17(8):1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83(2):247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann Rev Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28(4):161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Boseret G, Konkle AT, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci. 2008;27(4):801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Charlier TD, Barker JM, Yamamura T, Ball GF. Sex steroid-induced neuroplasticity and behavioral activation in birds. Eur J Neurosci. 2010;32(12):2116–2132. doi: 10.1111/j.1460-9568.2010.07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, De Clerck A, Foidart A. Behavioral demasculinization of female quail is induced by estrogens: Studies with the new aromatase inhibitor, R76713. Horm Behav. 1992;26:179–203. doi: 10.1016/0018-506x(92)90041-s. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990a;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: An immunocytochemical study. J Comp Neurol. 1990b;301:276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the Japanese quail: behavior, morphology and intracellular metabolism of testosterone. Gen Comp Endocrinol. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Taziaux M, Holloway K, Ball GF, Cornil CA. Behavioral effects of brain-derived estrogens in birds. Ann N Y Acad Sci. 2009;1163:31–48. doi: 10.1111/j.1749-6632.2008.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28(1):82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- Bardet SM, Cornil CA, Balthazart J. Testosterone recruits new aromatase-immunoreactive cells in neonatal quailbrain. Neuroreprot. 2010;21:376–380. doi: 10.1097/WNR.0b013e3283378edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylé JD, Ramade F, Oliver J. Stereotaxic topography of the brain of the quail. J Physiol (Paris) 1974;68:219–241. [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135(24):4165–4177. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- Borregon A, Boya J, Calvo JL, Lopez-Munoz F. Immunohistochemical study of the pineal glial cells in the postnatal development of the rat pineal gland. J Pineal Res. 1993;14(2):78–83. doi: 10.1111/j.1600-079x.1993.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Boya J, Calvo JL. Immunohistochemical study of the pineal astrocytes in the postnatal development of the cat and dog pineal gland. J Pineal Res. 1993;15(1):13–20. doi: 10.1111/j.1600-079x.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the song control system in adult birds. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge: Cambridge University Press; 2008. pp. 332–349. [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron-Curry P, Aste N, Viglietti-Panzica C, Panzica GC. Immunocytochemical distribution of glial fibrillary acidic protein in the central nervous system of the Japanese quail (Coturnix coturnix japonica) Anat Embryol. 1991;184:571–581. doi: 10.1007/BF00942579. [DOI] [PubMed] [Google Scholar]
- Cao J, Wenberg K, Cheng MF. Lesion induced new neuron incorporation in the adult hypothalamus of the avian brain. Brain Res. 2002;943(1):80–92. doi: 10.1016/s0006-8993(02)02537-4. [DOI] [PubMed] [Google Scholar]
- Carere C, Ball GF, Balthazart J. Sex differences in projections from preoptic area aromatase cells to the periaqueductal gray in Japanese quail. J Comp Neurol. 2007;500:894–907. doi: 10.1002/cne.21210. [DOI] [PubMed] [Google Scholar]
- Castagna C, Viglietti-Panzica C, Panzica GC. Protein S100 immunoreactivity in glial cells and neurons of the Japanese quail brain. J Chem Neuroanat. 2003;25(3):195–212. doi: 10.1016/s0891-0618(03)00009-7. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental species differences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav Evol. 2008;72(4):295–306. doi: 10.1159/000184744. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental origins of mosaic brain evolution: Morphometric analysis of the developing zebra finch brain. J Comp Neurol. 2009a;514(2):203–213. doi: 10.1002/cne.22005. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental basis for telencephalon expansion in waterfowl: enlargement prior to neurogenesis. Proc Biol Sci. 2009b;276:3421–3427. doi: 10.1098/rspb.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Bonder EM, Cheng MF. Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J Neurobiol. 2006;66(6):537–551. doi: 10.1002/neu.20247. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168(3):415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103(21):8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25(9–10):1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29(2):82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kemperman G, Song H. Adult neurogenesis. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2007. [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171(3):769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Gotz M, Hartfuss E, Malatesta P. Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res Bull. 2002;57(6):777–788. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Guichard A, Scheib D, Haffen K, Mignot TM, Cedard L. Comparative study in steroidogenesis by quail and chick embryonic gonads in organ culture. J Steroid Biochem. 1980;12:83–87. doi: 10.1016/0022-4731(80)90254-x. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386(1):2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Harada N. Cloning of a complete cDNA encoding human aromatase: Immunochemical identification and sequence analysis. Biochem Biophys Res Commun. 1988a;156:725–732. doi: 10.1016/s0006-291x(88)80903-3. [DOI] [PubMed] [Google Scholar]
- Harada N. Novel properties of human placental aromaase as cytochrome P-450: purification and characterization of a unique form of aromatase. J Biochem. 1988b;103:106–113. doi: 10.1093/oxfordjournals.jbchem.a122213. [DOI] [PubMed] [Google Scholar]
- Harada N, Yamada K, Foidart A, Balthazart J. Regulation of aromatase cytochrome P-450 (estrogen synthetase) transcripts in the quail brain by testosterone. Mol Brain Res. 1992;15:19–26. doi: 10.1016/0169-328x(92)90146-3. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229(1):15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1980;196:519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14(6):629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- Kemperman G. Stem cells and neuronal development in the adult brain. New York: Oxford University Press; 2011. Adult neurogenesis. [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505(2):209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294(5549):2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, Masson M. A stereotaxic atlas of the brain of the chick (Gallus domesticus) Baltimore: The Johns Hopkins University Press; 1988. pp. 1–166. [Google Scholar]
- LaVail JH, Cowan WM. The development of the chick optic tectum. II Autoradiographic studies. Brain Res. 1971;28:421–441. [PubMed] [Google Scholar]
- Ling CY, Zuo MX, Alvarez-Buylla A, Cheng MF. Neurogenesis in juvenile and adult ring doves. J Comp Neurol. 1997;379:300–312. [PubMed] [Google Scholar]
- Liu Y, Namba T, Liu J, Suzuki R, Shioda S, Seki T. Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus. Neuroscience. 2010;166(1):241–251. doi: 10.1016/j.neuroscience.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Macklis JD. Immunocytochemical analysis of neuronal differentiation. Methods Mol Biol. 2008;438:345–352. doi: 10.1007/978-1-59745-133-8_26. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25(2):143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32(12):2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457(1):44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Newman AE, MacDougall-Shackleton SA, An YS, Kriengwatana B, Soma KK. Corticosterone and dehydroepiandrosterone have opposing effects on adult neuroplasticity in the avian song control system. J Comp Neurol. 2010;518(18):3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- Nikolakopoulou AM, Parpas A, Panagis L, Zikopoulos B, Dermon CR. Early post-hatching sex differences in cell proliferation and survival in the quail telencephalic ventricular zone and intermediate medial mesopallium. Brain Res Bull. 2006;70(2):107–116. doi: 10.1016/j.brainresbull.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: Cyclical anatomical changes in song-control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The discovery of replaceable neurons. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge: Cambridge University Press; 2008. pp. 425–448. [Google Scholar]
- Ottinger MA, Pitts S, Abdelnabi MA. Steroid hormones during embryonic development in Japanese quail: Plasma, gonadal, and adrenal levels. Poult Sci. 2001;80(6):795–799. doi: 10.1093/ps/80.6.795. [DOI] [PubMed] [Google Scholar]
- Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, Garcia-Verdugo JM, Kuo CT. Ank3-Dependent SVZ Niche Assembly Is Required for the Continued Production of New Neurons. Neuron. 2011;71(1):61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemçani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Calcagni M, Anselmetti GC, Schumacher M, Balthazart J. Sexual differentiation and hormonal control of the sexually dimorphic preoptic medial nucleus in quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30(3):343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010;31(9):1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S. The chick brain in stereotaxic coordinates. Amsterdam: Academic Press; 2007. 272 pp. [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22(1):53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Reiner AD, Perkel J, Bruce L, Butler A, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske-Nielsen E, Oster S, Reintoft I. Astrocytes in the prenatal central nervous system. From 5th to 28th week of gestation. An immunohistochemical study on paraffin-embedded material. Acta Pathol Microbiol Immunol Scand A. 1987;95(6):339–346. [PubMed] [Google Scholar]
- Rousselot P, Heintz N, Nottebohm F. Expression of brain lipid binding protein in the brain of the adult canary and its implications for adult neurogenesis. J Comp Neurol. 1997;385(3):415–426. [PubMed] [Google Scholar]
- Sadgrove MP, Laskowski A, Gray WP. Examination of granule layer cell count, cell density, and single-pulse BrdU incorporation in rat organotypic hippocampal slice cultures with respect to culture medium, septotemporal position, and time in vitro. J Comp Neurol. 2006;497(3):397–415. doi: 10.1002/cne.21000. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J Neurobiol. 2005;64(2):192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- Scheib D, Guichard A, Mignot TM, Reyss-Brion M. Early sex differences in hormonal potentialities of gonads from quail embryos with a sex-linked pigmentation marker. An in vitro radioimmunoassay study. Gen Comp Endocrinol. 1985;60:266–272. doi: 10.1016/0016-6480(85)90323-5. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Hendrick JC, Balthazart J. Sexual differentiation in quail: critical period and hormonal specificity. Horm Behav. 1989;23:130–149. doi: 10.1016/0018-506x(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Sulon J, Balthazart J. Changes in serum concentrations of steroids during embryonic and post-hatching development of male and female Japanese quail (Coturnix coturnix japonica) J Endocrinol. 1988;118:127–134. doi: 10.1677/joe.0.1180127. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Barbas H, Dermon CR. Late granule cell genesis in quail cerebellum. J Comp Neurol. 2004;474(2):173–189. doi: 10.1002/cne.20066. [DOI] [PubMed] [Google Scholar]
- Striedter GF, Charvet CJ. Developmental origins of species differences in telencephalon and tectum size: morphometric comparisons between a parakeet (Melopsittacus undulatus) and a quail (Colinus virgianus) J Comp Neurol. 2008;507(5):1663–1675. doi: 10.1002/cne.21640. [DOI] [PubMed] [Google Scholar]
- Striedter GF, Keefer BP. Cell migration and aggregation in the developing telencephalon: pulse-labeling chick embryos with bromodeoxyuridine. J Neurosci. 2000;20(21):8021–8030. doi: 10.1523/JNEUROSCI.20-21-08021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo: I. Neuronal birthdates of telencephalic compartments in situ. J Comp Neurol. 1981a;198(2):275–292. doi: 10.1002/cne.901980207. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo: II. Dynamics of neuronal migration, displacement, and aggregation. J Comp Neurol. 1981b;198(2):293–306. doi: 10.1002/cne.901980208. [DOI] [PubMed] [Google Scholar]
- Vellema M, van der Linden A, Gahr M. Area-specific migration and recruitment of new neurons in the adult songbird brain. J Comp Neurol. 2010;518(9):1442–1459. doi: 10.1002/cne.22281. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Bakker J. Postnatal and adult exposure to estradiol differentially influences adult neurogenesis in the main and accessory olfactory bulb of female mice. FASEB J. 2011;25(3):1048–1057. doi: 10.1096/fj.10-172635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietti-Panzica C, Panzica GC, Fiori MG, Calcagni M, Anselmetti GC, Balthazart J. A sexually dimorphic nucleus in the quail preoptic area. Neurosci Ltrs. 1986;64:129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J Chem Neuroanat. 2007;33(2):75–86. doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Weston JA. Sequential expression and role of Hu RNA-binding proteins during neurogenesis. Development. 1997;124(17):3449–3460. doi: 10.1242/dev.124.17.3449. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44(10):1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56(1):97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192(2):251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Yuan TF, Arias-Carrion O. Adult neurogenesis in the hypothalamus: evidence, functions, and implications. CNS Neurol Disord Drug Targets. 2011;10(4):433–439. doi: 10.2174/187152711795563985. [DOI] [PubMed] [Google Scholar]
- Yurkewicz L, Lauder JM, Marchi M, Giacobini E. 3H-thymidine long survival autoradiography as a method for dating the time of neuronal origin in the chick embryo: the locus coeruleus and cerebellar Purkinje cells. J Comp Neurol. 1981;203:257–267. doi: 10.1002/cne.902030207. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20(5):588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]