Summary
There are many parallels between DNA replication in yeast and humans. Now, two recent studies extend this relationship by dissecting key conserved interactions necessary for initiation of the replisome.
Initiation of DNA replication involves an intricate cascade of cell cycle dependent steps of protein recruitment and activation. The process begins in early G1 phase when the ORC proteins localize to potential origins of replication. ORC then recruits Cdc6 and Cdt1, which facilitate the loading of the MCM2-7 replicative helicase complex to form a pre-replicative complex at a licensed origin. In order for replication to occur only once, loading of these factors onto chromatin must be temporally separate: ORC, Cdc6, Cdt1 and the MCM complex can only be loaded onto DNA in the absence of Cyclin dependent kinase (CDK) activity during G1[1]. Activation of the MCM complex and formation of the active helicase requires the recruitment of the replication proteins Cdc45 and GINS complex to origins, which occurs when CDK activity rises to high levels at the transition to S-phase[2]. While much is known about how the elevated CDK activity deters the licensing of origins that have already fired to prevent re-replication, till recently the mechanism by which CDK activates the MCM complex was not very clear. In yeast, it has been shown that CDK-mediated phosphorylation of Sld2 promotes binding to the replication protein Dpb11[3, 4] (Fig. 1). Sld2 in turn brings GINS to the origin through Pol ε as part of the pre-loading complex (pre-LC) [5]. In addition, CDK mediated phosphorylation of Sld3, which associates with Cdc45, leads to the association of Sld3 with Dpb11 and thus brings Cdc45 to the complex of proteins at the origin [6]. CDK initiates these important interactions of Sld2 and Sld3 with Dpb11 by phosphorylating Sld2 and Sld3[3, 4]. In both cases the phosphorylated proteins can then bind to the BRCT repeat domains of Dbp11. The interactions between phospho-Sld2, Dbp11, and phospho-Sld3 are critical for replication initiation, most likely because this is how Cdc45 and GINS are brought to the MCM 2-7 complex to form the active helicase, composed of Cdc45, MCM2-7 and GINS (CMG)[2].
Figure 1. Replicative helicase activation in yeast and humans.
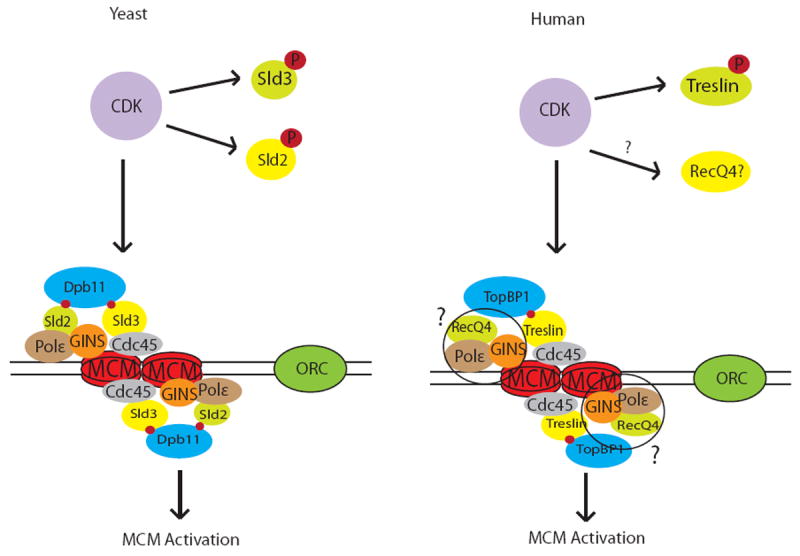
Left: In yeast, CDK phosphorylates Sld2 and Sld3 leading to their interaction with the BRCT repeats of Dpb11 and recruitment of Cdc45, Pol ε and GINS to origin sites to activate the MCM helicase. Right: In humans, CDK phosphorylates Treslin, which then binds TopBP1, leading to recruitment of Cdc45 and activation of the MCM helicase. The Sld2 homologue, RecQ4 does not appear to be regulated by CDK phosphorylation at this time, and its role in recruiting Pol ε and GINS remains unclear (indicated by question marks).
Unlike many replication factors, such as the ORC subunits, Cdt1, Cdc6 and MCMs, the presumptive equivalents of Dpb11, Sld2 and Sld3 in higher eukaryotes have very little sequence similarity with their yeast counterparts. TopBP1 has been identified as the human homologue of Dpb11, containing 8 BRCT repeats, and is required for the initiation of DNA replication[7]. The homologs of Sld2 and Sld3 are less well established, as there are no proteins with obvious sequence similarity and some of their functions may have been assumed by other proteins. For example, the BRCT repeats of TopBP1 can directly bind Cdc45 itself[8]. RecQ4 appears to be a good candidate for the human Sld2 homolog, possessing similarity in its N-terminal region, which is required for initiation of replication to occur. Oddly, unlike Sld2, RecQ4 is not required for GINS recruitment, and thus affects helicase initiation at some other step [9, 10]. While multiple TopBP1 interacting proteins have been identified, Treslin, also known as Ticrr, has been considered the most likely metazoan homologue of Sld3 as it interacts with TopBP1, has distant sequence homology with yeast Sld3[11], and is required for the recruitment of Cdc45 and for DNA replication in human cells [12].
Two groups have now characterized Treslin’s interaction with TopBP1 and demonstrate that the CDK-dependent regulation of the Treslin-TopBP1 interaction recapitulates that of Sld3- Dbp11 from budding yeast,[13, 14]. Both groups identify a central region of Treslin located between amino acids 790-1056 as strongly interacting with the 2 N-terminal BRCT repeats of TopBP1 homologous to those in Dpb11 that bind Sld3. Curiously, Kumagai et al also showed a weak interaction between TopBP1 with the N-terminus of Treslin, indicating there may be other domains in Treslin that also contribute to this recruitment. Digging further into the regulation of the interaction of Treslin with TopBP1, both publications demonstrate that phosphorylation by CDK of two conserved residues on Treslin, T969 and S100, is required for the interaction with TopBP1. CDK phosphorylation at these sites is functionally important as a T969A/S1001A mutant of Treslin is unable to rescue the inhibition of DNA replication when endogenous Treslin is depleted by siRNA. Boos et al. go on to demonstrate that the Treslin-TopBP1 interaction is inhibited by Chk1 activation following interruption of S-phase by hydroxyurea, much like Rad53 kinase regulates Sld3 in budding yeast[15, 16]. Thus, while the Sld2 and Sld3 proteins otherwise appear to evolve rapidly, conservation of the residues and domains that contribute to the interaction of the putative homologues of Dpb11 and Sld3 in higher eukaryotes demonstrates the essential nature of this step in activation of DNA replication. In addition, inhibition of Treslin function may be an important step by which the intra-S phase checkpoint inhibits origin firing.
While the Sld3-Dbp11 interaction is conserved between yeast and humans, the significance of the large differences between Treslin (which contains 1910 amino acids) and Sld3 (which contains 688 amino acids) still remains to be discovered. Amongst the 70 predicted CDK sites in Treslin, only 2 contribute to its interaction with TopBP1 and are required for its role in activating DNA replication. While CDK phosphorylation at additional sites still occurs in the S1001A/T969A mutant, it is unknown whether such sites have any regulatory potential. Additionally, there are other players involved in MCM activation in humans whose roles are not yet clear. GEMC1 and DUE-B are additional TopBP1 interacting proteins whose roles in Cdc45/GINS loading have yet to be dissected[17, 18]. Thus, it is likely that Treslin works with additional components to fulfill the function of yeast Sld3.
These two papers present convincing evidence in support of the assertion that Treslin is the functional ortholog of Sld3. It interacts with TopBP1 in a manner analogous to Sld3’s interaction with Dbp11. In further support of its role as the Sld3 ortholog, Kumagai et al. demonstrated that Treslin interacts with Cdc45 when overexpressed in human cells, as well as in nuclear lysates incubated with Xenopus laevis egg extracts[13]. Many questions still remain, however, regarding the similarities and differences between helicase activation in yeast and humans. Still, a perfect match for Sld2 in humans has not yet been determined, leaving the issue of how Pol ε and GINS are recruited to origins unclear, and further study of how RecQ4, GEMC1, and DUE-B are required for initiation is needed. Most significantly, the mechanism of how the recruitment of Cdc45 and GINS leads to helicase activity remains unknown and represents the next large step in understanding replication initiation in yeast and humans.
References
- 1.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 2.Ilves I, Petojevic T, Pesavento JJ, Botchan MR. Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Mol Cell. 2010;37:247–258. doi: 10.1016/j.molcel.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zegerman P, Diffley JF. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature. 2007;445:281–285. doi: 10.1038/nature05432. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature. 2007;445:328–332. doi: 10.1038/nature05465. [DOI] [PubMed] [Google Scholar]
- 5.Muramatsu S, Hirai K, Tak YS, Kamimura Y, Araki H. CDK-dependent complex formation between replication proteins Dpb11, Sld2, Pol (epsilon}, and GINS in budding yeast. Genes Dev. 2010;24:602–612. doi: 10.1101/gad.1883410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki H. Cyclin-dependent kinase-dependent initiation of chromosomal DNA replication. Curr Opin Cell Biol. 2010;22:766–771. doi: 10.1016/j.ceb.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010;24:1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt U, Wollmann Y, Franke C, Grosse F, Saluz HP, Hanel F. Characterization of the interaction between the human DNA topoisomerase IIbeta-binding protein 1 (TopBP1) and the cell division cycle 45 (Cdc45) protein. Biochem J. 2008;409:169–177. doi: 10.1042/BJ20070872. [DOI] [PubMed] [Google Scholar]
- 9.Sangrithi MN, Bernal JA, Madine M, Philpott A, Lee J, Dunphy WG, Venkitaraman AR. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund-Thomson syndrome. Cell. 2005;121:887–898. doi: 10.1016/j.cell.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Matsuno K, Kumano M, Kubota Y, Hashimoto Y, Takisawa H. The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase alpha in the initiation of DNA replication. Mol Cell Biol. 2006;26:4843–4852. doi: 10.1128/MCB.02267-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Pulido L, Diffley JF, Ponting CP. Homology explains the functional similarities of Treslin/Ticrr and Sld3. Curr Biol. 2010;20:R509–510. doi: 10.1016/j.cub.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai A, Shevchenko A, Dunphy WG. Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell. 2010;140:349–359. doi: 10.1016/j.cell.2009.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumagai A, Shevchenko A, Dunphy WG. Direct regulation of Treslin by cyclin-dependent kinase is essential for the onset of DNA replication. J Cell Biol. 2011;193:995–1007. doi: 10.1083/jcb.201102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boos D, Sanches-Pulido L, Rappas M, Pearl LH, Oliver AW, Ponting CP, Diffley JFX. Regulation of DNA replication through Sld3-Dpb11 interaction is conserved from yeast to humans. Current Biology. 2011 doi: 10.1016/j.cub.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 15.Zegerman P, Diffley JF. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature. 2010;467:474–478. doi: 10.1038/nature09373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature. 2010;467:479–483. doi: 10.1038/nature09377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balestrini A, Cosentino C, Errico A, Garner E, Costanzo V. GEMC1 is a TopBP1-interacting protein required for chromosomal DNA replication. Nat Cell Biol. 2010;12:484–491. doi: 10.1038/ncb2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury A, Liu G, Kemp M, Chen X, Katrangi N, Myers S, Ghosh M, Yao J, Gao Y, Bubulya P, et al. The DNA unwinding element binding protein DUE-B interacts with Cdc45 in preinitiation complex formation. Mol Cell Biol. 2010;30:1495–1507. doi: 10.1128/MCB.00710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


