Summary
It is indispensable to thoroughly characterize each animal model in order to distinguish between primary and secondary effects of genetic changes. The present study analyzed Nod1 and Nod2 double deficient (Nod1/2 DKO) mice under physiological and inflammatory conditions. Nod1 and Nod2 are members of the Nucleotide-binding domain and Leucine-rich repeat containing Receptor (NLR) family. Several inflammatory disorders, such as Crohn's disease and asthma, are linked to genetic changes in either Nod1 or Nod2. These associations suggest that Nod1 and Nod2 play important roles in regulating the immune system.
Three-month-old wildtype (Wt) and Nod1/2 DKO mice were sacrificed, body and organ weight were determined, and blood was drawn. Except for lower liver weight in Nod1/2 DKO mice, no differences were found in body/organ weight between both strains. Leukocyte count and composition was comparable. No significant changes in analyzed plasma biochemical markers were found. Additionally, intestinal and vascular permeability was determined. Nod1/2 DKO mice show increased susceptibility for intestinal permeability while vascular permeability was not affected. Next we induced septic shock and organ damage by administering LPS+PGN intraperitoneally to Wt and Nod1/2 DKO mice and sacrificed animals after 2 and 24 hours. The systemic inflammatory and metabolic response was comparable between both strains. However, renal response was different as indicated by partly preserved kidney function and tubular epithelial cell damage in Nod1/2 DKO at 24 hours. Remarkably, renal inflammatory mediators Tnfα, KC and Il-10 were significantly increased in Nod1/2 DKO compared with Wt mice at 2 hours.
Systematic analysis of Nod1/2 DKO mice revealed a possible role of Nod1/2 in the development of renal disease during systemic inflammation.
Key words: Innate immune receptors, Kidney, Knockout mouse, Phenotype, Systemic inflammation
Introduction
Nod1 and Nod2 are members of the nucleotide-binding domain and leucine-rich repeat containing receptor (NLR) family, which are intracellular pattern recognition receptors (PRRs) involved in the rapid response against invading bacterial pathogens. High structural homology is found among NLR members, i.e. a C-terminal leucine-rich repeat domain, and a central Nod (also known as NACHT) domain. The N-terminal region of Nod1 and Nod2 is composed of one or two caspase-recruitment domains (CARD) respectively. Different sub-structures of peptidoglycan (PGN), a component of the bacterial cell wall, can signal via Nod1 and Nod2 to induce the production of pro-inflammatory cytokines via NFκB activation (Inohara et al., 1999; Iwanaga et al., 2003; Ogura et al., 2001). Nod1 senses diaminopimelic acid (DAP)-containing PGN, which is found mainly in Gram− bacteria (Chamaillard et al., 2003; Girardin et al., 2003a), while Nod2 senses muramyl dipeptide (MDP) a component of PGN present in Gram− and Gram+ bacteria (Girardin et al., 2003b; Inohara et al., 2003). Expression of both NLRs is found in leukocytes and various tissues including lung, spleen, liver, and kidney (Inohara et al., 1999; Iwanaga et al., 2003; Ogura et al., 2001). Because of their high similarity, it is not surprising that Nod1 and Nod2 contribute in a redundant manner to the immune response following infection (Geddes et al., 2010; Kim et al., 2008; Park et al., 2009).
Several groups have generated and used knockout mice to unravel the role of Nod1 and Nod2 under normal circumstances, in inflammatory disorders and during bacterial infection (Le Bourhis et al., 2007). The analysis of phenotypic abnormalities in these mice already provided some clues to the physiological role of Nod1 or Nod2. Nod1−/− mice have reduced numbers of isolated lymphoid follicles in the distal ileum and colon and additionally the total bacterial flora was expanded 100-fold in the ileum (Bouskra et al., 2008). Recently, Clarke et al. observed that neutrophils from Nod1−/− mice showed defects in basal level of bacterial killing (Clarke et al., 2010). Although Nod2−/− mice displayed macroscopically no abnormalities and no overt symptoms of intestinal inflammation (Barreau et al., 2007; Kobayashi et al., 2005; Petnicki-Ocwieja et al., 2009), abnormal development and function of the Peyer's patches, characterized by an exaggerated immune response and increased intestinal permeability, was observed (Barreau et al., 2007; Barreau et al., 2010). In addition, increased bacterial loads were present in the terminal ileum of Nod2−/− mice (Petnicki-Ocwieja et al., 2009). These results indicate that normal functionality of Nod1 and Nod2 in the intestine is important in maintaining homeostasis between microbiota and the host immune system. Nod1 and Nod2 double knockout (Nod1/2 DKO) have, however, not yet been characterized under physiological conditions.
NLRs are genetically linked to inflammatory disorders. A well known association is the one between Nod2 and Crohn's disease, a chronic idiopathic inflammatory bowel disease. Reduced or lost ability to sense MDP and subsequently activate NFκB has been reported for some Crohn's disease-associated Nod2 mutations (Inohara et al., 2003; Ogura et al., 2003). In addition, several missense mutations of Nod2, resulting in constitutive NFκB activation and enhanced response to MDP, cause Blau syndrome (Miceli-Richard et al., 2001) and early-onset sarcoidosis (Kanazawa et al., 2005), two autosomal dominant disorders characterized by early-onset granulomatous inflammation involving the skin, eyes, and joints. Graft-versus-host disease has also been reported to be more severe in patients bearing certain Nod2 polymorphisms (Penack et al., 2010); however, this could not be confirmed in a Japanese population (Tanabe et al., 2011). Several Nod1 polymorphisms have been associated with susceptibility to asthma (Hysi et al., 2005), atopic eczema (Weidinger et al., 2005), and allergy (Eder et al., 2006). These associations suggest that Nod1 and Nod2 play an important role in regulating the immune system.
The ability of Nod1 or Nod2 agonist to induce an inflammatory response in vivo is under debate. Several studies reported the production of pro-inflammatory cytokines upon administration of PGN, MDP or synthetic Nod1 or Nod2 agonists that was abolished in Nod1 and/or Nod2 deficient mice (Cartwright et al., 2007; Masumoto et al., 2006; Rosenzweig et al., 2008; Werts et al., 2007). However, others have shown that stimulation with Nod1 or Nod2 agonist alone does not induce the production of cytokines, but co-administration of lipopolysaccharide and an agonist for Nod1 or Nod2 enhances TLR-mediated responses (Chedid et al., 1982; Kim et al., 2008; Kobayashi et al., 2005; Murch et al., 2008; Park et al., 2009; Ribi et al., 1979). The reason for these divergent results likely stems from the fact that different cell populations respond to Nod stimulation with different sensitivity thresholds.
In the present study we thoroughly analyzed Nod1/2 DKO mice in order to gain insight into the possible physiological significance of the simultaneous presence of Nod1 and Nod2 under basal conditions. In addition we analyzed systemic inflammation occurring after co-administering LPS and PGN into Wt and Nod1/2 DKO mice and focused on associated acute kidney disease as this is the major cause of mortality during sepsis (Chvojka et al., 2010).
Results
Weight, macroscopy and microscopy of Nod1/2 DKO mice
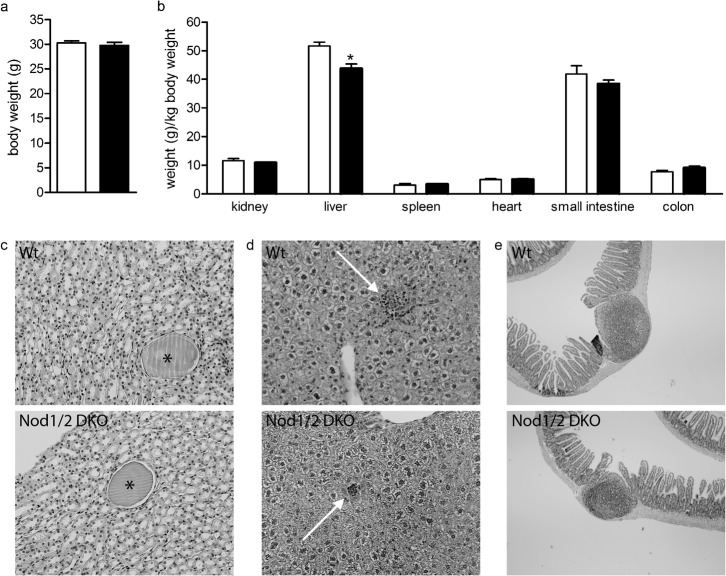
In order to characterize Nod1/2 DKO mice, we analyzed 3-month-old Nod1/2 DKO mice and compared them with Wt mice that were housed under the same circumstances. Body weight of both mouse strains was comparable (Wt: 30.3±0.4 g; Nod1/2 DKO: 29.8±0.6 g) (Fig. 1a).
Fig. 1.
Body (a) and organ (b) weight of Wt (white bars) and Nod1/2 DKO (black bars) mice at the age of 3 months revealed no differences except for liver weight, which was lower in Nod1/2 DKO compared with Wt mice. (c) PasD-stained kidney sections (magnification 20×) of Wt (upper) and Nod1/2 DKO (lower) mice showing small cysts (asterisk). (d) HE-stained liver sections (magnification 20×) of Wt (upper) and Nod1/2 DKO (lower) mice showing small inflammatory infiltrates (white arrow). (e) HE-stained small intestinal sections (magnification 4×) of Wt (upper) and Nod1/2 DKO (lower) mice including Peyer's patches. Data are expressed as mean ± sem, n = 7. *P<0.05 compared with Wt.
Upon sacrifice macroscopic analysis revealed no abnormalities or differences between Wt and Nod1/2 DKO mice. Kidney, spleen, heart, small intestine and colon weight (per body weight) were comparable between both mouse strains (Fig. 1b). Liver weight was significantly lower in Nod1/2 DKO compared to Wt (Wt: 51.7±1.3 g/kg body weight; Nod1/2 DKO: 43.7±1.6 g/kg body weight, P<0.05) (Fig. 1b).
PasD-stained kidney and HE-stained spleen, liver, mesenteric lymph nodes and intestinal (divided roughly into jejunum, duodenum, ileum, and colon) sections were analyzed microscopically. Typical hallmarks of aging were observed in several sections of both Wt and Nod1/2 DKO mice, such as small cysts in the kidney and small foci of inflammatory infiltrates in the liver (Fig. 1c,d). On histological level no differences in phenotype were observed between Nod1/2 DKO and Wt mice. Notably, in the intestines we did not observe a difference in the number of isolated lymphoid follicles or Peyer's patches as has been shown before in Nod1 KO (Bouskra et al., 2008) and Nod2 KO (Barreau et al., 2007; Barreau et al., 2010) mice respectively. In line with our results, Biswas et al. observed similar numbers of Peyer's patches in naive mice between Wt and Nod2 KO (Biswas et al., 2010). Examples of sections of a small intestine from Wt and Nod1/2 DKO mice are depicted in Fig. 1e.
Peripheral leukocyte count and composition of Nod1/2 DKO mice
Total peripheral blood leukocyte number was comparable between Wt and Nod1/2 DKO mice (Fig. 2a). In addition, no differences between the numbers of lymphocytes, granulocytes or monocytes between both strains was observed (Fig. 2a). Although absolute numbers of leukocyte populations were similar, there was a slight but significant lower percentage of granulocytes in Nod1/2 DKO mice while lymphocyte and monocyte percentages were similar between both strains (Fig. 2b).
Fig. 2.
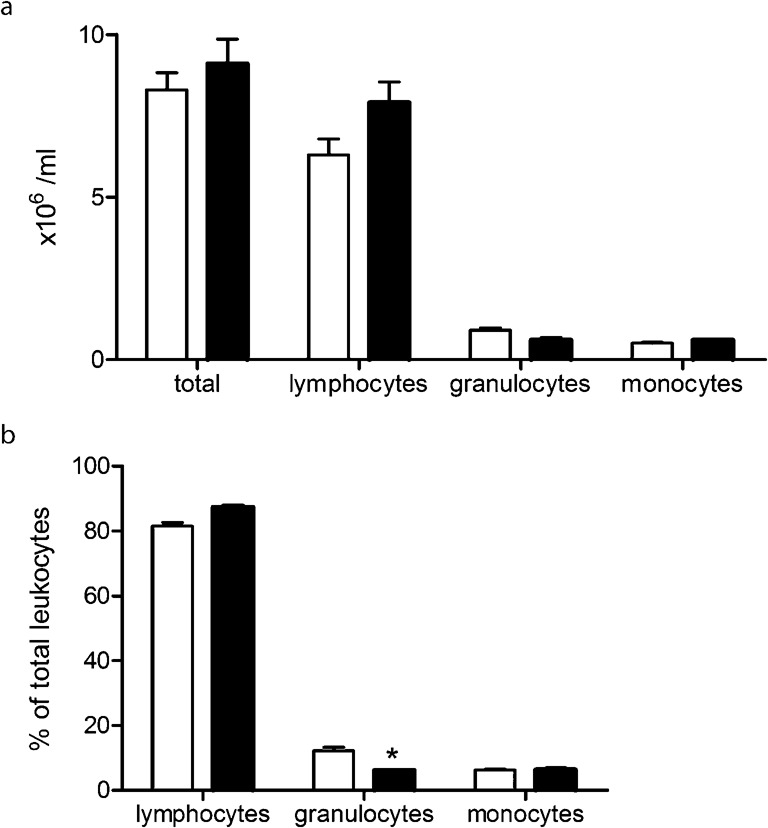
Absolute number of leukocytes (a) and relative numbers of the different leukocyte subsets as a percentage of total leukocyte count (b) in blood of 3-month-old Wt (white bars) and Nod1/2 DKO mice (black bars). No differences between both strains were observed in the total number of leukocytes. Nod1/2 DKO mice had slightly lower percentage of granulocytes as compared with Wt mice. Data are expressed as mean ± sem, n = 7. *P<0.05 compared with Wt.
Plasma biochemical analysis of Nod1/2 DKO mice
Plasma of Wt and Nod1/2 DKO mice was used to determine several biochemical parameters that are predictors of a number of pathological conditions (Table 1). Lactate dehydrogenase (LDH), a marker of general organ damage, was comparable between Wt and Nod1/2 DKO mice. The enzyme ASAT (aspartate aminotransferase), which is found in the liver, heart muscle, kidney, brain and red blood cells, can be elevated in plasma when one of these organs is damaged. No significant differences in plasma ASAT levels were observed between both strains. Levels of plasma ALAT (alanine aminotransferase), an enzyme predominantly found in the liver, are indicative of liver damage. In line with LDH and ASAT, comparable levels of ALAT were present in plasma of Wt and Nod1/2 DKO mice. Elevated alkaline phosphatase is associated with a number of pathological conditions such as liver and bone disease. No difference in plasma alkaline phosphatase was observed between Wt and Nod1/2 DKO mice. Amylase, a marker of pancreatitis, is not significantly different between Wt and Nod1/2 DKO mice. The renal function parameters ureum and creatinine were similar between both strains. Albumin is the most abundant protein in plasma and is important in the maintenance of an oncotic pressure difference between plasma and the interstitial space. Low albumin levels can indicate liver or kidney disease, inflammation, shock, or malnutrition while high albumin levels usually reflect dehydration. In line with the aforementioned damage markers, plasma albumin was comparable between Wt and Nod1/2 DKO mice. Non-fasting plasma glucose and triglyceride levels were not significant different between Wt and Nod1/2 DKO mice. Total cholesterol levels were not detectable in 3 out of 7 Wt and 6 out of 7 Nod1/2 DKO mice.
Table 1. Biochemical analysis of Wt and NOD1/2 DKO plasma.
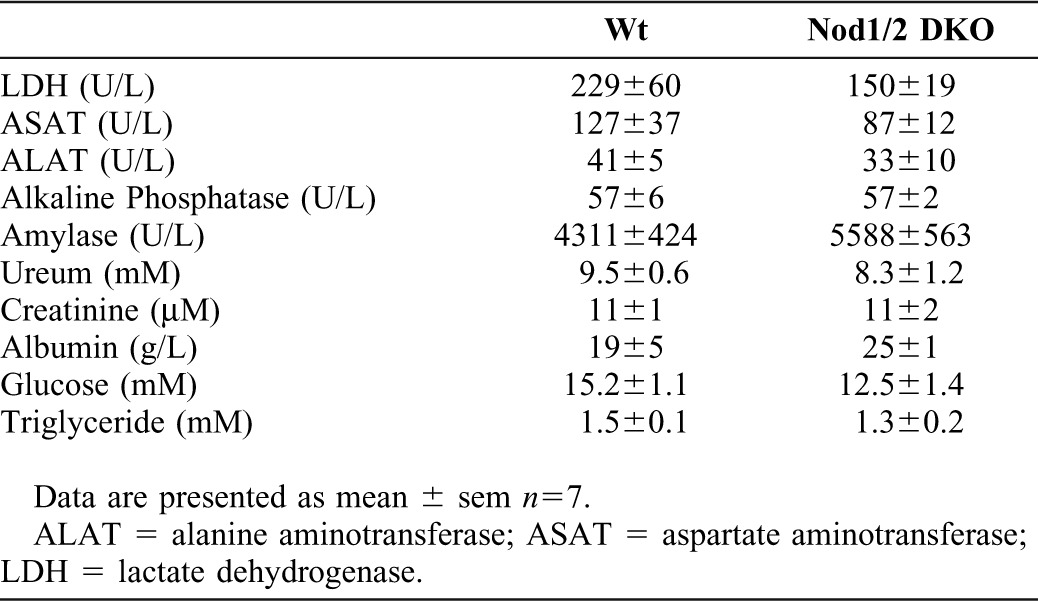
Overall, in plasma no evidence of organ damage due to a deficiency for Nod1/2 was found.
Intestinal and vascular permeability of Nod1/2 DKO mice
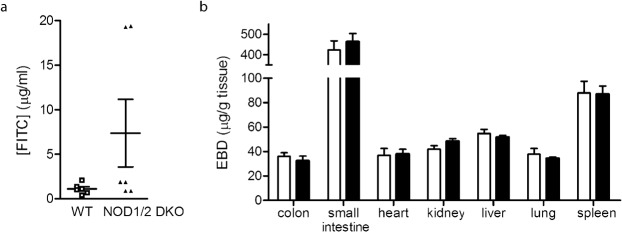
Recently Barreau et al. have shown increased intestinal permeability in mice deficient for Nod2 (Barreau et al., 2010). In Nod1 deficient mice intestinal permeability was not altered under basal conditions (Chen et al., 2008). To investigate whether intestinal permeability is changed in Nod1/2 DKO mice, we determined FITC-dextran in plasma after oral gavage. As FITC-dextran is not actively absorbed by the gut, the amount of fluorescence detected in the serum is a direct measure of intestinal permeability in vivo. Wt mice showed marginal intestinal permeability as indicated by low levels of FITC in plasma (Fig. 3a). Interestingly, 2 out of 6 Nod1/2 DKO mice showed markedly increased intestinal permeability while the rest of the Nod1/2 DKO mice had levels comparable to Wt mice. This might indicate that the Nod1/2 DKO mice are more susceptible to increased intestinal permeability.
Fig. 3.
(a) Intestinal permeability was determined by the concentration of FITC ([FITC]) in plasma of Wt (white bars) and Nod1/2 DKO (black bars) 4 hours after oral administration of 4 kDa dextran-FITC. Low levels of FITC were detected in Wt plasma. Two out of 6 Nod1/2 DKO mice showed markedly increased plasma FITC levels indicating an increased intestinal permeability as compared with Wt mice. (b) Vascular permeability was determined 1 hour after i.v. injection of 20 mg/kg Evan's Blue Dye (EBD). No differences between Wt (white bars) and Nod1/2 DKO (black bars) mice was observed in the extravasation of EBD into various organs. Data are expressed as mean ± sem, n = 6 (a) or n = 9 (b).
In addition we have determined the vascular permeability in several organs by quantifying the amount of Evan's Blue Dye (EBD) per gram tissue 1 hour after intravenous administration. EBD binds to albumin in the plasma and when extravasated is a determinant of protein leakage and hence vascular permeability. All examined organs had comparable levels of EBD between Wt and Nod1/2 DKO mice indicating that vascular permeability was similar (Fig. 3b).
Nod1/2 DKO mice and septic shock
To evaluate the role of Nod1 and Nod2 in septic shock, we subjected Wt and Nod1/2 DKO mice to a model of severe systemic inflammation resulting in multiple organ failure. First we determined whether intraperitoneal administration of PGN was sufficient to cause organ damage in Wt mice. Although plasma LDH levels were increased 2 hours after injection, mice showed no signs of discomfort and did not develop acute kidney injury (AKI) after 24 hours as determined by plasma ureum and creatinine levels (data not shown). We therefore co-administered LPS and PGN to induce systemic inflammation and organ damage in Wt and Nod1/2 DKO mice.
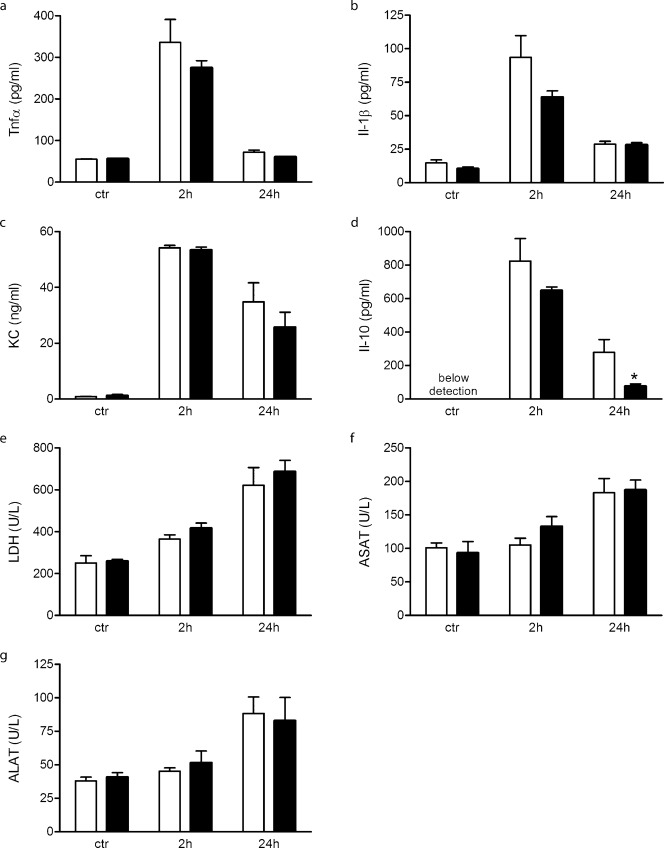
To investigate the systemic inflammatory response, plasma cytokine levels were determined. Two hours after LPS+PGN injection Tnfα, Il-1β, and KC were significantly increased in Wt and Nod1/2 DKO mice and declined after 24 hours, no difference between both strains was observed (Fig. 4a–c). Two hours after LPS+PGN injection comparable Il-10 levels were observed in Wt and Nod1/2 DKO mice, while after 24 hours Nod1/2 DKO had significantly less Il-10 compared with Wt mice (Fig. 4d).
Fig. 4.
Plasma analysis of Wt (white bars) and Nod1/2 DKO (black bars) mice 2 hours and 24 hours after i.p. injection of LPS+PGN or saline (control). Pro-inflammatory cytokines Tnfα (a), Il-1β (b) and KC (c) were significantly increased upon LPS+PGN injection and returned to control levels after 24 hours, no differences between both strains was observed. The anti-inflammatory cytokine Il-10 (d) was not detectable in plasma of control mice; however, it was present 2 and 24 hours after LPS+PGN challenge with a significant lower level Nod1/2 DKO mice at 24 hours as compared with Wt mice. Plasma damage marker LDH (e) was significant increased 2 hours after LPS+PGN injection and increased further at 24 hours. ASAT (f) and ALAT (g), two other organ damage markers, were significantly increased 24 hours upon LPS+PGN administration. All three damage markers were comparable between Wt and Nod1/2 DKO mice at all examined time-points. Data are expressed as mean ± sem, n = 5–8. *P<0.05
General organ damage was determined by plasma LDH. Two and 24 hours after LPS+PGN injection plasma LDH levels were significantly increased in Wt and Nod1/2 DKO mice, no difference between both strains was observed (Fig. 4e). Twenty-four hours following LPS+PGN injection, plasma ASAT and ALAT levels were significantly increased in both strains compared to control, however, no difference between both strains was observed (Fig. 4f,g).
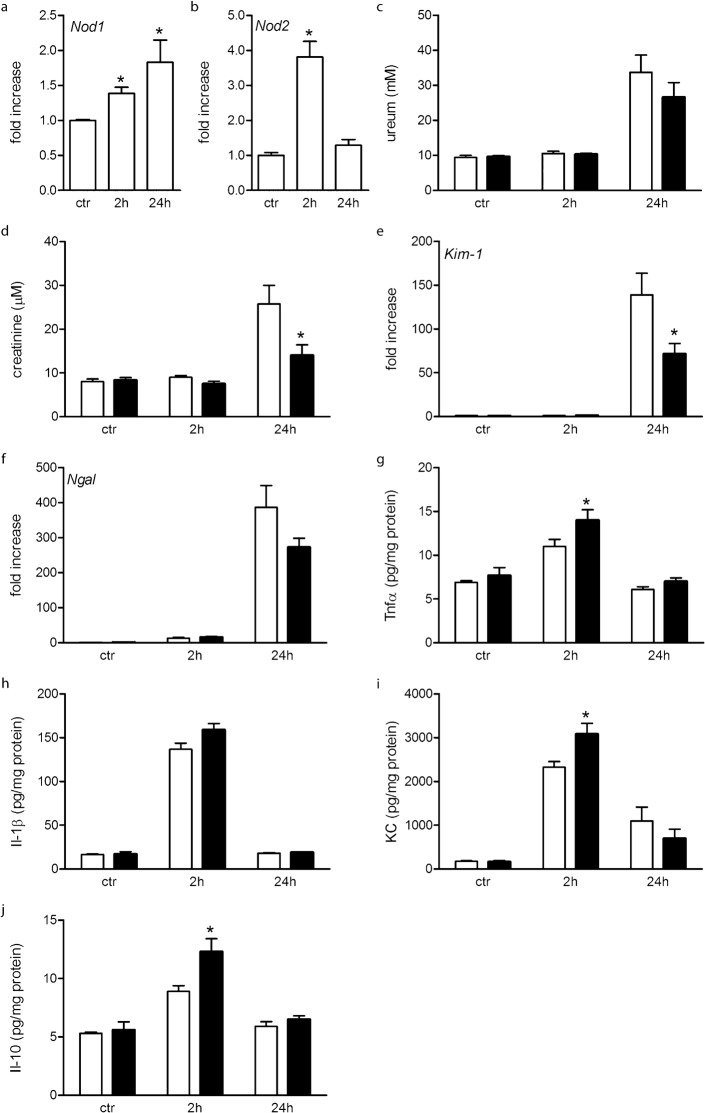
Since AKI is the major cause of mortality in severe sepsis, with a prevalence of 20–50% (reviewed by Chvojka et al., 2010), we investigated the role of Nod1 and Nod2 in sepsis-induced AKI in more detail. First we determined the renal expression of Nod1 and Nod2 2 and 24 hours following LPS+PGN injection. Nod1 mRNA is significantly increased at both examined time-points, while Nod2 mRNA is significantly increased 2 hours following LPS+PGN injection and has returned to control levels at 24 hours (Fig. 5a,b). Next we determined whether renal function was differentially influenced by inducing septic shock in Wt and Nod1/2 DKO mice. Renal function was determined by ureum and creatinine plasma levels. Ureum was significantly increased 24 hours after LPS+PGN injection in both strains, no significant difference between Wt and Nod1/2 DKO mice was observed (Fig. 5c). Interestingly, creatinine was significantly increased in Wt and not in Nod1/2 DKO mice 24 hours after injection. As a consequence serum creatinine was significantly higher in Wt compared to Nod1/2 DKO mice at this time-point (Fig. 5d). Thus renal function was partly preserved in Nod1/2 DKO mice after inducing systemic inflammation. PasD-stained kidney sections were examined for histological signs of renal damage. Although renal function was impaired in LPS+PGN treated mice, no histological changes were present 2 or 24 hours following treatment compared with control mice. This is in line with previous reports showing only mild non-specific renal histological changes in human and animal sepsis even when renal dysfunction is evident (Langenberg et al., 2008). Since it is difficult to demonstrate renal damage on histological level in this mouse model, we analyzed the renal expression of kidney injury molecule-1 (Kim-1), and neutrophil gelatinase-associated lipocalin (Ngal) both sensitive biomarkers for AKI (Ko et al., 2010). Both Kim-1 and Ngal are remarkably increased 24 hours following LPS+PGN injection (Fig. 5e,f). Additionally, we observed significant lower Kim-1 expression after 24 hours in Nod1/2 DKO mice compared to Wt mice (Fig. 5e).
Fig. 5.
Analysis of renal parameters in Wt (white bars) and Nod1/2 DKO (black bars) 2 and 24 hours after LPS+PGN or 24 hours after saline (control) administration. In Wt mice the renal expression of Nod1 (a) and Nod2 (b) was determined. Nod1 and Nod2 mRNA was significantly increased 2 hours after LPS+PGN injection, Nod1 mRNA increased further at 24 hours while Nod2 expression returned to control levels. Renal function was determined by plasma ureum (c) and creatinine (d). Twenty-four hours following LPS+PGN injection Wt mice showed elevated levels of ureum and creatinine indicating a decrease in renal function. Plasma creatinine was not significantly increased in Nod1/2 DKO mice following LPS+PGN injection and was significantly lower as compared to Wt mice at 24 hours following LPS+PGN injection. Renal mRNA expression of Kim-1 (e), and Ngal (f) was significantly increased 24 hours following LPS+PGN compared with control, indicative of acute kidney injury. Significantly lower Kim-1 levels were observed in Nod1/2 DKO mice compared with Wt mice at 24 hours after LPS+PGN injection. Renal pro-inflammatory cytokine protein levels of Tnfα (g), Il-1β (h) and KC (i) and the anti-inflammatory cytokine Il-10 (j) were significantly increased after 2 hours and returned to control levels after 24 hours in both strains. Significantly higher levels of Tnfα, KC, and Il-10 were observed in Nod1/2 DKO mice as compared with Wt mice after 2 hours. Data are expressed as mean ± sem, n = 5–8. *P<0.05 compared with control (a,b) or Wt (c–j).
Since kidney function and tubular damage were partly preserved in Nod1/2 DKO mice, we determined whether there was a difference in renal pro- and anti-inflammatory cytokines following LPS+PGN injection. In line with plasma cytokine levels Tnfα, KC, Il-1β, and Il-10 were significantly increased after 2 hours and returned to control levels after 24 hours (Fig. 5g–j). Interestingly, Tnfα, KC and Il-10 were significantly higher in Nod1/2 DKO compared with Wt mice 2 hours following LPS+PGN injection (Fig. 5g,i,j).
In addition the influx of granulocytes and lymphocytes was determined by scoring Ly6 and CD3ε stained kidney sections respectively. Two hours following LPS+PGN administration a slight influx of granulocytes was observed that was similar between both mouse strains. After 24 hours no infiltrating granulocytes could be detected in kidneys of both mouse strains (data not shown). Interstitial lymphocytes were not increased following treatment at all examined time-points (data not shown).
Although no significant differences in the systemic response between Wt and Nod1/2 DKO were found upon LPS+PGN injection, we did observe an altered response in the kidney in the absence of Nod1/2.
Discussion
Prior to subjecting knockout mice to disease models, it is important to first assess the phenotype of these animals in order to distinguish between the primary and secondary effects of genetic changes. Systemically analysis of over 100 mutant mice revealed new and unexpected phenotypes in 96% of these mutants, approximately one-third of these mouse lines had no previously described phenotype, suggesting that phenotypes have been overlooked even in mouse models that have been studied extensively (Beckers et al., 2009). The present study is the first that analyzed the phenotype of Nod1/2 DKO mice extensively, and although our phenotypic screening is just a starting point, it provides us already with essential information.
An interesting observation is the lower liver weight in Nod1/2 DKO mice while no differences in other organs or total body weight were observed. Importantly, we observed no macro- or microscopical abnormalities in all examined organs and, except for increased number of Peyer's Patches in two studies (Barreau et al., 2007; Barreau et al., 2010), so far no microscopical abnormalities are described for Nod2 KO mice (Barreau et al., 2007; Body-Malapel et al., 2008). Recently Schertzer et al. reported lower liver weight in Nod1/2 DKO mice after 16 weeks on a high fat diet as compared to Wt mice (Schertzer et al., 2011). They, however, do not report on the liver weights of mice receiving a non-high fat diet. Interestingly, a role for Nod proteins in diet-induced metabolic syndrome has been proposed by Schertzer et al. (Schertzer et al., 2011).
Exciting discoveries in the last few years support a role for gut microbiota as responsible for, and involved in, the perpetuation of both insulin resistance and low-grade chronic inflammation (Petruzzelli and Moschetta, 2010). Vijay-Kumar et al. were the first to provide evidence of the direct relationship between development of the metabolic syndrome, malfunction of the innate immune system, and changes in the composition of the gut microbiota (Vijay-Kumar et al., 2010). Mice deficient in the PRR Tlr5, a main component of the innate immune system in the gut mucosa, presented increased adiposity, which was associated with clinical signs of metabolic syndrome such as elevated serum triglycerides and cholesterol levels, increased blood pressure, and insulin resistance (Vijay-Kumar et al., 2010). Since Nod1/2 DKO mice have a lower risk at developing metabolic syndrome (Schertzer et al., 2011), this might imply that in the gut Tlr and Nod signaling have opposite effects. Indeed, it seems that Nod signaling is important to control the inflammatory response against commensal microbiota in the gut, as loss of function results in inflammatory bowel disease (Strober et al., 2008). Moreover, recently PGN is proposed as a potential trigger for metabolic syndrome (Schertzer et al., 2011).
The role for Nods, especially Nod2, in the maintenance of intestinal and microbiotic homeostasis has been described by several groups. Nod2 is important in the maintenance of intestinal epithelial integrity as has been shown by increased intestinal permeability (Barreau et al., 2007; Barreau et al., 2010) in Nod2 KO mice. In the present study we demonstrate an increased intestinal permeability in one-third of the Nod1/2 DKO mice. We did not observe this in all our Nod1/2 DKO mice, which implies that intestinal permeability is not a gradual phenomenon and might need another trigger besides Nod1/2 deficiency to occur. Next we questioned whether vascular integrity was also affected by Nod1/2 deficiency. Expression of Nod1 and Nod2 in endothelial cells has been shown previously (Davey et al., 2006; Oh et al., 2005; Opitz et al., 2005; Scurrell et al., 2009); however, their role in endothelial integrity has not been described. We did not observe an altered vascular permeability in mice deficient for Nod1/2. This indicates that although Nod1/2 plays a role in epithelial cell integrity, as demonstrated by intestinal permeability, endothelial cell integrity is likely not regulated by Nod1/2.
To investigate the role of Nod1/2 during systemic inflammation we used the bacterial cell wall components PGN and LPS to mimic the inflammatory response taking place during sepsis. In our hands PGN alone did not induce a significant inflammatory response resulting in organ damage – in contrast with studies in rat, which show that PGN from S. aureus caused moderate but significant increases in various plasma pro-inflammatory and damage markers (Myhre et al., 2004; Wang et al., 2004). This discrepancy can be explained by the different PGN formulation we have used (E. coli versus S. aureus), or by a difference in response among species (mouse versus rat). However, in line with our results, others did not observe a systemic effect of PGN administration alone (De Kimpe et al., 1995). Several studies have reported a synergistic effect of Nod agonist in combination with TLR agonist, either bacterial or viral (Chamaillard et al., 2003; Fritz et al., 2006; Kim et al., 2011; Park et al., 2007). Therefore we induced systemic inflammation by co-administration of LPS and PGN. Although we did not observe any differences between systemic responses of Wt and Nod1/2 DKO mice, we did see significant differences in the kidney indicating that there are tissue specific effects of Nod1/2 signaling. LPS+PGN injection resulted in a rapid increase in Nod1 and Nod2 mRNA expression in the kidney. Surprisingly the inflammatory response, both pro- and anti-inflammatory, was significantly higher in Nod1/2 DKO compared with Wt mice. This seemingly paradoxical result was also observed in a uveitis model, where the inflammatory response was increased in the absence of Nod2 (Rosenzweig et al., 2008). Our results may imply that the presence of Nod1/2 is critical to suppress an inflammatory response in the kidney. Although we observed an increased early renal inflammatory response, at 24 hours renal outcome was better in Nod1/2 DKO mice as demonstrated by preserved renal function and less renal damage. A possible explanation would be that the enhanced inflammatory response results in a faster recovery of the kidney. Alternatively, Nod1/2 deficiency could have a beneficial effect on renal outcome by affecting cell damage in a direct manner. Indeed, signaling via Nod1 leads to induction of apoptosis (da Silva Correia et al., 2007).
Overall, our phenotypic screening confirmed a role for Nod1/2 in protecting intestinal integrity. Most parameters of inflammation and organ damage were, however, not affected by Nod1/2 deficiency under physiological or systemic inflammatory conditions. One striking exception is the renal response upon systemic inflammation; our results clearly indicate a role for Nod1 and/or Nod2 in sepsis-induced acute renal disease. Further research is needed to identify the role of Nods in other renal disorders.
Materials and Methods
Mice
C57Bl/6 (Wt) mice were purchased from Janvier (Le Genest, France). Nod1 and Nod2 double knockout (Nod1/2 DKO) mice were generated as described before (Geddes et al., 2010; Schertzer et al., 2011), and bred in the animal facility of the Academic Medical Center in Amsterdam. Mutant mice were backcrossed at least 10 generations into the C57Bl/6 background. Age-matched male mice were used in all experiments. The Animal and Use Committee of the University of Amsterdam approved all experiments.
Phenotype analysis
Three-month-old mice were used to perform phenotype analysis (n = 7). Wt mice were purchased at the age of 7 weeks and maintained in our animal facility until sacrifice. At the time of sacrifice weight was determined and mice were anesthetized by inhalation of 3% isofluran, 0.2% N2O and 2% O2. Blood was collected by heart puncture in heparin-containing tubes. Liver, spleen, kidneys, intestines (divided into jejunum, duodenum, ileum, and colon), and mesenteric lymph nodes were removed and stored o/n in 10% formalin prior to embedding in paraffin. The intestinal parts were cut open longitudinal and rolled up before embedding in paraffin.
Histology
Formalin-fixed tissue was embedded in paraffin using standard procedures. Four-µm thick sections were cut and used for all stainings. For examining renal histology, sections were stained with periodic acid-Schiff reagents after diastase digestion (PasD). Histology of all other organs was examined on Haematoxylin/Eosin (HE) stained sections. PasD and HE stained sections were analyzed in a blinded fashion by a pathologist.
Leukocyte analysis
Count and composition of leukocytes was determined in whole blood using a Coulter Ac T diff2 (Beckman Coulter, Mijdrecht, The Netherlands).
Plasma biochemical analysis
Using standard autoanalyzer methods plasma levels of ureum, creatinine, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), albumin, alkaline phosphatase, amylase, glucose, total cholesterol, and triglyceride were determined by our hospital research facility.
Intestinal permeability
Three-month-old Wt and Nod1/2 DKO mice (n = 6) were fasted o/n (water ad libitum). The following morning 500 mg/kg FITC-dextran (4 kD; Sigma–Aldrich, Zwijndrecht, The Netherlands) was administered orally. After 4 hours mice were sacrificed and blood was collected by heart puncture in heparin-containing tubes. The concentration of FITC in plasma was measured with a Synergy HT Multi-Mode microplate reader (BioTek, Bad Friedrichshall, Germany).
Vascular permeability
One hour after intravenous administration of 20 mg/kg Evan's Blue Dye (Sigma–Aldrich), 3-month-old Wt and Nod1/2 DKO mice (n = 9) were sacrificed by perfusion through the left cardiac ventricle with ice-cold PBS. Colon, ileum, heart, kidney, liver, lung, and spleen were removed, dried and weighed. Tissues were homogenized in 4 ml/g formamide, and EBD was extracted by incubation for 48 hours at room temperature. Tissue debris was removed by spinning down for 30 min at 5000 g, concentration of EBD in the supernatant was determined at 620 nm. Extravasation is expressed as µg EBD per g tissue (dry weight).
Systemic inflammation
Eight to 12-week-old Wt and Nod1/2 DKO mice (n = 6–8) received an intraperitoneal injection of 7.5 mg/kg LPS (from E. coli O111:B4; Sigma) and 1 mg/kg PGN (from E. coli K12; InvivoGen, San Diego, CA, USA) in saline. Two and 24 hours after LPS+PGN injection mice were sacrificed, blood was collected by heart puncture in heparin-containing tubes and kidneys were removed. Kidneys were snap frozen in liquid nitrogen and stored at −80°C or fixed in 10% formalin o/n prior to further processing. Control mice were sacrificed 24 hours after intraperitoneal injection of saline.
RNA isolation and quantitative real-time RT-PCR
Total RNA was isolated from snap frozen kidney using the TRIzol® reagent (Invitrogen, Breda, the Netherlands) method. Complementary DNA (cDNA) was synthesized using the M-MLV RT enzyme kit (Invitrogen), oligo dT primers (Sigma) and RNase inhibitor (Applied Biosystem, Nieuwerkerk a/d Ijssel, the Netherlands) according to the manufacturer's protocol. Primer sequences were designed based on Primer3 software (Rozen and Skaletsky, 2000): Nod1 forward 5′-tcagactcagcgtcaaccag-3′ and reverse 5′-taaacccaggaacgtcacga-3′, Nod2 forward 5′-gggagatgttggagtggaac-3′ and reverse 5′-agcgaagagcacactcaacc-3′, Kim-1 forward 5′-tggttgccttccgtgtctct-3′ and reverse 5′-tcagctcgggaatgcacaa-3′, Ngal forward 5′-gcctcaaggacgacaacatc-3′ and reverse 5′-ctgaaccattgggtctctgc-3′, and Hprt forward 5′-tcctcctcagaccgctttt-3′ and reverse 5′-cctggttcatcatcgctaatc-3′. All primers were synthesized by Eurogentec (Maastricht, the Netherlands).
Quantitative real-time RT-PCR was performed on a LightCycler® 480 System (Roche) using LightCycler® 480 SYBR Green I Master mix (Roche). Forty-five cycles were carried out at an annealing temperature of 58°C. The starting concentrations of RNAs were calculated by the LinRegPCR program (version 9.30 beta) as described previously (Ramakers et al., 2003). The expression of genes was normalized towards the housekeeping gene Hprt. The data are presented as fold increase compared with Wt control kidney.
ELISA
Frozen kidneys were blended in PBS containing 1% Triton X-100, 1 mM EDTA and 1% protease inhibitor cocktail II (Sigma–Aldrich). KC, Il-1β, Il-10, and Tnfα DuoSet ELISA kits (R&D Systems, Abingdon, UK) were performed according to the supplied protocols. Renal cytokine levels were corrected for protein content per sample using Bio-Rad Protein Assay (Bio-Rad, Veenendaal, The Netherlands).
Statistical analyses
All statistical analyses were performed using Graphpad 4 software (San Diego, CA, USA). Data were analyzed using the non-parametric Mann–Whitney U-test and these results are expressed as mean ± standard error of the mean (sem). Values of P≤0.05 were considered statistically significant.
Acknowledgments
I.S. developed concepts/approach, performed experiments, and wrote the manuscript. L.M.B., N.C., G.J.T., and S.J.R. performed experiments. S.E.G. contributed reagents and edited the manuscript. S.F. and J.C.L. developed concepts/approach and wrote the manuscript. This study is financially supported by the Dutch Kidney Foundation (to I.S. and L.M.B.). S.E.G. is supported by funding from the Canadian Institutes of Health Research (C.I.H.R.).
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Barreau F., Meinzer U., Chareyre F., Berrebi D., Niwa–Kawakita M., Dussaillant M., Foligne B., Ollendorff V., Heyman M., Bonacorsi S.et al. (2007). CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS ONE 2, e523 10.1371/journal.pone.0000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F., Madre C., Meinzer U., Berrebi D., Dussaillant M., Merlin F., Eckmann L., Karin M., Sterkers G., Bonacorsi S.et al. (2010). Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer's patches. Gut 59, 207–217 10.1136/gut.2008.171546 [DOI] [PubMed] [Google Scholar]
- Beckers J., Wurst W., de Angelis M. H. (2009). Towards better mouse models: enhanced genotypes, systemic phenotyping and envirotype modelling. Nat. Rev. Genet. 10, 371–380 10.1038/nrg2578 [DOI] [PubMed] [Google Scholar]
- Biswas A., Liu Y. J., Hao L., Mizoguchi A., Salzman N. H., Bevins C. L., Kobayashi K. S. (2010). Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc. Natl. Acad. Sci. USA 107, 14739–14744 10.1073/pnas.1003363107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body–Malapel M., Dharancy S., Berrebi D., Louvet A., Hugot J. P., Philpott D. J., Giovannini M., Chareyre F., Pages G., Gantier E.et al. (2008). NOD2: a potential target for regulating liver injury. Lab. Invest. 88, 318–327 10.1038/labinvest.3700716 [DOI] [PubMed] [Google Scholar]
- Bouskra D., Brézillon C., Bérard M., Werts C., Varona R., Boneca I. G., Eberl G. (2008). Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 10.1038/nature07450 [DOI] [PubMed] [Google Scholar]
- Cartwright N., Murch O., McMaster S. K., Paul–Clark M. J., van Heel D. A., Ryffel B., Quesniaux V. F., Evans T. W., Thiemermann C., Mitchell J. A. (2007). Selective NOD1 agonists cause shock and organ injury/dysfunction in vivo. Am. J. Respir. Crit. Care Med. 175, 595–603 10.1164/rccm.200608-1103OC [DOI] [PubMed] [Google Scholar]
- Chamaillard M., Hashimoto M., Horie Y., Masumoto J., Qiu S., Saab L., Ogura Y., Kawasaki A., Fukase K., Kusumoto S.et al. (2003). An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4, 702–707 10.1038/ni945 [DOI] [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. (1982). Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect. Immun. 35, 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Y., Shaw M. H., Redondo G., Núñez G. (2008). The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 68, 10060–10067 10.1158/0008-5472.CAN-08-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvojka J., Sýkora R., Karvunidis T., Raděj J., Kroužecký A., Novák I., Matějovič M. (2010). New developments in septic acute kidney injury. Physiol. Res. 59, 859–869. [DOI] [PubMed] [Google Scholar]
- Clarke T. B., Davis K. M., Lysenko E. S., Zhou A. Y., Yu Y., Weiser J. N. (2010). Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 10.1038/nm.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Correia J., Miranda Y., Leonard N., Hsu J., Ulevitch R. J. (2007). Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 14, 830–839 10.1038/sj.cdd.4402070 [DOI] [PubMed] [Google Scholar]
- Davey M. P., Martin T. M., Planck S. R., Lee J., Zamora D., Rosenbaum J. T. (2006). Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc. Res. 71, 103–107 10.1016/j.mvr.2005.11.010 [DOI] [PubMed] [Google Scholar]
- De Kimpe S. J., Kengatharan M., Thiemermann C., Vane J. R. (1995). The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92, 10359–10363 10.1073/pnas.92.22.10359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder W., Klimecki W., Yu L., von Mutius E., Riedler J., Braun–Fahrländer C., Nowak D., Holst O., Martinez F. D.the ALEX–Team (2006). Association between exposure to farming, allergies and genetic variation in CARD4/NOD1. Allergy 61, 1117–1124 10.1111/j.1398-9995.2006.01128.x [DOI] [PubMed] [Google Scholar]
- Fritz J. H., Ferrero R. L., Philpott D. J., Girardin S. E. (2006). Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7, 1250–1257 10.1038/ni1412 [DOI] [PubMed] [Google Scholar]
- Geddes K., Rubino S., Streutker C., Cho J. H., Magalhaes J. G., Le Bourhis L., Selvanantham T., Girardin S. E., Philpott D. J. (2010). Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect. Immun. 78, 5107–5115 10.1128/IAI.00759-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin S. E., Boneca I. G., Carneiro L. A., Antignac A., Jéhanno M., Viala J., Tedin K., Taha M. K., Labigne A., Zähringer U.et al. (2003a). Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300, 1584–1587 10.1126/science.1084677 [DOI] [PubMed] [Google Scholar]
- Girardin S. E., Boneca I. G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D. J., Sansonetti P. J. (2003b). Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 10.1074/jbc.C200651200 [DOI] [PubMed] [Google Scholar]
- Hysi P., Kabesch M., Moffatt M. F., Schedel M., Carr D., Zhang Y., Boardman B., von Mutius E., Weiland S. K., Leupold W.et al. (2005). NOD1 variation, immunoglobulin E and asthma. Hum. Mol. Genet. 14, 935–941 10.1093/hmg/ddi087 [DOI] [PubMed] [Google Scholar]
- Inohara N., Koseki T., del Peso L., Hu Y., Yee C., Chen S., Carrio R., Merino J., Liu D., Ni J.et al. (1999). Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274, 14560–14567 10.1074/jbc.274.21.14560 [DOI] [PubMed] [Google Scholar]
- Inohara N., Ogura Y., Fontalba A., Gutierrez O., Pons F., Crespo J., Fukase K., Inamura S., Kusumoto S., Hashimoto M.et al. (2003). Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278, 5509–5512 10.1074/jbc.C200673200 [DOI] [PubMed] [Google Scholar]
- Iwanaga Y., Davey M. P., Martin T. M., Planck S. R., DePriest M. L., Baugh M. M., Suing C. M., Rosenbaum J. T. (2003). Cloning, sequencing and expression analysis of the mouse NOD2/CARD15 gene. Inflamm. Res. 52, 272–276. [DOI] [PubMed] [Google Scholar]
- Kanazawa N., Okafuji I., Kambe N., Nishikomori R., Nakata–Hizume M., Nagai S., Fuji A., Yuasa T., Manki A., Sakurai Y.et al. (2005). Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-κB activation: common genetic etiology with Blau syndrome. Blood 105, 1195–1197 10.1182/blood-2004-07-2972 [DOI] [PubMed] [Google Scholar]
- Kim Y. G., Park J. H., Shaw M. H., Franchi L., Inohara N., Núñez G. (2008). The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 28, 246–257 10.1016/j.immuni.2007.12.012 [DOI] [PubMed] [Google Scholar]
- Kim Y. G., Park J. H., Reimer T., Baker D. P., Kawai T., Kumar H., Akira S., Wobus C., Núñez G. (2011). Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe 9, 496–507 10.1016/j.chom.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko G. J., Grigoryev D. N., Linfert D., Jang H. R., Watkins T., Cheadle C., Racusen L., Rabb H. (2010). Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am. J. Physiol. Renal Physiol. 298, F1472–F1483 10.1152/ajprenal.00619.2009 [DOI] [PubMed] [Google Scholar]
- Kobayashi K. S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R. A. (2005). Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 10.1126/science.1104911 [DOI] [PubMed] [Google Scholar]
- Langenberg C., Bagshaw S. M., May C. N., Bellomo R. (2008). The histopathology of septic acute kidney injury: a systematic review. Crit. Care 12, R38 10.1186/cc6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourhis L., Benko S., Girardin S. E. (2007). Nod1 and Nod2 in innate immunity and human inflammatory disorders. Biochem. Soc. Trans. 35, 1479–1484 10.1042/BST0351479 [DOI] [PubMed] [Google Scholar]
- Masumoto J., Yang K., Varambally S., Hasegawa M., Tomlins S. A., Qiu S., Fujimoto Y., Kawasaki A., Foster S. J., Horie Y.et al. (2006). Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J. Exp. Med. 203, 203–213 10.1084/jem.20051229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli–Richard C., Lesage S., Rybojad M., Prieur A. M., Manouvrier–Hanu S., Häfner R., Chamaillard M., Zouali H., Thomas G., Hugot J. P. (2001). CARD15 mutations in Blau syndrome. Nat. Genet. 29, 19–20 10.1038/ng720 [DOI] [PubMed] [Google Scholar]
- Murch O., Abdelrahman M., Kapoor A., Thiemermann C. (2008). Muramyl dipeptide enhances the response to endotoxin to cause multiple organ injury in the anesthetized rat. Shock 29, 388–394 10.1097/SHK.0b013e3181453e59 [DOI] [PubMed] [Google Scholar]
- Myhre A. E., Stuestøl J. F., Dahle M. K., Øverland G., Thiemermann C., Foster S. J., Lilleaasen P., Aasen A. O., Wang J. E. (2004). Organ injury and cytokine release caused by peptidoglycan are dependent on the structural integrity of the glycan chain. Infect. Immun. 72, 1311–1317 10.1128/IAI.72.3.1311-1317.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Inohara N., Benito A., Chen F. F., Yamaoka S., Nunez G. (2001). Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276, 4812–4818 10.1074/jbc.M008072200 [DOI] [PubMed] [Google Scholar]
- Ogura Y., Saab L., Chen F. F., Benito A., Inohara N., Nuñez G. (2003). Genetic variation and activity of mouse Nod2, a susceptibility gene for Crohn's disease. Genomics 81, 369–377 10.1016/S0888-7543(03)00027-2 [DOI] [PubMed] [Google Scholar]
- Oh H. M., Lee H. J., Seo G. S., Choi E. Y., Kweon S. H., Chun C. H., Han W. C., Lee K. M., Lee M. S., Choi S. C.et al. (2005). Induction and localization of NOD2 protein in human endothelial cells. Cell. Immunol. 237, 37–44 10.1016/j.cellimm.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Opitz B., Förster S., Hocke A. C., Maass M., Schmeck B., Hippenstiel S., Suttorp N., Krüll M. (2005). Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ. Res. 96, 319–326 10.1161/01.RES.0000155721.83594.2c [DOI] [PubMed] [Google Scholar]
- Park J. H., Kim Y. G., McDonald C., Kanneganti T. D., Hasegawa M., Body–Malapel M., Inohara N., Núñez G. (2007). RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–2386. [DOI] [PubMed] [Google Scholar]
- Park J. H., Kim Y. G., Núñez G. (2009). RICK promotes inflammation and lethality after gram-negative bacterial infection in mice stimulated with lipopolysaccharide. Infect. Immun. 77, 1569–1578 10.1128/IAI.01505-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penack O., Holler E., van den Brink M. R. (2010). Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood 115, 1865–1872 10.1182/blood-2009-09-242784 [DOI] [PubMed] [Google Scholar]
- Petnicki–Ocwieja T., Hrncir T., Liu Y. J., Biswas A., Hudcovic T., Tlaskalova–Hogenova H., Kobayashi K. S. (2009). Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl. Acad. Sci. USA 106, 15813–15818 10.1073/pnas.0907722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M., Moschetta A. (2010). Intestinal ecology in the metabolic syndrome. Cell Metab. 11, 345–346 10.1016/j.cmet.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Ramakers C., Ruijter J. M., Deprez R. H., Moorman A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66 10.1016/S0304-3940(02)01423-4 [DOI] [PubMed] [Google Scholar]
- Ribi E. E., Cantrell J. L., Von Eschen K. B., Schwartzman S. M. (1979). Enhancement of endotoxic shock by N-acetylmuramyl-L-alanyl-(L-seryl)-D-isoglutamine (muramyl dipeptide). Cancer Res. 39, 4756–4759. [PubMed] [Google Scholar]
- Rosenzweig H. L., Martin T. M., Planck S. R., Galster K., Jann M. M., Davey M. P., Kobayashi K., Flavell R. A., Rosenbaum J. T. (2008). Activation of NOD2 in vivo induces IL-1beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J. Leukoc. Biol. 84, 529–536 10.1189/jlb.0108015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386. [DOI] [PubMed] [Google Scholar]
- Schertzer J. D., Tamrakar A. K., Magalhaes J. G., Pereira S., Bilan P. J., Fullerton M. D., Liu Z., Steinberg G. R., Giacca A., Philpott D. J.et al. (2011). NOD1 activators link innate immunity to insulin resistance. Diabetes 60, 2206–2215 10.2337/db11-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurrell E., Stanley R., Schöniger S. (2009). Immunohistochemical detection of NOD1 and NOD2 in the healthy murine and canine eye. Vet. Ophthalmol. 12, 269–275 10.1111/j.1463-5224.2009.00698.x [DOI] [PubMed] [Google Scholar]
- Strober W., Kitani A., Fuss I., Asano N., Watanabe T. (2008). The molecular basis of NOD2 susceptibility mutations in Crohn's disease. Mucosal Immunol. 1Suppl 1, S5–S9 10.1038/mi.2008.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Yamaguchi N., Matsuda K., Yamazaki K., Takahashi S., Tojo A., Onizuka M., Eishi Y., Akiyama H., Ishikawa J.et al. (2011). Association analysis of the NOD2 gene with susceptibility to graft-versus-host disease in a Japanese population. Int. J. Hematol. 93, 771–778 10.1007/s12185-011-0860-5 [DOI] [PubMed] [Google Scholar]
- Vijay–Kumar M., Aitken J. D., Carvalho F. A., Cullender T. C., Mwangi S., Srinivasan S., Sitaraman S. V., Knight R., Ley R. E., Gewirtz A. T. (2010). Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. E., Dahle M. K., Yndestad A., Bauer I., McDonald M. C., Aukrust P., Foster S. J., Bauer M., Aasen A. O., Thiemermann C. (2004). Peptidoglycan of Staphylococcus aureus causes inflammation and organ injury in the rat. Crit. Care Med. 32, 546–552 10.1097/01.CCM.0000109775.22138.8F [DOI] [PubMed] [Google Scholar]
- Weidinger S., Klopp N., Rummler L., Wagenpfeil S., Novak N., Baurecht H. J., Groer W., Darsow U., Heinrich J., Gauger A.et al. (2005). Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J. Allergy Clin. Immunol. 116, 177–184 10.1016/j.jaci.2005.02.034 [DOI] [PubMed] [Google Scholar]
- Werts C., le Bourhis L., Liu J., Magalhaes J. G., Carneiro L. A., Fritz J. H., Stockinger S., Balloy V., Chignard M., Decker T.et al. (2007). Nod1 and Nod2 induce CCL5/RANTES through the NF-κB pathway. Eur. J. Immunol. 37, 2499–2508 10.1002/eji.200737069 [DOI] [PubMed] [Google Scholar]