Abstract
Background
Muscle strains are one of the most common injuries treated by physicians. Standard conservative therapy for acute muscle strains usually involves short-term rest, ice, and non-steroidal anti-inflammatory medications, but there is no clear consensus on how to accelerate recovery.
Hypothesis
Local delivery of platelet-rich plasma (PRP) to injured muscles hastens recovery of function.
Study Design
Controlled laboratory study. We used an established animal model of injury to test the effects of autologous platelet-rich plasma PRP on recovery of contractile function.
Methods
In vivo, the tibialis anterior muscles (TA) of anesthetized Sprague-Dawley rats were injured by a single (large strain) lengthening contraction or multiple (small strain) lengthening contractions, both of which result in a significant injury. The TA was injected with either PRP, PPP (platelet-poor plasma, as a sham treatment), or received no treatment.
Results
Both injury protocols yield a similar loss of force. The PRP only had a beneficial effect at one time point after the single contraction injury protocol. However, PRP had a beneficial effect at several time points after the multiple contraction injury protocol, and resulted in a faster recovery time to full contractile function. The sham injections had no effect compared to no treatment.
Conclusion
Local delivery of PRP can shorten recovery time after a muscle strain injury. Recovery of muscle from the high repetition protocol has already been shown to require myogenesis, whereas recovery from a single strain does not. This difference in mechanism of recovery may explain why PRP was more effective in the high repetition protocol, as PRP is rich in growth factors that can stimulate myogenesis. Since autologous blood products are safe, PRP may be a useful product to use in clinical treatment of muscle injuries.
Keywords: injury, strain, muscle strain, muscle damage, skeletal muscle, muscle regeneration
Introduction
Muscle injury occurs from either acute or repetitive trauma and results in a decreased ability to produce force, which does not recover after a short period of rest. When an activated muscle lengthens because the external load exceeds the tension generated by the muscle contraction, this is termed a lengthening (“eccentric”) contraction. Submaximal lengthening contractions are used in everyday life, but it is well known that high force lengthening contractions are associated with muscle damage and pain.13, 18, 30 The force generated during a maximal lengthening contraction is approximately twofold the force developed during a maximal isometric contraction.9, 18
The generation of high force by muscles is a goal of strength training; this is evident in training protocols that use lengthening contractions, or “negatives”, to increase strength. Although lengthening contractions are common and often occur without causing damage, high force lengthening contractions are more likely to produce damage than either isometric or concentric contractions, resulting in pain and damage.16, 18, 23, 30 In clinical lexicon, the injury resulting from a high force lengthening contraction is termed a “muscle strain”; such strains are the most common cause of muscle injuries.10, 37
In order to conduct a well controlled study, we developed an animal model of muscle injury and a reproducible mechanism for generating muscle strain. We recently reported that recovery of contractile function following injury by a single, large-strain lengthening contraction involves repair of damaged sarcolemma with minimal myogenesis, whereas recovery from multiple, small-strain lengthening contractions requires myogenesis, with minimal sarcolemmal repair.25 Here we used both protocols in our established injury model to test the effects of autologous platelet-rich plasma (PRP) on recovery in the tibialis anterior muscle of rats. PRP is isolated by a technique involving centrifugation of whole blood, allowing extraction of the specific part of the plasma containing a high concentration of platelets. These platelets are rich in growth factors that can stimulate myogenesis,7, 27, 32, 36 and mitigate inflammation.8, 26 We hypothesize that the local delivery of PRP to injured skeletal muscles accelerates recovery and we present data that from experiments that support this hypothesis.
Materials and Methods
Injury Model
All protocols were approved by the University [blinded copy] Institutional Animal Care & Use Committee (IACUC). Adult male Sprague-Dawley rats (n = 72) weighing 341 ± 21 gm (approximately 4months of age) were anesthetized with isoflurane (2% with oxygen flow rate of 0.5 L/min). The Sprague-Dawley rats were inbred, allowing us to consider them syngeneic. The injury model results in a significant and reproducible injury and has been described previously.11, 23–25, 33 In brief, anesthesia was confirmed by lack of response to a normally painful stimulus (pinching the foot); the left hindlimb was stabilized and the foot was secured onto a footplate. The axis of the footplate was attached to a stepper motor (T8904 NMB Technologies), a potentiometer to record angular position, and a torque sensor (QWFK-8M, Sensotec) to measure torque. The fibular nerve was stimulated via subcutaneous needle electrodes (Harvard Apparatus 723742, Cambridge, MA) and proper electrode position was determined by a series of isometric twitches. In addition to visual confirmation of isolated dorsiflexion, an increase in twitch torque in response to increasing voltage indicated that opposing plantarflexor muscles were not being simultaneously stimulated.3 A custom program was used (Labview version 8.5, National Instruments, Austin, TX) to synchronize contractile activation and onset of ankle rotation. Impulses generated by an S48 square pulse stimulator (Grass Instruments, West Warwick, RI) were 1 ms in duration and passed through a PSIU6 stimulator isolation unit (Grass Instruments, West Warwick, RI).
To induce injury in the tibialis anterior muscle (TA), we superimposed a lengthening contraction onto a maximal isometric contraction (Figure 2), using either a single repetition (large strain) or multiple repetitions (small strain). Specifically, a maximal isometric contraction was obtained in the dorsiflexors and after 200 ms they were lengthened through an arc of motion at 900°/sec. The majority of torque produced by the dorsiflexors is from the TA15 and we have shown previously that this model results in injury to this muscle.11, 23–25 The TA remained stimulated throughout lengthening, and was injured using one of two protocols: a single lengthening contraction through a 90° arc, or 45 lengthening contractions through a 60° arc. For multiple repetitions, the lengthening contractions were spaced 2 mins apart.
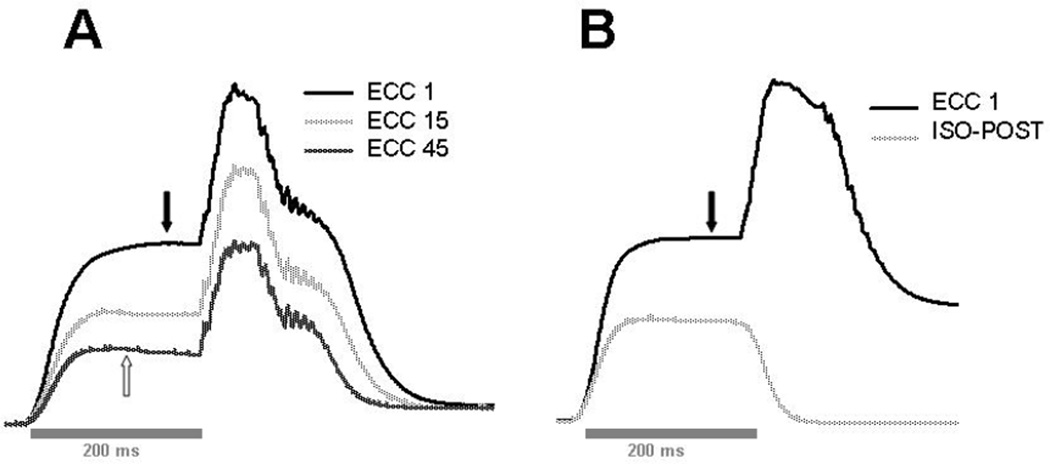
Figure 2.
Representative trace recordings of torque from the lengthening contractions. For both single and multiple repetitions, muscles were stimulated for 200 ms to induce a peak isometric contraction prior to lengthening by the foot plate. Maximal isometric torque (without lengthening) was measured before injury (not shown, but equal to the plateau of the isometric portion of the trace recordings, indicated by filled arrows). A: superimposed recordings from the multiple repetition protocol (a 60° arc of motion) showing the first (ECC 1), middle (ECC 15), and last (ECC 45) eccentric contractions. Note that even with injury, the eccentric torque is still relatively high compared to isometric torque (the peak isometric torque measured after injury is not shown, but is similar to the isometric portion of the last trace recording, indicated by open arrow) B: superimposed recordings of the single repetition protocol (ECC 1, a 90° arc of motion) and the isometric contraction to measure torque loss after injury (ISO-POST).
Outcome measures
For both protocols, a maximal isometric contraction (200 ms duration) of the dorsiflexors was used to measure maximal torque before injury. For each animal, maximal isometric torque was also measured 4 mins post injury (to measure force lost due to injury). Animals were returned to their cages after recovery from anesthesia and maximal isometric torque was retested under anesthesia at selected time points (3, 7, 14, and 21 days after injury). All isometric contractions were performed at the same point in the range of motion (with the foot orthogonal to the tibia, considered 0°).
After functional data were collected, tibialis anterior muscles were harvested from the anesthetized rat, snap frozen in liquid nitrogen, and stored at −80° C. The animal was then euthanized by carbon dioxide inhalation or with pentobarbital sodium (200mg/kg) administered intraperitoneally.
PRP
20 ml of whole blood was collected from 5 adult male Sprague-Dawley rats (withdrawn from the femoral vein, renal vein, or cardiac puncture). The syngeneic nature of the Sprague-Dawley inbred rat allowed us to consider the blood of one animal autologous to blood from another animal.36 Autologous platelet-rich plasma (PRP) was then separated from the blood using the Symphony™ II Platelet Concentration System (Depuy, a Johnson & Johnson Company). The centrifugation results in the formation of two layers within the plasma: a platelet-poor plasma component (PPP), and a platelet-rich plasma component (PRP). The PPP supernatant was carefully removed and used as a control vehicle. The remaining PRP was conditioned using 10 secs of high frequency ultrasound to lyse platelets and release growth factors, thereby enriching the PRP prior to injection.36 One-hundred µl of PRP were injected into the TA of the injured hind limb in each rat of the treated group at days 0 (day of injury), 3, 5, and 7. Both the conditioned PRP and the PPP were refrigerated and used within a few days.
ELISA assays (R & D Systems, Minneapolis) were performed following the manufacturer’s instructions, to determine if PRP is enriched in myogenic growth factors. Both PRP and PPP were separated and subjected to enzyme-linked immunosorbent assays (ELISAs) to detect and to quantify the presence of platelet-derived growth factor (PDGF) and insulin growth factor-1 (IGF-1). In addition, we also assayed “conditioned” plasma, as described in the methods.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Two µg of total RNA were isolated from frozen rat tibialis anterior muscle with TRIzol (Invitrogen, Carlsbad, CA) and was reversed-transcribed with Superscript II First strand Synthesis System (Invitrogen), per the manufacturer’s instructions. The resulting cDNA was used as a template for PCR amplification. The primers for rat MyoD were (FW) 5’- CACTCCTCCAATTGTCC -3’ and (REV) 5’- CTTATTTCCAACACCTGAGC -3’. The primers for rat myogenin were 5’- CACCTTCCCAGATGAAACC -3’ and 5’- AAGAAGTCACCCCAAGAGC- 3’. The primers for rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were 5’- ACGACCCCTTCATTGACC- 3’ and 5’- ATCACGCCACAGCTTTCCC- 3’. The NCBI reference numbers are M24393, M017008, and M84176 for MyoD, myogenin, and GAPDH, respectively. PCR products were run on 1% agarose gels, stained with ethidium bromide, and scanned. The intensity of bands was quantified using NIH Image J software and relative expression was quantified based on the total GAPDH expressed in a particular muscle sample.
SDS PAGE and Immunoblotting
Western blot analysis was used to assess semi-quantitative changes in the levels of MyoD and myogenin proteins. Extracts of unfixed TA muscles were snap frozen in liquid nitrogen, pulverized, and homogenized with a PowerGen 125 homogenizer (Fisher Scientific, Waltham MA) at a w/v ratio of 0.05 in homogenate buffer (10 mM NaPO4, 2 mM EDTA, 140 mM NaCl, 1% NP40, pH 7.4) with protease inhibitors (Complete Protease Inhibitor Tablets, Roche Diagnostics). Samples were boiled and centrifuged and the protein concentration of the supernatant was determined using a Bradford assay. Samples were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 4–12% gradient gel and transferred onto nitrocellulose electrophoretically. The nitrocellulose was blocked in 3% milk-PTA, washed, and then incubated with anti-myoD (sc 760) or anti-myogenin (sc 576) polyclonal [rabbit] antibodies (Santa Cruz Biotechnology, Inc.) × 6 h. Excess antibodies were washed off and the nitrocellulose was incubated with donkey anti-rabbit secondary antibodies conjugated to alkaline phosphatase (Jackson Laboratories). The excess secondary antibodies were then washed off and the bands were visualized by a chemiluminescent assay method (Tropix, Bedford, MA).
HE Staining
Tissue was frozen in isopentane-cooled liquid nitrogen and transverse sections were cut on a cryostat (10 µm thickness). Sections were collected onto glass slides (Superfrost Plus; VWR, West Chester, PA) and stained with hematoxylin and eosin for counting centrally nucleated fibers. Sections were randomized and viewed at 100X magnification in a Zeiss Axioskop light microscope and pictures were taken with a digital camera (AxioCam HR using AxioVision 3.0). Each optical field contained an average of 38 ± 7 fibers and more than 45 fields were counted per muscle.
Statistics
Contractile data from each experiment were analyzed using a single factor analysis of variance (ANOVA, Sigma-Stat, San Rafael, CA). When a significant ratio was found, a Tukey post-hoc analysis was performed to determine where significant differences had occurred (P < 0.05). Each ELISA contained three replicates and the results were analyzed with a one-way ANOVA test. For statistical analysis of RT-PCR, densitometry was performed for mRNA of myoD or myogenin and GAPDH for each blot. mRNA/GADPH ratios were calculated and analyzed using the Holm-Sidak pairwise multiple comparison test.
Results
Assessment of Growth Factors
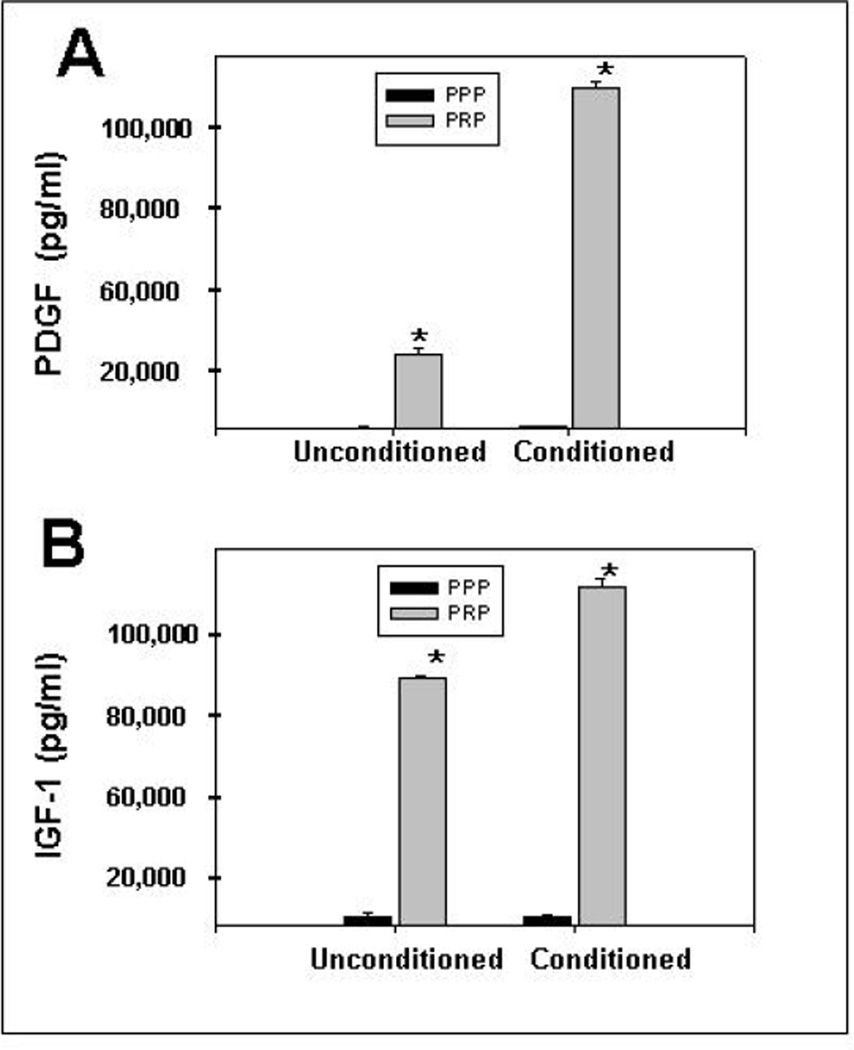
We quantified growth factors such as PDGF and IGF-1, both known to stimulate myogenesis, in PRP and PPP. We examined conditioned (sonicated) and unconditioned PRP and PPP using enzyme-linked immunosorbent assay (ELISAs) kits. Figure 1 shows that the concentrations of PDGF and IGF-1 (20,745 ± 520 pg/ml and 65,550 ± 500 pg/ml, respectively) in PRP were significantly higher than in PPP (P < .05), and markedly increased (a five-fold increase in PDGF and a 27% increase in IGF-1) by mechanical perturbation of the platelets (see methods). Because of the significant increase of growth factors with conditioning, we tested the effects of conditioned PRP on muscle injury, as stated in the methods.
Figure 1.
Results of enzyme-linked immunosorbent (ELISA) assays to confirm that platelet-rich plasma (PRP) is enriched in myogenic growth factors PDGF and IDF-1. Both PRP and platelet-poor plasma (PPP) were separated and subjected to ELISAs to detect and to quantify the presence of growth factors, such as platelet-derived growth factor (PDGF, grey bar to left in A) and insulin growth factor-1 (IGF-1, grey bar to left in B), both known to stimulate myogenesis. In addition, we also assayed “conditioned” plasma (grey bars to right), as described in the methods. The PRP was clearly rich in the 2 tested growth factors, and was further enriched by conditioning. Results are shown compared to ELISA assays of platelet-poor plasma (PPP).
* = P < .05
Functional Recovery
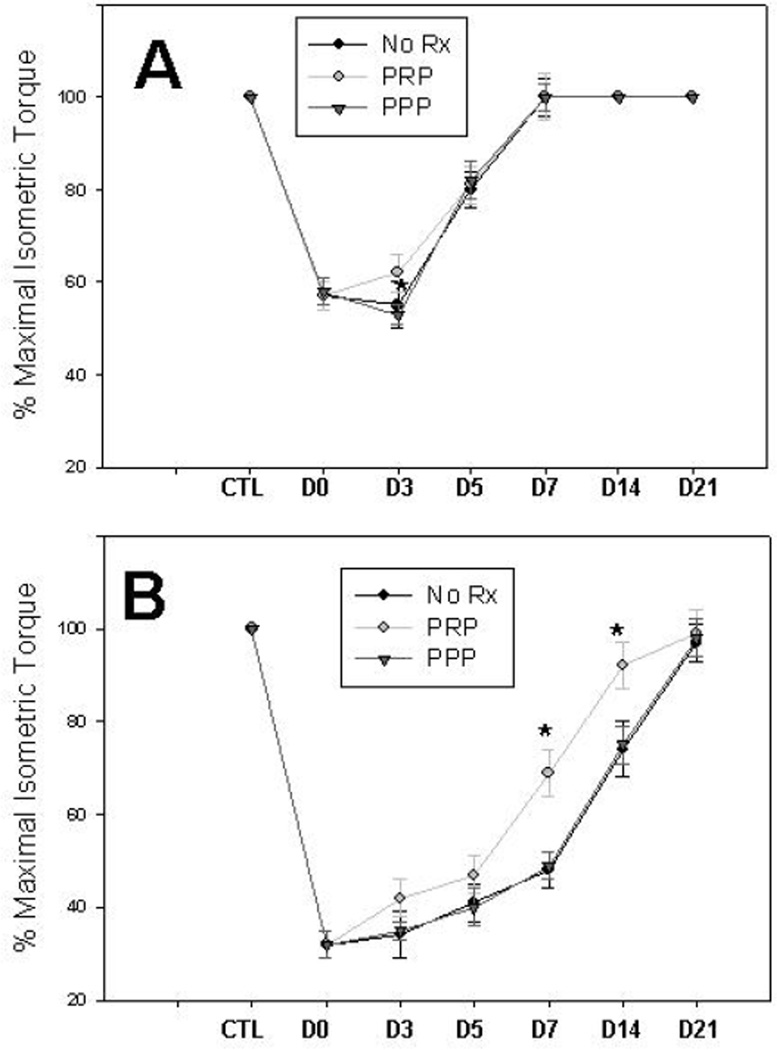
We induced injury and studied recovery of function in the whole ankle dorsiflexor group. We harvested and then examined TA muscles, which account for most of the torque generated by this muscle group.15 The peak isometric torque generated by each animal was measured prior to injury and was considered “100%” for that animal (the mean ± SD torque for all animals was 44 ± 5 Nmm). For each animal, peak isometric torque was also measured after injury. The torque measurements immediately after injury and during recovery were expressed as the “percent of maximal isometric torque” (out of 100%) for a given animal. The mean percentage of recovery at each time point is presented in Figure 3. Both injury protocols resulted in a significant loss of muscle function followed by gradual recovery (Figures 2 and 3). The multiple repetition protocol results in a larger force deficit and takes longer to recover.25 PRP treatment had little effect on the single repetition protocol, but did ameliorate the force loss at one time point (day 3, Figure 3A), even though the animals had received only one injection by his time point (injections were immediately after injury and on days 3, 5, and 7 post injury). In the multiple repetition protocol, PRP treatment resulted in a marked improvement in contractile function at several time points (days 7 and 14), effectively shortening the time to full recovery from 21 days to 14 days (Figure 3B).
Figure 3.
Maximal torque was measured in each animal before injury (CTL) and immediately after injury (D0), as well as at selected time points after injury (days 3, 5, 7, 14, and 21). “100%” represents peak torque before injury. The percentage of recovery for each animal was calculated and the mean is expressed as the “percent of maximal isometric torque” (out of 100%) at each time point. A: after a single repetition through a 90° arc of motion, there is a significant drop in torque and gradual recovery to full contractile function by day 7. PRP had a significant effect only on day 3 (n = 8 animals each group). B: after multiple repetitions through a 60° arc of motion, there is a significant loss of torque followed by a gradual recovery by day 21. PRP had a significant effect on days 7 and 14, by which time the injured muscle had returned to pre-injury level of strength (n = 8 animals each group).
“No Rx” = injury only (no injections)
* = P < .05
Muscle Regeneration
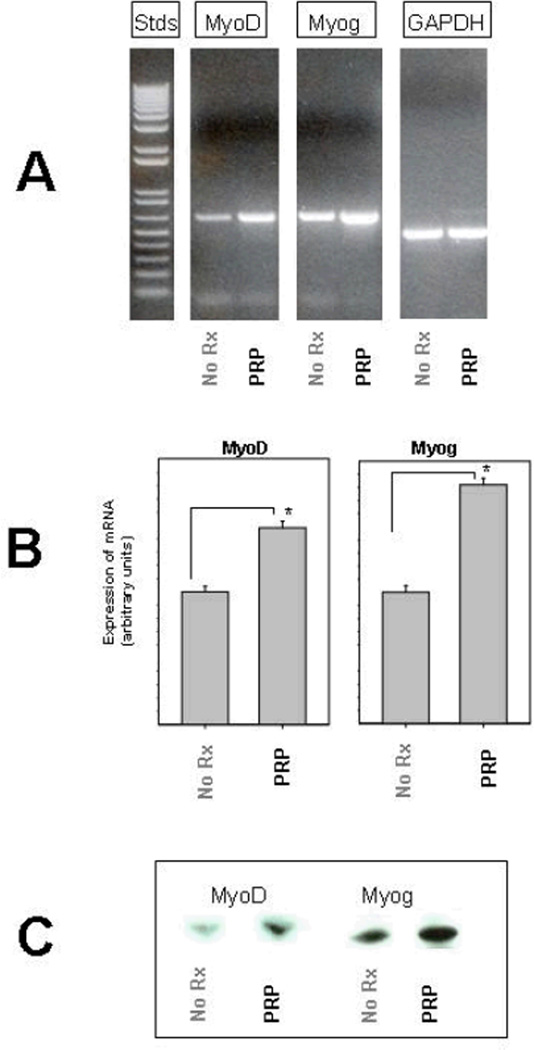
TA muscles were harvested from at least 2 animals at each time point for each protocol. We used two different markers to assay muscle regeneration. The first was to assay levels of myoD and myogenin. These are major muscle regulatory factors (MRFs), and are only expressed when satellite cells are activated to proliferate.6 Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The RT-PCR data (Figure 4A) show that mRNA transcripts for both of these muscle-specific transcription factors were present 7 days after injury, but clearly elevated in the muscles treated with PRP compared to sham treated muscles (PPP) or controls (not shown). Muscle tissue from other time points was also analyzed (data not shown), but the most obvious differences in mRNA levels occurred on day 7. The GAPDH transcript was tested and did not show altered transcription after muscle injury. Densitometry was performed and normalized to GAPDH to compare relative expression of myoD and myogenin (Figure 4B). In order to confirm that changes were not just in the expression of mRNA, we performed western blotting and probed for myoD and myogenin. The results confirm that these two markers of muscle regeneration were increased in muscle samples injected with PRP (Figure 4C).
Figure 4.
Myogenesis after the multiple repetition injury. A: 2 µg of total RNA was isolated from frozen rat tibialis anterior muscle and reverse transcriptase polymerase chain reaction (RT-PCR) was performed at various time points after injury using primers for two different genes involved in muscle regeneration (myoD and myogenin), as well as a gene used as an internal control (GAPDH). The gel shows representative PCR products from muscles injected with PPP or PRP 7 days after injury. B: Densitometry of the bands was performed and the results quantified relative to expression of the total GAPDH expressed in a particular muscle sample, as described in the Materials and Methods section. Thus, the histogram shows the mRNA transcript levels of myoD and myogenin from muscles injected with PPP or PRP (n = 3). C: Muscle samples from the same time point (day 3) were homogenized and proteins separated by electrophoresis. Immunoblots confirmed the increase protein expression of myoD (38 kD) and myogenin (36 kD).
* = P < .05
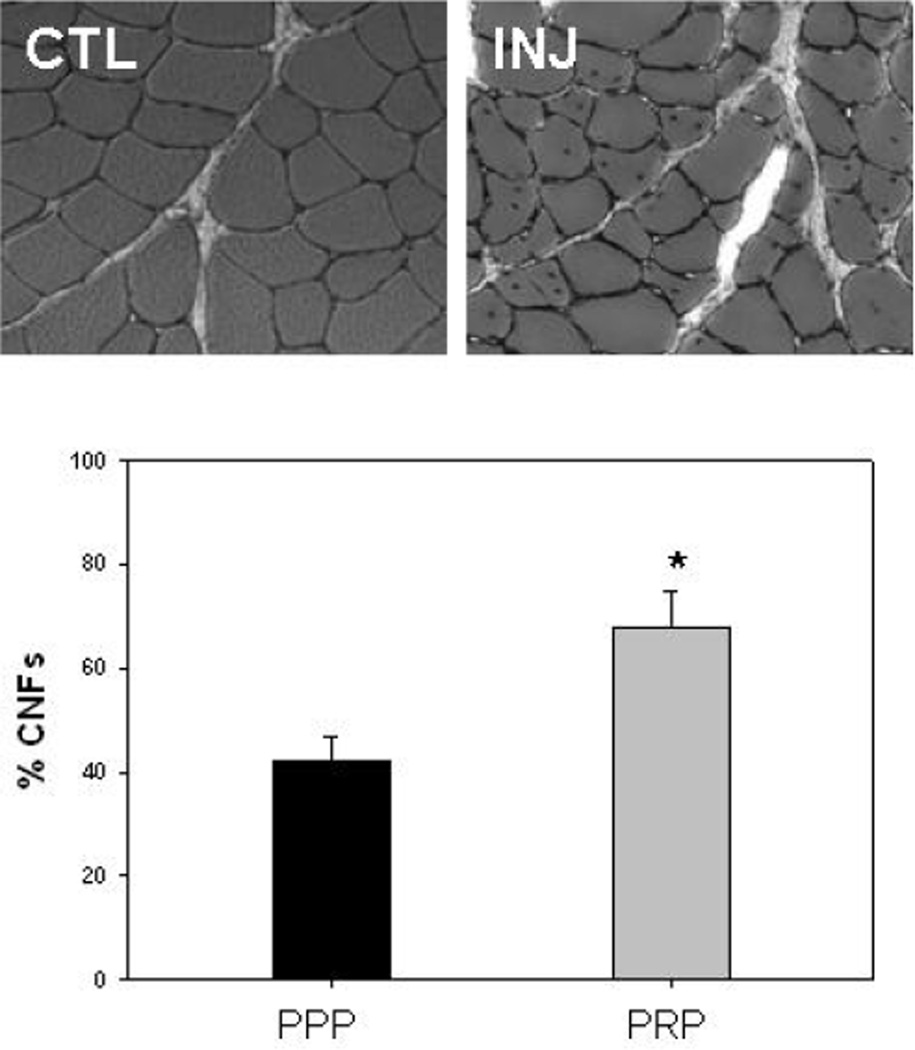
The second assay we used to detect myogenesis was quantification of centrally nucleated fibers (CNFs). Muscle fibers are multi-nucleated with the nuclei located at the periphery of the fibers, but within weeks after injury, nuclei are observed in the cytoplasm (Figure 5A) and these CNFs are widely accepted as a marker of muscle regeneration.6, 12, 16 As shown previously,25 the number of fibers with CNFs was higher in the multiple repetition protocol, peaking at about 2 weeks after injury (the single repetition protocol does not result in a significant increase in the number of CNFs25 – data not shown). PRP-treated TAs had an even higher number of CNFs than injured TAs with a sham treatment (PPP) or no treatment (not shown), indicating that the PRP was effective in stimulating further myogenesis (Figure 5B).
Figure 5.
Tibialis anterior muscles were harvested at various time points after injury. The micrograph (top) shows representative cross-sections of non-injured control muscle (CTL) and of injured muscle (INJ) 14 days after the multiple repetition injury, when some of the normally peripherally located nuclei (CTL) are seen in the middle of the fiber (INJ). These centrally nucleated fibers (CNFs) are a marker of muscle regeneration and the peak in CNFs occurred on day 14. The histogram (bottom) shows the percentage of fibers that had CNFs at this time point in the injured TA muscles injected with PPP (black bar) and PRP (grey bar).
* = P < .05
Discussion
Muscle strains are one of the most common complaints treated by physicians10, 17 and account for the majority of all sports-related injuries.5, 37 Except for complete ruptures of muscles, displaced avulsions, and recalcitrant symptoms from myositis ossificans, almost all muscle injuries are uniformly treated with non-operative therapy. Standard conservative therapy for either acute muscle injuries usually involves rest, ice, compression, and elevation (RICE). Beyond the principle of short-term rest and ice, there is no clear consensus on treatment of muscle injuries.5
In the laboratory setting, investigators have used toxins, lacerations, freeze damage, and contusions to study muscle damage, but by far the majority of muscle injuries during sports are attributable to excessive strain of an activated muscle (e.g., forceful lengthening, or “eccentric”, contractions).5, 10, 17 Because muscle strains are so common, we use an animal model of contraction-induced injury. Some models that remove part or all of the muscle to perform ‘load to failure’ or other such tests have certainly yielded useful information, but this is less representative of normal physiology. We used an in vivo model where the neurovascular supply and anatomical attachments are undisturbed, inducing a muscle strain injury under conditions that are as similar to the clinical scenario as possible, while preserving control over the biomechanical parameters in order to yield a consistent injury. One of the reasons muscle injury is difficult to study is that there are so many models of injury. Even with the in vivo model, the response to injury can vary widely based on the timing of activation, the amount of strain, the number of repetitions, and level of activation.13, 20, 24, 25
We compared the effects of conditioned PRP on two in vivo protocols of contraction-induced injury. The 90° arc of plantarflexion utilizes a large part of the available range of motion, but this magnitude of stretch is required to induce a detectable and reliable injury after just a single contraction, which we used to mimic an acute strain.24 The 60° arc of plantarflexion yields a significant and reliable injury, but only with multiple repetitions. Despite the different protocols, they yield a similar force loss,25 presumably because the amount of active strain (“arc of motion” in this study) is a key determinant of muscle injury.20
Previously, we found that recovery of muscle contractile function following injury by a single, large-strain lengthening contraction involves sarcolemma repair, whereas recovery from multiple, small-strain lengthening contractions requires muscle regeneration, with minimal sarcolemmal repair.25 Here we used both protocols to test the effects of autologous platelet-rich plasma (PRP) on recovery. In the single repetition protocol, use of PRP did show some improvement in the ability of the muscle to generate force, but only at the day 3 time point. Otherwise, the overall recovery—and time to full return of function—was not altered. Alternatively, in the multiple repetition protocol, use of PRP resulted in significant improvement at several time points, as well as a quicker return to full function. This is most likely due to the enhancement of myogenesis, a process required to recover from this protocol.
Myogenesis is not restricted to prenatal development, but also occurs in regenerating muscle after some injuries. A large body of evidence suggests that individual growth factors play a role during muscle regeneration/myogenesis. IGF-1, FGF-2, HGF, and TGF-β1 are thought to be key regulators for myogenesis. For example, IGF-1 is able to stimulate the proliferation and differentiation of myoblasts (precursors of muscle cells) and improves muscle regeneration in mouse skeletal muscles.27 In vivo, FGF-2 enhances the diameter and number of regenerating fibers.19 In vitro, HGF is able to activate quiescent satellite cells,1 the stem cells of skeletal muscle committed to a myogenic lineage. TGF-β1 supports other growth factors, specifically platelet-derived growth factor (PDGF), which stimulates satellite cell activation.14, 28, 31 Satellite cells are dormant in healthy skeletal muscle, but can be stimulated by injury to proliferate or differentiate into mononucleated myoblasts, which then fuse to form multinucleated myotubes. These myotubes can then form new skeletal muscle, replacing damaged or lost tissue.4, 16, 34
PRP contains up to 8 times the concentration of platelets found in whole blood7 and these platelets contain α-granules, which can release a multitude of growth factors, such as PDGF, IGF-1, TGF-β, FGF, VEGF, EGF, and HGF.7, 8, 32 The fact that PRP contains several different growth factors, present in physiological proportions, is an appealing benefit compared to using isolated growth factors. Other advantages are that it is relatively simple and easy to obtain PRP from a human blood sample, and there is little risk of developing an immune response from autologous PRP.
Given the common nature of muscle strain injuries, a treatment that can improve recovery time could have a tremendous impact in athletics. PRP can be isolated from a centrifuge about the size of a small microwave oven; this can increase the convenience and feasibility of using this treatment method in an athletic training room. After an injury, whole blood could be drawn from an athlete, centrifuged, and autologous PRP could be retrieved and injected at the site of injury. This would be a convenient and cost effective way to administer concentrated growth factors locally. Injection of isolated growth factors, even if effective, could be prohibitively more expensive. A potential limitation to the use of PRP in the clinical setting, at least with elite athletes, is the concern expressed by the World Anti-Doping Association (WADA) regarding the use of growth factors in sports.7 One possible solution for professional athletes is to obtain an exemption from a WADA-approved Anti-Doping Organization for therapeutic use.
The mechanisms for the improved muscle recovery resulting from the use of PRP after injury need to be elucidated. We have shown via RT-PCR analysis and counts of CNFs that myogenesis is enhanced with the use of PRP; however this does not preclude other possibilities. PRP may alter cytokine release, limit inflammation, or have other effects not yet examined. Although we did not attempt to quantify inflammation, the inflammatory process is likely altered in the presence of PRP. This may explain the improvement seen only at day 3 of recovery after the single repetition protocol, as this is when inflammation peaks after a muscle strain.29 Another possibility is that muscle fiber membrane damage and/or repair is altered, or that damage and/or repair of contractile and or structural proteins is altered by the use of PRP. These and other hypotheses have not been rigorously tested.
We operationally defined muscle injury as a loss in the ability of the muscle to produce force. Torque of a muscle is represented by the equation: T = F * d, where T is torque, F is muscle force, and d is the moment arm of the muscle. Since we use a maximal tetanic contraction and we measured torque at a fixed ankle position, our measure of torque ultimately reflects muscle force.2, 21, 22, 35 After the initial injury, there is sometimes another drop in force, as seen after our single repetition protocol (Figure 3A). This “secondary injury” is thought to be due to the increase in inflammation that occurs several days after the muscle strain, and is also the presumed cause of delayed onset muscle soreness (DOMS).9 We did not see a secondary injury with the multiple repetition protocol. Although it is not clear why, it could be that we missed a further decline in force production due to our selected time points. For example, force in the multiple repetition protocol may have dropped even further than the initial injury, but within 24 or even 48 hours, well before our day 3 measurement.
An animal model provides several obvious advantages, such as control over force of contraction, type of contraction, lengthening velocity, diet, activity level, and access to tissues for analysis. Animal models also provide control over many threats to internal validity of a study, such as ‘history’, ‘selection bias’, and ‘maturation’. Yet, this study has several limitations. The first and most obvious one is that findings from animal studies are not always applicable to humans. Second, the high level of control over experimental parameters (timing of contraction, arc of motion, etc) is less representative of the wide array of injuries that occur in humans. For example, we use maximal stimulation to recruit all motor units within the muscle (to obtain a consistent injury), but this is not representative of the graded recruitment of motor units that occurs in humans. A third possible limitation is that we did not identify which components of PRP are responsible for the improvements we found. However, because PRP is easily obtainable through several commercial centrifuge devices and no negative side-effects have been reported, it may not be necessary to isolate the specific growth factor(s) within PRP that account for the enhanced recovery from injury.
Like any experiment, this work raises new questions, which we hope to address in future work. We did not examine the effects of conditioned PPP. ELISA assays indicate no discernible difference with conditioning (Figure 1), but the ELISA results are based on only 2 representative growth factors. It is possible that a small fraction of platelets persisted in the PPP, containing other growth factors that could potentially affect recovery from injury. Because of the significant increase in growth factors with high frequency ultrasound (conditioning), our “PRP treatment” consisted of conditioned PRP. This allowed us to minimize the number of animals needed for the study and improved our chances of finding an effect. It is likely that the platelets from the unconditioned PRP would rupture during injection and still release their contents, either immediately or soon after injection, but we did not test this.
Another question is what type of strain injury is PRP appropriate for? Our results do not determine if PRP would be beneficial for an acute strain versus an overuse type injury in humans. It is clear that injuries that require myogenesis are better candidates for PRP therapy, but muscle injury is highly variable between subjects. Finally, we do not know what the optimal dose and delivery method should be. We did not test various permutations of frequency, amount, and duration of injections, and it is likely that other delivery methods may work as well, or better than repeated injections.
To our knowledge, this is the first study to use PRP in a model of muscle strain injury. In summary, we demonstrated that PRP extract can hasten recovery from a muscle strain injury, and that enhanced myogenesis is the probable mechanism underlying this effect. Delivery of growth factors at the site of injury is a potential therapy to treat muscle injuries, and since autologous blood products are safe, PRP may be a useful product to use in treatment of muscle injuries.
Footnotes
No potential conflict of interest declared.
References
- 1.Allen RE, Sheehan SM, Taylor RG, et al. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 2.Ashton-Miller JA, He Y, Kadhiresan VA, et al. An apparatus to measure in vivo biomechanical behavior of dorsi- and plantarflexors of mouse ankle. J Appl Physiol. 1992;72:1205–1211. doi: 10.1152/jappl.1992.72.3.1205. [DOI] [PubMed] [Google Scholar]
- 3.Barash IA, Mathew L, Ryan AF, et al. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- 4.Best TM, Hunter KD. Muscle injury and repair. Phys Med Rehabil Clin N Am. 2000;11:251–266. [PubMed] [Google Scholar]
- 5.Chan YS, Li Y, Foster W, et al. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- 6.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 7.Creaney L, Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med. 2008;42:314–320. doi: 10.1136/bjsm.2007.040071. [DOI] [PubMed] [Google Scholar]
- 8.El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther. 1993;73:911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- 10.Garrett WE., Jr Muscle strain injuries. Am J Sports Med. 1996;24:S2–S8. [PubMed] [Google Scholar]
- 11.Hakim M, Hage W, Lovering RM, et al. Dexamethasone and Recovery of Contractile Tension after a Muscle Injury. Clin Orthop Relat Res. 2005;439:235–242. doi: 10.1097/01.blo.0000177716.70404.f9. [DOI] [PubMed] [Google Scholar]
- 12.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A:822–832. [PubMed] [Google Scholar]
- 13.Hunter KD, Faulkner JA. Pliometric contraction-induced injury of mouse skeletal muscle: effect of initial length. J Appl Physiol. 1997;82:278–283. doi: 10.1152/jappl.1997.82.1.278. [DOI] [PubMed] [Google Scholar]
- 14.Husmann I, Soulet L, Gautron J, et al. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- 15.Ingalls CP, Warren GL, Zhang JZ, et al. Dihydropyridine and ryanodine receptor binding after eccentric contractions in mouse skeletal muscle. J Appl Physiol. 2004;96:1619–1625. doi: 10.1152/japplphysiol.00084.2003. [DOI] [PubMed] [Google Scholar]
- 16.Jarvinen TA, Jarvinen TL, Kaariainen M, et al. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 17.Kirkendall DT, Garrett WE., Jr Clinical perspectives regarding eccentric muscle injury. Clin Orthop Relat Res. 2002:S81–S89. doi: 10.1097/00003086-200210001-00010. [DOI] [PubMed] [Google Scholar]
- 18.LaStayo PC, Woolf JM, Lewek MD, et al. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther. 2003;33:557–571. doi: 10.2519/jospt.2003.33.10.557. [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur JP, Sebille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol. 1995;57:85–91. doi: 10.1016/0165-5728(94)00166-l. [DOI] [PubMed] [Google Scholar]
- 20.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1993;74:520–526. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- 21.Lieber RL, Schmitz MC, Mishra DK, et al. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J Appl Physiol. 1994;77:1926–1934. doi: 10.1152/jappl.1994.77.4.1926. [DOI] [PubMed] [Google Scholar]
- 22.Lieber RL, Shah S, Friden J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop. 2002:S90–S99. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- 23.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovering RM, Hakim M, Moorman CT, III, et al. The contribution of contractile pre-activation to loss of function after a single lengthening contraction. J Biomech. 2005;38:1501–1507. doi: 10.1016/j.jbiomech.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovering RM, Roche JA, Bloch RJ, et al. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch Phys Med Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Meijer H, Reinecke J, Becker C, et al. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res. 2003;52:404–407. doi: 10.1007/s00011-003-1197-1. [DOI] [PubMed] [Google Scholar]
- 27.Menetrey J, Kasemkijwattana C, Day CS, et al. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br. 2000;82:131–137. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen-Hamilton M. Transforming growth factor-beta and its actions on cellular growth and differentiation. Curr Top Dev Biol. 1990;24:95–136. [PubMed] [Google Scholar]
- 29.Pizza FX, Peterson JM, Baas JH, et al. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol. 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33:98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 32.Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 33.Stone MR, O'Neill A, Lovering RM, et al. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci. 2007;120:3999–4008. doi: 10.1242/jcs.009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Willems ME, Stauber WT. Force deficits after repeated stretches of activated skeletal muscles in female and male rats. Acta Physiol Scand. 2001;172:63–67. doi: 10.1046/j.1365-201X.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 36.Wright-Carpenter T, Opolon P, Appell HJ, et al. Treatment of muscle injuries by local administration of autologous conditioned serum: animal experiments using a muscle contusion model. Int J Sports Med. 2004;25:582–587. doi: 10.1055/s-2004-821303. [DOI] [PubMed] [Google Scholar]
- 37.Zarins B, Ciullo JV. ACute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2:167–182. [PubMed] [Google Scholar]