Abstract
Chronic inflammation is becoming a hallmark of several neurodegenerative disorders and accordingly, interleukin-1 beta (IL-1β), a proinflammatory cytokine, is implicated in the pathogenesis of neurodegenerative diseases. While IL-1β binds to its high-affinity receptor, interleukin-1 receptor (IL-1R), and upregulates proinflammatory signaling pathways, interleukin-1 receptor antagonist (IL-1Ra) adheres to the same receptor and inhibits proinflammatory cell signaling. Therefore, upregulation of IL-1Ra is considered important in attenuating inflammation. The present study underlines a novel application of gemfibrozil, an FDA-approved lipid-lowering drug, in increasing the expression of IL-1Ra in primary mouse and human neurons. Gemfibrozil alone induced an early and pronounced increase in the expression of IL-1Ra in primary mouse cortical neurons. Activation of type IA p110α phosphatidylinositol 3-kinase (PI3-K) and Akt by gemfibrozil and abrogation of gemfibrozil-induced upregulation of IL-1Ra by inhibitors of PI3-K and Akt indicate a role of the PI3-K – Akt pathway in the upregulation of IL-1Ra. Gemfibrozil also induced the activation of cAMP response element-binding (CREB) via the PI3-K – Akt pathway and siRNA attenuation of CREB abolished the gemfibrozil-mediated increase in IL-1Ra. Furthermore, gemfibrozil was able to protect neurons from IL-1β insult. However, siRNA knockdown of neuronal IL-1Ra abrogated the protective effect of gemfibrozil against IL-1β suggesting that this drug increases the defense mechanism of cortical neurons via upregulation of IL-1Ra. Together, these results highlight the importance of the PI3-K – Akt – CREB pathway in mediating gemfibrozil-induced upregulation of IL-1Ra in neurons and suggest gemfibrozil as a possible therapeutic treatment for propagating neuronal self defense in neuroinflammatory and neurodegenerative disorders.
Keywords: Neurons, Gemfibrozil, IL-1Ra, Neuroinflammation, Neurodegeneration, PI3-kinase, Akt, CREB, Apoptosis
Introduction
Alzheimer’s disease (AD), the most common form of dementia in the world and the 6th leading cause of death in the United States, is a neurodegenerative disorder characterized by compounded neuronal loss and cognitive deficits (1–3). These malicious alterations have continually been attributed to the accumulation of stable beta-amyloid, hyperphosporylation of the cytoskeletal protein tau and pro- and anti-neuroinflammatory imbalance (4, 5). Despite the immense amount of research toward developing a therapeutic solution for AD, there is currently no cure available.
Many of the histopathological changes associated with AD have, in part, been attributed to an upregulation of physiological interleukin-1 beta (IL-1β), a vital proinflammatory cytokine capable of inducing the expression of other proinflammatory molecules (6, 7). During neurodegenerative insult, microglia-mediated IL-1β release is increased thus contributing to neurotoxicity (6). IL-1β is a prominent member of the IL-1 family of cytokines, a group which also includes interleukin-1 receptor antagonist (IL-1Ra aka IL-1rn). IL-1Ra binds competitively with IL-1β to the biologically active interleukin-1 receptor isoform (IL-1R1) and disrupts the proinflammatory intracellular signaling cascade (8). Indeed, proper balance between these two cytokines within the brain plays a vital role in the vulnerability to and severity of a number of neuroinflammatory states including but not limited to AD (9, 10). For example, decreased IL-1Ra levels have been described in cerebrospinal fluid (CSF) of AD patients (11) and a polymorphism in the IL-1 gene cluster leading to increased IL-1Ra has also been shown to be protective for dementia severity (12). Experimental allergic encephalomyelitis (EAE) is an animal model of multiple sclerosis (MS). It has been found that HSV-1-mediated IL-1Ra gene therapy ameliorates MOG35–55-induced chronic EAE in mice (13). Therefore, characterization of intracellular pathways required to transduce the signal from the cell surface to the nucleus to upregulate IL-1Ra is an active area of investigation, since compounds capable of agonizing such signaling steps may have therapeutic effects in IL-1-mediated pathophysiological conditions. However, such mechanisms are poorly understood.
Gemfibrozil, known as ‘Lopid’ in the pharmacy, has often been prescribed to patients to lower triglyceride levels (14, 15). This drug decreases the risk of coronary heart disease by increasing high-density lipoprotein (HDL) cholesterol and decreasing low-density lipoprotein (LDL) cholesterol (16). Earlier, we have shown that gemfibrozil inhibits the expression of inducible nitric oxide synthase (iNOS) in human astrocytes (17) and protects mice from experimental allergic encephalomyelitis (EAE), an animal model of multiple sclerosis (18). Here, we explore another novel function of gemfibrozil. We describe the ability of gemfibrozil to significantly and effectively upregulate the expression of IL-1Ra in fetal mouse cortical neurons. Via step-by-step analysis, we demonstrate that gemfibrozil upregulates the expression of IL-1Ra in neurons via phosphatidylinositol 3-kinase (PI3-K) – Akt-mediated activation of cAMP response element binding (CREB). Furthermore, we present evidence that gemfibrozil suppresses IL-1β-mediated neuronal apoptosis via upregulation of IL-1Ra. These results suggest that gemfibrozil, an approved drug for hyperlipidemia in humans, may further extend its therapeutic use to neurodegenerative disorders.
Materials and Methods
Reagents
Neurobasal medium and B27/B27-AO supplement were purchased from Invitrogen (Carlsbad, CA) and fetal bovine serum (FBS) was obtained from Atlas Biologicals (Fort Collins, CO). L-Glutamine, DMEM/F-12 50/50 1x, Hank’s balanced salt solution (HBSS) and 0.05% trypsin were purchased from Mediatech (Washington, DC). Antibiotic-antimycotic, Akt inhibitor (Akt-i), forskolin and gemfibrozil were obtained from Sigma (St. Louis, MO). Wortmannin and LY294002 were acquired from Calbiochem (Darmstadt, Germany).
Isolation of Primary Human Neurons
Human primary neurons were prepared as described by us earlier (19, 20). All of the experimental protocols were reviewed and approved by the Institutional Review Board of the Rush University Medical Center. Briefly, 11–17-week-old fetal brains obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA) were dissociated by trituration and trypsinization (0.25% trypsin in PBS at 37 °C for 15 min). The trypsin was then neutralized with 10% heat-inactivated fetal bovine serum. Dissociated cells were filtered through 380- and 140-μm meshes (Sigma) and pelleted by centrifugation. The pellet was washed once with 1x PBS and once with Neurobasal medium containing 2% B27 and 1% antibiotic-antimycotic mixture (Sigma). Neurons were enriched by allowing the cells (3 × 106/ml) to adhere to poly-D-Lysine-coated coverslips for 5 min. Nonadherent cells were removed, and adherent cells were further treated with 10μM Ara-C (Sigma) to prevent the proliferation of dividing cells. After 10 days of Ara-C treatment, cells were deemed ready for treatment.
Animals
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animal maintenance and experiments were in accordance with National Institute of Health guidelines and were approved by the Institutional Animal Care and Use committee (IACUC) of the Rush University Medical Center, Chicago, IL.
Fetal cortical cell culture
Fetal (E18-E16) mouse cortical neurons were prepared as previously described (21) with modifications. Whole brains were removed and cortices dissected in serum free Neurobasal media. Cortical cells were then spun into pellet 3 times at 1500 rpm for 10 min, dissociated and plated in 6-, 12- or 24-well plates or 8-well chamber slides pre-treated for at least 2 hr with Poly-D-Lysine (Sigma, St. Louis, MO). After 4 min, the non-adherent cell suspension was discarded and 500ml complete Neurobasal media supplemented with 2% B27 was added to each well. Cells were incubated for 3–5 days and analyzed for confluence prior to experimentation. Treatment of cells was conducted in Neurobasal media supplemented with 2% B27 minus antioxidants (Invitrogen).
Reverse Transcriptase-Coupled Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from fetal mouse primary neurons using RNA-Easy Qiagen (Valencia, CA) kit following manufactures protocol. Semi-quantitative RT-PCR was carried out as described earlier (22) using oligo (dT) 12–18 as primer and moloney murine leukemia virus reverse transcriptase (MMLV-RT, Invitrogen) in a 20μl reaction mixture. The resulting cDNA was appropriately amplified using Promega Master Mix (Madison, WI) and the following primers (Invitrogen) for murine genes:
| CREB (324 bp): | Sense, 5′-TGC AGC TGC CAC TCA GCC GGG-3′ Antisense, 5′-TGC CAA GCC AGT CCA TTC TCC AC-3′ |
| IL-1Ra (365bp): | Sense, 5′-GCA GCA CAG GCT GGT GAA TGA C-3′ Antisense, 5′-TGC CCC CGT GGA TGC CCA AG-3′ |
| IL-1β (563 bp): | Sense, 5′-ATG GCA ACT GTT CCT GAA CTC AAC T-3′ Antisense, 5′-CAG GAC AGG TAT AGA TTC TTT CCT TT-3′ |
| IL-1R1 (360 bp): | Sense, 5′-GGT GCC TCT GCT GTC GCT GG-3′ Antisense, 5′-CGC TGT GGG AAG GTG GCC TG-3′ |
| GAPDH (300bp): | Sense, 5′-GGT GAA GGT CGG AGT CAA CG-3′ Antisense, 5′-GTG AAG ACG CCA GTG GAC TC-3′ |
Amplified products were electrophoresed on 2% agarose (Invitrogen) gels and visualized by ethidium bromide (Invitrogen) staining. Response of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as a loading control to ascertain that an equivalent amount of cDNA was synthesized from each sample.
Real-Time qPCR
mRNA quantification was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) using iTaq™ Fast Supermix With ROX (Bio-Rad, Hercules, CA) and the following 6-FAM/ZEN/IBFQ-labeled primers for murine genes: IL-1Ra (Applied Biosystems), CREB and GAPDH (Integrated DNA Technologies Coralville, IA). The mRNA expression of the targeted genes was normalized to the level of GAPDH mRNA and data was processed by the ABI Sequence Detection System 1.6 software.
Immunostaining
Immunocytochemistry was performed as described earlier (23). Briefly, coverslips containing neurons cultured to 70–80% confluence were fixed with chilled Methanol (Fisher Scientific, Waltham, MA) overnight, followed by two brief rinses with filtered PBS. Samples were blocked with 2% BSA (Fisher Scientific) in PBS containing Tween 20 (Sigma) and Triton X-100 (Sigma) for 30 min and incubated at room temperature under shaking conditions for 2 hr in PBS containing the following anti-mouse primary antibodies: IL-1Ra (1:100; Santa Cruz Biotech, Santa Cruz, CA), p-Akt (1:600; Cell Signaling, Beverly, MA), p-CREB (1:600; Cell Signaling), GFAP, (1:100; Santa Cruz), CD11b (1:500; Cedarlane Laboratories, Burlington, NC) and MAP-2 (1:50; Santa Cruz). After four 15 min washes in filtered PBS, slides were further incubated with Cy2-, Cy3- or Cy5-labeled secondary antibodies (all 1:200; Jackson ImmunoResearch, West Grove, PA) for 1 hr under similar shaking conditions. Following four 15 minute washes with filtered PBS, cells were incubated for 4–5 min with 4′,6-diamidino-2-phenylindole (DAPI, 1:10,000; Sigma). For negative controls, a set of culture slides was incubated under similar conditions void of primary antibodies. The samples were run in an EtOH and Xylene (Fisher) gradient, mounted and observed under a Bio-Rad MRC1024ES confocal laser-scanning microscope (24).
IL-1Ra assay
Supernatants were collected post-treatment and the presence of IL-1Ra protein was analyzed using high-sensitivity sandwich ELISA kits (R&D Systems, Minneapolis, MN) according to the protocol outlined by the manufacturer. Plates were analyzed spectrophotometrically with the Thermo-Fisher Multiskanskan MCC plate reader (Fisher).
Transfection
Primary neurons were transfected with Lipofectamine, PLUS® (Invitrogen) and Nupherin™-neuron (Enzo Life Sciences/Biomol, Farmington, NY) according to manufacturer’s protocol and as described earlier (21). Briefly, neurons cultured in 12-well plates were transfected with 0.25μg/well of control or IL-1Ra siRNA (Santa Cruz) complexed with Nupherin™ peptide, Lipofectamine and PLUS® in serum-free DMEM/F containing L-glutamine (Invitrogen) for 5 hr. After 5 hr, 20% FBS was added at a volume of 1:1 with the transfection media and cells were further incubated for 19 hrs.
MTT assay
Mitochondrial activity was measured with the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay from Sigma. Cells were cultured in 24-well plates with 500 μl of medium and treated for 2.5 hrs with various reagents according to the protocol outlined by the manufacturer. At the end of the treatment period, 300 μl of culture medium was removed from each well, and 20 μl of MTT solution (5 mg/ml) was added and incubated for 1 hr. After distribution to 96-well plates, absorbance was measured at 570 nm with the Thermo-Fisher Multiskanskan MCC plate reader (Fisher).
Lactate Dehydrogenase Measurement
The activity of lactate dehydrogenase (LDH) was likewise measured using the Sigma LDH kit. Briefly, half the original culture medium was removed to Eppendorf tubes and LDH Mixture was added in a volume equal to 1.5 that of the supernatant. The reaction was carried out for 30 min at room temperature in the dark and stopped with 1N HCl (Fisher). Resultant absorbance was measured at 490 nm with the Thermo-Fisher Multiskanskan MCC plate reader.
Fragment End Labeling of DNA
Fragmented DNA was detected in situ by the terminal deoxynucleotidyltransferase-mediated binding of 3′-OH ends of DNA fragments generated in response to IL-1β, using the TdT FragEL™ kit from Calbiochem. Briefly, cover slips were treated with 20 μg/ml proteinase K for 15 min at room temperature, equilibrated for 30 min in 1x TdT buffer and washed with PBS prior to terminal deoxynucleotidyltransferase and DAPI (1:10,000; Sigma) staining. After visualizing with a Bio-Rad (Hercules, CA) MRC1024ES confocal laser-scanning microscope, stereological counting was performed.
Immunoblotting
Western blotting was conducted as described earlier (25) with modifications. Briefly, cells were scraped in lysis buffer, transferred to microfuge tubes and spun into pellet. The supernatant was collected and analyzed for protein concentration via the Bradford method (Bio-Rad). SDS sample buffer was added to 40–60 μg total protein and boiled for 5 min. Denatured samples were electrophoresed on NuPAGE® Novex® 4–12% Bis-Tris gels (Invitrogen) and proteins transferred onto a nitrocellulose membrane (Bio-Rad) using the Thermo-Pierce Fast Semi-Dry Blotter. The membrane was then washed for 15 min in TBS plus Tween 20 (TBST) and blocked for 1 hr in TBST containing BSA. Next, membranes were incubated overnight at 4°C under shaking conditions with the following 1° antibodies; Akt, p-Akt, CREB, p-CREB, Histone H3 (all 1:600; Cell Signaling), β-actin (1:800; Abcam, Cambridge, MA), p110α, p110β, p110γ, IL-1Ra (all 1:200; Santa Cruz) and TrkB (1:500; R&D Systems, Minneapolis, MN). The next day, membranes were washed in TBST for 1 hr, incubated in 2° antibodies against 1° antibody hosts (all 1:10,000; Li-Cor Biosciences) for 1 hr at room temperature, washed for one more hour and visualized under the Odyssey® Infrared Imaging System (Li-COR, Lincoln, NE).
Densitometric Analysis
Protein blots were analyzed using ImageJ (NIH, Bethesda, MD) and bands were normalized to their respective β-actin loading controls. Data are representative of the average fold change with respect to control for three independent experiments.
Cellular Membrane Extraction
Neuronal membranes were isolated to determine the recruitment of various membrane associated proteins to the membrane. Cells were washed with PBS and scraped in phenol-red-free HBSS to 5 mL ultracentrifuge tubes. The solution was then diluted with 100 mM sodium bicarbonate buffer (pH 11.5) and spun in an ultracentrifuge at 40,000 rpm for 1 hr at 4°C. The resultant supernatant was aspirated and the pellet was immersed in double-distilled H20 and SDS and stored at −80°C overnight. The following day, the pellet was resuspended by repeated grinding and boiling.
Assay of transcriptional activity
Transcriptional activities of CREB were analyzed using the protocol previously outlined by us (26) with some modification. Briefly, cells plated at 50 to 60% confluence in 12-well plates were transfected with 0.25 μg/well pCRE-Luc (a CREB-dependent reporter construct), pNF-κB-Luc (a NF-κB-dependent reporter construct) and pAP-1-Luc (a AP-1-dependent reporter construct) using Nupherin™ peptide and Lipofectamine PLUS® as described above. These reporter constructs were obtained from Stratagene, Santa Clara, CA. After 24 hr of transfection, cells were stimulated with various reagents and firefly luciferase activity was recorded in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) by analyzing total cell extract according to standard instructions provided in the Dual Luciferase Kit (Promega).
Nuclear extraction and gel shift
DNA-binding activities of CREB and NF-κB were analyzed by non-radioactive electrophoretic mobility shift assay (EMSA). After treatment, cells were washed with HBSS, scraped into 1.5 mL tubes and centrifuged in 4°C for 5 min at 500 rpm. The supernatant was aspirated and the pellet was resuspended in a membrane lysis buffer comprised of (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES, pH 8.0), MgCl2, KCl, dithiothreitol (DTT) and protease/phosphatase inhibitors (Sigma), vortexed and centrifuged in 4°C at 720 × g for 5 minutes. Again, the supernatant was aspirated and the pellet was resuspended in a high salt, nuclear envelope lysis buffer comprised of HEPES (pH 8.0), MgCl2, glycerol, NaCl, ethylenediaminetetraacetic acid (EDTA), DTT and protease/phosphatase inhibitors, rotated vigorously in 4°C for 15 min and centrifuged in 4°C at 13,000 rpm for 15 minutes. The resultant supernatant was complexed with a cocktail of binding buffer (Tris-HCl, KCl, EDTA, DTT, 10x TGE, glycerol and Triton-X 100), custom designed fluorescent CREB-specific or NF-κB-specific probes (Li-Cor Biosciences), and salmon sperm DNA (Invitrogen) for 15 min at room temperature and electrophoresed on custom-cast 6% polyacrylamide-TGE gels in 1X TGE for 2 hrs. Supershift was performed by incubating nuclear extracts with 2 μg ChIP-grade CREB antibody or IgG (Millipore) for 30 min prior to addition of the probe. The shift was visualized under the Odyssey® Infrared Imaging System (Li-Cor).
Chromatin immunoprecipitation
Recruitment of CREB to the IL-1Ra promoter was determined using the EZ ChIP kit from Millipore according to manufactures instructions. Briefly, 1 × 106 cortical neurons were treated with gemfibrozil and after 3 h of stimulation, cells were fixed by adding formaldehyde (1% final concentration), and cross-linked adducts were resuspended and sonicated. ChIP was performed on the cell lysate by overnight incubation at 4°C with 2 μg of Abs against CREB and RNA polymerase II followed by overnight incubation with protein G-agarose (Santa Cruz Biotechnology). The beads were washed and incubated with elution buffer. To reverse the cross-linking and purify the DNA, precipitates were incubated in a 65°C incubator overnight and digested with proteinase K. DNA samples were then purified, precipitated, and precipitates were washed with 75% ethanol, air-dried, and resuspended in Tris-EDTA buffer. The following primers were used to amplify fragments flanking the only CRE in the mouse IL-1Ra promoter: sense: 5′-CACAAGTGGCCTTTGTTTGGC-3′, antisense: 5′-GTGTTCACCGGA AACAAACCG-3′. PCR products were electrophoresed on 2% agarose gels.
Cell Counting
Four independent images were taken from each chamber slide well. The image field (20x magnification, 20μm) was divided into 16 equal sections and the number of TUNEL and DAPI positive cells were counted. Statistical analysis was then conducted based on the mean number of cells across four images taken from each chamber slide well.
Statistics
Values are expressed as means ± SD of at least three independent experiments. Statistical analyses for differences were performed via one-way ANOVA followed by Tukey’s or Scheffe’s post-hoc tests using SPSS 19 (IBM Corporation, Armonk, NY). The criterion for statistical significance was p < 0.05.
Results
Gemfibrozil upregulates IL-1Ra expression in fetal mouse cortical neurons (fMCNs)
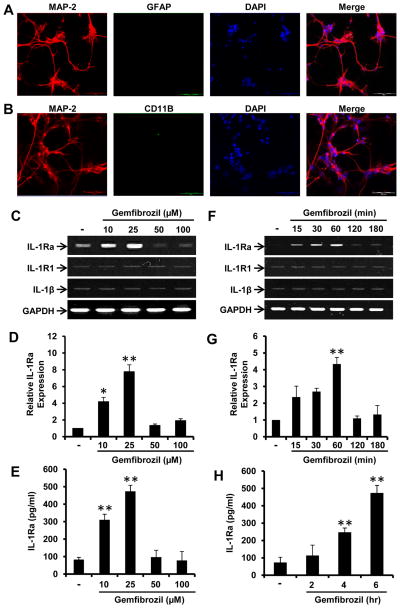
Increasing IL-1Ra in neurons could be an important defense mechanism for cells vulnerable to inflammatory insult. We examined if gemfibrozil (gem) could upregulate IL-1Ra in fMCNs. Prior to experimentation, we examined the purity of neuronal cultures. Double-label immunofluorescence with MAP-2 (neuronal marker) and either GFAP (astroglial marker) (Fig. 1A) or CD11b (microglial marker) (Fig. 1B) reveals more than 97% homogenous cultures. Interestingly, within 1 h of treatment, gem dose-dependently increased the mRNA expression of IL-1Ra as evident from RT-PCR (Fig. 1C) and real-time PCR (Fig. 1D). Gem was most efficient in increasing IL-1Ra at lower doses, demonstrating maximum effect at 25μM (Fig. 1C & D). However, the increase was absent at higher doses (Fig. 1C & D). Importantly, the upregulation of IL-1Ra was not accompanied by concordant increases in the expression of IL-1β and IL-1R1 (Fig. 1C). To understand whether neuronal IL-1Ra is secreted or remains cell-bound, we performed ELISA from gem-treated and untreated supernatants. ELISA results (Fig. 1E) support our mRNA finding and suggest that IL-1Ra can be released from gem-treated neurons.
Figure 1. Gemfibrozil upregulates the expression of IL-1Ra mRNA and protein in mouse cortical neurons.
In order to determine the purity of neuronal cultures, chamber slides were double-labeled for MAP-2 and either GFAP (A) or CD11b (B). Fetal mouse cortical neurons (fMCNs) were treated with different concentrations of gemfibrozil for 1 hr followed by monitoring mRNA expression of IL-1Ra by semi-quantitative RT-PCR (C) and real-time PCR (D). fMCNs were treated with different concentrations of gemfibrozil for 6 hrs followed by measuring IL-1Ra protein in supernatants by sandwich ELISA (E). fMCNs were treated with 25μM gemfibrozil for 15 min, 30 min, 1 hr, 2 hr and 3 hr followed by monitoring the mRNA expression of IL-1Ra by semi quantitative RT-PCR (F) and real time PCR (G). fMCNs were treated with 25μM gemfibrozil for 2, 4 and 6 hrs followed by measuring IL-1Ra protein in supernatants by sandwich ELISA (H). Results are mean ± SD of three independent experiments. Significance is indicated by *p < 0.05 and **p < 0.01. Scale bar = 20μm.
Next we analyzed the time-dependent induction of IL-1Ra in fMCNs. The effect of gem was rapid, as gem was able to upregulate the mRNA expression of IL-1Ra as early as 15 min (Fig. 1F & G). This effect was highest at 60 min and decreased thereafter (Fig. 1F & G). Again, gem did not increase the expression of IL-1β and IL-1R1 at different time points (Fig. 1F). Time-dependent IL-1Ra protein expression was then monitored by ELISA. Although gem-induced production of IL-1Ra was significant at 2 h, a strong upregulation of IL-1Ra protein was observed at 4 and 6 hrs of treatment (Fig. 1H). These results suggest that gem is capable of inducing the expression of the anti-inflammatory cytokine IL-Ra without altering IL-1R1 or IL-1β expression in fMCNs.
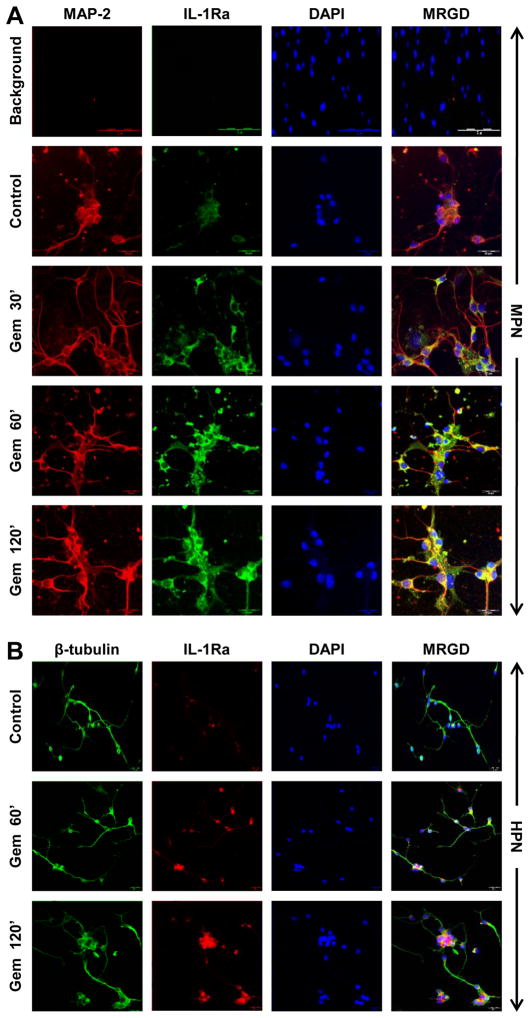
To confirm the findings further, we examined the upregulation of IL-1Ra protein in fMCNs by immunofluorescence. Control and gem-treated fMCNs were double-labeled for MAP-2 (red) and IL-1Ra (green) (Fig. 2A). Again, we observed a strong time-dependent increase in IL-1Ra protein expression, localized to the neuronal cell body, after gem treatment. Because results obtained in mice do not always translate to humans, we examined if gem was capable of upregulating IL-1Ra in primary human neurons. As evident from figure 2B, gem also induced the level of IL-1Ra in fetal human neurons.
Figure 2. Gemfibrozil upregulates IL-1Ra in fMCNs and human embryonic neurons.
(A) fMCNs incubated with 25μM gemfibrozil for 30 min, 1 hr and 2 hrs were analyzed for IL-1Ra expression by immunofluorescence. Background fluorescence (A; top panel) and specificity (data not shown) of the IL-1Ra antibody was also monitored. (B) Primary human neurons treated with 25μM gemfibrozil for 1 and 2 hrs were analyzed for IL-1Ra expression by immunofluorescence. Scale bar = 20μm.
Gem requires activation of phosphatidylinositol 3-kinase (PI3-K) to upregulate IL-1Ra
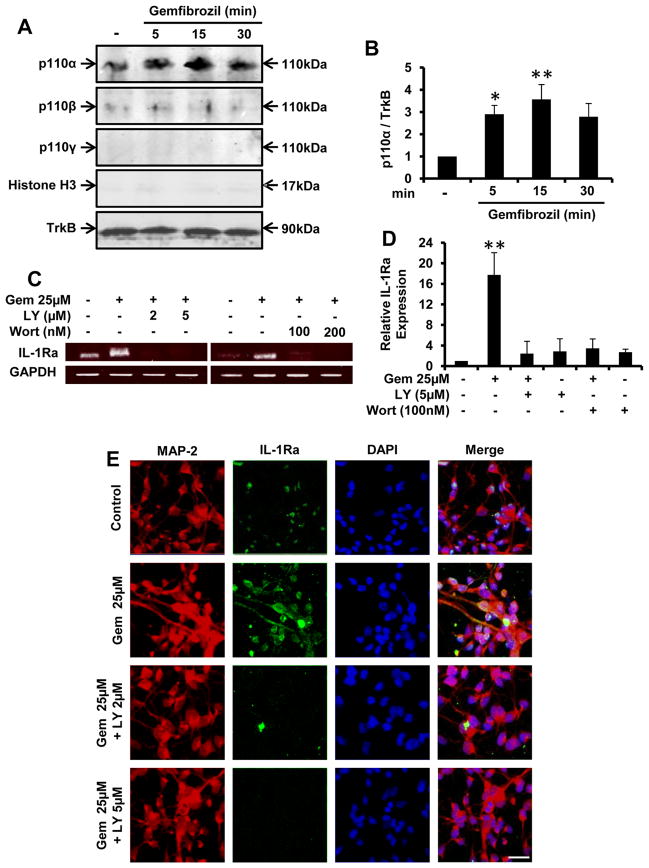
Next, we set out to identify signaling pathway by which gem induces IL-1Ra in neurons. Since gem-induced neuronal upregulation of IL-1Ra was very rapid, and in our earlier study (27) gem induced the activation of PI3-K in microglia within minutes, we were prompted to investigate the involvement of PI3-K in gem-mediated increase in IL-1Ra. PI3-K, a dual protein and lipid kinase, transduces signals for multiple biological processes (28). Class IA PI3-K, which is regulated by receptor tyrosine kinases, consists of a heterodimer of a regulatory 85-kDa subunit and a catalytic 110-kDa subunit (p85:p110α/p110β). In contrast, class IB PI3-K consists of a dimer of a 101-kDa regulatory subunit and a p110γ catalytic subunit (p101:p110γ). While in resting condition, subunits of PI3-K are located mainly in cytoplasm. Upon activation, these are translocated to the plasma membrane (28, 29). Therefore, we monitored the activation of class IA and IB PI3-K by the recruitment of p110α, p110β and p110γ to the membrane. As expected, immunoblot analysis of fMNC membrane fractions showed the presence of TrkB, but not histone H3 (Fig. 3A). Western blotting of membrane fractions for p110 subunits suggests that gem specifically induces the recruitment of p110α, but neither p110β nor p110γ, to the plasma membrane (Fig. 3A). Densitometric analysis of the p110α response under increasing exposure to gemfibrozil indicates significant activation of PI3-K at 15 min (Fig. 3B). These results suggest that gem specifically activates type IA PI3-K p110α in fMCNs. Next we examined if gem required PI3-K for the upregulation of IL-1Ra in fMCNs. Abrogation of the gem-induced upregulation of IL-1Ra mRNA in fMCNs by LY294002 and wortmannin (Fig. 3C for RT-PCR and Fig. 3D for real-time PCR) indicates the involvement of PI3-K in neuronal upregulation of IL-1Ra. This was further confirmed by IL-1Ra immunofluorescence in fMCNs (Fig. 3E).
Figure 3. Gemfibrozil requires the activation of PI3-K to induce the upregulation of IL-1Ra in fMCNs.
(A) Cells treated with 25μM gemfibrozil for 5, 15 and 30 min were analyzed for the presence of histone H3 and TrkB and recruitment of p110α, p110β and p110γ to the cellular membrane via western blot. (B) Densitometric analysis of time dependent recruitment of p110α to the plasma membrane. Cells preincubated with different concentrations of LY294002 and wortmannin for 30 min were treated with 25μM gemfibrozil for 1 hr followed by monitoring the expression of IL-1Ra mRNA by semi-quantitative RT-PCR (C) and real time PCR (D). The effects of LY294002 pretreatment on gemfibrozil-mediated induction of IL-1Ra protein were subsequently analyzed via immunofluorescence (E). Results are mean ± SD of three independent experiments. Significance is indicated by *p < 0.05 and **p < 0.01. Scale bar = 20μm.
Involvement of Akt in gem-mediated upregulation of IL-1Ra in fMCNs
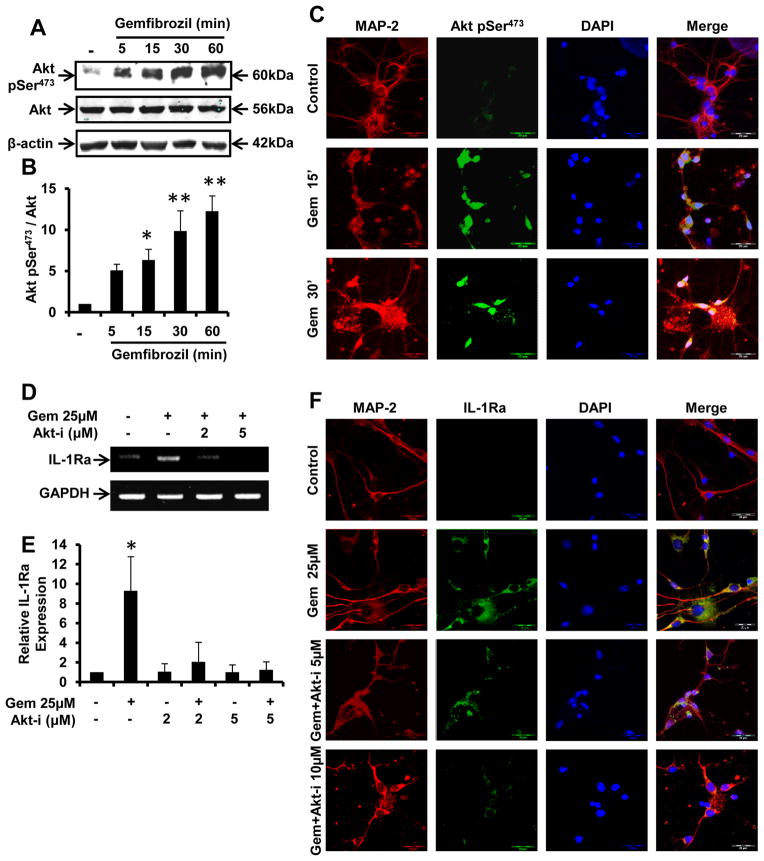
Since PI3-K is known to activate the downstream kinase Akt, we investigated if Akt was involved in gem-induced upregulation of IL-1Ra. First, we examined if gem alone was capable of inducing the activation of Akt by monitoring levels of phosphorylated Akt (p-Akt) using antibodies against Akt-p-Ser473. While gem time-dependently induced the phosphorylation of Akt, the level of total Akt (t-Akt) was unchanged (Fig. 4A). Densitometric analysis of p-Akt indicated that gem was capable of inducing the phosphorylation of Akt as early as 5 min and that significant elevation of pAkt was observed at 15, 30 and 60 min of gem treatment (Fig. 4B). To further confirm the activation of Akt, we immunostained fMCNs for MAP-2 (red) and p-Akt (green). Again, we observed an increase in p-Akt at 15 and 30 min of gem exposure relative to control (Fig. 4C). These results suggest gem alone is capable of inducing the activation of Akt in fMCNs.
Figure 4. Gemfibrozil induces IL-1Ra expression in fMCNs via activation of Akt.
(A) Western blot analysis of phosphorylated Akt (p-Akt) and total Akt (t-Akt) in fMCNs treated with 25μM gemfibrozil for 5, 15, 30 and 60 min. (B) Densitometric analysis of time dependent increase in p-Akt by gemfibrozil. (C) Cells were treated with 25μM gemfibrozil for 15 and 30 min under the same culture conditions followed by monitoring the level of p-Akt by double-label immunofluorescence. Prior to treatment with gemfibrozil, neurons were preincubated with different concentrations of Akt-i for 30 min followed by monitoring the expression of IL-1Ra mRNA by semi-quantitative RT-PCR (D) and real time PCR (E) and protein by immunofluorescence (F). Results are mean ± SD of three independent experiments. Significance is indicated by *p < 0.05 and **p < 0.01. Scale bar = 20μm.
Next, to monitor the involvement of Akt in gem-induced upregulation of IL-1Ra, we used Akt-i, a specific inhibitor of Akt. RT-PCR (Fig. 4D) and real-time PCR (Fig. 4E) analyses indicate an increase in IL-1Ra mRNA expression in the presence of gem alone. This increase in IL-1Ra mRNA was abrogated when fMCNs were preincubated with Akt-i (Fig. 4D–E). To further confirm this observation, we performed double-label immunofluorescence for MAP-2 (red) and IL-1Ra (green). As evident from figure 4F, Akt-i markedly inhibited gem-induced upregulation of IL-1Ra in fMCNs. These results suggest an obligatory role for Akt in the gem-mediated upregulation of IL-1Ra in neurons.
CREB is required for gem to induce IL-1Ra expression
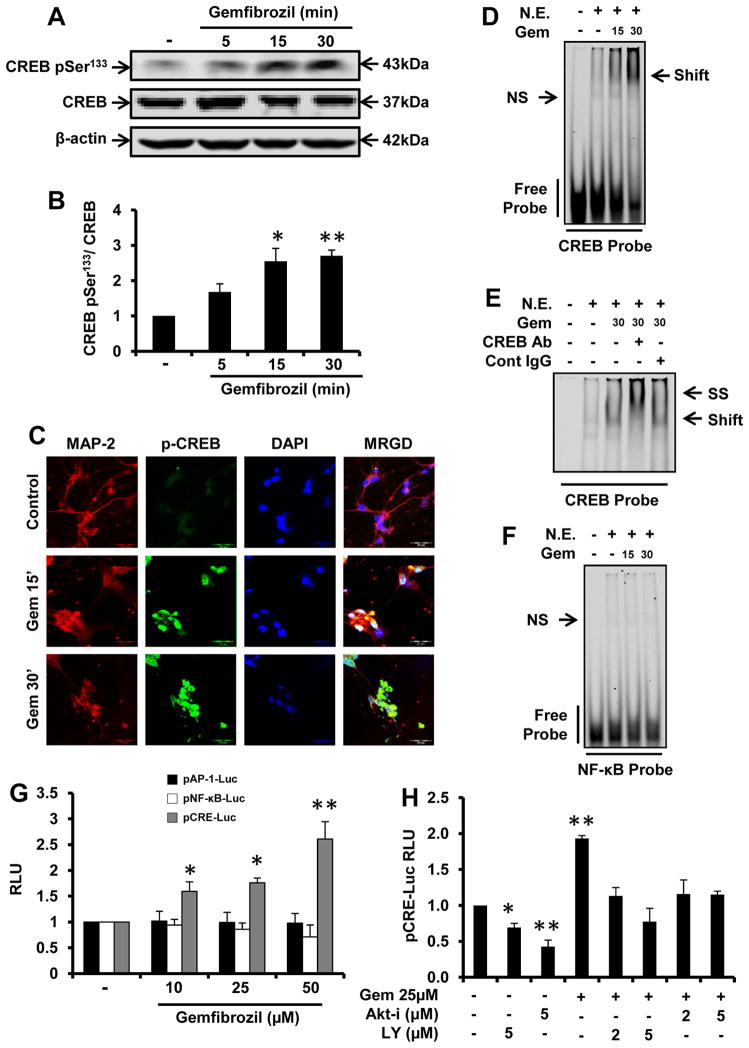
Next we investigated mechanisms by which PI3-K – Akt pathway coupled IL-Ra upregulation in gem-treated neurons. Upon analysis of the IL-1Ra promoter using MatInspector, we observed binding sites for many transcription factors including one consensus cAMP response element (CRE) near the transcriptional start site. Furthermore, CREB plays multiple roles in neuronal health and survival (30, 31). Therefore, we were prompted to investigate if gem required CREB for the transcription of IL-1Ra in neurons. First, we examined if gem alone induced the activation of CREB in neurons by monitoring levels of phosphorylated CREB (p-CREB), DNA-binding activity by EMSA and transcriptional activity using a luciferase reporter construct. Gem alone induced the phosphorylation of CREB as depicted by Western blot (Fig. 5A & B) and immunofluorescence analyses (Fig. 5C). On the other hand, we did not see any significant change in the level of total CREB (t-CREB) (Fig. 5A). Next we examined the DNA-binding activity of CREB. As seen in figure 5D, gem treatment induced a slower migrating band, which was supershifted by antibody against CREB, but not control IgG (Fig. 5E), confirming the presence of CREB in the protein-nucleic acid complex. In contrast, gem was unable to induce the activation of NF-κB (Fig. 5F), indicating the specificity of the effect. When we analyzed transcriptional activities, we also observed that gem specifically induced the transcriptional activity of CREB, but not other transcription factors like NF-κB and AP-1, in fMCNs (Fig. 5G). Next, we examined if gem required PI3-K – Akt pathway for the activation of CREB. As evident from figure 5H, both LY294002 and Akt-i markedly suppressed the gem-induced transcriptional activation of CREB, suggesting the involvement of the PI3-K – Akt pathway for the activation of CREB.
Figure 5. Gemfibrozil induces the activation of CREB in fMCNs via PI-3 kinase – Akt pathway.
(A) Western blot analysis of phosphorylated CREB and total CREB in fMCNs treated with 25μM gemfibrozil for 5, 15 and 30 min. (B) Densitometric analysis of time dependent increase in phosphorylated CREB by gemfibrozil. (C) Cells were treated with 25μM gemfibrozil for 15 and 30 min followed by monitoring the level of phosphorylated CREB by double-label immunofluorescence. Cells were treated with 25μM gemfibrozil for 15 and 30 min followed by monitoring the DNA-binding activity of CREB (D) and NF-κB (F) by EMSA. (E) For supershift assay, nuclear extracts were incubated with 2 μg CREB antibody or control IgG for 30 min followed by EMSA. (G) Cells were transfected with different luciferase constructs (pNF-κB-Luc, pAP-1-Luc and pCRE-Luc) and 24 h after transfection, cells were stimulated with gemfibrozil. After 4 h, activity of firefly luciferase was measured in total cell extracts as described in Materials and Methods. (H) Cells were transfected with pCRE-Luc and after 24 h of transfection, cells were treated with different concentrations of LY294002 or Akt-i for 30 min followed by stimulation with gemfibrozil and luciferase assay. Results are mean ± SD of at least three independent experiments. Significance is indicated by *p < 0.05 and **p < 0.01. Scale bar = 20μm. N.E. = nuclear extract.
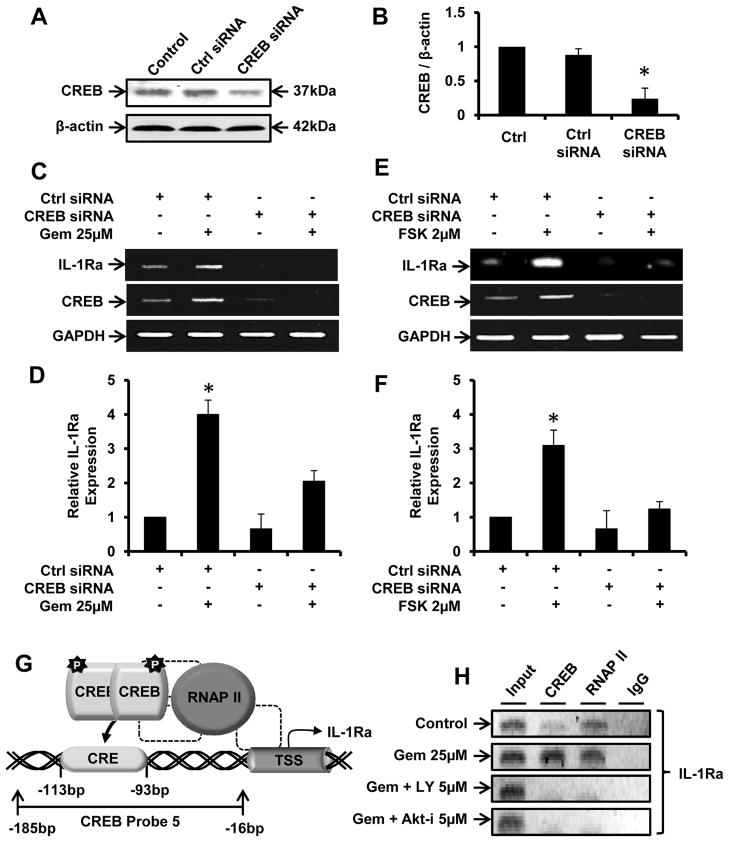
Next, we investigated if gem required CREB for the upregulation of IL-1Ra in neurons. At first, we examined if antisense knockdown of CREB was capable of suppressing the expression of CREB protein in fMCNs. As evident from figure 6A and B, CREB siRNA, but not control siRNA, decreased the expression of CREB protein in fMCNs. Accordingly, CREB siRNA, but not control siRNA, also decreased the expression of CREB mRNA in control and gem-treated neurons (Fig. 6C) and abrogated gem-mediated upregulation of IL-1Ra mRNA (Fig. 6C & D). These results suggest that gem induces the activation of CREB via the PI3-K – Akt signaling pathway and that CREB is required for increased transcription of IL-1Ra. We next investigated if forskolin, a prototypic activator of CREB, also induced the upregulation of IL-1Ra. In this instance as well, forskolin alone increased the mRNA expression of IL-1Ra (Fig. 6E & F) and siRNA knockdown of CREB suppressed the expression of IL-1Ra in forskolin-treated neurons (Fig. 6E & F), indicating an important role of CREB in neuronal IL-1Ra upregulation.
Figure 6. Involvement of CREB in gemfibrozil-induced transcription of IL-1Ra in fMCNs.
Cells transfected with either control or CREB siRNA were analyzed for CREB inhibition efficiency via western blot (A) and subsequent densitometry (B). Forty-eight hours after transfection, cells were treated with either 25μM gemfibrozil (C & D) or 2μM forskolin (E & F) for 30 min followed by monitoring the mRNA expression of CREB and IL-1Ra by RT-PCR (C & E) and real time PCR (D & F). Results are mean ± SD of three independent experiments. Significance is indicated by *p < 0.05. (G) Graphical representation of the CRE located 93 to 113 base pairs upstream of the IL-1Ra transcription start site (TSS). (H) Cells preincubated with inhibitors of PI-3 kinase and Akt for 30 min were stimulated with gemfibrozil. After 2 h of stimulation, ChIP assay was performed as described in Materials and Methods.
To further confirm the role of CREB in gem-induced transcription of IL-1Ra, we monitored the recruitment of CREB to the IL-1Ra promoter. Mouse IL-1Ra promoter harbors one CRE between 93 and 113 base pairs upstream of the transcriptional start site (Fig. 6G). At first, we used ChIP analysis to study if gem induced the recruitment of CREB to this CRE. After immunoprecipitation of gem-treated fMNCs chromatin fragments by Abs against CREB, we were able to amplify 169-bp fragment flanking the CRE (Fig. 6H). We also observed the recruitment of RNA polymerase II at this site and this recruitment was stimulated by gem treatment (Fig. 6H). These results suggest that gem alone is capable of increasing the recruitment of both CREB and RNA polymerase II to the mouse IL-1Ra promoter. Therefore, next we examined if gem stimulated this recruitment via PI-3 kinase – Akt pathway. Consistent to the inhibition of IL-1Ra mRNA expression, both LY and Akt-i inhibited the recruitment of both CREB and RNA polymerase II to the IL-1Ra promoter in gem-treated fMNCs (Fig. 6H). In contrast, no amplification product was observed in any of the immunoprecipitates obtained with control IgG (last lanes of Fig. 6H), suggesting the specificity of these interactions. These results demonstrate that gem increases the recruitment of CREB and RNA polymerase II to the IL-1Ra promoter in fMNCs via PI-3 kinase – Akt pathway.
Gem attenuates IL-1β induced apoptosis in fMCNs
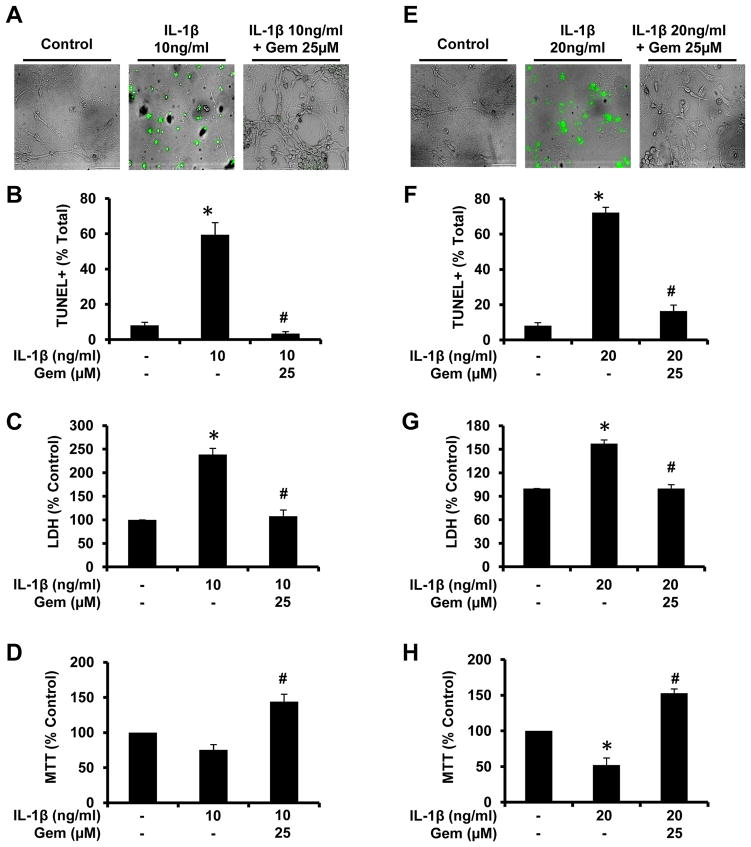
Since we have shown that gem upregulates IL-1Ra, and IL-1Ra is the endogenous inhibitor of IL-1β, we examined the effects of gem on IL-1β-mediated cytotoxicity in vitro. Earlier it has been shown that brief exposure (<2 hrs) to IL-1β stimulates N-Methyl-D-aspartic acid receptor (NMDAR)-mediated excitotoxicity in homogenous neuronal cultures (32). Therefore, fMCNs preincubated with low doses of gem for 1 hr were insulted by exposure to either 10ng/ml (Fig. 7A) or 20ng/ml (Fig. 7E) IL-1β for 2 hr followed by monitoring apoptosis via TUNEL assays. IL-1β treatment markedly induced the death of fMCNs as evident from increase TUNEL staining (Fig. 7A & E). Cell counting was then performed to quantify the percentage of TUNEL-positive to DAPI-positive cells and results indicate significant increases in dead/dying neurons in the presence of IL-1β (Fig. 7B & F). However, gem pretreatment markedly suppressed IL-1β-induced apoptosis of fMCNs (Fig. 7). To confirm this finding from another angle, we monitored cell viability by lactic dehydrogenase (LDH) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assays. Consistent with TUNEL results, IL-1β treatments alone markedly increased LDH release (Fig. 7C & G) and decreased mitochondrial activity as monitored by MTT assay (Fig. 7D & H). However, this IL-1β-induced cytotoxicity could be reduced to near-control levels if fMCNs were preincubated with gem before IL-1β insult (Fig. 7C, D, G & H). These results suggest that gem is able to attenuate apoptosis and protect neurons from IL-1β-mediated inflammatory insult.
Figure 7. Gemfibrozil protects fMCNs from IL-1β-induced apoptosis.
Cells preincubated with 25μM gemfibrozil for 1 hr were exposed to either 10ng/ml (A, B, C, & D) or 20ng/ml (E, F, G, & H) IL-1β. After 2 hrs, cell death was monitored by phase-contrast TUNEL immunostaining (A & E), counting of TUNEL-positive cells (B & F), LDH release (C & G), and MTT (D & H). Results are mean ± SD of three independent experiments. Significance is indicated by *p < 0.05 relative to control and #p < 0.05 relative to the IL-1β. Scale bar = 20μm.
Gem is unable to abate IL-1β-induced apoptosis if IL-1Ra is abrogated
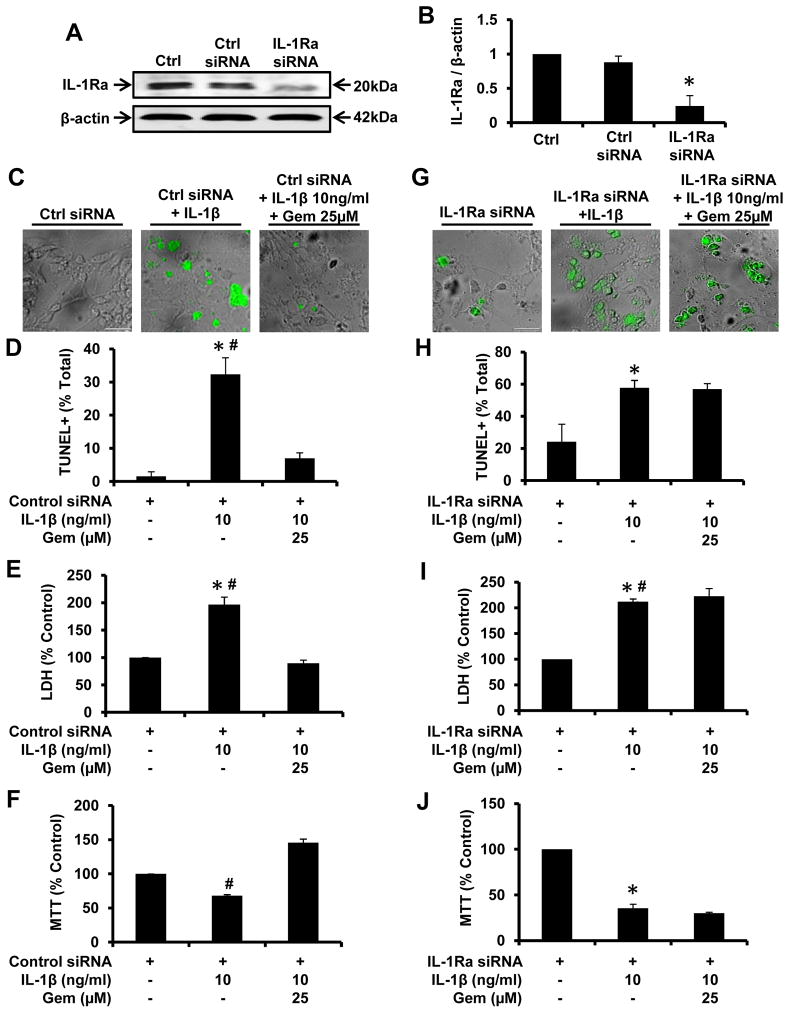
Since gem induces the upregulation of IL-1Ra, we investigated if gem exhibited the protection of fMNCs from IL-1β-induced cell death via IL-1Ra. We examined if antisense knockdown of IL-1Ra was capable of suppressing the expression of IL-1Ra protein in fMCNs. As evident from figure 8A and B, IL-1Ra siRNA, but not control siRNA, decreased the expression of IL-1Ra protein in fMCNs. While gem markedly protected control siRNA-transfected fMCNs from IL-1β-induced apoptosis (Fig. 8C & D), siRNA knockdown of IL-1Ra abrogated this protective effect of gem (Fig. 8G & H) almost completely.
Figure 8. siRNA knockdown of IL-1Ra abrogates the protective efficacy of gemfibrozil from IL-1β-induced apoptosis in fMCNs.
Cells were transfected with either control siRNA or IL-1Ra siRNA and monitored for IL-Ra inhibition efficiency via western blot (A) and subsequent densitometry (B). Cells transfected with either control siRNA (C, D, E & F) or IL-1Ra siRNA (G, H, I & J) were incubated with 25μM gemfibrozil for 1 hr followed by exposure to 10ng/ml IL-1β. After 2 hrs, cell death was monitored by phase-contrast TUNEL immunostaining (C & G), counting of TUNEL-positive cells (D & H), LDH release (E & I), and MTT (F & J). Results are mean ± SD of six independent experiments. Significance is indicated by *p < 0.05 relative to control and #p < 0.05 relative to the IL-1β. Scale bar = 20μm.
To further confirm these results, we monitored cell viability using LDH and MTT assays. As expected, IL-1β increased the release of LDH (Fig. 8E & I) and decreased MTT (Fig. 8F & J), indicating the induction of cell death by IL-1β insult. Gem treatment markedly protected control siRNA-transfected neurons from this IL-1β insult as evident from LDH release (Fig. 8E) and MTT (Fig. 8F). Consistent to that observed with TUNEL assays, siRNA knockdown of IL-1Ra abrogated this protective effect of gem in IL-1β-treated neurons as depicted by LDH release (Fig. 8I) and MTT (Fig. 8J). Taken together, these results indicate that gemfibrozil mediates neuronal protection via upregulation of IL-1Ra.
Discussion
Chronic inflammation is becoming a hallmark of human neurodegenerative disorders including AD (33–35). Although microglia, prototypical central nervous system (CNS) macrophages, play a vital role in immune surveillance, phagocytosis and neuroprotection (36–38), persistent activation and recruitment can become detrimental (39–41). For example, prolonged microglial activation results in elevated IL-1β production, a proinflammatory cytokine known to contribute to the degeneration of neurons (42, 43). Under normal physiological conditions, IL-1β promotes long term potentiation (LTP) and memory formation (44). Shaftel and coworkers (45) have also shown that hippocampal overexpression of IL-1β in an AD transgenic mouse model results not in the expected exacerbation of the amyloid beta plaque deposition common to AD, but instead in plaque amelioration. On the other hand, consistent with the elevated inflammatory response and memory impairments seen in neurodegenerative disease, increased IL-1β inhibits synaptic strength and LTP in vivo (46, 47) and is neurotoxic in vitro (32). Therefore, the development and implementation of a novel mechanism by which healthy neurons can be protected from inflammatory insults is an important target. IL-1Ra is a physiologically-occurring negative regulator of inflammation that defends cells from insult. Results described in this manuscript clearly demonstrate that gemfibrozil, an FDA-approved lipid-lowering drug, is able to dose- and time-dependently upregulate the expression of the anti-inflammatory cytokine IL-1Ra in neurons and protect neurons from IL-1β-mediated cell death. Abrogation of gem-mediated protection of neurons from IL-1β insult by siRNA knockdown of neuronal IL-1Ra suggests that this drug armors neurons via IL-1Ra. Because IL-1Ra has continually been implicated as a vital anti-inflammatory molecule (8, 48–50), these results highlight an important neuroprotective function of gem.
Though it is well-known that IL-1Ra produces its anti-inflammatory effects by competitively binding to IL-1R1, the intracellular signaling cascade by which IL-1Ra production is regulated in neurons remains to be elucidated (9, 51). Phosphatidylinositol 3-kinase (PI3-K) is a key signaling molecule implicated in the regulation of a broad array of biological responses including receptor-stimulated mitogenesis, oxidative burst and cell survival (28). For class IA PI3-K, the p85 regulatory subunit acts as an interface by interacting with the IRS-1 through its SH2 domain and thus recruits the p110 catalytic subunit to the cell membrane (28). On the other hand, for class IB PI3-K, p110γ is activated by the engagement of G-protein coupled receptors. p110γ then catalyzes the reaction to release phosphatidylinositol (3,4,5)-triphosphate as the second messenger, using phosphatidylinositol (4,5)-bisphosphate as the substrate, and activates downstream signaling molecules like Akt/protein kinase B and p70 ribosomal S6 kinase (29). Prior research in our lab has indicated that the anti-inflammatory effects of gem in microglia are mediated by the activation of PI3-K (27). Therefore, it was logical to determine if gemfibrozil could similarly propagate the activation of PI3-K in neurons. Here we demonstrate that gem induces the activation of p110α, but neither p110β nor p110γ, suggesting the specific activation of type IA p110α PI3-K in neurons. This is in contrast to our earlier observation (27), where we found the activation of type IA p110β PI-3 kinase by gem in microglia. Earlier, Learn et al (52) explained the requirement of the PI3-K pathway in the upregulation of IL-1Ra in LPS-stimulated leukocytes. However, in this instance, the types of PI3-K and associated downstream signaling pathways that are needed for LPS-induced upregulation of IL-1Ra have not been described. Consistent with the fact that Akt is a downstream target of PI3-K (29, 53), we also observed the phosphorylation of Akt by gem in neurons. Furthermore, abrogation of gem-induced expression of IL-1Ra in neurons by inhibitors of PI3-K and Akt suggest that gem induces IL-1Ra in neurons via the PI3-K – Akt pathway. However, at present, we do not know mechanisms by which gem induces the p85α-associated p110α PI3-K signaling pathway in neurons. In general, p85α-associated PI3-K is activated via growth factor receptors. Tyrosine phosphorylation of growth factor receptors creates docking sites for binding of p85α through its SH2 domains. Because gem induces the activation of PI3-K within minutes, it may not be surprising if gem uses any of these growth factor receptors to activate type IA PI3-K in neurons.
Up to this point, we have identified the requirement of PI3-K – Akt signaling pathway for gem-induced upregulation of IL-1Ra in neurons. However, it remains to be elucidated how the PI3-K – Akt pathway couples the transcription of IL-1Ra in neurons. Recently, Tamassia et al (54) have delineated that IL-10 potentiates IL-1Ra transcription in LPS-stimulated monocytes via enhanced recruitment of NF-κB to the IL-1Ra promoter. However, gem suppresses the activation of NF-κB (27), ruling out the possible involvement of NF-κB in gem-mediated upregulation of IL-1Ra in neurons. It is well-known that Akt activity modulates a myriad of downstream kinases and transcription factors implicated in a number of cellular processes (55). Interestingly, the neuroprotective Akt pathway has been shown to activate CREB, a transcription factor directly implicated in neuronal survival, plasticity, viability, and development (30, 31, 56, 57). In order to determine if CREB was a plausible target, we analyzed the IL-1Ra promotor using the Genomatix Software Suite. Indeed, genomic analysis indentified one cAMP response element (CRE) between 93 and 113 base pairs upstream of the IL-1Ra open reading frame (ORF), prompting us to investigate whether CREB was required for gem-mediated upregulation of IL-1Ra. Activation of CREB by gem alone and abrogation of gem-mediated CREB induction by inhibitors of PI3-K and Akt suggest that gem propagates the activation of CREB in neurons via the PI3-K – Akt pathway. Furthermore, siRNA knockdown of CREB abated the gem-induced upregulation of IL-1Ra and inhibitors of PI-3 kinase – Akt pathway suppressed gem-mediated recruitment of CREB and RNA polymerase II to the IL-1Ra promoter. Together, these results indicate an obligatory role of the PI-3 kinase – Akt – CREB pathway in gem-induced transcription of IL-1Ra in neurons.
Pharmacokinetics of gemfibrozil must be considered prior to clinical experimentation, as higher doses and prolonged exposure times in cultured neurons correspond with minimal changes in IL-1Ra expression relative to control. We have previously documented the ability of gemfibrozil to cross the blood brain barrier (BBB) as measured by HPLC in mouse brain tissue. After 7 d of feeding of chow containing 0.2% gemfibrozil, the level of gemfibrozil in the brain reached to 17.2 ± 5.09 μg/g tissue (18). In human, peak plasma levels of gemfibrozil have been documented at 1 to 2 hours following oral administration (58). The typical 1200mg/d dose of gemfibrozil administered to patients with hyperlipidemia corresponds to peak plasma levels of 40μg/ml. However, there are no data on the bioavailability of gemfibrozil in human brain, which warrants further investigation.
In summary, we have delineated that gemfibrozil, an FDA-approved lipid-lowering drug, upregulates the anti-inflammatory cytokine IL-1Ra in neurons via the p110α PI3-K – Akt – CREB signaling pathway. Although the in vitro situation of mouse cortical neurons in culture and its treatment with IL-1β may not truly resemble the in vivo situation of neurons in the brain of patients with neurodegenerative disorders, our results identify gem as a possible therapeutic agent to increase neuronal self defense against inflammatory insult via upregulation of IL-1Ra.
Acknowledgments
This study has been supported by grants from National Institutes of Health (AT6681, NS64564 and NS71479). GTC is supported by a grant from the NIA (T32 AG00269).
References
- 1.Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Jana A, Pahan K. Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer’s disease. J Neurosci. 30:12676–12689. doi: 10.1523/JNEUROSCI.1243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–17. doi: 10.1212/wnl.51.1_suppl_1.s2. discussion S65–17. [DOI] [PubMed] [Google Scholar]
- 5.Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol Aging. 2001;22:903–908. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 7.Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 9.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 10.Floyd RA. Neuroinflammatory processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic Biol Med. 1999;26:1346–1355. doi: 10.1016/s0891-5849(98)00293-7. [DOI] [PubMed] [Google Scholar]
- 11.Tarkowski E, Liljeroth AM, Nilsson A, Minthon L, Blennow K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12:314–317. doi: 10.1159/000051276. [DOI] [PubMed] [Google Scholar]
- 12.Bosco P, Gueant-Rodriguez RM, Anello G, Romano A, Namour B, Spada RS, Caraci F, Tringali G, Ferri R, Gueant JL. Association of IL-1 RN*2 allele and methionine synthase 2756 AA genotype with dementia severity of sporadic Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1036–1038. doi: 10.1136/jnnp.2003.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlan R, Bergami A, Brambilla E, Butti E, De Simoni MG, Campagnoli M, Marconi P, Comi G, Martino G. HSV-1-mediated IL-1 receptor antagonist gene therapy ameliorates MOG(35-55)-induced experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther. 2007;14:93–98. doi: 10.1038/sj.gt.3302805. [DOI] [PubMed] [Google Scholar]
- 14.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 15.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 16.Roy A, Pahan K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol Immunotoxicol. 2009;31:339–351. doi: 10.1080/08923970902785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahan K, Jana M, Liu X, Taylor BS, Wood C, Fischer SM. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J Biol Chem. 2002;277:45984–45991. doi: 10.1074/jbc.M200250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol. 2007;72:934–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- 19.Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. J Biol Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saha RN, Ghosh A, Palencia CA, Fung YK, Dudek SM, Pahan K. TNF-alpha preconditioning protects neurons via neuron-specific up-regulation of CREB-binding protein. J Immunol. 2009;183:2068–2078. doi: 10.4049/jimmunol.0801892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 25.Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2009;29:13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 29.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 30.Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 31.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 32.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 34.Giulian D. Microglia and the immune pathology of Alzheimer disease. Am J Hum Genet. 1999;65:13–18. doi: 10.1086/302477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 37.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 41.Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 42.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 43.Simi A, Lerouet D, Pinteaux E, Brough D. Mechanisms of regulation for interleukin-1beta in neurodegenerative disease. Neuropharmacology. 2007;52:1563–1569. doi: 10.1016/j.neuropharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- 45.Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 47.Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- 48.Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 51.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 52.Learn CA, Boger MS, Li L, McCall CE. The phosphatidylinositol 3-kinase pathway selectively controls sIL-1RA not interleukin-1beta production in the septic leukocytes. J Biol Chem. 2001;276:20234–20239. doi: 10.1074/jbc.M100316200. [DOI] [PubMed] [Google Scholar]
- 53.Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 54.Tamassia N, Castellucci M, Rossato M, Gasperini S, Bosisio D, Giacomelli M, Badolato R, Cassatella MA, Bazzoni F. Uncovering an IL-10-dependent NF-kappaB recruitment to the IL-1ra promoter that is impaired in STAT3 functionally defective patients. FASEB J. 24:1365–1375. doi: 10.1096/fj.09-145573. [DOI] [PubMed] [Google Scholar]
- 55.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 56.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 57.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 58.Kim CK, JJP, Hwang HR, Ban E, Maeng JE, Kim MK, Piao XL. Simple and sensitive HPLC method for determination of gemfibrozil in human plasma with fluorescence detection. J Liquid Chromatography & Related Technol. 2006;29:403–414. [Google Scholar]