Abstract
Objective
To study the expression and function of adiponectin and its receptors in mouse and human follicle cells and in early embryo development.
Design
Whole ovaries, granulosa cells and cumulus oocyte complexes were isolated from immature mice prior to and during hormone-induced ovulation and used to analyze the expression of adiponectin, its receptors and ovulation-related genes. Human cumulus cells and granulose cells were isolated from patients undergoing IVF procedures.
Patients
Women were in IVF programs in Japan and the United States.
Interventions
None
Main Outcome Measures
Expression of adiponectin receptors and fertility.
Setting
Adiponectin is a potent cytokine that is often at low levels in serum of women with polycystic ovarian syndrome (PCOS) compared to fertile women. Adiponectin may impact fertility and early embryo development by acting on ovarian cells.
Results
Adiponectin expression is absent/low in mouse and human granulosa cells and cumulus cells. Adiponectin receptors are hormonally regulated in mouse granulosa and cumulus cells in vivo and in culture. Adiponectin differentially alters the expression of Adipor1/Adipor2 as well as steroidogenic-, ovulation- and apoptosis related-genes in cumulus cells versus granulosa cells. Adiponectin enhances oocyte maturation and early embryo development in mouse and human IVF procedures.
Conclusion
Adiponectin can modulate not only follicle growth, but also embryo development in mouse and human.
Keywords: adiponectin, adiponectin receptors (Adipor1/r2), cumulus cells, granulosa cells, oocyte, IVF
INTRODUCTION
Adipose tissue is now known to be an important part of the endocrine system and releases potent hormones known as adipokines, such as leptin and adiponectin, that regulate energy homeostasis, lipid metabolism and neuroendocrine functions (1, 2). Although adiponectin has previously been thought to be expressed exclusively by adipocytes (1), recent reports have indicated that adiponectin and its receptors Adipor1 and Adipor2, may be expressed in ovarian cells (3–5). These observations raised the intriguing possibility that adiponectin might be regulated by pituitary and/or ovarian factors and exert endocrine or paracrine functions within the ovary (6).
Several reports in different species, including human, indicate that adiponectin can modulate granulosa cell steroidogenesis (3, 4, 7) and the expression of genes associated with ovulation (6, 7). There is also evidence that the functions of the two adiponectin receptors in granulosa cells may differ (8). Reducing ADIPOR1 led to apoptosis of human KGN granulosa cells whereas reduction of ADIPOR2 decreased steroidogenesis. Mutant mouse models indicate that depletion of adiponectin or adiponectin receptors decreases insulin sensitivity without reported changes in fertility whereas over-expression of adiponectin leads to increased insulin sensitivity and infertility or subfertility (6, 9–12). However, the mechanisms by which adiponectin modulates fertility and whether or not the disruption of ADIPOR1/R2 causes any changes in ovarian function have not yet been addressed. Of clinical relevance, adiponectin levels are reduced in women with polycystic ovarian syndrome (PCOS) compared to fertile women(13, 14), possibly associated with elevated androgens (15, 16), obesity and altered adipose tissue functions (17). Altered responses of ovarian cells to insulin and insulin-like growth factor 1 (IGF1) in PCOS patients may be linked, in part, to obesity and reduced levels of serum adiponectin (18).
Collectively, these observations provide evidence that adiponectin might impact metabolic homeostasis in granulosa and cumulus cells, thereby modulating the expression of factors that control steroidogenesis, ovulation and apoptosis. Among the many transcription factors that are regulated by the insulin/IGF and FSH pathways, FOXO1 and FOXO3 are expressed in mouse and human granulosa cells and appear be linked to granulosa cell metabolism and apoptosis, as well as steroidogenesis (19–25). Furthermore, FOXO1 has been shown to increase the expression of adiponectin in adipose cells (26) and Adipor2 in hepatic cells (27) providing evidence that FOXO1/3 may also modulate the response of granulosa cells to adiponectin by similar or different mechanisms.
Although some effects of adiponectin have been analyzed in granulosa cells, less is known about the role of the adipokine on cumulus cells and oocyte quality or the preimplantation embryo (28– 31). Therefore, we sought to determine if adiponectin alone or in conjunction with pituitary hormones could alter not only mouse granulosa cell functions but also COC functions in culture and if this was associated with changes in the expression of specific genes or oocyte quality, including fertilization and early stages of embryo development. We also sought to determine if there were any correlations among the levels of the adiponectin receptors (ADIPOR1 and ADIPOR2) and FOXO1 or FOXO3 in human granulosa cells or cumulus cells collected from IVF patients and the fertility outcome of these patients.
Materials and Methods
Animals
Immature (day 24 of age) C57BL/6 female mice were housed under a 16:8h light:dark schedule in the Center for Comparative Medicine at Baylor College of Medicine and Hiroshima University, and provided food and water ad libitum. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Baylor College of Medicine and Hiroshima University. For in vivo studies, immature (im) mice were treated with eCG ((equine chorionic gonadotropin; 4IU; intraperitoneally)(Calbiochem/EMD, Billerica, MA) to stimulate follicular development. Forty-eight hours later (designated 0h, hCG) mice were injected with human chorionic gonadotropin (hCG; 5IU)(Green Park Pharmacy, Houston, TX) to stimulate ovulation (16h) and luteinization (48h)(21). Whole ovaries (WO), granulosa cells (GC) and cumulus cell-oocyte complexes (COCs) were isolated.
Granulosa cell culture and treatment with adiponectin
Immature mice were injected IP with 4 IU eCG and sacrificed 24h later (21). Granulosa cells were cultured in serum free medium alone (controls) or were treated with adiponectin alone (globular; 20 µg/ml) (R & D Research, Minneapolis, MN), forskolin alone (10 µM; Calbiochem), or adiponectin and forskolin. Cells were used to prepare total RNA or protein. Media samples were saved for analyses of progesterone. Granulosa cells were also transfected with adenoviral vectors expressing GFP (control) or a stable, active form of FOXO1, FOXOA3 as described previously (21).
Cumulus cell-oocyte complex (COC) isolation and culture
Non-expanded COCs were isolated by needle puncture from preovulatory follicles of eCG-primed-mice (48h) and cultured (50/well) in defined medium with FSH or eCG with or without adiponectin (globular; 20 µg/ml) for 16 hr (32). Some COCs were then placed in 50µl drop of human tubal fluid (HTF) medium for in vitro fertilization and embryo development (32). Spermatozoa were collected from the cauda epididymis of adult mice into 500 µl of HTF medium. After 60 minutes, the spermatozoa were introduced into the fertilization medium at a final concentration of 1000 spermatozoa/µl. Twelve hr after insemination, the oocytes were washed thoroughly five times, and then examined for formation of pronuclei under a phase-contrast microscopy. The gametes were further cultured for an additional day in the developing medium (KSOM+AA, Millipore, Billerica, MA, USA) to check the cleavage rate and development to blastocyst stage (32).
mRNA extraction and real time RT-PCR
Total RNA was prepared from WO, granulosa cells and COCs using the Qiagen Kit. Real-time PCR was performed using the Rotor-Gene 6000 thermocycler (Qiagen, Valencia, CA) as described previously (21). The primers were designed using software Primer3 (33)(Table I). The relative levels of gene expression were calculated using Rotor-Gene 6.0 software through “Comparative Quantitation” and normalized to levels of ribosomal protein L19 mRNA (34).
Western blots
Granulosa cells (GC) were homogenized with RIPA buffer containing complete protease inhibitors (Roche, Palo Alto, CA). Western blots were performed utilizing cell lysates equivalent to 15µg of GC protein and antibodies to FOXO1 phospho-FOXO1 FOXO3, phosphoAKT, PTEN (Cell Signaling, Beverly, MA) and ACTB (Cytoskeleton, Inc, Denver, CO) as previously (35).
In situ hybridization
In situ hybridization was performed as previously shown in our laboratory (22) using [35S]UTP-labeled antisense and sense probes of a mouse adiponectin cDNA generated and subcloned in our laboratory by routine procedures.
Collection of human granulosa cells and cumulus cells from periovulatory follicles
Luteinized mural granulosa cells were obtained from 20 patients in the United States and from cumulus cells of COCs obtained from 21 patients in Japan undergoing in vitro fertilization (IVF). The study protocols were approved by the Cedars-Sinai Institutional Review Board (IRB) Los Angeles, CA, USA and Daigo Watanabe Clinic, Kyoto, Japan, and informed consent to participate in the study was obtained from each patient. In the USA samples, purified mural granulosa cells and total RNA were collected and analyzed as previously described using specific primers (Suppl Table I) (23, 36).
In the Japan study, ultrasound guided oocyte retrieval was performed and the cumulus-oocyte complexes (with a few mural cells) were obtained from the aspirates. Cumulus cells were recovered from the complexes after gentle hyaluronidase treatment. Total RNA was prepared and analyzedas described previously using specific primers (Suppl Table I) (32). During the IVF procedures in Japan the oocytes were cultured monitored for MII, fertilization, 2- cell stage, blastocyst /oocyte and blastocyst/2 cell.
Statistical Analyses
Mouse data
Quantitative real-time PCR experiments were carried out in triplicate, using specific primers (Suppl Table I) and the levels of amplified genes were expressed relative to L19 used as a control. The fold induction of each gene is expressed as the mean +/−SEM. Differences among groups were analyzed by Student’s t test. P<0.05 was considered significant.
Human samples
Prior to statistical analysis, all data were confirmed to follow a normal distribution by way of the Kolmogorov-Smirnov test. Linear regression analysis was used to evaluate relationships between the different Forkhead transcription factors, FOXO1 and FOXO3, and adiponectin receptors, ADIPOR1 and ADIPOR2 and PTEN. Furthermore, linear regression was performed to evaluate relationships between the factors identified in mural granulosa cells (forkhead transcription factors and adiponectin receptors) and oocyte function (oocyte number and fertilization rate (number of bipronuclear oocytes (2PN) retrieved vs. the number of oocytes for IVF). Influential data (e.g. outliers) were determined as data >2 standard deviations beyond the mean and all linear regression were repeated without any influential values to evaluate goodness of fit. For each linear regression, the R2 value was used to express the correlation efficiency. P-values less than 0.05 were considered significant.
RESULTS
Adiponectin receptors but not adiponectin are expressed and hormonally regulated in mouse ovarian cells
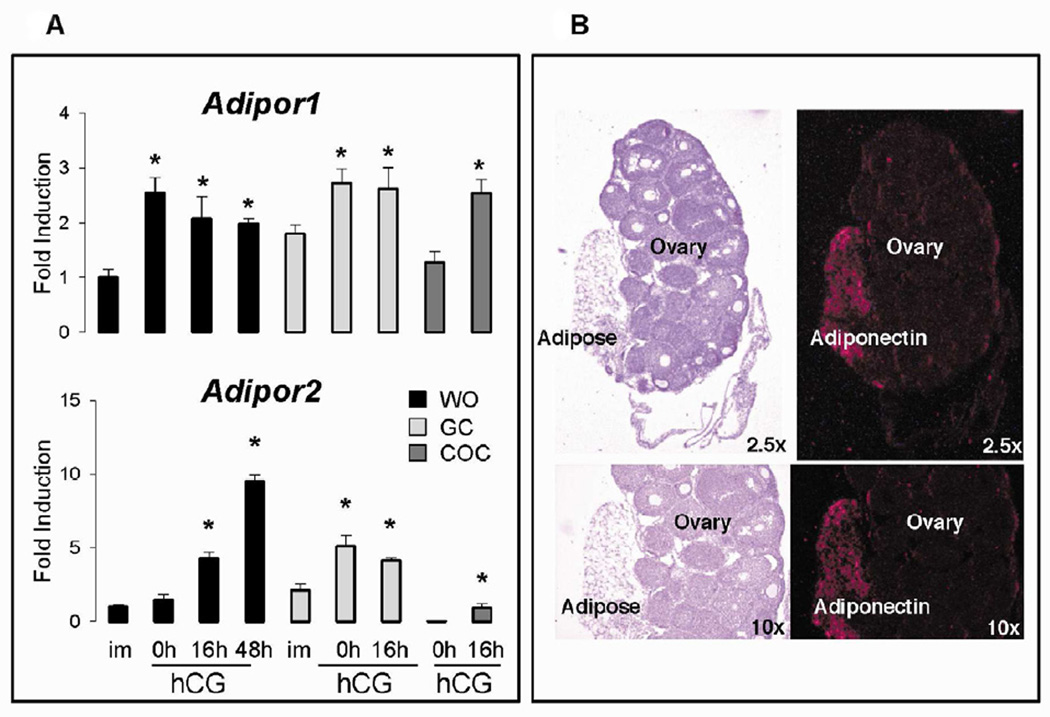
Adiponectin receptors Adipor1 and Adipor2 were expressed in whole ovarian (WO) samples of immature (im) mice (Figure 1A). Adipor1 mRNA increased significantly in response to eCG for 48h (0h, hCG) but not hCG whereas Adipor2 mRNA increased only slightly after exposure to eCG but was dramatically elevated in response to hCG at 16h and 48h. Adipor1 and Adipor2 mRNA levels in granulosa cells were higher than WO samples in immature mice and increased significantly in response to eCG but not hCG at 16h. In COCs collected from preovulatory follicles of eCG-primed mice, Adipor1 mRNA was similar to that observed in preovulatory granulosa cells whereas Adipor2 levels were much lower than those observed in granulosa cell or WO samples. However, mRNAs encoding both receptors increased significantly in ovulated COCs collected from the oviducts at 16h post-hCG. These results indicate that Adipor1 and Adipor2 are differentially regulated in granulosa cells and COCs and that the elevated levels of Adipor2 in WO samples likely reflect a high level of this receptor in luteal cells (Figure 1A).
Figure 1. Adiponectin receptors are differentially regulated by eCG and hCG in ovarian compartments.
Whole ovaries (WO). granulosa cells (GC) and cumulus cell-oocyte complexes (COC) were isolated from immature (im) mice prior to and after treatment with eCG for 48h (0h) and hCG for 16 and/or 48h. A) Total RNA was prepared and used for real-time RT-PCR analyses presented as fold-induction relative to that observed in immature mice for WO. B) In situ hybridization localized adiponectin mRNA to adipose tissue adherent to the ovary but not to any ovarian cells. Different letters denote P<0.05 between samples. Tissues from at least 3 mice were used.
Although adiponectin (ADIPOQ) is predominantly synthesized in and secreted from adipose tissue (1), recent studies have reported expression of adiponectin in ovarian cells of rat and chicken (3, 4) but not human granulosa cells (37). Expression of Adipoq mRNA was extremely low and variable in the WO samples and essentially undetectable in granulosa cells we obtained from hormonally-primed immature mice (data not shown). Therefore, to assess the cell specific expression of adiponectin in the mouse ovary, we used in situ hybridization. Adipoq mRNA was not detected in the ovary of immature mice (Figure 1B) or from immature mice primed with eCG for 48h (not shown). However, there was intense staining of the Adipoq probe to peritoneal adipose tissue associated with the ovary. These data indicate that small amounts of adherent adipose tissue in the in vivo samples likely account for the random and low expression levels of Adipoq mRNA observed by real-time RT-PCR analyses.
Adiponectin modulates the responses of granulosa cells and COCs to FSH
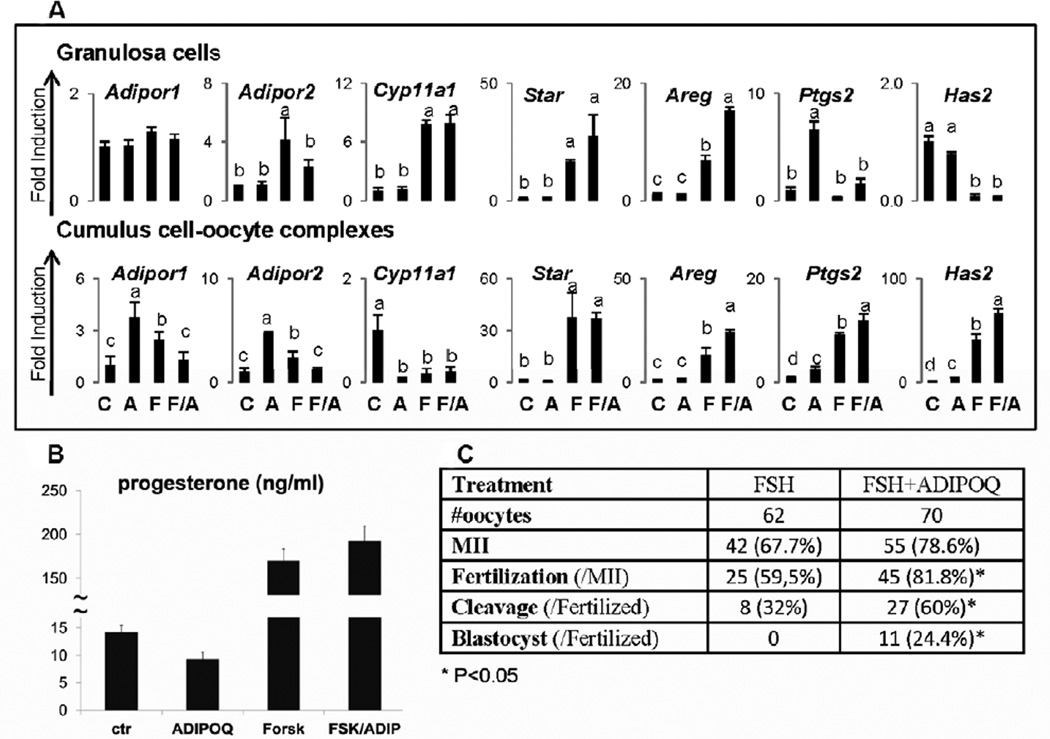
Based on the hormonal regulation of Adipor1 and Adipor2 in different ovarian cell compartments in vivo, we sought to determine if FSH and/or adiponectin modulated the expression of adiponectin receptors in cultured granulosa cells and COCs. Forskolin (used as a mimetic of FSH) significantly increased Adipor1 and Adipor2 mRNA in granulosa cells whereas adiponectin alone had no effect (Figure 2A). However, adiponectin partially suppressed the forskolin-induced increase in Adipor2 in granulosa cells. Similar results were obtained using full-length adiponectin and FSH (data not shown). By contrast, adiponectin alone and FSH alone increased Adipor1 and Adipor2 in COCs whereas adiponectin and FSH together failed to increase mRNA encoding these receptors (Figure 2A).
Figure 2. Regulated expression of selected genes by adiponectin, forskolin/FSH or the combination in granulosa cells and COCs.
Granulosa cells and COCs were cultured in the presence of adiponectin (20µg/ml), forskolin (10µM) or FSH (100ng/ml)(recombinant FSH from Organon) or the combination for 24h. A) Total RNA was prepared for real-time RT-PCR analyses. B) Media samples were collected for measuring progesterone. C) Analyses of IVF of oocytes and their development. All treatments and assays were run in triplicate. Different letters denote P<0.05 between samples. A= adiponectin, F=forskolin for granulosa cells and FSH for COCs. A/F means the combined treatment of adiponect/forskolin for granulosa cells and adiponectin/FSH for COCs.
Adiponectin has been shown to differentially regulate the expression of steroidogenic genes (Cyp11a1, Star and Cyp19a1) in ovarian cells of other species (3, 4, 7, 37, 38). Adiponectin did not significantly alter the basal or the forskolin (of FSH) induced levels of Cyp11a1 or Star mRNA in mouse granulosa cells (Figure 2A). However, adiponectin alone did reduce basal levels of progesterone in the culture medium but this was not statistically significant (p<0.085) (Figure 2B). By contrast, in COCs either adiponectin or FSH alone or together suppressed the expression of Cyp11a1 but not Star. (Figure 2A).
Because adiponectin has previously been shown to induce some ovulation related genes (7, 39–41), we analyzed the expression of Areg, Has2, Tnfaip6 and Ptgs2 in both granulosa cells and COCs. In granulosa cells, adiponectin alone markedly increased Ptgs2 and enhanced forskolin-mediated induction of Areg but did not alter the expression of Has2 Figure 2A). By contrast, in COCs, adiponectin alone stimulated significant increases in mRNA encoding Has2 and Ptgs2; albeit far less than that mediated by FSH (Figure 2A). Unlike FSH, adiponectin alone did not increase Areg or Tnaip6 not shown) but did enhance FSH mediated increases in Has2. FSH alone but not adiponectin alone stimulated COC expansion (data not shown).
In vitro fertilization analyses of oocytes retrieved from FSH- and FSH + adiponectin-treated COCs showed that the presence of adiponectin enhanced significantly the in vitro fertilization and development of the embryo to the blastocyst stage (Figure 2C). These results, coupled with the ability of adiponectin to regulate COC gene expression profiles, indicate that COCs are an important target of adiponectin.
Adiponectin regulates FOXO1 and FOXO3 protein
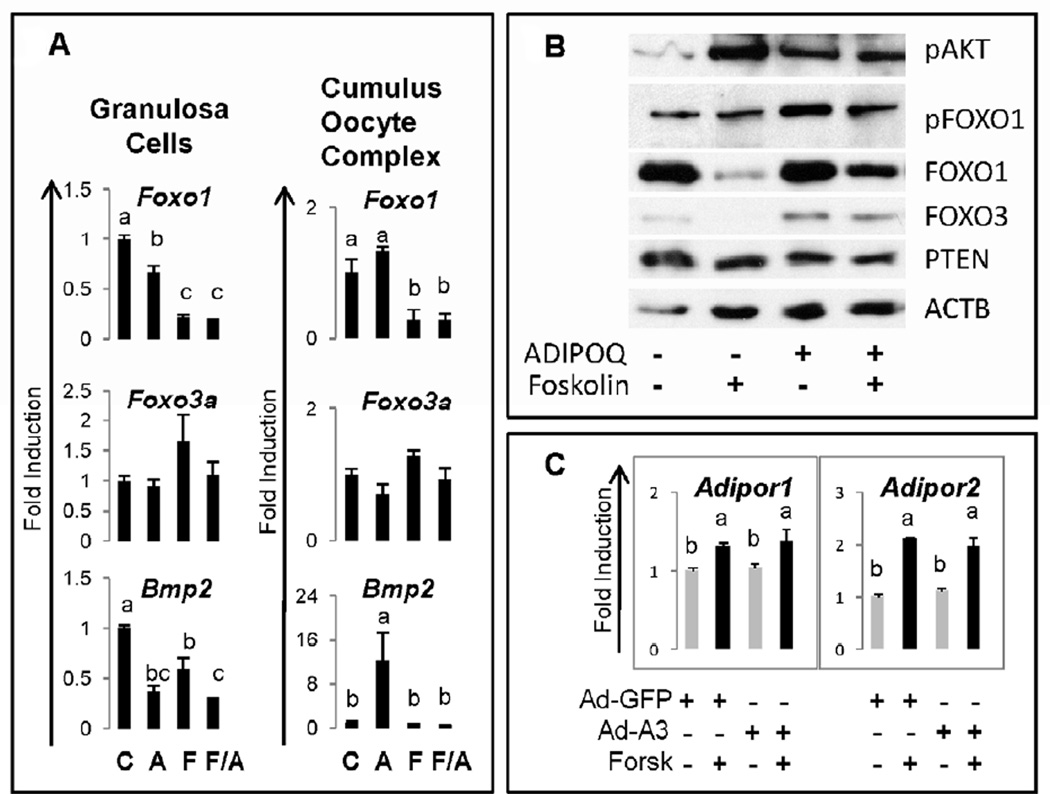
The induction of adiponectin and its receptors have been linked functionally to FOXO1 and FOXO3 activity in other tissues (26, 27). Because these transcription factors are expressed in granulosa cells and regulate metabolic as well as apoptotic pathways (21), we analyzed the expression of Foxo1 and Foxo3 mRNA and protein. Adiponectin alone slightly suppressed expression of Foxo1 but not Foxo3 mRNA in granulosa cells but had no effect on the expression of these genes in COCs (Figure 3A). Furthermore, adiponectin did not alter the dramatic down-regulation of Foxo1 that occurs in response to either forskolin or FSH in granulosa cells and COCs, respectively. Expression of Foxo3 mRNA was not markedly changed by any treatment in either granulosa cells or COCs. However, when we analyzed the levels of FOXO1 and FOXO3 protein, we observed that adiponectin partially, but consistently, prevented the forskolin-mediated loss of FOXO1 and FOXO3 protein (Figure 3B). Thus, adiponectin may act to stabilize these factors by post-translational mechanisms. Because Adipor2 is a target of, and up-regulated by, FOXO1 in hepatic cells, we sought to determine if this occurred in granulosa cells as well. Therefore, granulosa cells were transfected with an adenoviral vector expressing constitutively active, stable form of FOXO1 (FOXOA3) in which three serines are mutated to alanines. FOXOA3 failed to increase either Adipor1 or Adipor2 expression but did not alter the forskolin-mediated increases in these receptor mRNAs (Figure 3C).
Figure 3. Regulation of FOXO transcription factors by adiponectin.
Granulosa cells were cultured in the presence of adiponectin (ADIPOQ), forskolin or the combination for 24h. A) Total RNA (as in Figure 2) was prepared for real-time RT-PCR analyses and B) cell lysates were prepared for Western blots. Each assay was run at least three times. C) Granulosa cells were also transfected with adenoviral vectors for 4h, washed and cultured overnight as in Materials and Methods (21). Total RNA was prepared for analyzing the expression of Adipor1 and Adipor2 by quantitative RT-PCR analyses.
One direct target of FOXO1/3 in granulosa cells is Bmp2, a gene that encodes a member of the TGFβ family of growth factors (42. In the mouse ovary, Bmp2 is selectively expressed in granulosa cells of growing follicles that are undergoing apoptosis (43). In addition, adiponectin and its receptors have been shown to alter apoptosis in KGN cells (8). Therefore, we sought to determine if adiponectin might alter expression of Bmp2 in mouse granulosa cells or COCs (Figure 3A). As shown, adiponectin alone reduced the expression of Bmp2 more dramatically than forskolin in granulosa cells and the combined treatments of forskolin and adiponectin were more effective than either treatment alone. By contrast, adiponectin alone markedly increased Bmp2 mRNA in COCs. Although FSH alone did not alter basal Bmp2 levels, FSH completely blocked the adiponectin-mediated increase in Bmp2 Figure 3A).
Expression of ADIPOR1 and ADIPOR2 mRNAs in human granulosa/cumulus cells is associated with elevated expression of Foxo1 mRNA
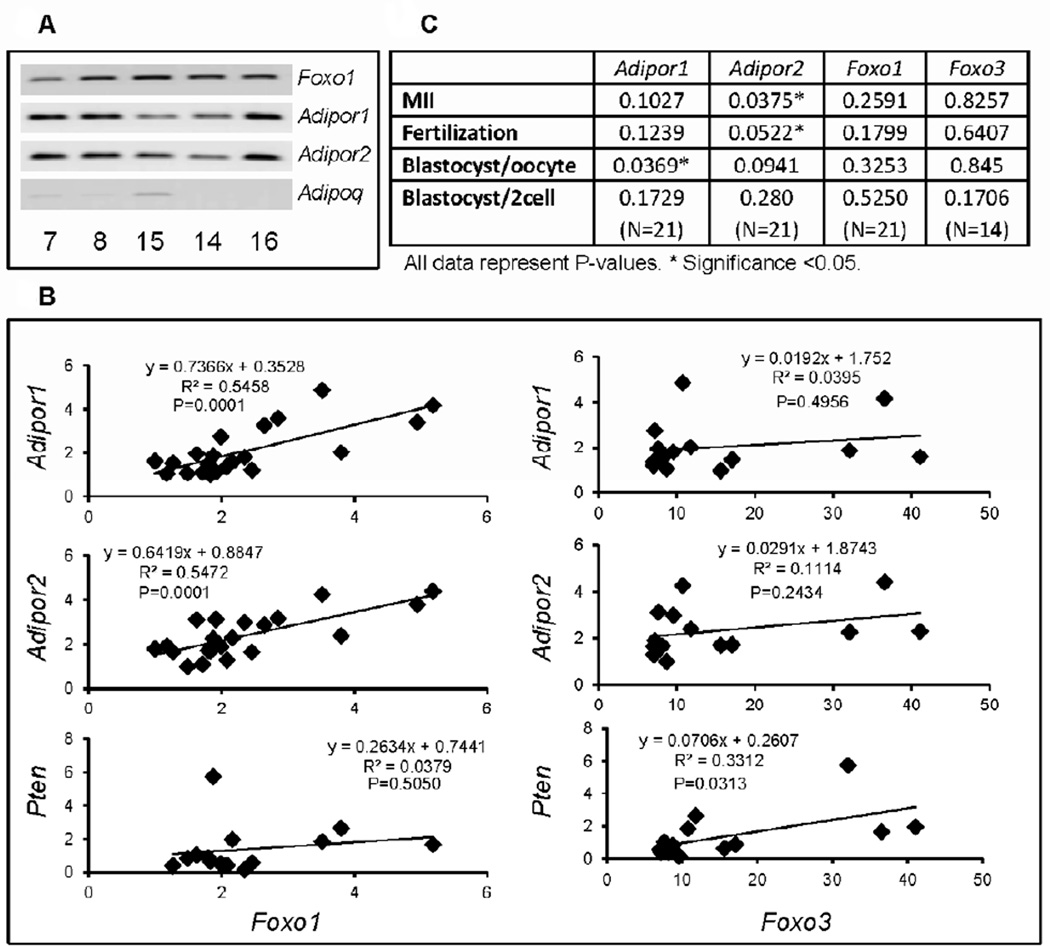
To determine if adiponectin or the adiponectin receptors were expressed in human cumulus cells and if their expression was related to that of FOXO1 or FOXO3, samples were collected from IVF patients. Cumulus cells express mRNA encoding FOXO1, ADIPOR1 and ADIPOR2 but express low to undectable levels of ADIPOQ mRNA (Figure 4A). Importantly, increased levels of FOXO1 mRNA in different cumulus cell samples exhibited significant positive correlations with increased levels of ADIPOR1 and ADIPOR2 mRNA but not with expression of PTEN Figure 4B). FOXO3 exhibited positive correlations with PTEN but not with ADIPOR1 or ADIPOR2 Figure 4B). Luteinized mural granulosa cells selectively showed a positive correlation (P<0.001) between FOXO3 and ADIPOR1 data not shown). There was no correlation between the expression of FOXO1 and FOXO3 in cumulus cells or granuolsa cells (data not shown). The expression of the genes in cumulus cells was also compared to oocyte quality as assessed by analyses of the number of metaphase II (MII) oocytes, fertilization, blastocyst/oocyte or blastocyst/2cell stages. Positive correlations were observed between the expression of ADIPOR2 (> ADIPOR1>FOXO1) and MII oocytes, fertilized oocytes and blastocyst/oocyte (Figure 4C).
Figure 4. Expression of ADIPOR1, ADIPOR2, FOXO1 and FOXO3 in cumulus cells samples obtained from human IVF patients.
A) Cumulus cell samples were collected from patients in Japan undergoing IVF procedures and total RNA was prepared. FOXO1, ADIPOR1 and ADIPOR2 were amplified by RT-PCR in selected samples (patient#7,8,15,14,16) using 50ng RNA, 35 cycles and Comparative Quantitation. These may not be in the linear range. Expression of ADIPOQ was low or undetectable. B) The expression levels of FOXO1 and FOXO3 mRNA in each sample were compared to expression of ADIPOR1, ADIPOR2 and PTEN using quantitative RT-PCR conditions in the linear range as described in methods. C) Correlation of gene expression levels of ADIPOR1 and ADIPOR2 in cumulus cells with oocyte maturation, fertilization and embryo development in the same patients. These data were collected in conjunction with the IVF samples in Japan. * denotes P<0.05.
DISCUSSION
These studies document that adiponectin is expressed at undetectable or very low levels in mouse or human granulosa cells, confirming results observed in human granulosa cells (37)(Figure 4). These results differ from other studies (3–5), indicating that there may be species-specific differences in ovarian expression of the adiponectin gene or that if any adipose tissue is inadvertently collected with ovarian tissue samples, it will provide a potent false positive (Figure 1B). High levels of adiponectin in serum may explain the elevated immuno-positive staining in the vascular regions of the theca layer and corpora lutea of rats (4).
Our studies provide novel evidence that COCs (cumulus cells) as well as granulosa cells express Adipor1 and Adipor2 but, in response to adiponectin, exhibit markedly different expression profiles of genes involved in metabolism, steroidogenesis, ovulation and apoptosis. For example, adiponectin alone increased Adipor1 and Adipor2 expression in COCs but not in granulosa cells whereas it blocked the ability of FSH to increase Adipor2 in both granulosa cells and cumulus cells. Adiponectin alone markedly suppressed the expression of Cyp11a1 in COCs but not in granulosa cells and did not alter forskolin (FSH)-mediated induction of Cyp11a1 or granulosa cell release progesterone. These results confirm those obtained in human granulose cells (37) but differ from other species (3–5, 7). Thus, adiponectin alone appears to exert a more potent effect on genes expressed in COCs than in granulosa cells. The biochemical and molecular mechanisms that underlie these differences remain to be determined bur may be species specific, cell context specific or related to the actions of globular versus full-length adiponectin (7, 29, 30).
Adiponectin alone increased significantly the expression of the ovulation-related genes, Ptgs2 and Has2 in COCs but did not stimulate COC expansion; most likely because the levels of Ptgs2 and Has2 that are induced by adiponectin alone remain far below those induced by FSH. Adiponectin enhanced FSH mediated induction of Areg, Has2 and Ptgs2 but not Tnfaip6 in COCs whereas the most dramatic effect of adiponectin alone in granulosa cells was its ability to increase Ptgs2, indicating again the selective responses of COCs to adiponectin (6).
Adiponectin, like FSH, potently down-regulated the apoptosis-related gene Bmp2 in granulose cells. By contrast, adiponectin but not FSH markedly increased the expression of this gene in COCs. Although the function of Bmp2 in cumulus cells remains to be determined, high levels of adiponectin may help to protect granulosa cells from undergoing apoptosis (21, 43). This is supported by recent studies showing that disruption of Adipor1 caused cell death and increased cell death-related mechanisms in the KGN granulosa cell line (8). Disruption of Adipor2 reduced FSH-mediated induction of Cyp11a1 and Cyp19a in the KGN cells, indicating that these receptors control different functions. Although disruption of adiponectin, Adipor1 and Adipor2 in mouse models in vivo alters insulin sensitivity the effects on fertility in these mice have not been extensively examined (6).
FOXO1 and FOXO3 are expressed in mouse and human granulosa cells (22, 23). Functional links between the AKT/FOXO pathway and the adiponectin/ADIPOR1/R2 pathways and metabolism have been observed in adipocytes and hepatocytes (26, 27) suggesting that similar mechanisms might operate in granulosa cells. Although adiponectin alone did not alter Foxo1 or Foxo3 mRNA in granulosa cells, it did prevent the decrease of FOXO1 and FOXO3 protein, indicating that adiponectin may modulate metabolism and apoptosis by the AKT/FOXO pathway. A possible regulatory link between the adiponectin pathway and Foxo1 is also observed in human cumulus cells collected from IVF patients. In these samples, levels of FOXO1 mRNA exhibited a significant correlation with levels of ADIPOR1 and ADIPOR2 transcripts whereas FOXO3 was correlated more with PTEN. Although these results do not confirm functional links, it is highly suggestive of coordinate regulation. Because levels of ADIPOR1 and ADIPOR2 in the human cumulus cell samples were significantly related to fertility outcome and because the addition of adiponectin to mouse COCs improved oocyte quality as assessed by fertilization and early stages of embryo growth, it is possible that the addition of adiponectin to in vitro maturation media might improve the developmental competence of in vitro matured oocytes in human infertility care. Because FOXO3 correlated more with ADIPOR1 in luteinized mural granulosa cells, it appears that human cumulus cells and luteal cells exhibit different expression patterns of these genes. The potential link between adiponectin and FOXO factors in COCs is consistent with those reported recently showing that SIRT1 and FOXO1 enhance the activity of the Adipor2 promoter (27), that knock-down of either Sirt or Foxo1 blunted the metabolic effect of a high fat diet (27) and that FOXO1 regulates Adipoq expression in adipocytes (26). Collectively, these results suggest that when serum levels of adiponectin decline, as is observed consistently in women with PCOS or in response to high androgens, the levels of FOXO1 and FOXO3 in ovarian cells might also decline leading to altered metabolic, steroidogenic and apoptotic activities.
In summary, adiponectin (globular) differentially regulates the expression of specific genes in granulosa cells and cumulus cells indicating that the effects of this cytokine are cell context specific and dependent on the stage of granulosa/cumulus cell differentiation. That adiponectin can enhance fertility in both mouse and human IVF models suggests that it impacts oocyte/embryo development and that additional target genes need to be identified. In this regard, our studies support recent reports in mouse and pig where full-length adiponectin enhanced embryo development (29, 30).
Supplementary Material
Acknowledgements
NIH-HD-16272 and 16229 (JSR), No.21688019, No.21028014, No. 21248032 from the Japan Society for the Promotion of Science (JSPS) (to M.S.) and The Helping Hands of Los Angeles, Inc. (MDP). We thank Dr. T Mori, Professor Emeritus of Kyoto University and the laboratory staff of Daigo-Watanabe Clinic to collect human granulose-cumulus cell complexes. Measurements of progesterone were performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of disclosure: With the exception of Dr. Shimada and Dr. Pisarska, the authors have nothing to disclose.
Contributor Information
JoAnne S. Richards, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston TX 77030.
Zhilin Liu, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston TX 77030
Tomoko Kawai, Laboratory of Reproductive Endocrinology, Graduate School of Biosphere Science, Hiroshima University, Higashi-Hiroshima, Hiroshima, 739-8528, Japan
Kei Tabata, Daigo-Watanabe Clinic, Kyoto, 601-1375, Japan
Hirohiko Watanabe, Daigo-Watanabe Clinic, Kyoto, 601-1375, Japan
Deepa Suresh, Department of Pediatrics, Section of Endocrinology & Metabolism, Baylor College of Medicine, Houston, Texas
Fang-Ting Kuo, Department of Obstetrics and Gynecology; Division of Reproductive Endocrinology and Infertility, Cedars-Sinai Medical Center, Los Angeles, CA 90048 and Department of Obstetrics and Gynecology; Division of Reproductive Endocrinology and Infertility, David Geffen School of Medicine at UCLA, Los Angeles, CA 90024
Margareta D. Pisarska, Department of Obstetrics and Gynecology; Division of Reproductive Endocrinology and Infertility, Cedars-Sinai Medical Center, Los Angeles, CA 90048 and Department of Obstetrics and Gynecology; Division of Reproductive Endocrinology and Infertility, David Geffen School of Medicine at UCLA, Los Angeles, CA 90024
Masayuki Shimada, Laboratory of Reproductive Endocrinology, Graduate School of Biosphere Science, Hiroshima University, Higashi-Hiroshima, Hiroshima, 739-8528, Japan
REFERENCES
- 1.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol (minireview) 2008;22:1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trujillo ME, Scherer PE. Adiponectin -- journey from the adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 3.Chabrolle C, Tosca L, Crochet S, Tesseraud S, Dupont J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: potential role in ovarian steroidogenesis. Domest Animal Endocrinol. 2007;33:480–487. doi: 10.1016/j.domaniend.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction. 2007;133:719–731. doi: 10.1530/REP-06-0244. [DOI] [PubMed] [Google Scholar]
- 5.Maillard V, Uzbekova S, Guignot F, Perreau C, Rame C, Coyral-Castel S, et al. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod Biol and Endocrinol. 2010;8:23. doi: 10.1186/1477-7827-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos DB, Palin M-F, Bordignon V, Murphy BD. The "beneficial adipolines in reproduction and fertility. Internat J Obesity. 2008;32:223–231. doi: 10.1038/sj.ijo.0803719. [DOI] [PubMed] [Google Scholar]
- 7.Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin M-F, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006;147:5178–5186. doi: 10.1210/en.2006-0679. [DOI] [PubMed] [Google Scholar]
- 8.Pierre P, Froment P, Negre D, Rame C, barateau V, Chabrolle C, et al. Role of adiponectin receptors, AdipoR1 and AdipoR2, in steroidogenesis of the human granulosa tumor cell lline, KGN. Hum Reprod. 2009;24:2890–2901. doi: 10.1093/humrep/dep292. [DOI] [PubMed] [Google Scholar]
- 9.Kubota n, Terauchi Y, Yamauchi T, Kubtia T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neoimtimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 11.Nawrocki AR, Rajala MW, Tomas E, UB P, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced resonsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 12.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 13.Adrdawi MS, Rouzi AA. Plasma adiponectin and insulin resistance in women with polycystic ovarian syndrome. Fertil Steril. 2005;83:1708–1716. doi: 10.1016/j.fertnstert.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 14.Panidis D, Farmakiotis D, Rousso D, Koliakos G, Kaltsas T, Krassas G. Decrease in adiponectin levels in women with polycystic ovarian synddrome after an orla glucose tolerance test. Fertil Steril. 2005;83:232–234. doi: 10.1016/j.fertnstert.2004.05.105. [DOI] [PubMed] [Google Scholar]
- 15.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated theca cells form polycystic ovaries. J Clin Endocrinol Metab. 1994;79:1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- 16.Nelson VL, Qin K-N, Rosenfield RL, Wood JR, Penning TM, Legro RS, et al. The biochemical basis for increased testosterone production in theca calls propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 17.Barber TM, McCarthy MI, Wass JAH, S F. Obesity and polycystic ovary syndrome. Clin Endocinol (Oxf) 2006;65:137–145. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 18.Sator BM, Dickey RP. Polycystic ovarian syndrome and the metabolic syndrome. Am J Med Sci. 2005;330:336–342. doi: 10.1097/00000441-200512000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 20.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Rudd MD, I H-G, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, et al. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthesis pathways in granulosa cells. Mol Endocrinol. 2009;23:649–661. doi: 10.1210/me.2008-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1 and AFX genes in the rodent ovary: Evidence for regulation by IGF-1, estrogen and the gonadotropins. Mol Endocrinol. 2002;16:580–599. doi: 10.1210/mend.16.3.0806. [DOI] [PubMed] [Google Scholar]
- 23.Pisarska MD, Kuo FT, Tang D, Zarrini P, Khan S, Ketefian A. Expression of forkhead transcription factors in human granulosa cells. Fertil Steril. 2009;91:1392–1394. doi: 10.1016/j.fertnstert.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan HY, Liu Z, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, et al. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005;280:9135–9148. doi: 10.1074/jbc.M409486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A, Liu M, Liu X, Dong LQ, Glickman RD, Slaga TJ, et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J Biol Chem. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang X, Hu M, Rogers CO, Shen Z, You M. Role of SIRT1-FOXO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanoladministered mice. Antioxid Redox Signal. 2011;15:425–435. doi: 10.1089/ars.2010.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim ST, Marquard K, Stephens S, Louden E, Allsworth J, Moley KH. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum Reprod. 2011;26:82–95. doi: 10.1093/humrep/deq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Čikoš Š, Burkuš J, Bukovská A, Fabian D, Rehák P, Koppel J. Expression of adiponectin receptors and effects of adiponectin isoforms in mouse preimplantation embryos. Human Reproduction. 2010;25:2247–2255. doi: 10.1093/humrep/deq193. [DOI] [PubMed] [Google Scholar]
- 30.Chappaz E, Albornoz MS, Campos D, Che L, Palin M-F, Murphy BD, et al. Adiponectin enhances in vitro development of swine embryos. Domestic Animal Endocrinology. 2008;35:198–207. doi: 10.1016/j.domaniend.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt T, Fischer S, Tsikolia N, Navarrete Santos A, Rohrbach S, Ramin N, et al. Expression of adipokines in preimplantation rabbit and mice embryos. Histochemistry and Cell Biology. 2008;129:817–825. doi: 10.1007/s00418-008-0409-8. [DOI] [PubMed] [Google Scholar]
- 32.Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TRL2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 33.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Richards JS. IL6: anautocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2008;150:3360–3368. doi: 10.1210/en.2008-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Fan HY, Y W, Richards JS. Targeted disruption of Mapk14 (p38MAPKa) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue cell-oocyte complex expansion and maintain fertility. Mol Endocrinol. 2010;24:1794–1804. doi: 10.1210/me.2010-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo F-T, Fan K, P M, Ketefian A, Bentsi-Barnes IK, Pisarska MD. Relative expression of genes encoding SMAD signal transduction proteins in human granulosa cells is correlated with oocyte quality. J Assist Reprod Genet. 2011;28:931–938. doi: 10.1007/s10815-011-9609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chabrolle C, Tosca L, Rame C, P L, Royere D, Dupont J. Adiponectin increases insulin-like growth factor 1-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril. 2009;92:1988–1996. doi: 10.1016/j.fertnstert.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. m. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008;284:38–45. doi: 10.1016/j.mce.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Shimada M, Gonzalez-Robayna I, Hernandez-Gonzalez I, Richards JS. Paracrine and autocrine regulation of EGF-like factors in cumulus oocyte complexes and granulosa cells: key role for prostaglandin synthase 2 (Ptgs2) and progesterone receptor (Pgr) Mol Endocrinol. 2006;20:348–364. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez-Gonzalez I, Gonzalez-Robayna IJ, Shimada M, Wayne CM, Ochsner SA, White L, et al. Gene expression profiles of cumulus cell oocyte complexes (COCs) during ovulation reveal cumulus cells express neuronal and immune-related genes: Does this expand their role in the ovulation process? Mol Endocrinol. 2006;20:1300–1321. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- 41.Richards JS. Genetics of Ovulation. Seminars in Reproductive Medicine. 2006;25:235–242. doi: 10.1055/s-2007-980217. [DOI] [PubMed] [Google Scholar]
- 42.Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, et al. A FoxO-Smad synexpression group in human keratinocytes. Proceedings of the National Academy of Sciences. 2006;103:12747–12752. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrin. 2003;1:1–20. doi: 10.1186/1477-7827-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.