Abstract
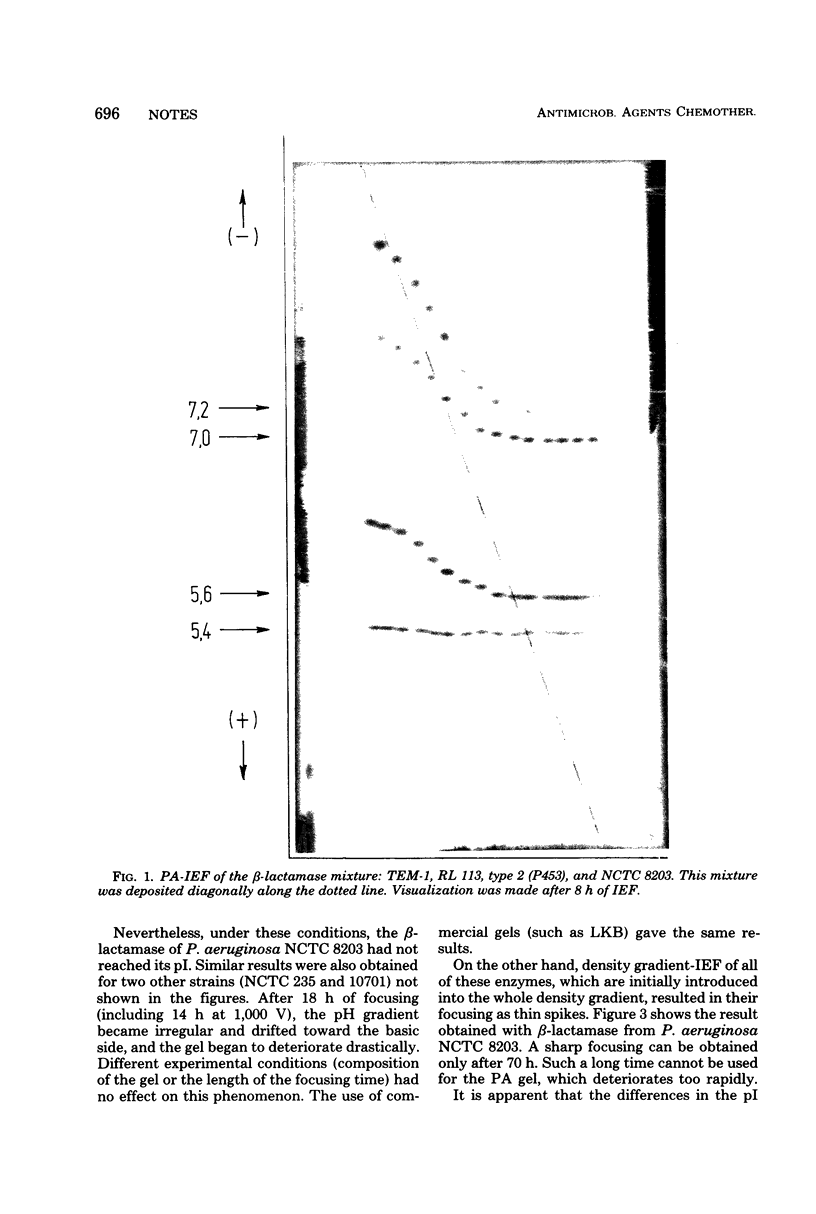
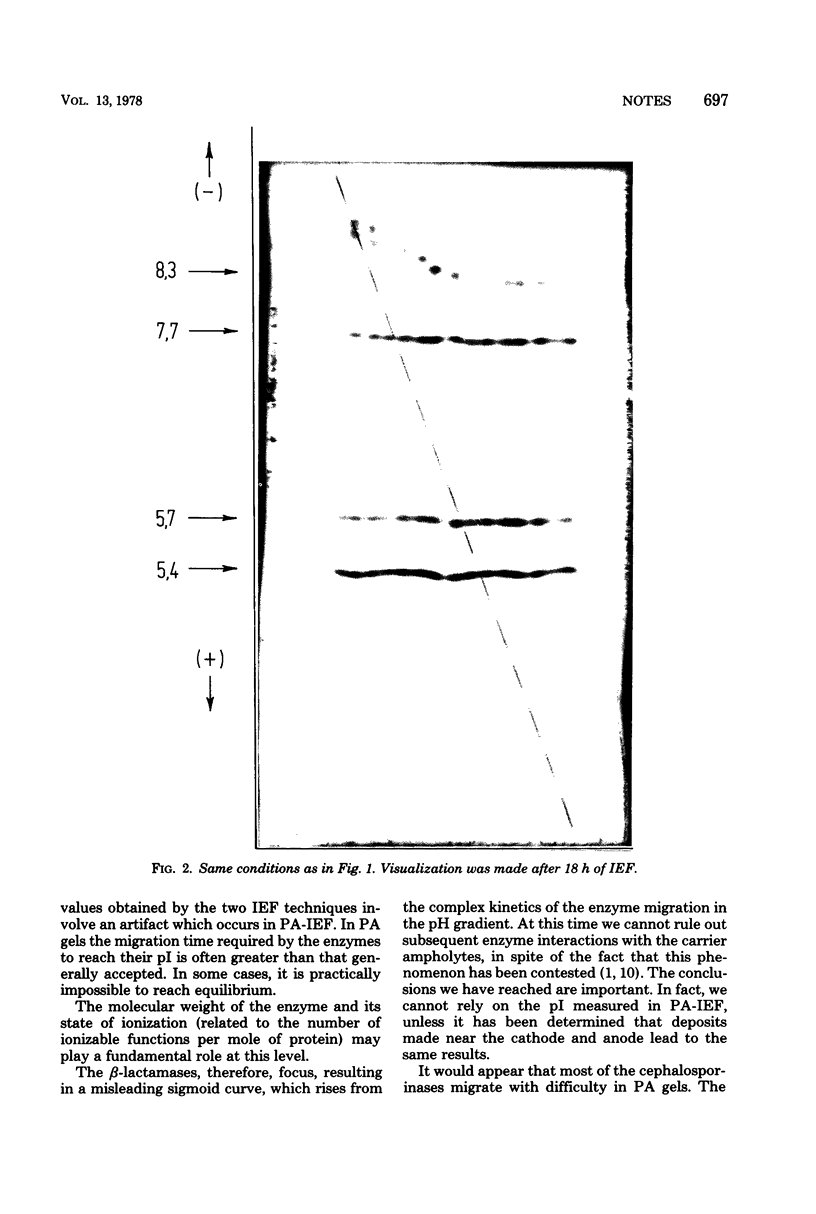
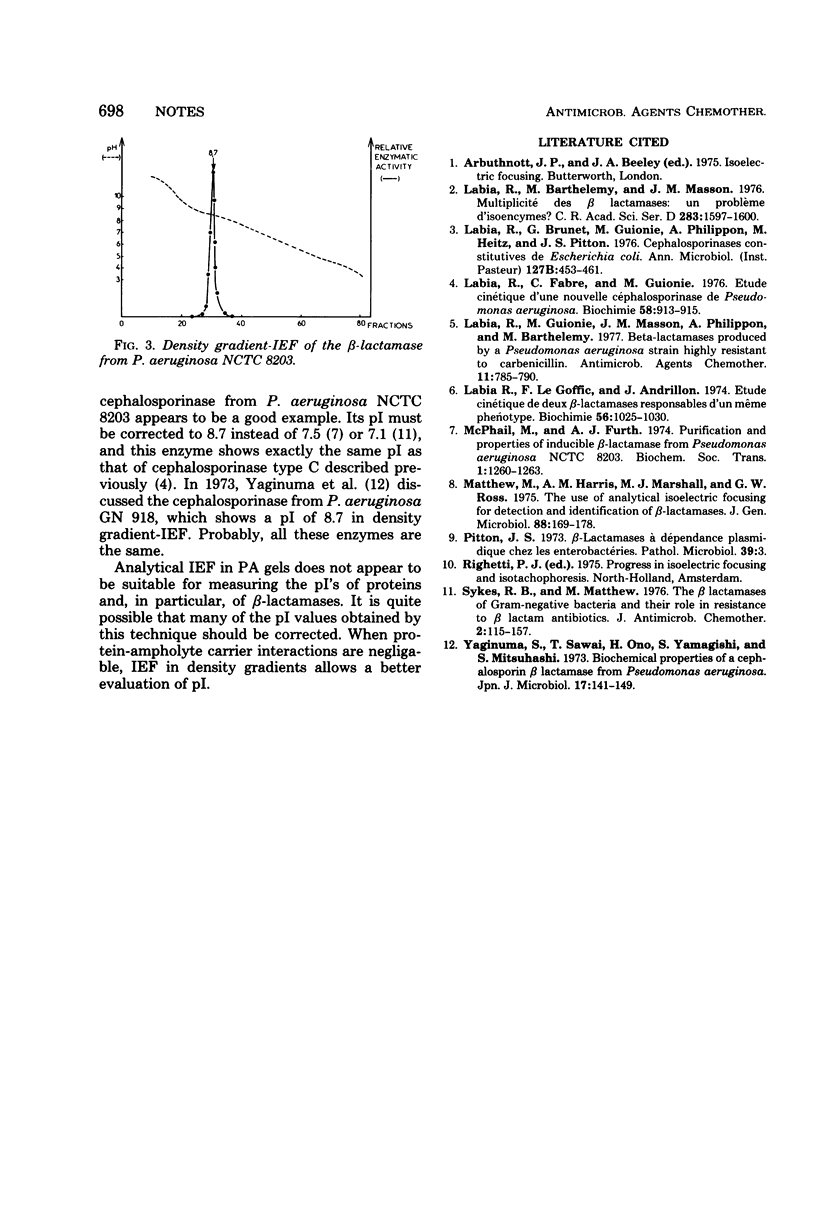
Important discrepancies in isoelectric points (pI) of β-lactamases were observed depending on the experimental procedure used for their determination: isoelectric focusing (IEF) in sucrose density gradients or analytical IEF in thin-layer polyacrylamide gels (PA). The variations, negligable in the case of TEM-like β-lactamases, appeared to be important in the case of cephalosporinases and are related to an artifact which appears in PA-IEF. This has been clearly shown with β-lactamase preparations from the following bacterial strains: Pseudomonas aeruginosa NCTC 8203 (pI = 8.7), P. aeruginosa NCTC 10701 (pI = 9.4), and Proteus morganii NCTC 235 (pI = 8.3). The data previously obtained by PA-IEF were much lower.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Labia R., Barthélémy M., Masson J. M. Multiplicité des beta lactamases: un probléme d'isoenzymes. C R Acad Sci Hebd Seances Acad Sci D. 1976 Nov 29;283(14):1597–1600. [PubMed] [Google Scholar]
- Labia R., Brunet G., Guionie M., Philippon A., Heitz M., Pitton J. S. Céphalosporinases constitutives de Escherichia coli. Ann Microbiol (Paris) 1976 Nov-Dec;127B(4):453–461. [PubMed] [Google Scholar]
- Labia R., Fabre C., Guionie M. Etude cinétique d'une nouvelle céphalosporinase de Pseudomonas aeruginosa. Biochimie. 1976;58(8):913–915. doi: 10.1016/s0300-9084(76)80279-9. [DOI] [PubMed] [Google Scholar]
- Labia R., Guionie M., Masson J. M., Philippon A., Barthelemy M. Beta-lactamases produced by a Pseudomonas aeruginosa strain highly resistant to carbenicillin. Antimicrob Agents Chemother. 1977 May;11(5):785–790. doi: 10.1128/aac.11.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labia R., Le Goffic F., Andrillon J. Etude cinétique de deux beta lactamases responsables d'un møeme phénotype. Biochimie. 1974;56(8):1025–1030. doi: 10.1016/s0300-9084(74)80092-1. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Yaginuma S., Sawai T., Ono H., Yamagishi S., Mitsuhashi S. Biochemical properties of a cephalosporin beta-lactamase from Pseudomonas aeruginosa. Jpn J Microbiol. 1973 Mar;17(2):141–149. doi: 10.1111/j.1348-0421.1973.tb00718.x. [DOI] [PubMed] [Google Scholar]





