Abstract
GTP cyclohydrolase 1 (GTPCH1) is the rate-limiting enzyme in de novo synthesis of tetrahydrobiopterin (BH4), an essential cofactor for endothelial nitric oxide synthase (eNOS) dictating at least partly, the balance of nitric oxide (NO) and superoxide (O2•−) produced by this enzyme. The aim of this study was to determine the effect of acute inhibition of GTPCH1 on BH4, eNOS function, and blood pressure in vivo. Exposure of bovine or mouse aortic endothelial cells to GTPCH1 inhibitors (DAHP or NAS) or GTPCH1- siRNA significantly reduced BH4 and NO levels, but increased superoxide (O2•−) levels. This increase was abolished by sepiapterin (BH4 precursor) or L-NAME (non-selective NOS inhibitor). Incubation of isolated murine aortas with DAHP or NAS impaired acetylcholine-induced endothelium-dependent relaxation, but not endothelium-independent relaxation. Aortas from GTPCH1 siRNA-injected mice, but not their control-siRNA injected counterparts, also exhibited impaired endothelium-dependent relaxation. BH4 reduction induced by GTPCH1 siRNA injection was associated with increased aortic levels of O2•−, 3-nitrotyrosine, and adhesion molecules (ICAM1 and VCAM1) as well as a significantly elevated systolic, diastolic, and mean blood pressure in C57BL6 mice. GTPCH1 siRNA was unable to elicit these effects in eNOS−/− mice. Sepiapterin supplementation, which had no effect on high blood pressure in eNOS−/− mice, partially reversed GTPCH1 siRNA-induced elevation of blood pressure in wild type mice. In conclusion, GTPCH1 via BH4 maintains normal blood pressure and endothelial function in vivo by preserving NO synthesis by eNOS.
Keywords: Hypertension, Nitric Oxide, Superoxide Anions, Endothelial Nitric Oxide Synthase, Tetrahydrobiopterin, GTP-cyclohydrolase 1, Oxidative Stress
Introduction
Nitric oxide (NO) produced by endothelial nitric oxide synthase (eNOS) is essential for cardiovascular homeostasis owing to its anti-inflammatory, anti-thrombotic, anti-proliferative, and antioxidant effects.1,2 A critical determinant of eNOS activity is the availability of tetrahydrobiopterin (BH4).3 Recent studies4,5 indicate that eNOS must be fully saturated with BH4 to completely couple NADPH oxidation to NO production. Under conditions of limited BH4, eNOS functions in an “uncoupled” state in which NAD(P)H-derived electrons are added to molecular oxygen, rather than L-arginine, leading to the production of superoxide (O2•−) and H2O2.6 In this uncoupled state, eNOS exacerbates oxidative stress initiated by other reactive oxygen species (ROS)-generating enzymes (e.g., NADPH oxidase). eNOS uncoupling has been implicated in a number of vascular diseases, such as hypertension,7 atherosclerosis,8 and diabetes,4 and is often accompanied by a reduction in BH4 levels. BH4 supplementation in vessel rings from animals with atherosclerosis, diabetes, or hypertension reduces endothelial dysfunction.9–11 In addition, BH4 administration augments NO-mediated vasodilatation in diabetic patients12 and smokers.13 These findings support the hypothesis that intracellular BH4 concentrations dictate, at least in part, the balance of NO and O2•− produced by eNOS in diseased blood vessels. Although the concept that BH4 deficiency uncouples eNOS is well established, how cardiovascular risk factors results in BH4 deficiency remains poorly defined.
Intracellular BH4 levels are regulated through de novo synthesis of BH4 from GTP cyclohydrolase 1 (GTPCH1), a homodecameric protein consisting of 25-kDa subunits in mammalian cells.14 This enzyme catalyzes the rearrangement of GTP to dihydroneopterin triphosphate, a species subsequently converted to BH4 through the sequential action of 6-pyruvoyltetrahydrobiopterin synthase and sepiapterin reductase.15 GTPCH1 is the rate-limiting enzyme in most tissues, making it the major determinant of intracellular BH4 content. Although several studies have investigated neuronal regulation of the BH4-dependent enzyme tyrosine hydroxylase by GTPCH, the role of GTPCH1 in maintaining eNOS function has not yet been investigated in vivo. Several in vitro studies have shown that GTPCH1 gene transfer reverses BH4 deficiency, increases NO synthesis, and restores eNOS function in both endothelial cells and vessels isolated from diabetic rats16 and rats with low-renin hypertension.17 However, these studies did not establish a cause-effect relationship between GTPCH1 and eNOS uncoupling in vivo because the amount of BH4 generated from GTPCH1 overexpression system are several folds higher than those in physiological conditions, which might increase eNOS function or NO bioactivity by scavenging superoxide anions (O2•−). In addition, the role of GTPCH1 in the regulation of blood pressure and vascular tone were poorly characterized. The current study was undertaken to investigate the effect of acute GTPCH1 inhibition on BH4 deficiency, eNOS function and blood pressure in vivo. Our results demonstrate that acute inhibition of GTPCH1 causes high blood pressure via BH4 deficiency/eNOS uncoupling.
Methods and Materials
A full description of materials, animals, and methods used, including cell culture, blood pressure measurement, measurement of BH4, superoxide anions, and nitric oxide (NO), organ chamber, western blot, reverse transcription–polymerase chain reaction (RT-PCR) and immunohistochemistry, can be found in the online-only Data Supplement. Please see http://hyper.ahajournals.org.
Blood pressure measurement
To measure the blood pressure in mice, a PE-10 catheter was inserted into the right carotid artery. Blood pressure was monitored with a pressure transducer and recorded with a software in computer after anaesthesia.
Measurement of BH4, superoxide anions, and NO
The levels of BH4 and total biopterins were determined by HPLC. O2•− production in cultured cells or mice aortas was detected by using dihydroethidium (DHE) fluorescence/HPLC assay. NO release in cultured cells was detected by using the DAF fluorescent probe.
Organ chamber
Mice aorta rings were mounted in organ bath in Kreb’s solution. Contractile response was evoked by U46619. At the plateau of contraction, accumulative ACh or SNP was added into the organ bath to induce the endothelium dependent or independent relaxation.
Statistical analysis
Data are reported as mean±SEM. All data were analyzed using a one- or two-way ANOVA, followed by Bonferroni’s post test analysis. P<0.05 was considered significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
GTPCH1 inhibition reduces total biopterin and BH4 levels in endothelial cells
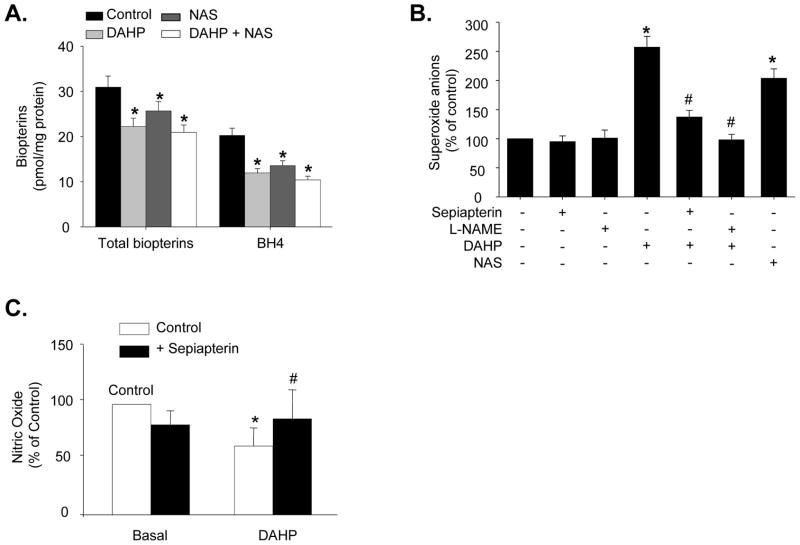
We first determined if pharmacological inhibition of GTPCH1 alters the levels of both total biopterins and BH4 in BAECs. Treatment of BAECs with the chemical inhibitors DAHP (10 mM) or NAS (1 mM) for 24 h did not alter cell viability (data not shown). However, both treatments, applied alone or in combination, reduced both total biopterins (BH4 + BH2 + other biopterins) and BH4 levels, compared to the control treatment (Figure 1A). In addition, neither DAHP nor NAS altered the ratios of BH2 to BH4 (data not shown), a common index for oxidative BH4 reductin.18 Overall, our results suggest that GTPCH1 inhibition by DAHP or NAS caused BH4 deficiency mainly by inhibiting de novo synthesis of biopterins (BH4 plus BH2).
Figure 1.
Pharmacological inhibition of GTPCH1 decreases NO release and increases eNOS-derived O2•− in cultured bovine aortic endothelial cells (BAECs). (A) Total biopterins and BH4 levels following a 24 h exposure to DAHP and/or NAS. N=5 *P<0.05 control vs. NAS/DHAP or both; (B) Effect of L-NAME or sepiapterin on induction of O2•− production by DAHP or NAS (24 h exposure). N=5, *P<0.05 NAS or DAHP vs. control; #P<0.05 DAHP treated vs. DAHP plus L-NAME or sepiapterin. (C) NO production following treatment with DAHP or DAHP+sepiapterin. Data are expressed as mean±SEM. N=5, *P<0.05 DAHP vs. control; #P<0.05 DAHP treated vs. DAHP plus sepiapterin.
Pharmacological inhibition of GTPCH1 uncouples eNOS in endothelial cells
Recent studies19–22 in transgenic mice and endothelial cells have shown that an imbalance between intracellular eNOS levels and BH4 availability leads to uncoupling of eNOS, resulting in greater O2•− production and diminished NO release. As shown in Figure S1, exposure of BAECs to DAHP (10 mM) for 24 h increased O2•− production by 52.36%, as detected by DHE fluorescence. DAHP (5 and 10 mM) simultaneously induced 26.70% and 39.58% decreases in NO release. Treatment of BAECs with the non-selective eNOS inhibitor, L-NAME, did not alter baseline O2•− levels but reduced DAHP-induced O2•− release by 61.87%, showing that DAHP-induced O2•− production was eNOS-dependent (Figure 1B).
Uncoupling of eNOS by GTPCH1 inhibition is reversed by sepiapterin in endothelial cells
To determine whether the reduced NO release observed in DAHP-treated BAECs was due to BH4 deficiency, we treated BAECs with sepiapterin, a compound that can be converted to BH4 through a GTPCH-independent salvage pathway. Sepiapterin (10 μM) almost restored NO release in DAHP-treated BAECs, but did not alter NO release in the absence of DAHP (Figure 1C). Similarly, sepiapterin abolished DAHP-triggered O2•− production, but had no effect on O2•− levels in non DAHP-treated BAECs (Figure 1B).
Gene silencing of GTPCH1 uncouples eNOS in endothelial cells
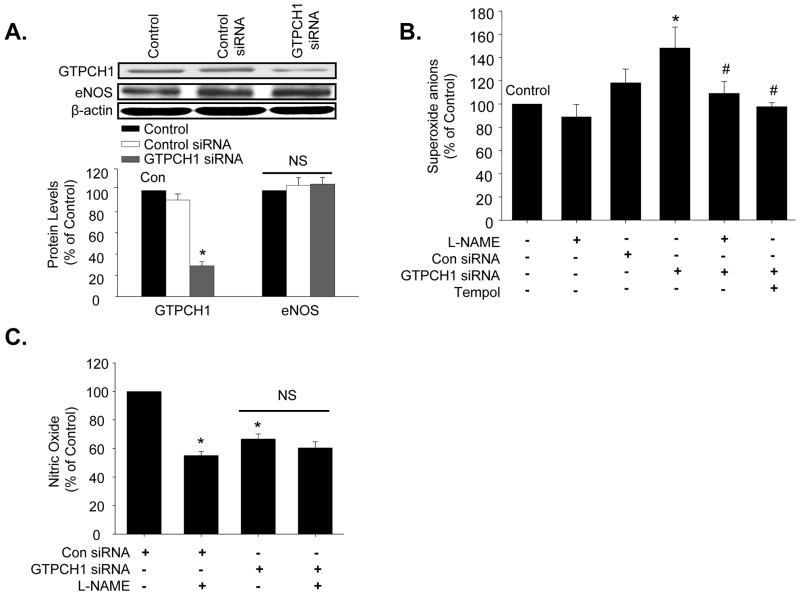
To exclude non-specific effects of DAHP and NAS, we tested the effect of GTPCH1 small interference RNA (siRNA) on O2•− and NO production. As no specific siRNA for bovine GTPCH1 exists, MAECs were used in these experiments. As shown in Figure 2A, transfection of GTPCH1 siRNA, but not control siRNA, reduced GTPCH1 protein levels by 68.73%. Levels of eNOS were not affected by GTPCH1 siRNA. Analysis of O2•− production in these cells revealed that control siRNA did not alter O2•− levels. In contrast, GTPCH1 siRNA increased O2•− levels by 25.40%, with this increase being completely blocked by either L-NAME or Tempol (1 mM), a SOD mimetic (Figure 2B). GTPCH1 siRNA-induced O2•− production was accompanied by 42.23% reduction in NO release (Figure 2C), compared with control siRNA. In addition, L-NAME inhibited the NO release from control siRNA transfection MAECs, but not in GTPCH1 siRNA-transfection MAECs (Figure 2C).
Figure 2.
GTPCH1-specific siRNA decreases NO release and increases eNOS-derived O2•− in isolated mouse aortic endothelial cells (MAECs). (A) Western blot analysis of GTPCH1 and eNOS in control and siRNA-transfected cells. The blot is representative of three blots from three independent experiments. N=3 *P<0.05 GTPCH1 siRNA vs. control or control siRNA, NS indicates P>0.05. (B) Effect of GTPCH1 siRNA on O2•− production. A subset of siRNA-transfected MAECs were treated with the eNOS inhibitor, L-NAME, or Tempol, a SOD mimetic. MAECs were incubated with 1 mM L-NAME or 1 mM Tempol for 24 h after the siRNA transfection. N=3 *P<0.05 GTPCH1 siRNA vs. control or control siRNA; n=5 #P<0.05 GTPCH1 siRNA vs. GTPCH1-siRNA plus L-NAME or plus Tempol; (C) NO release in siRNA transfected MAECs incubated with L-NAME or without L-NAME. Data are expressed as mean±SEM. N=3, *P<0.05 vs. control siRNA, NS indicates P>0.05.
GTPCH1 inhibition or gene silencing results in an impairment of endothelium-dependent relaxation in isolated aortas that is sepiapterin-reversible
The effect of NAS and DAHP on endothelium-dependent and endothelium-independent vasorelaxation was determined in isolated aortas. Aortas were incubated with DAHP (10 mM) or NAS (1 mM) for 24 h in EBM at 37°C prior to the vasomotor reactivity assays. As shown in Figure S2A, Ach-induced relaxation (endothelium-dependent vasorelaxation) was lower in DAHP-treated aortas (42.12±9.25%) than in vehicle-treated aortas (86.96±8.04%, P<0.05). NAS, like DAHP, suppressed Ach-induced endothelium-dependent relaxation (54.53±11.07 vs. 86.96±8.04%, P<0.05, Figure S2B). In contrast, SNP-induced relaxation (endothelium-independent vasorelaxation) was not altered by the presence of DAHP or NAS (Figures S2C and S2D).
Inclusion of sepiapterin in the chamber fluid did not affect endothelium-dependent relaxation (data not shown). However, sepiapterin partially restored relaxation in the presence of DAHP, as evidenced by an Emax of 67.23±6.33% in sepiapterin+DAHP-treated aortas and 42.12±9.21% in DAHP-treated aortas (P<0.05, Figure S2A). Sepiapterin had a similar effect in NAS-treated aortas (Emax: 65.96±6.70% vs 54.53±11.07%, P<0.05, Figure S2B).
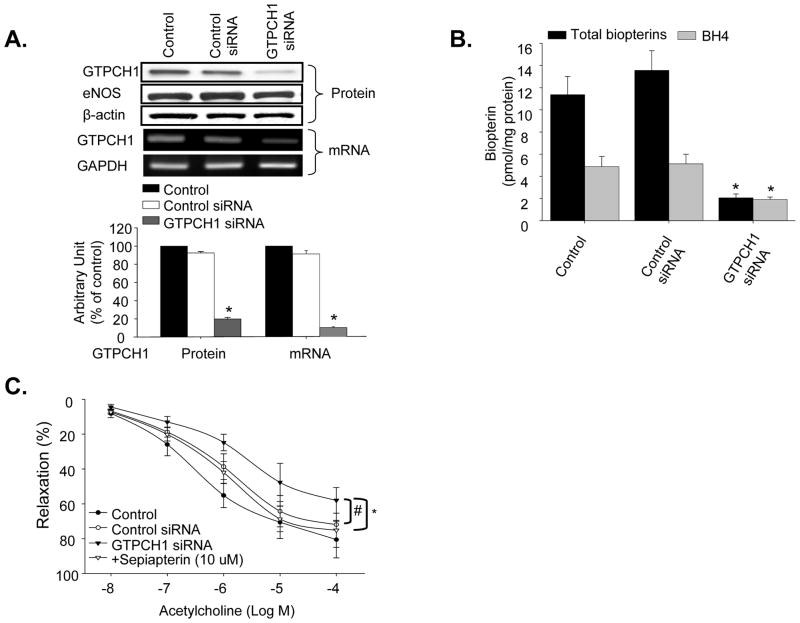
Next, the effects of chemical GTPCH1 inhibition on endothelium-dependent relaxation were confirmed using in vivo GTPCH1 gene silencing. Retro-orbital injection of GTPCH1 siRNA decreased aortic levels of GTPCH1 protein by 80%, while control siRNA had no effect (Figure 3A). Neither GTPCH1 siRNA nor control siRNA altered aortic expression of eNOS. The gene silence of GTPCH1 in vivo by GTPCH1 siRNA injection was further confirmed in RT-PCR detection of GTPCH mRNA. As shown in Figure 3A, GTPCH1 siRNA specially decreased GTPCH1 mRNA level, compared to control siRNA. Analysis of total biopterin and BH4 levels revealed that GTPCH1 knockdown reduced total biopterin levels in the aorta to 32.07% of the levels in the control siRNA group (P<0.05, Figure 3B). Aortic BH4 was also reduced by GTPCH1 knockdown, with levels in this group being 60.06% of the levels in the control-siRNA group (P<0.05, Figure 3B).
Figure 3.
In vivo GTPCH1 knockdown decreases both BH4 and total biopterins. (A) Western blot and RT-PCR analysis of GTPCH1 or eNOS in aorta from control siRNA- and GTPCH1 siRNA-injected mice. N=5, *P<0.05 GTPCH1 siRNA vs. control siRNA or control, NS indicates P>0.05. (B) Total biopterins and BH4 levels in aortas from siRNA-transfected mice. N=5, *P<0.05 GTPCH1 siRNA vs. control siRNA or control; (C) Endothelium–dependent relaxation in isolated aortas from control and GTPCH1 siRNA-injected mice. In a subset of experiments, aortas were incubated with sepiapterin for 1 h. Data are expressed as mean±SEM. N=5, *P<0.05 GTPCH1 siRNA vs. control siRNA or control; n=5 #P<0.05 GTPCH1 siRNA vs. GTPCH1 treated with sepiapterin.
Vasoreactivity assays of aortas from siRNA-injected mice revealed that Ach-induced relaxation was lower in aortas from GTPCH1 siRNA-injected mice (Emax: 58.01±7.38%) than in those from control siRNA-injected animals (Emax: 71.92±2.47%, P<0.05, Figure 3C). Relaxation in aortas from GTPCH1 siRNA-injected mice was improved by the presence of sepiapterin (Emax: 75.16±9.80%, P<0.05), while vasorelaxation in the control siRNA group was unaffected by this treatment (Figure 3C). SNP-induced relaxation was identical in aortas from both groups (data not shown).
In vivo GTPCH1 gene silencing generates aortic oxidative stress via eNOS-uncoupling
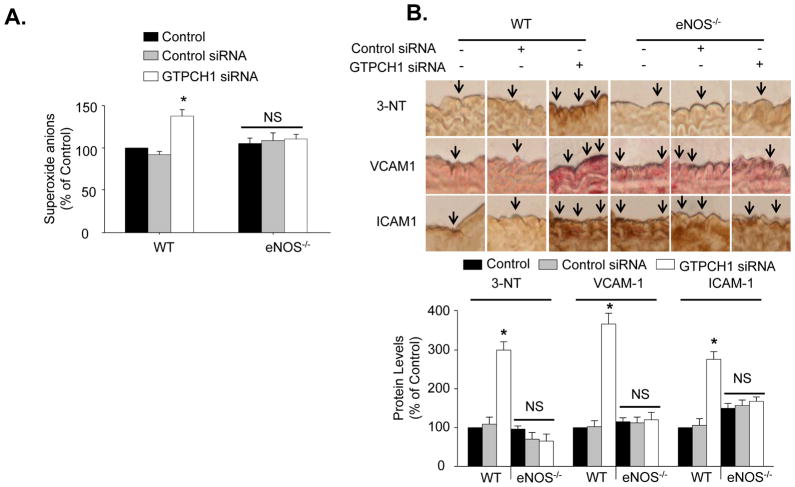
GTPCH1 siRNA increased aortic O2•− levels by 30.26%, as compared with aortic O2•− levels in the control siRNA-treated group (Figure 4A). Accordingly, GTPCH1 siRNA, but not control siRNA, increased immunohistochemical staining for 3-nitrotyrosine (by 200%), a footprint for reactive nitrogen species such as ONOO− (a potent oxidant formed by O2•− and NO).23 Staining for ICAM1 and VCAM1 were also increased (by 267% and 176%) by GTPCH siRNA, with control siRNA having no affect on the expressions of these proteins (Figure 4B). These results were further confirmed in western blot analysis (Figure S3).
Figure 4.
In vivo GTPCH1 knockdown induces eNOS-dependent increases in superoxide anions, ICAM-1, VCAM-1 and 3-nitrotyrosine (3-NT). (A) Aortic O2•− production in WT and eNOS−/− mice injected with GTPCH1 siRNA or control siRNA. N=5 *P<0.05, control siRNA vs. GTPCH1-siRNA, NS indicates P>0.05. (B) Immunocytochemical staining of 3-NT, VCAM1, and ICAM1 in wild type and eNOS−/− mice treated with control siRNA or GTPCH1-specific siRNA. N=5 *P<0.05, control siRNA vs. GTPCH1-siRNA, NS indicates P>0.05.
Next, the contribution of eNOS uncoupling in vascular oxidant stress and inflammation was investigated using eNOS-null mice. In contrast to aorta from wild type mice, eNOS-null aorta did not exhibit increased levels of O2•− or 3-nitrotyrosine in response to GTPCH1 siRNA (Figure 4A and 4B). The expression of both ICAM-1 and VCAM-1 was slightly higher in eNOS-null mice than in wild type mice. However, GTPCH1 siRNA did not alter levels of either protein in eNOS-null animals (Figure 4B).
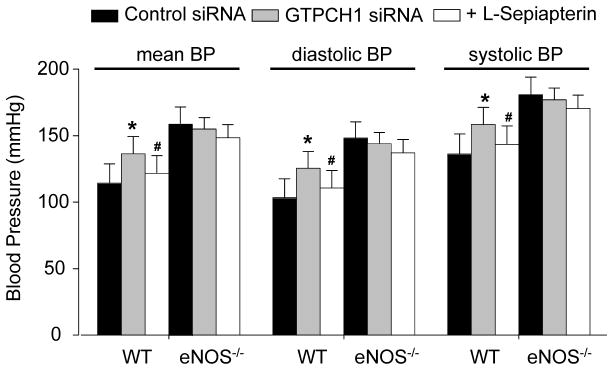
In vivo GTPCH1 gene silencing elevates systemic blood pressure in an eNOS-dependent and sepiapterin-reversible manner
NO is known to regulate vascular tone, and eNOS depletion results in spontaneous hypertension.24–27 Thus, we analyzed the effect of in vivo GTPCH1 gene silencing on systemic blood pressure. As shown in Figure 5, mean blood pressure (BP) was greater in GTPCH1 siRNA-treated wild type mice (136.81±2.45 mmHg) than those in the control siRNA-treated counterparts (114.28±4.48 mmHg, P<0.05). Systolic pressure and diastolic pressure also showed significant differences between control siRNA-treated and GTPCH1 siRNA-treated WT mice. The mean, systolic, and diastolic blood pressure were significantly elevated in eNOS-null mice compared to those in C57BL6 wild type animals (Figure 5), similar to Huang et al’s results.24 As expected, administration of neither GTPCH1 siRNA nor control siRNA altered the mean, systolic, and diastolic blood pressure in in eNOS-null mice.
Figure 5.
In vivo GTPCH1 knockdown elevates arterial blood pressure in an eNOS-dependent manner. Mean blood pressure (BP), systolic blood pressure, and diastolic blood pressure in control or GTPCH1 siRNA-injected wild type (WT) or eNOS−/− mice supplementation with or without sepiapterin (10 mg/kg/day for 7 days, I.P). The blood pressure was measured by a carotid catheter method. Data are expressed as mean±SEM (n=4 or 5). *P<0.05 GTPCH1 siRNA vs. control siRNA, #P<0.05 GTPCH1 siRNA vs. GTPCH1 siRNA treated with sepiapterin.
Administration of sepiapterin had no effects on the mean, systolic, and diastolic BP in control siRNA- or GTPCH1 siRNA-treated eNOS-null mice (Figure 5). Similar results were obtained in untreated or control siRNA-treated wild type mice (data not shown). However, in wild type animals receiving GTPCH1 siRNA, sepiapterin reduced the mean, systolic, and diastolic BP by 15.46% (P<0.05, Figure 5).
Discussion
The major finding of the present study is that GTPCH1 likely plays an essential role in maintaining normal blood pressure. In wild type mice, eNOS uncoupling induced by GTPCH1 inhibition significantly elevated systemic blood pressure. Sepiapterin partially reversed this effect in these animals, but did not alter blood pressure in eNOS-null mice. These findings indicate that the blood pressure-lowering effect of sepiapterin is eNOS-dependent and that sepiapterin has no direct effect on blood pressure. Because conduit arteries like aorta do not regulate total peripheral resistance, the hypertensive phenotype of mice administered GTPCH1 siRNA and the blood pressure lowering effect of sepiapterin in these animals may be attributable to changes in resistance artery structure and function. Indeed, initial studies of the vascular phenotype in the hyperphenylalaninemic mouse mutant (hph-1), which displays a 90% GTPCH1 deficiency, of hph-1 mouse have demonstrated that BH4 deficiency causes pulmonary hypertension, even under normoxic conditions, and greatly increases susceptibility to hypoxia-induced pulmonary hypertension. In contrast, augmentation of endothelial BH4 synthesis through targeted transgenic overexpression of GTP-cyclohydrolase I (GTPCH1) prevents hypoxia-induced pulmonary hypertension.28 Similarly, restoration of endothelial BH4 in hph-1 mice by crossing these animals with GTPCH1 transgenic mice rescues pulmonary hypertension induced by systemic BH4 deficiency.28 In this study, lung BH4 availability dose-dependently controlled pulmonary vascular tone, right ventricular hypertrophy, and vascular structural remodeling under both normoxic and hypoxic conditions. We have found that GTPCH inhibition has dual effects (i.e., increased oxidative stress and decreased NO release) that lead to high blood pressure in vivo. This finding is in line with a recent study in humans showing that the common GTPCH1 variant, C+243T, predicted NO excretion, with the most extreme diastolic and systolic blood pressure values occurring in females independently of catecholamine secretion.29 In contrast, genetic variation in eNOS (Glu298Asp) does not influence the renal NO excretion (P>0.1).30 Our results are consistent with these reports, as they suggest that GTPCH1 is the rate-limiting enzyme determining in vivo NO biosynthesis and consequently, vascular tone. Thus, GTPCH1 might play essential role in maintaining endothelial function through regulation of eNOS function.
Perspectives
It has been observed in animal models of cardiovascular diseases including hypertension and diabetes that the endothelial nitric oxide synthase (eNOS), crucial in maintaining endothelium homeostasis, has been transformed from a protective enzyme to a contributor of oxidative stress, known as eNOS uncoupling. It is generally agreed that lack of tetrahydrobiopeterin (BH4), the essential co-factor of eNOS, plays a causal role in the development of eNOS uncoupling. However, how BH4 shortage is developed is poorly understood. In this publication, we have discovered that selective inhibition of GTP-cyclohydrolase, a rate-limiting enzyme in BH4 de novo synthesis, causes hypertension and vascular inflammation. The clinical implications of the present study is supported by a recent study29 shows a common GTPCH1 variant C+243T in the 3′-untranslated region (3′-UTRs) predicts NO excretion with the most extreme BP values of diastolic and systolic BP in females though not catecholamine secretion. Our results support human data that GTPCH1 is a key player in maintaining eNOS function and systolic blood pressure. The finding that GTPCH1 inhibition increases systemic blood pressure through eNOS uncoupling may have broad applications for cardiovascular diseases, since endothelial GTPCH1 protein levels, GTPCH-1 activity, and BH4 levels are reduced in a number of vascular diseases including atherosclerosis31,32 and diabetes.16,33–35 GTPCH1 inhibition might be a common mechanism for vascular endothelial dysfunction in vascular diseases including hypertension, diabetes, and atherosclerosis.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants (HL079584, HL074399, HL080499, and HL089920), a research award from the American Diabetes Association, a research award from the Juvenile Diabetes Research Foundation, a research award from the Oklahoma Center for Advancement of Science and Technology, Research Award from Research Management Group, and Travis endowed chair in Endocrinology, University of Oklahoma Health Sciences Center. Dr. Jian Xu is supported by postdoctoral fellowship from American Heart Association.
Footnotes
Disclosures
None
References
- 1.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 2.Sandrim VC, Yugar-Toledo JC, Desta Z, Flockhart DA, Moreno HJ, Tanus-Santos JE. Endothelial nitric oxide synthase haplotypes are related to blood pressure elevation, but not to resistance to antihypertensive drug therapy. J Hypertens. 2006;24:2393–2397. doi: 10.1097/01.hjh.0000251899.47626.4f. [DOI] [PubMed] [Google Scholar]
- 3.Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res. 1999;43:274–278. doi: 10.1016/s0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- 4.Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F1144–1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 6.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 7.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukai T. Endothelial GTPCH in eNOS uncoupling and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1493–1495. doi: 10.1161/ATVBAHA.107.148239. [DOI] [PubMed] [Google Scholar]
- 9.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 10.Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol. 2003;140:701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–332. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- 12.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 13.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 14.Franscini N, Bachli EB, Blau N, Fischler M, Walter RB, Schaffner A, Schoedon G. Functional tetrahydrobiopterin synthesis in human platelets. Circulation. 2004;110:186–192. doi: 10.1161/01.CIR.0000134281.82972.57. [DOI] [PubMed] [Google Scholar]
- 15.Kolinsky MA, Gross SS. The mechanism of potent GTP cyclohydrolase I inhibition by 2,4-diamino-6-hydroxypyrimidine: requirement of the GTP cyclohydrolase I feedback regulatory protein. J Biol Chem. 2004;279:40677–40682. doi: 10.1074/jbc.M405370200. [DOI] [PubMed] [Google Scholar]
- 16.Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, Wood MK, Wade LA, Wu G. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. Faseb J. 2004;18:1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- 17.Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation. 2003;108:1238–1245. doi: 10.1161/01.CIR.0000089082.40285.C3. [DOI] [PubMed] [Google Scholar]
- 18.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 19.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 20.Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, Yokoyama M, Kawashima S, Channon KM. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 21.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, Verhaar MC, Joles JA. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 23.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 24.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 25.Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mice with targeted deletion of eNOS develop hyperdynamic circulation associated with portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1074–1081. doi: 10.1152/ajpgi.00145.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kubis N, Richer C, Domergue V, Giudicelli JF, Levy BI. Role of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS3pt−/−) J Hypertens. 2002;20:1581–1587. doi: 10.1097/00004872-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto Y, Saito Y, Kajiyama N, Yoshimura M, Shimasaki Y, Nakayama M, Kamitani S, Harada M, Ishikawa M, Kuwahara K, Ogawa E, Hamanaka I, Takahashi N, Kaneshige T, Teraoka H, Akamizu T, Azuma N, Yoshimasa Y, Yoshimasa T, Itoh H, Masuda I, Yasue H, Nakao K. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension. 1998;32:3–8. doi: 10.1161/01.hyp.32.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–2133. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Rao F, Zhang K, Khandrika S, Das M, Vaingankar SM, Bao X, Rana BK, Smith DW, Wessel J, Salem RM, Rodriguez-Flores JL, Mahata SK, Schork NJ, Ziegler MG, O’Connor DT. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dell’Omo G, Penno G, Pucci L, Fotino C, Lucchesi D, Del Prato S, Pedrinelli R. Lack of association between endothelial nitric oxide synthase gene polymorphisms, microalbuminuria and endothelial dysfunction in hypertensive men. J Hypertens. 2007;25:1389–1395. doi: 10.1097/HJH.0b013e3281268548. [DOI] [PubMed] [Google Scholar]
- 31.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 32.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 33.Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 34.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 35.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.