Abstract
Multiple mechanisms contribute to a photoreceptor's ability to adapt to ambient light conditions. The mosquito Aedes aegypti expresses the long-wavelength rhodopsin Aaop1 in all R1–R6 photoreceptors and most R8 photoreceptors. These photoreceptors alter the cellular location of Aaop1 and reorganize their photosensitive rhabdomeric membranes on a daily basis. During daylight periods, Aaop1 is excluded from the light-sensitive rhabdomeres and localized to multivesicular bodies (MVBs) within the photoreceptor cytoplasm. In the dark, Aaop1 accumulates in the rhabdomeres and no Aaop1-containing MVBs are present in the cytoplasm. Manipulation of light treatments shows the cellular movement of Aaop1 in and out of the rhabdomere is directly controlled by light. In a separate process, the photoreceptors reduce Aaop1 protein content during a time period spanning from late afternoon into the first 2 h of the dark period. Aaop1 levels then gradually increase through the dark period and remain high following movement of Aaop1 to the cytoplasm at dawn. These results demonstrate that mosquito photoreceptors control rhodopsin availability during the daily light-dark cycle by novel mechanisms not discerned from analysis of traditional invertebrate models. These mechanisms will maximize a photoreceptor's light sensitivity range and therefore may be common in organisms active in low light conditions.
Introduction
The mosquito Aedes aegypti is the vector for transmission of the flaviviruses that cause yellow fever and dengue fever. There are 10 different rhodopsins found in the Ae. aegypti genome (Nene et al., 2007). Similar sets of rhodopsins (Holt et al., 2002), and similar retinal organization (Hu et al., 2009) are found in other mosquitoes, including the malaria vector Anopheles gambiae lineage that diverged from Ae. aegypti approximately 150 million years ago (Nene et al., 2007). Conservation within these visual systems implies that these species possess visual processing capabilities similar to those of the common ancestor and that these capabilities continue to be important to the fitness of the extant species.
The compound eye of the adult Ae. aegypti mosquito contains 300–400 ommatidial units, and each ommatidium contains eight photoreceptor cells (R1–R8). Every photoreceptor cell contains a single rhabdomere, which is the light-sensitive organelle composed of 60,000 microvilli generated by infoldings of the plasma membrane. These cells and their rhabdomeres are organized such that the R7 cell has an apical rhabdomere positioned on top of the closed rhabdom generated by the rhabdomeres of the R1–R6 and R8 cell (Brammer, 1970; Hu et al., 2009). The fused rhabdom structural organization increases sensitivity at the expense of visual field resolution and therefore provides an advantage for insects active in low light environments (Land et al., 1999).
In Ae. aegypti, the long-wavelength Aaop2 and UV-sensitive Aaop8 rhodopsins are expressed in nonoverlapping patterns of R7 cells (Hu et al., 2009). The expression of these rhodopsins in the R7 cell divides the retina into four distinct domains, these being the dorsal region, the central region, the ventral stripe, and the ventral region. The coexpression of another rhodopsin, Aaop9, in these R7 photoreceptors enhances light sensitivity in the low-light conditions at dawn and dusk in which Ae. aegypti mosquitoes are most active (Hu et al., 2011).
In this report we identify another mechanism used by mosquito photoreceptors to increase light sensitivity. We show that the Aaop1 rhodopsin is expressed in all R1–R6 and most R8 cells that form the closed rhabdom structure. In daylight conditions, most Aaop1 is located within multivesicular bodies (MVBs) in the cytoplasm and thus unable to trigger phototransduction. When mosquitoes are placed in dark conditions, Aaop1 moves back to the rhabdomeric membranes and hence is positioned for light reception. A distinct process results in the daily depletion of Aaop1 in the late afternoon and early evening periods. Aaop1 is restored to normal levels during the subsequent night period. These findings show that diurnal management of rhodopsin trafficking may play a major role in modulation of visual system sensitivity.
Materials and Methods
Mosquito management.
The Wh (a white-eyed) strain of Ae. aegypti was reared in a 12 h light/dark cycle at 27°C and 85% humidity. A 1 h transition period was used to gradually increase the light from 0 to 100 lux between ZT0 (zeitgeber time 0) and ZT1 and from 100 to 0 lux between ZT12 and ZT13 to mimic dawn and dusk light transitions, respectively. We manipulated the light treatments by evoking the same dawn and dusk transitions 6 h before dusk (at ZT6), by continuing dark treatment of mosquitoes beyond the ZT0 dawn period, or by continuing light treatment of mosquitoes beyond the ZT12 dusk period. All mosquitoes used in the study were mated, non-blood-fed females, at 2–10 d of age.
Antibody production.
A peptide corresponding to the sequence KFPALSCTDAPAASNSD found in the C-terminal domain of the Aaop1 rhodopsin was used to produce and affinity purify antibody against Aaop1 (Biomatik). The Aaop8 mouse polyclonal antibody was previously described (Hu et al., 2009).
Protein blot analysis.
Mosquito heads and bodies were homogenized in 1× lysis buffer (30 mm Tris-HCl, pH 6.8, 10% SDS, 0.0002% bromophenol blue, 5% β-mercaptoethanol, 10% glycerol). Proteins from approximately one Ae. aegypti head or one body were loaded and fractionated on a NuPAGE Novex 4–12% Bis-Tris gel (Invitrogen) and transferred to a polyvinylidene difluoride membrane. Membranes were blocked and probed with a 1:1000 dilution of rabbit anti-Aaop1 serum. In some experiments, a 1:2000 dilution of mouse anti-β-actin mAb JLA 20 (Developmental Studies Hybridoma Bank) was used to confirm equal sample loading. The primary antibodies were detected by horseradish peroxidase-linked goat anti-rabbit or anti-mouse IgG (1:2000, GE Healthcare Life Sciences) and developed with the ECL Western Blotting Detection System (GE Healthcare Life Sciences) according to the manufacturer's protocol. The digitized images obtained from these protocols were adjusted for contrast and brightness using Adobe Photoshop CS5 software.
Immunostaining and electron microscopy.
To prepare retinas for whole-mount observation, adult mosquito heads were bisected, leaving one eye undamaged, and fixed overnight on ice in 2% paraformaldehyde (Electron Microscopy Sciences, stock 16% solution) in PBS. Isolation and fixation of heads in the dark was done under dim red light using a 650 nm long-pass filter. Heads were transferred to PBS the following morning and kept at 4°C for a period up to 1 week. Retinas were dissected in PBS and incubated with the primary antibodies (anti-Aaop1 and/or anti-Aaop8 polyclonal antisera) diluted 1:100 in BNT (1× PBS/0.1% BSA/0.1% Triton X-100/250 mm NaCl) 12–24 h at 4°C. After three 10 min washes with PBS, retinal tissues were incubated with secondary antibodies (Alexa Fluor 488 goat anti- rabbit IgG and/or Alexa Fluor 594 goat anti-mouse IgG: 1:500 in BNT) and/or Alexa Fluor 594 phalloidin (1:40 dilution in BNT) for 4 h at room temperature. After three 10 min washes with PBS, retinal tissues were mounted in 7–8 μl of Vectashield (Vector Labs). A glass ring slide with tape strips held the coverslip slightly above the slide to prevent damage to the retinal tissue. Retinas were visualized using a Nikon A1R confocal microscope. The cross section confocal images shown in this article were prepared by overlaying three optical sections imaged 1 μm apart. The z-axis projections shown below in Figure 6C–E were generated from a larger series of optical sections using the Nikon Elements software. Determination of Aaop1 levels in different cellular compartments was performed using MetaMorph software. The signal intensity and area of three ommatidial regions, these being (1) the fused rhabdom area, (2) the R1–R6 cell body area, and (3) the cell body of the R7 cell, were mapped within each image. To control for background signal, the signal density (intensity/area) within the R7 cell body was subtracted from the densities of other regions. The adjusted signal intensities were then used to compute the percentage of the total signal present in the rhabdom and cell body compartments. For electron microscopy, mosquito heads were embedded and stained using a standard Drosophila protocol (Ahmad et al., 2007). Digital photomicrographs were adjusted for contrast and brightness using Adobe Photoshop CS5 software.
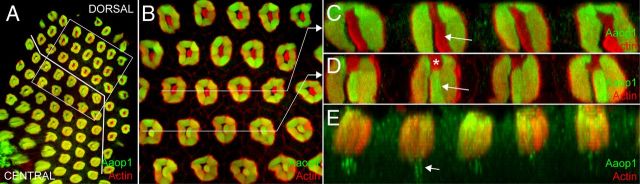
Figure 6.
Aaop1 is expressed in most R8 photoreceptor cells. A, A retina at ZT14 is imaged for Aaop1 (green) and actin (red). The white line identifies the border between the dorsal region in which the R8 cells lack Aaop1 expression and the rest of the retina in which R8 cells express Aaop1. B, The boxed region in A is enlarged to highlight ommatidia in the border region to distinguish the two classes of ommatidia. The top three rows of ommatidia lack Aaop1 expression in the central R8 rhabdomere, whereas the lower two rows show Aaop1 expression in the R8 rhabdomere. C, D, A z-plane section through the ommatidia identified by the line in B. Arrows point to the area occupied by the R8 rhabdomere. The R8 rhabdomere is Aaop1-positive in the ommatidia from the section shown in D but not in the ommatidia from the section shown in C. The asterisk in D identifies the area of the rhabdom occupied by the R7 rhabdomere. E, A z-plane section of ommatidia from a retina at ZT13, immediately after the dusk dimming period. At this time point, the R8 cell body located centrally below the rhabdom contains many Aaop1-labeled cytoplasmic structures (arrow).
Results
Aaop1 is expressed in mosquito R1–R6 photoreceptor cells
Aaop1 rhodopsin (Gene name GPRop1, AAEL006498) shares high overall sequence homology with 5 other long-wavelength opsins of Ae. aegypti (Nene et al., 2007). To create an antibody that identified Aaop1, we generated polyclonal antisera against a peptide antigen within the C-terminal domain of the Aaop1 protein. This peptide sequence is a region showing high variation among the members of the long-wavelength opsin family (Fig. 1A). The resulting antisera recognized a major protein in Ae. aegypti heads, but not in bodies, of an apparent molecular weight of 35 kDa near the predicted Aaop1 size of 41.5 kDa (Fig. 1B). Protein blot analysis of transgenic flies expressing Aaop2, Aaop3 and Aaop7 long-wavelength rhodopsins showed that the antisera did not detect these proteins, hence the antisera were determined to be specific for the Aaop1 rhodopsin.
Figure 1.
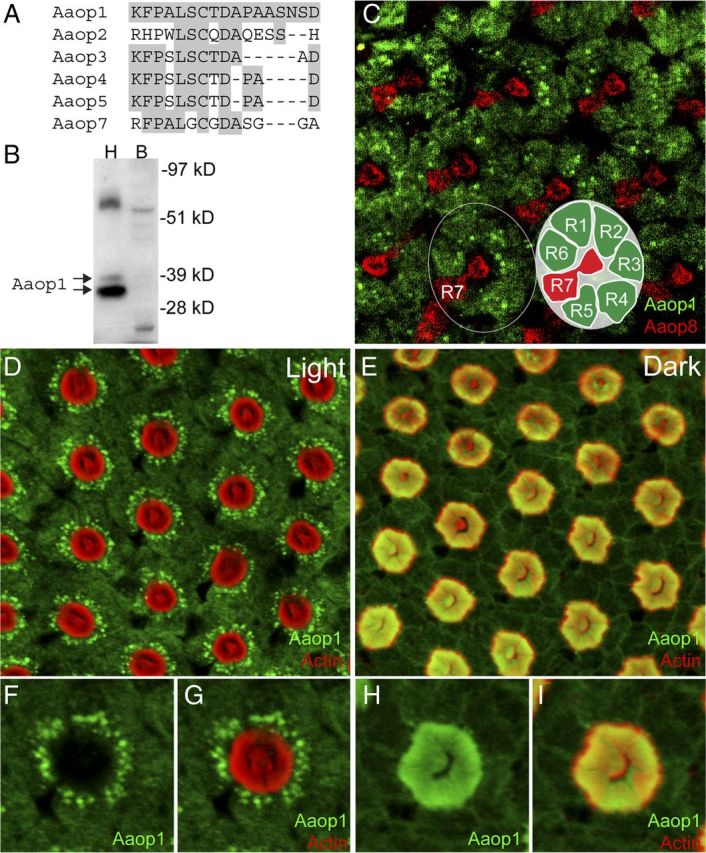
Aaop1 is expressed in Ae. aegypti R1–R6 photoreceptor cells. A, The C-terminal domain sequence of Aaop1 used for production of antisera is compared with the corresponding sequences of other Ae. aegypti long-wavelength rhodopsins. For all sequences, the amino acids identical to the Aaop1 sequence are shaded gray. B, Protein blot analysis shows that the Aaop1 antisera identify two proteins in the 35–37 kDa range in Ae. aegypti head (H) samples (arrows). These proteins are not found in Ae. aegypti bodies (B). C, Confocal image of a distal section to visualize the R7 rhabdomere shows multiple ommatidia in the central region of a light-treated (ZT6–ZT8) retina labeled for Aaop1 (green) and Aaop8 (red). Aaop1 localizes to the R1–R6 photoreceptor cell bodies. Aaop8, but not Aaop1, is expressed in the R7 central cell. The circle encloses a single ommatidium consisting of eight photoreceptors (R1–R8). The inset diagram shows the layout of the R1–R6 and R7 photoreceptors. The R8 photoreceptor lies below the centrally located R7 rhabdomere and is not visible in this image. D, In a light-treated retina at ZT11, Aaop1 (green) displays localization within punctate cytoplasmic structures and is largely absent from the rhabdomeric membranes (actin staining in red). E, In the dark-adapted retina at ZT14, Aaop1 colocalizes with actin within the rhabdomeric membranes. F, G, A single ommatidium from the light-adapted retinal image presented in D is shown for only Aaop1 labeling and for both Aaop1 and actin labeling. Aaop1 is found within punctate cytoplasmic structures and is not detected within the rhabdom area. H, I, A single ommatidium from the dark-adapted retinal image presented in E, is shown for only Aaop1 labeling and for both Aaop1 and actin labeling. Aaop1 is detected within the rhabdom area and not within punctate cytoplasmic structures.
We used these antisera to carry out immunostaining of retinal preparations. Figure 1C shows ommatidia within the central region of a light-treated retina colabeled for Aaop1 (green) and Aaop8 (red). Aaop8 is expressed in R7 cells (Hu et al., 2009). A single ommatidium is circled in Figure 1C and the seven individual photoreceptor cells visible at this level are depicted in the inset diagram. The Aaop1 antisera labeled the R1–R6 peripheral photoreceptors cells of the ommatidia but not the R7 cell.
The rhabdomeres of invertebrate photoreceptors are extensive infoldings of microvillar membranes that contain rhodopsin and other phototransduction components. To determine Aaop1 localization relative to the rhabdomeres, a fluorescently tagged phalloidin (red) was used to label the actin-rich rhabdomeric membranes of Aaop1 immunostained retinas. The results in Figure 1D show that before dusk (ZT11), Aaop1 (green) is predominantly localized within punctate cytoplasmic structures. However, in retinas obtained from mosquitoes 2 h after dusk (ZT14), Aaop1 is found within the rhabdomeric membranes (Fig. 1E). Figure 1F–I shows enlarged views of a single ommatidium to highlight the redistribution of Aaop1. Detection of Aaop1 is extremely low within the rhabdomeric membranes in the light-treated retina. Intensity analysis indicated that only 1.1 ± 0.8% (n = 7) of the Aaop1 signal is found within the rhabdom area. In contrast, none of the punctate cytoplasmic structures labeled by Aaop1 in the light are present under the dark conditions, and the majority of the Aaop1 is localized to the rhabdoms (69.1 ± 2.9%, n = 7).
Light triggers rapid cellular redistribution of Aaop1
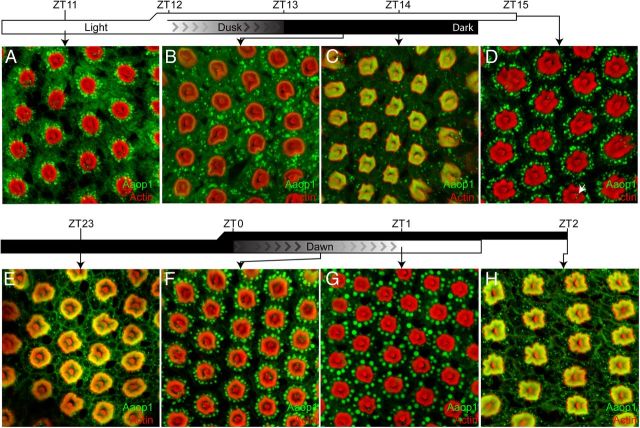
To determine how quickly Aaop1 redistributes within the photoreceptor cell, we examined the movement of Aaop1 during a set of time points surrounding the dawn and dusk light transitions. At 1 h before onset of dusk (Fig. 2A), Aaop1 is localized to punctate cytoplasmic structures lying adjacent to the rhabdomeres of the R1–R6 cells. At this time, Aaop1 is not detected in R1–R6 rhabdomeres. The dusk period consists of a 1 h transition in which lights are gradually dimmed from 100 lux to 0 lux (complete darkness). Thirty minutes after this dimming period (ZT13.5), Aaop1 can be detected in the R1–R6 rhabdomeres, although the majority of Aaop1 remains in the cytoplasm (Fig. 2B). The transition to rhabdomeric localization is nearly complete within the first hour of darkness (ZT14, Fig. 2C), with very few cytoplasmic structures now containing Aaop1.
Figure 2.
Light drives Aaop1 from the fused rhabdom to the cytoplasm. A–C, Mosquitoes were transitioned from light to dark conditions at ZT12. Lighting during the day was 100 lux and dimmed from 100 to 0 lux in the 1 h period from ZT12 to ZT13. Retinas were imaged for Aaop1 (green) and actin (red). Images show retinas dissected in the light 1 h before dusk (A), and 30 min (B) and 60 min (C) after complete darkness. D, Mosquitoes were retained in light conditions through the dusk time period and the retina was imaged for Aaop1 (green) and actin (red) at ZT15. The arrow marks one of the Aaop1-containing vesicles within the cytoplasm of an R8 photoreceptor. E–G, Mosquitoes were transitioned from dark to light conditions at ZT0. Whole-mount retinas were imaged for Aaop1 (green) and actin (red) 1 h before the lights gradually increase to 100 lux at dawn (E), and 30 min (F) and 60 min (G) after the dawn period. H, Mosquitoes were retained in dark conditions through the dawn time period and a retina imaged for Aaop1 (green) and actin (red) at ZT2, an hour after the time point shown in G for a mosquito subjected to the dawn conditions.
The dark to light transition at dawn reverses this process (Fig. 2E–G). One hour before onset of light (ZT23), Aaop1 is localized to the fused rhabdom, but is relocated into the cytoplasm by the end of the 1 h period in which lights were gradually increased from 0 lux to 100 lux (ZT1). The relocation from the rhabdomere to the cell body results in the initial formation of Aaop1-labeled cytoplasmic structures that appear larger than those seen later in the day (compare Fig. 2A,G).
To determine whether the Aaop1 movement into the cytoplasm is triggered directly by light, as opposed to the circadian clock, we performed a series of experiments in which the mosquitoes were subjected to non-circadian light and dark treatments. In one experiment, the mosquitoes were maintained under constant light past the normal ZT12 time point. Figure 2D shows that light treatment during the expected dark period results in cytoplasmic localization of Aaop1. The converse experiment, retaining mosquitoes in the dark until the ZT2 time point, results in rhabdomeric localization of Aaop1 (Fig. 2H). These data show that the light stimulus initiates the relocation of Aaop1 from the rhabdomere to the cytoplasm, and in the absence of light, Aaop1 remains in the rhabdomere. An influence of the circadian clock was not evident.
Cytoplasmic Aaop1 correlates with appearance of multivesicular bodies
The prevalence and large size of the cytoplasmic Aaop1 vesicular units in light-treated samples suggested that we could determine the morphological identity of these structures. Toward this goal, retinal sections were examined by transmission electron microscopy. Figure 3A shows a single ommatidial unit, cross-sectioned at the level of the fused rhabdom, from a mosquito at ZT23. This is 1 h before the dawn transition and a time point at which Aaop1 is localized within the rhabdomeric membranes (Fig. 2E). The photoreceptor cells are characterized by loosely packed, frayed rhabdomeric membranes.
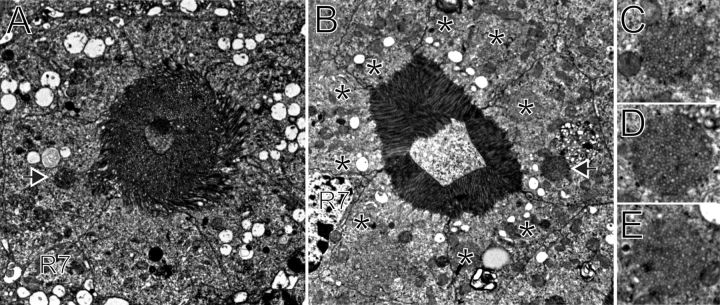
Figure 3.
Multivesicular bodies are common only in light-treated photoreceptors. A, A micrograph from a dark-adapted mosquito at the ZT23 time point for which Aaop1 is located within the rhabdom. An electron-dense spherical body is present (arrow); however, the MVBs present at the ZT1.5 time point are absent. B, Transmission electron micrograph of a cross-sectioned mosquito at the ZT1.5 time point for which Aaop1 is located in the cytoplasm. Many MVBs (asterisks) are located adjacent to the rhabdomeres of the photoreceptor cells. An electron-dense spherical body is also seen (arrow). The images in A and B are from similar retinal depths (the R7 cell nucleus is labeled “R7” in both images) to facilitate comparison of the photoreceptor ultrastructure at these two time points. Rhabdomeric membranes occupy a larger area and are less organized during the dark period. C–E, Micrographs of individual MVBs from retinas at the ZT1.5 time point.
Figure 3B shows a single ommatidial unit, cross-sectioned at the level of the fused rhabdom, from a mosquito retina at ZT1.5, soon after dawn. At this time point, Aaop1 is found within cytoplasmic vesicular compartments. The micrograph reveals that the membrane organization of the photoreceptor cell is distinguished from the dark-treated sample. The rhabdomeric membranes are well organized, tightly packed, and smaller in volume. The cytoplasm of the photoreceptors contains numerous MVBs (Fig. 3B, asterisks) positioned near the base of the rhabdomeric membranes. Magnified views of three of these bodies are shown in Figure 3C–E. These MVB structures are absent in the dark-treated retina, although darker (electron-dense) spherical bodies are less frequently seen in both light- and dark-treated retinas (arrow, Fig. 3A,B).
Aaop1 protein levels drop during the light-to-dark transition period
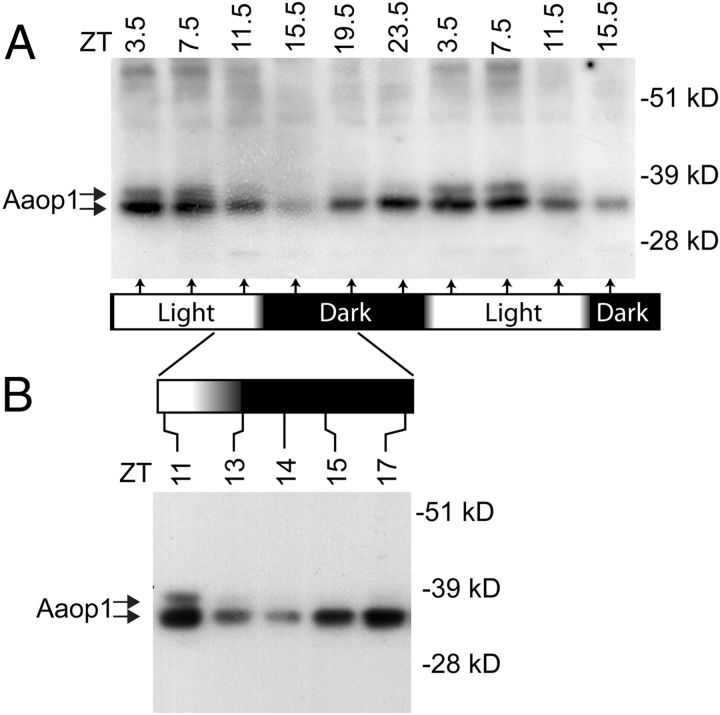
Protein blots were used to examine the overall levels of Aaop1 at 4 h intervals through a 36 h period (Fig. 4A). During the dawn period, as Aaop1 moves into vesicular compartments within the cytoplasm, Aaop1 levels remain high. In late afternoon, before dusk, the levels begin to decline and are markedly reduced by 2.5 h into the dark period (ZT15.5). Additional time points were examined around dusk to confirm these results and further resolve the time course (Fig. 4B). The time point with the lowest level of Aaop1 content is ZT14, 1 h into the dark period, with Aaop1 content gradually increasing at later time points.
Figure 4.
Aaop1 levels fluctuate throughout a 24 h period. A, A protein blot assesses the Aaop1 protein levels in samples obtained every 4 h for a 36 h period from mosquitoes reared under a normal light-dark cycle. The protein extracts equivalent to one mosquito head was loaded in each lane. The levels of Aaop1 start to decline before the dusk period and reach a minimum near the ZT15.5 time point. The membrane was reprobed for actin to confirm that similar amounts of protein were loaded in each lane. B, A protein blot assesses the changes in Aaop1 protein levels in heads harvested at selected times encompassing the dusk period. The decrease is most pronounced at the ZT14 time point.
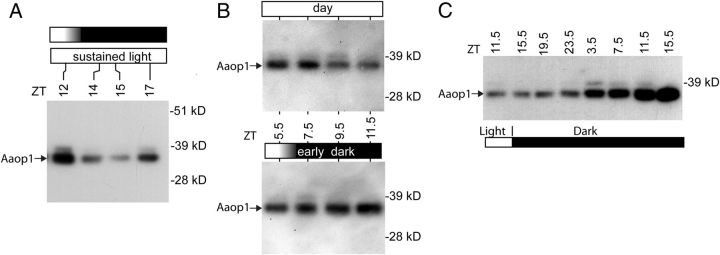
The results of Figure 4A show Aaop1 levels start to decline during the latter part of the daylight cycle, before the dusk period, suggesting that the process is not triggered by the reduction of light at dusk. To confirm this result, we examined Aaop1 protein levels in mosquitoes maintained in light past the normal dusk period. Figure 5A shows that the decline and subsequent increase in Aaop1 levels still occurs even when mosquitoes are subjected to continuous light.
Figure 5.
Aaop1 levels in mosquitoes subjected to altered light conditions. A, A protein blot assesses Aaop1 levels at the indicated times in mosquitoes exposed to sustained light beyond the dusk period. The decline in Aaop1 is not dependent on the decrease in light at the dusk period. B, Protein blots compare the Aaop1 levels in late afternoon in mosquitoes under normal light conditions (top) and subjected to an early dusk period initiated at ZT6 (bottom). Aaop1 levels remain high when mosquitoes are placed in darkness before the expected time of the Aaop1 decrease. C, A protein blot profile of Aaop1 levels in Ae. aegypti subjected to extended dark conditions. Aaop1 levels continue to rise when mosquitoes were retained in dark conditions.
To further examine the mechanisms involved in the Aaop1 reduction, we subjected mosquitoes to an early dark treatment in the middle of daylight period (ZT6), and performed protein blots to examine the levels of Aaop1 at different time points (Fig. 5B). Mosquitoes exposed to a normal light regime showed a decrease in Aaop1 levels at ZT9.5 and ZT11.5 (top). However, subjecting the mosquitoes to dark treatment prevented the decline in Aaop1 levels (bottom). Because the early dark treatment results in rhabdomeric localization of Aaop1, these results suggest that cytoplasmic location of Aaop1 is a necessary precondition for initiating the decline in Aaop1 levels. Consistent with this interpretation, mosquitoes subjected to continuous darkness through an entire 24 h period, hence retaining Aaop1 in the rhabdomere, accumulate increasing levels of Aaop1 (Fig. 5C).
Aaop1 is expressed in all R8 photoreceptors except within the dorsal region
The central area of the fused rhabdom in each ommatidial unit is formed by the rhabdomeres of the R7 and R8 photoreceptors. The R7 photoreceptor projects a small apical rhabdomere that forms the distal-most part, while the R8 photoreceptor cell is the major contributor of the central rhabdom structure. Throughout the set of experiments reported here, labeling within this central area was observed in some ommatidia but not in others. One example of this type of labeling is evident in the micrograph previously shown in Figure 1E, in which ommatidia in the upper region lacks staining in the central area of the fused rhabdom.
Several whole-mount retinal preparations of dark-adapted mosquitoes at ZT14 proved useful in understanding the presence and absence of Aaop1 labeling within the central rhabdom area. Figure 6A shows the dorsal region of a dark-adapted retina. The ommatidia not labeled by Aaop1 lie within this dorsal region, an area differentiated previously by expression of Aaop2 in the R7 cell (Hu et al., 2009). The boxed area in Figure 6A is shown at a higher resolution in Figure 6B.
From the image data of Figure 6B, two z-axis images were generated that show neighboring ommatidial units lacking Aaop1 expression (Fig. 6C) or possessing Aaop1 expression (Fig. 6D) within the central area. These images reveal that the presence or absence of labeling is maintained throughout the length of the rhabdom (arrows, Fig. 6C,D). This is the predicted pattern for Aaop1 expression in the R8 cells. A second approach confirmed this result. We examined Aaop1 localization in the retina at the time of transition from light to dark conditions at ZT13. At this time point, ommatidia of the central retina possess R1–R6 cells with the majority of Aaop1 in the rhabdom. These ommatidia also possess Aaop1-containing cytoplasmic vesicles lying underneath each fused rhabdom in the area occupied by the cytoplasm of the R8 (Fig. 6E, arrow). In more distal sections, a few Aaop1-containing cytoplasmic vesicles are often detected within the R8 cell body in light-treated retinas (arrow, Fig. 2D).
The R7 rhabdomere lies on top of the R8 rhabdomere. The z-axis section in Figure 6D shows the rhabdom area contributed by R7 rhabdomere is not labeled by Aaop1 (asterisk). This result confirms the conclusion of the earlier analysis of light-adapted mosquitoes (Fig. 1C) that Aaop1 is not expressed in the R7 photoreceptor.
Discussion
Ae. aegypti Aaop1 rhodopsin movement is a diurnal light-adaptive process
The capacity of retinal systems to adapt to ambient light conditions is a well studied phenomenon. Across the animal kingdom, a large number of mechanisms are involved that represent two basic types of strategies. The first group consists of anatomical changes that modulate the light flux to the photoreceptors through the use of pigment migration, pupillary, or other structural movements involving other cell types of the retina. Anatomical changes that increase light flux to photoreceptors during dark periods have been described for mosquitoes (Land et al., 1999). The second group is the adaptive responses occurring within the photoreceptor itself that shift the working range of the phototransduction response to match environmental light conditions. Both groups of mechanisms will contribute to the ability of visual systems to adapt to ambient light intensities and occur in the subsecond-to-minutes time range.
Here we characterize a slow adaptation process operational in the mosquito Ae. aegypti. At dawn, Aaop1 is removed from the rhabdomeric membranes and sequestered into cytoplasmic compartments such that the Aaop1 remaining in the rhabdomere is nearly undetectable. The extensive light-triggered endocytosis of a rhodopsin reveals a new mechanism for achieving extreme daily transformations in a photoreceptor's light sensitivity. Given that the light-triggered reduction in rhabdomeric Ae. aegypti Aaop1 rhodopsin is estimated to be reduced to 1%, this process in the absence of other influences would cause a 100-fold change in light sensitivity within the Aaop1-containing photoreceptors.
Brammer et al. (1978) documented a 50% decrease in rhabdomeric membrane volume of Ae. aegypti photoreceptors following light treatment. Thus, the loss of Aaop1 rhodopsin from these membranes is much greater than expected solely from the loss of rhabdomeric volume. This is similar to the situation in Limulus in which a 50% rhodopsin loss from the rhabdomeres at dawn (Katti et al., 2010) is greater than the 10% loss in rhabdom volume (Sacunas et al., 2002).
The endocytic processes responsible for internalization are likely similar to those described for other G-protein-coupled receptors (Claing et al., 2002). In Drosophila photoreceptors, rhodopsin-containing MVBs are generated by endocytosis of rhodopsin from rhabdomeric membranes following light treatment (Satoh et al., 2005). Previous studies using Ae. aegypti larval eyes (White, 1968) and adult compound eyes (Brammer et al., 1978) documented the appearance of MVBs following light treatment, and predicted that these MVBs would contain rhodopsin. We have confirmed this expectation by showing that extensive light-driven movement of Aaop1 results in Aaop1 being sequestered into cytoplasmic structures that share both the morphology and cellular location of the MVBs.
It seems likely that cytoplasmic rhodopsin sequestration is a mechanism contributing to the daily changes in photoreceptor light sensitivity for other invertebrates. A dramatic 1000-fold change in sensitivity between the day and night phases has been documented for photoreceptors of the nocturnal scorpion Androconus australis (Fleissner, 1977). Only in the horseshoe crab Limulus has both the movement of rhodopsin to a cytoplasmic compartment (Katti et al., 2010) and a diurnal change in light sensitivity (Powers and Barlow, 1985; Pieprzyk et al., 2003) been documented. The light-driven movement of the Limulus Ops1–2 results in a 50% decline in the levels of rhabdomeric rhodopsin (Katti et al., 2010), not as extreme as shown here for a white-eyed strain of Ae. aegypti. There is a dramatic reduction in light flux reaching the Limulus photoreceptors during the day due to screening pigmentation and anatomical changes within the retina (Barlow et al., 1989). Hence it is possible that Limulus photoreceptors are capable of more extensive Ops1–2 movement. It is also the case that these Limulus photoreceptors coexpress a second rhodopsin, Ops5, which does not show as dramatic of a light-driven decrease. Thus, the relative abundance of Ops1–2 and Op5 in the rhabdomere changes during the day-night cycle and this may affect spectral and other properties of the photoreceptor (Katti et al., 2010). A similar situation may exist in Ae. aegypti, as the expression pattern of four other long-wavelength rhodopsins (Aaop3, Aaop4, Aaop5 and Aaop7) and one other rhodopsin (Aaop12) have not yet been characterized.
Aaop1 is degraded on a daily cycle
In addition to the daily light-driven movement of Aaop1, we documented a decrease in the overall levels of Aaop1 during the late afternoon and evening time periods. The decline in Aaop1 levels is initiated before the dusk period, indicating that the reduction in light intensity does not trigger the process. A genome-wide survey to identify transcripts cycling during a 24 h period failed to detect cycling of the Aaop1 mRNA (Ptitsyn et al., 2011). Thus it is likely that a post-transcriptional mechanism is involved.
In principle, the decline at dusk could result from a period of decreased Aaop1 synthesis or enhanced Aaop1 degradation. Our analysis suggests that the major mechanism is increased degradation. The reduction in Aaop1 levels occurs as expected when mosquitoes are maintained in the light through the expected dusk period. However, keeping the mosquitoes in the dark through the expected dusk period prevents the reduction in Aaop1 levels. This result shows that cytoplasmic location of the existing Aaop1 is required and is best explained by a mechanism in which the reduction is due to increased degradation of cytoplasmic Aaop1. The alternative mechanism, that Aaop1 synthesis is curtailed during this period, does not account for the protective effect of rhabdomeric localization.
The finding of a diurnal control of rhodopsin degradation is a novel finding that was not anticipated from analysis of other invertebrates. Drosophila does not show dramatic diurnal changes in rhabdomere volume (Sapp et al., 1991) or rhodopsin levels (Hartman et al., 2001). Other invertebrate species show daily changes in membrane shedding (Blest, 1978; Barlow et al., 1989; Sacunas et al., 2002). The possibility that the overall rhodopsin levels cycle in these other invertebrates has not been investigated.
Patterned rhodopsin expression in the mosquito eye
There are 10 rhodopsins encoded in the Ae. aegypti genome. The analysis of Aaop1 places at least one rhodopsin in every photoreceptor of the compound eye. Hu et al. (2009) previously showed that expression of Aaop2 and Aaop8 rhodopsins in distinct sets of R7 photoreceptors divides the retina into four distinct regions. The Aaop9 rhodopsin is coexpressed in all R7 photoreceptors and also within the R8 cells of the dorsal region (Hu et al., 2011). We show in this report that Aaop1 is expressed in the R8 cell found in all other retinal regions. The advantages provided by the R7 and R8 cell patterned expression of rhodopsins in these different regions is not known. In Drosophila, the R7 and R8 cells support both color vision (Yamaguchi et al., 2010) and detection of polarized light (Wernet et al., 2012).
Aaop1 is the first rhodopsin in Ae. aegypti identified that is expressed in the R1–R6 photoreceptors. The use of a long-wavelength rhodopsin in the major class of photoreceptors is common in both vertebrates and invertebrates. The R1–R6 photoreceptors generate most of the volume of the fused rhabdom structure that maximizes light sensitivity. Further, given that all R1–R6 cells of the retina express the same visual pigment, these cells are capable of forming a large and coherent visual field, providing the best opportunity for image detection. This also provides the best opportunity for motion detection, known to be mediated by R1–R6 photoreceptors in Drosophila (Yamaguchi et al., 2008).
Footnotes
This work was supported by National Institutes of Health Grant EY006808 (to J.E.O.). We thank Bronwen Mitchell and Yeona Chun for help with immunostaining analysis, Bill Archer, the Notre Dame Integrated Imaging Facility, Barbara Nagel, and the Research Microscopy Core, Saint Louis University, for assistance with the EM analysis.
References
- Ahmad ST, Natochin M, Artemyev NO, O'Tousa JE. The Drosophila rhodopsin cytoplasmic tail domain is required for maintenance of rhabdomere structure. FASEB J. 2007;21:449–455. doi: 10.1096/fj.06-6530com. [DOI] [PubMed] [Google Scholar]
- Barlow R, Chamberlan S, Lehman H. Circadian rhythms in the invertebrate retina. In: Stavenga DG, Hardie RC, editors. Facets of vision. New York: Springer; 1989. pp. 257–280. [Google Scholar]
- Blest A. The rapid synthesis and destruction of photoreceptor membrane by a dinopid spider: a daily cycle. Proc R Soc Lond B Biol Sci. 1978;200:463–483. [Google Scholar]
- Brammer JD. The ultrastructure of the compound eye of a mosquito Aedes aegypti L. J Exp Zool. 1970;175:181–196. [Google Scholar]
- Brammer J, Stein P, Anderson R. Effect of light and dark adaptation upon the rhabdom in the compound eye of the mosquito. J Exp Zool. 1978;206:151–156. [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Fleissner G. The absolute sensitivity of the median and lateral eyes of the scorpion Androctonus australis L. (Buthidae, Scorpiones) J Comp Physiol. 1977;118:109–120. [Google Scholar]
- Hartman SJ, Menon I, Haug-Collet K, Colley NJ. Expression of rhodopsin and arrestin during the light-dark cycle in Drosophila. Mol Vision. 2001;7:95–100. [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Hu X, England JH, Lani AC, Tung JJ, Ward NJ, Adams SM, Barber KA, Whaley MA, O'Tousa JE. Patterned rhodopsin expression in R7 photoreceptors of mosquito retina: implications for species-specific behavior. J Comp Neurol. 2009;516:334–342. doi: 10.1002/cne.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Whaley MA, Stein MM, Mitchell BE, O'Tousa JE. Coexpression of spectrally distinct rhodopsins in Aedes aegypti R7 photoreceptors. PloS One. 2011;6:e23121. doi: 10.1371/journal.pone.0023121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti C, Kempler K, Porter ML, Legg A, Gonzalez R, Garcia-Rivera E, Dugger D, Battelle BA. Opsin co-expression in Limulus photoreceptors: differential regulation by light and a circadian clock. J Exp Biol. 2010;213:2589–2601. doi: 10.1242/jeb.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF, Gibson G, Horwood J, Zeil J. Fundamental differences in the optical structure of the eyes of nocturnal and diurnal mosquitoes. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:91–103. [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieprzyk AR, Weiner WW, Chamberlain SC. Mechanisms controlling the sensitivity of the Limulus lateral eye in natural lighting. J Physiol. 2003;189:643–653. doi: 10.1007/s00359-003-0437-8. [DOI] [PubMed] [Google Scholar]
- Powers MK, Barlow R.B. Behavioral correlates of circadian rhythms in the Limulus visual system. Biol Bull. 1985;169:578–591. [Google Scholar]
- Ptitsyn AA, Reyes-Solis G, Saavedra-Rodriguez K, Betz J, Suchman EL, Carlson JO, Black WC. Rhythms and synchronization patterns in gene expression in the Aedes aegypti mosquito. BMC Genomics. 2011;12:153. doi: 10.1186/1471-2164-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacunas RB, Papuga MO, Malone MA, Pearson AC, Jr, Marjanovic M, Stroope DG, Weiner WW, Chamberlain SC, Battelle BA. Multiple mechanisms of rhabdom shedding in the lateral eye of Limulus polyphemus. J Comp Neurol. 2002;449:26–42. doi: 10.1002/cne.10263. [DOI] [PubMed] [Google Scholar]
- Sapp RJ, Christianson J, Stark WS. Turnover of membrane and opsin in visual receptors of normal and mutant Drosophila. J Neurocytol. 1991;20:597–608. doi: 10.1007/BF01215267. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O'Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Velez MM, Clark DA, Baumann-Klausener F, Brown JR, Klovstad M, Labhart T, Clandinin TR. Genetic dissection reveals two separate retinal substrates for polarization vision in Drosophila. Curr Biol. 2012;22:12–20. doi: 10.1016/j.cub.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RH. The effect of light and light deprivation upon the ultrastructure of the larval mosquito eye. 3. Multivesicular bodies and protein uptake. J Exp Zool. 1968;169:261–277. doi: 10.1002/jez.1401690302. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Desplan C, Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc Natl Acad Sci U S A. 2010;107:5634–5639. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]