Abstract
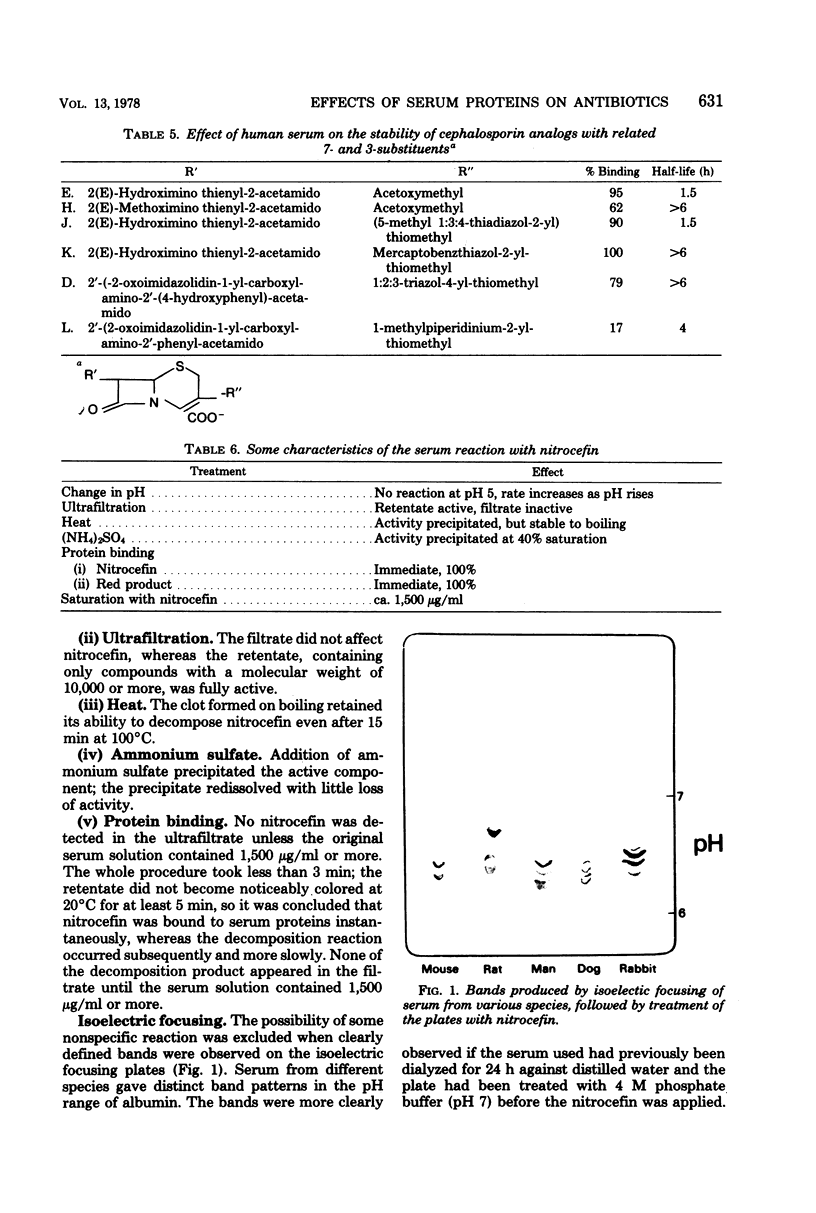
The chromogenic cephalosporin nitrocefin (87/312) demonstrates rapid and visible instability to serum from many species. This phenomenon was distinct from serum binding, being significantly slower. Destruction of another cephalosporin, 10485, by serum appeared to account for some anomalous results during investigation into its human pharmacokinetics. Many cephalosporins of very different structures also showed serum instability, unrelated to their degrees of serum binding as measured by plate assay. Extrapolation could not be made from one species to another with regard to either binding or instability. Small changes in the chemical structures of the 3- and 7-substituents of the cephalosporins made profound changes in their susceptibility to serum attack. The decomposition is pH dependent, occurring more slowly at acid pH, and is due to a high-molecular-weight component of serum that resists boiling for several minutes. Isoelectric focusing of serum from several animal species gave various species-specific bands that decomposed nitrocefin. The inactivation of nitrocefin was not entirely parallel with that of 10485 and was inhibited by it. All other β-lactam compounds tested also inhibited the reaction, much greater concentrations usually being necessary when the inhibitor was stable to serum. The complex that causes breakdown of the β-lactam compounds is not necessarily the same as the one causing serum binding. It is postulated that serum may affect most other β-lactam antibiotics in a similar way, although in most cases, this only occurs to a very slight extent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole M., Kenig M. D., Hewitt V. A. Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid in man and its significance in assessing penicillin absorption. Antimicrob Agents Chemother. 1973 Apr;3(4):463–468. doi: 10.1128/aac.3.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord R. D. Cefuroxime: human pharmacokinetics.. Antimicrob Agents Chemother. 1976 May;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Tootill J. P., Robinson W. D. A new approach to the study of serum concentrations of orally administered cephalexin. J Pharm Pharmacol. 1971 Jan;23(1):50–57. doi: 10.1111/j.2042-7158.1971.tb12779.x. [DOI] [PubMed] [Google Scholar]
- Ryan D. M., O'Callaghan C., Muggleton P. W. Cefuroxime, a new cephalosporin antibiotic: activity in vivo. Antimicrob Agents Chemother. 1976 Mar;9(3):520–525. doi: 10.1128/aac.9.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]



