Abstract
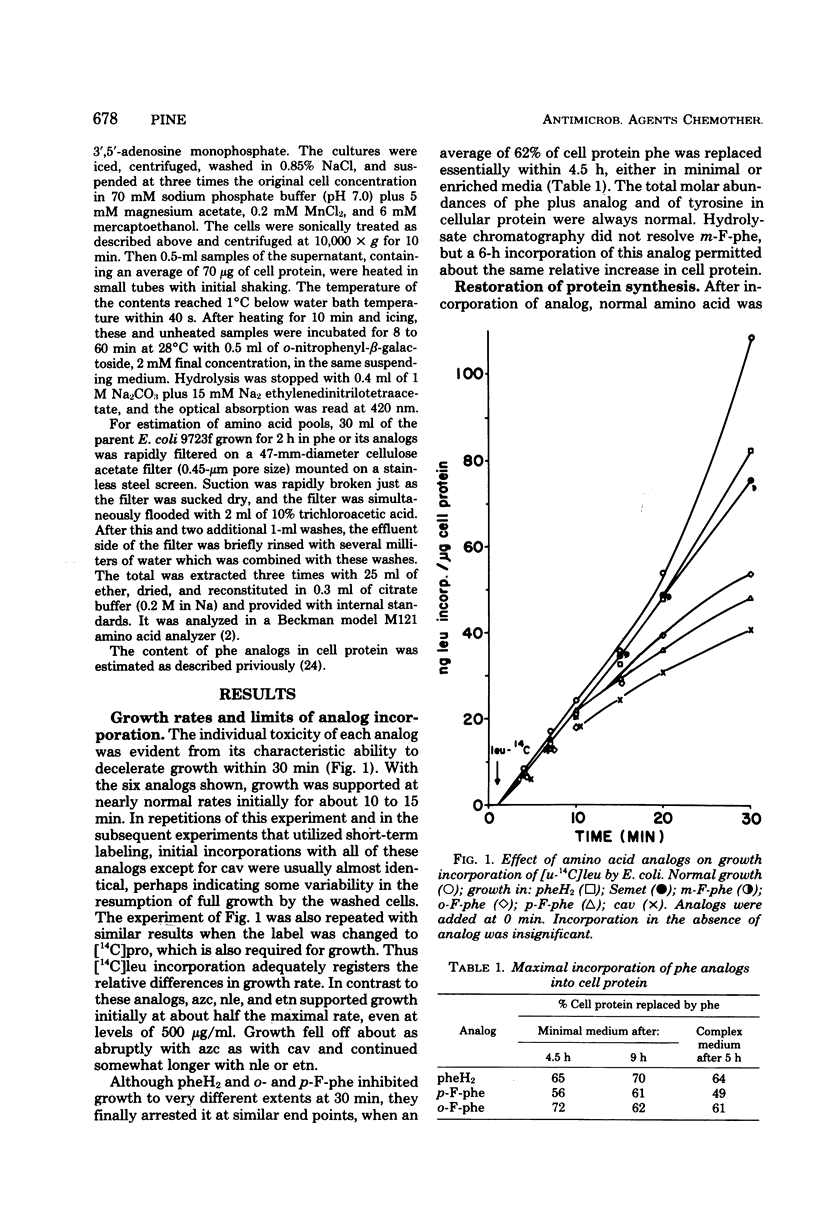
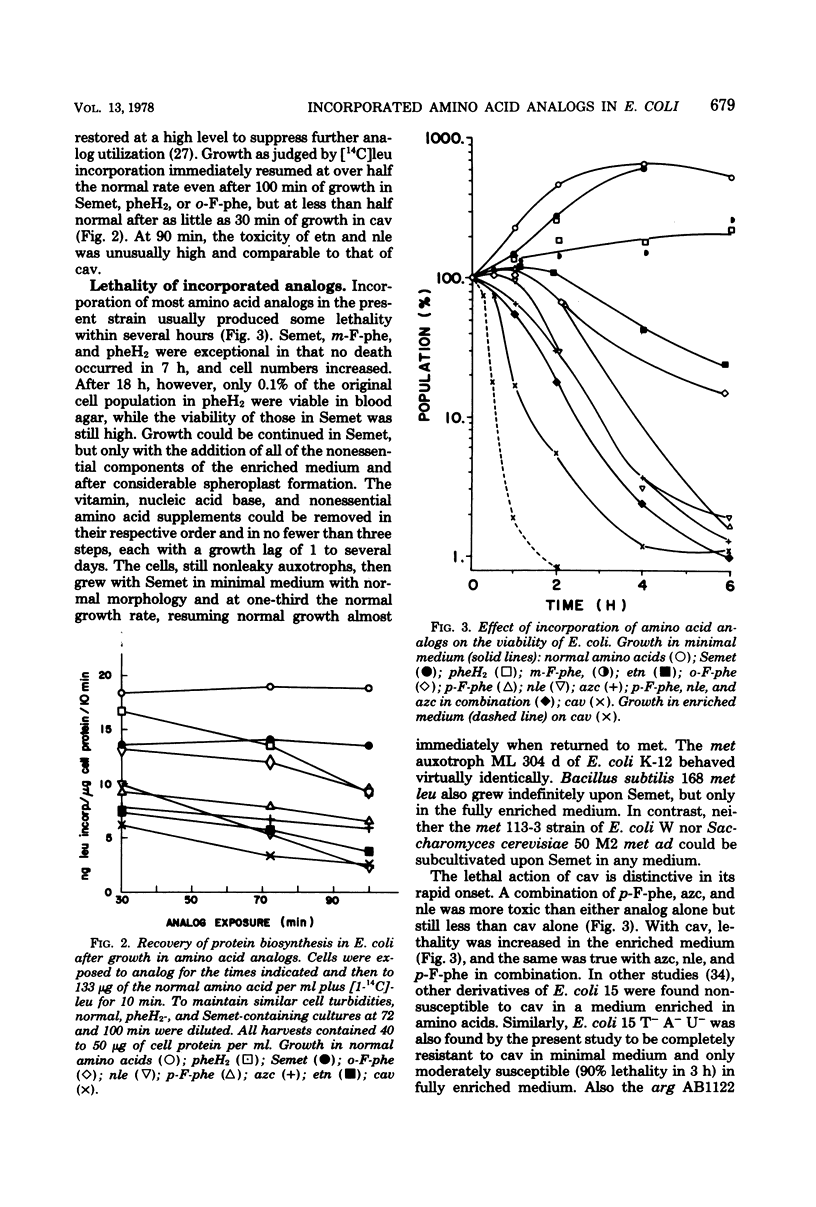
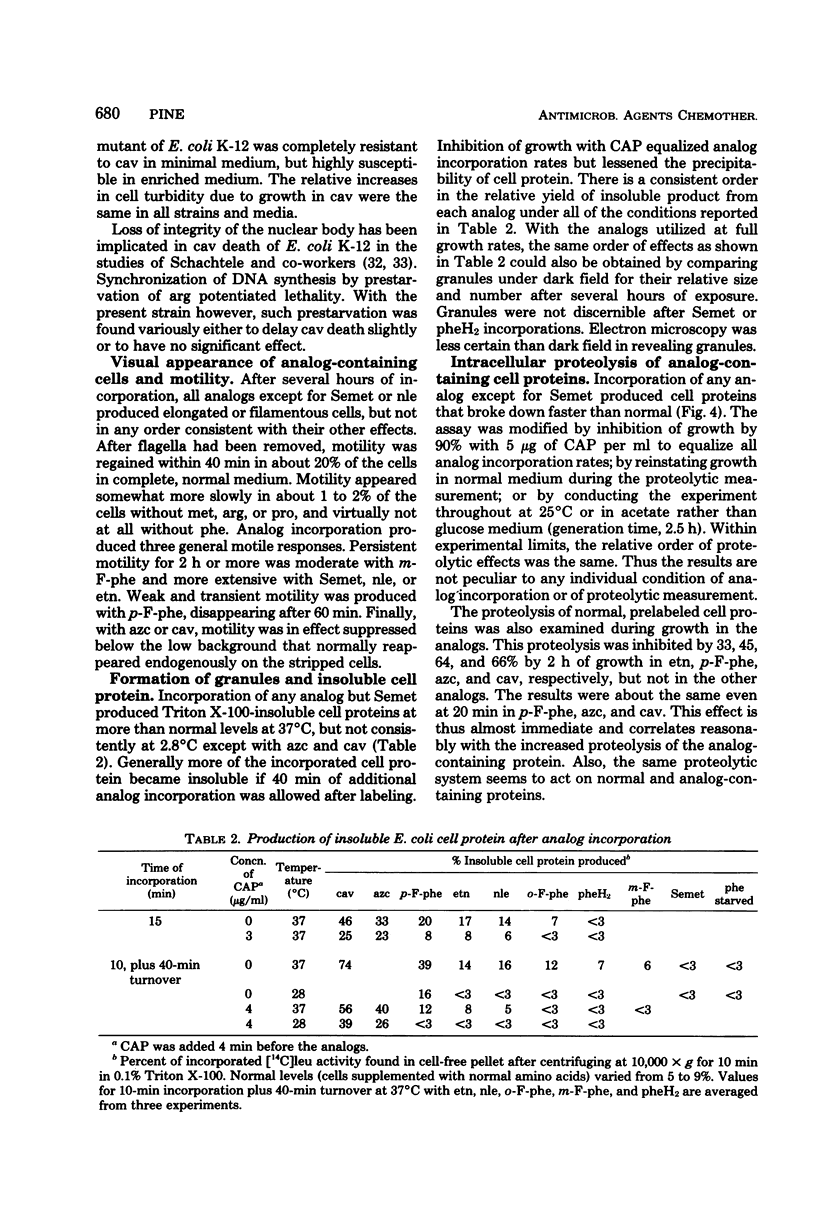
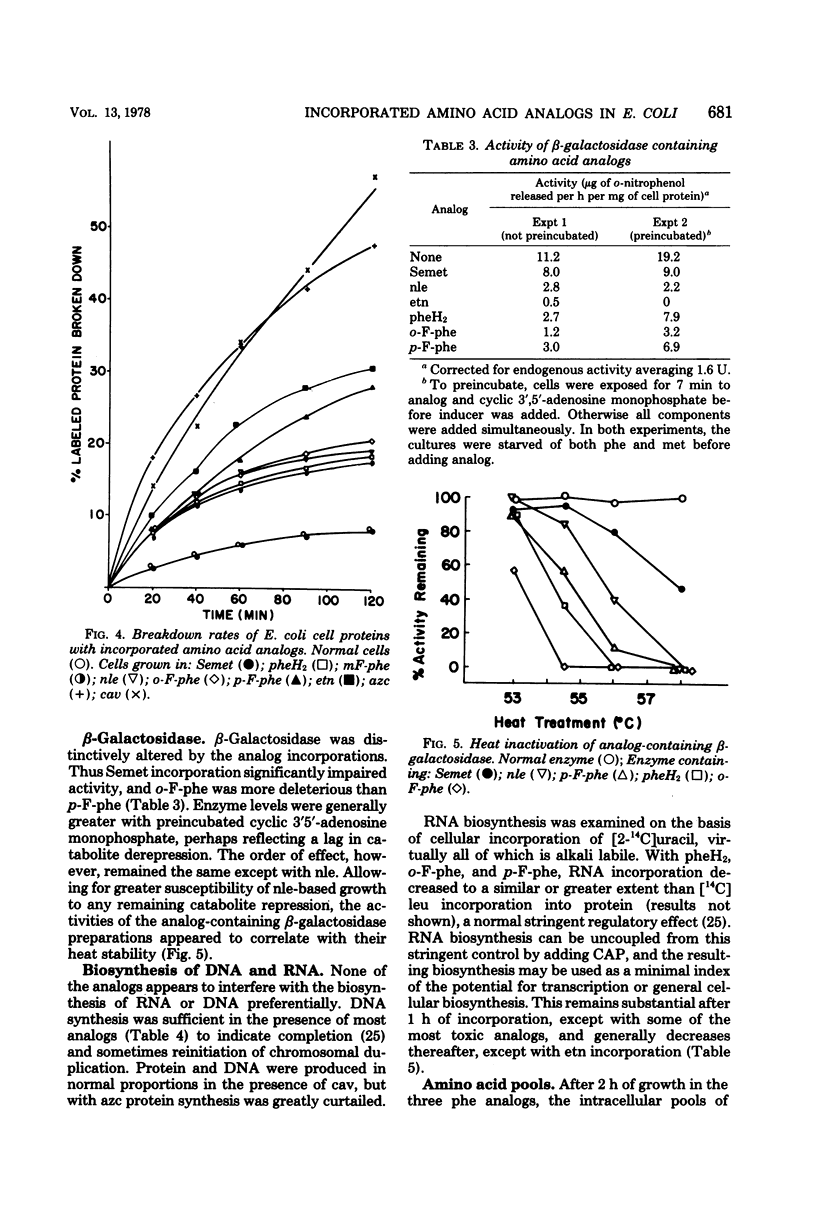
The relative toxicities of several incorporated analogs of phenylalanine, methionine, arginine, and proline were assessed by a variety of criteria in a derivative of Escherichia coli 15 requiring the antagonized amino acids. Toxicity of the analog-substituted cell protein was most consistently indicated by its insolubility at graded temperatures, its increased breakdown, the relative suppression of further cell growth, and lethality. The relative toxicity of poorly utilized analogs could be judged clearly only by the first two criteria. Toxicity generally increased as follows: selenomethionine < 2,5-dihydrophenylalanine and m-fluorophenylalanine < o-fluorophenylalanine and norleucine < ethionine < p-fluorophenylalanine < azetidine-2-carboxylate < canavanine. The overall perturbation of cell protein structure indicated by the toxicity of the methionine and phenylalanine analogs correlated with their alteration of charge and bulk and was greatly modified by minor positional modifications of fluorine. Among the more specific functional impairments, the activity and heat stability of β-galactosidase were lowered in parallel by substitutions of phenylalanine and methionine analogs, but not in the usual order of toxicity. Flagella were transiently motile with p-fluorophenylalanine, moderately motile with m-fluorophenylalanine, and fully motile with all methionine analogs. Usually the analog incorporations were no more than bacteriostatic in E. coli strains, canavanine killing only the E. coli 15 substrain extensively in minimal media. Selenomethionine supported indefinite growth of procaryotes such as Bacillus subtilis and certain E. coli strains, but only upon supplementation, at least initially, with many nonessential metabolites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., COWIE D. B. Remplacement total de la méthionine par la sélénométhionine dans les protéines d'Escherichia coli. C R Hebd Seances Acad Sci. 1957 Jan 28;244(5):680–683. [PubMed] [Google Scholar]
- COWIE D. B., COHEN G. N. Biosynthesis by Escherichia coli of active altered proteins containing selenium instead of sulfur. Biochim Biophys Acta. 1957 Nov;26(2):252–261. doi: 10.1016/0006-3002(57)90003-3. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E., McConomy J., Franzen B., Marroquin F., Stewart G. A., Magee P. N. Interaction between ethionine and rat liver ribonucleic acid and protein in vivo. Cancer Res. 1967 Oct;27(10):1761–1772. [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- KECK K. An ultramicro technique for the determination of deoxypentose nucleic acid. Arch Biochem Biophys. 1956 Aug;63(2):446–451. doi: 10.1016/0003-9861(56)90059-5. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERRIDGE D. Synthesis of flagella by amino acid-requiring mutants of Salmonella typhimurium. J Gen Microbiol. 1959 Aug;21:168–179. doi: 10.1099/00221287-21-1-168. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of amino acid analogues on the synthesis of bacterial flagella. Biochim Biophys Acta. 1959 Feb;31(2):579–581. doi: 10.1016/0006-3002(59)90048-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacLean S. J., Huber R. E. The effects of DL-p-fluorophenylalanine and L-3-nitrotyrosine on the growth and biochemistry of the Taper liver tumor. Cancer Res. 1971 Nov;31(11):1669–1672. [PubMed] [Google Scholar]
- McMahon D., Langstroth P. The effects of canavanine and of arginine starvation on macromolecular synthesis in Chlamydomonas reinhardi. J Gen Microbiol. 1972 Nov;73(2):239–250. doi: 10.1099/00221287-73-2-239. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE M. J., HARZEWSKI E., WISSLER F. C. ACTION OF THE PHTHALANILIDE DRUGS ON ESCHERICHIA COLI. Cancer Res. 1963 Jul;23:932–937. [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Incorporation of L-2,5-dihydrophenylalanine into cell proteins of Escherichia coli and sarcoma 180. Antimicrob Agents Chemother. 1975 May;7(5):601–605. doi: 10.1128/aac.7.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Regulation of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Jul;115(1):107–116. doi: 10.1128/jb.115.1.107-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Stringent control of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Dec;116(3):1253–1257. doi: 10.1128/jb.116.3.1253-1257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty W. F., Karnovsky M. J., Goldberg A. L. Degradation of abnormal proteins in Escherichia coli. Formation of protein inclusions in cells exposed to amino acid analogs. J Biol Chem. 1975 Feb 10;250(3):1112–1122. [PubMed] [Google Scholar]
- RENNERT O. M., ANKER H. S. ON THE INCORPORATION OF 5',5',5'-TRIFLUOROLEUCINE INTO PROTEINS OF E. COLI. Biochemistry. 1963 May-Jun;2:471–476. doi: 10.1021/bi00903a013. [DOI] [PubMed] [Google Scholar]
- Rabinovitz M., Finkleman A., Reagan R. L., Breitman T. R. Amino acid antagonist death in Escherichia coli. J Bacteriol. 1969 Jul;99(1):336–338. doi: 10.1128/jb.99.1.336-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. L., Elliott J. A. Fluorophenylalanine inhibition of tumors in mice on a phenylalanine-deficient diet. Arch Biochem Biophys. 1968 Jun;125(3):797–801. doi: 10.1016/0003-9861(68)90516-x. [DOI] [PubMed] [Google Scholar]
- Scannell J. P., Pruess D. L., Demny T. C., Williams T., Stempel A. L-3-(2,5-dihydrophenyl)alanine, an antimetabolite of L-phenylalanine produced by a streptomycete. Jpn J Antibiot. 1970 Dec;23(6):618–619. doi: 10.7164/antibiotics.23.618. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Anderson D. L., Rogers P. Mechanism of canavanine death in Escherichia coli. II. Membranes-bound canavanyl-protein and nuclear disruption. J Mol Biol. 1968 May 14;33(3):861–872. doi: 10.1016/0022-2836(68)90324-0. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Mechanism of canavanine death in Escherichia coli. I. Effect of canvainine on macromolecular synthesis. J Mol Biol. 1968 May 14;33(3):843–860. doi: 10.1016/0022-2836(68)90323-9. [DOI] [PubMed] [Google Scholar]
- Simonnet G. M., Chapeville F. Action de la canavanine sur la synthèse du RNA chez E. coli. Eur J Biochem. 1969 Jun;9(2):199–206. doi: 10.1111/j.1432-1033.1969.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Snow M. L., Lauinger C., Ressler C. 1,4-cyclohexadiene-1-alanine (2,5-dihydrophenylalanine) a new inhibitor of phenylalanine for the rat and Leuconostoc dextranicum 8086. J Org Chem. 1968 May;33(5):1774–1780. doi: 10.1021/jo01269a016. [DOI] [PubMed] [Google Scholar]


