Abstract
Background and Purpose
As several new devices for mechanical thrombectomy have become available, the outcomes of patients undergoing endovascular treatment for acute ischemic stroke are expected to improve in the United States. We performed this analysis to evaluate trends in utilization of endovascular treatment and associated rates of death and disability among acute ischemic stroke patients over a six year period, including further assessment within age strata.
Methods
We obtained data for patients admitted to hospitals in the United States from 2004 to 2009 with a primary diagnosis of ischemic stroke using a large national database. We determined the rate and pattern of utilization, and associated in-hospital outcomes of endovascular treatment among ischemic stroke patients and further analyzed trends within age strata. Outcomes were classified as minimal disability, moderate to severe disability, and death based on discharge disposition and compared between two time periods: 2004-2007 (post MERCI) and 2008-2009 (post Penumbra) approvals
Results
Of the 3,292,842 patients admitted with ischemic stroke, 72,342 (2.19%) received intravenous thrombolytic treatment, and 13,799 (0.41%) underwent endovascular treatment. There was a 6 fold increase in patients who underwent endovascular treatment (0.1% of ischemic strokes in 2004 vs. 0.6% in 2009, p < 0.001), with the patients >85 years old having the lowest rate of utilization (0.2%). The rates of intracranial hemorrhage remained unchanged throughout the 6 years. In multivariate logistic regression analysis, after adjusting for age, gender, presence of hypertension, congestive heart failure, renal failure, and secondary intracranial hemorrhages, there was no difference in the rate of minimal disability between the two study intervals (2004-2007 versus 2008-2009), odds ratio (OR) 0.8, 95% confidence interval (CI) (0.7-1.04, p=0.11). Mortality decreased while moderate to severe disability increased for patients treated during 2008-2009; OR 0.7(95% CI 0.6-0.9, p=0.007) and OR of 1.4 (95% CI 1.2-1.7, p=0.0002), respectively.
Conclusion
There has been a significant increase in the proportion of acute ischemic stroke patients receiving endovascular treatment over the 6 years and reduction in in-hospital mortality. Our results highlight the need to implement endovascular techniques in a balanced manner across various age groups that also result in the reduction of disability in addition to mortality.
Keywords: acute ischemic stroke, endovascular treatment, trend, cerebral infarction, outcomes
1. Introduction
Until 2004, the only FDA-approved treatment option for patients with acute ischemic stroke was intravenous (IV) tissue plasminogen activator (tPA) administration if patients presented within 3 hours of symptoms onset1. Endovascular treatment has concurrently existed at a very limited scale for acute ischemic stroke. In general, endovascular treatment could be sub-divided into pharmacological modalities such as intra-arterial (IA) tPA, or mechanical modalities such as the snare device, balloon angioplasty, stent placement, and microcatheter/wire manipulation.2-6 FDA approval of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) concentric retriever (Concentric Medical, Mountain View, California) in August 2004 introduced endovascular treatment including thrombectomy of acute ischemic stroke in practice and resulted in new trials of mechanical thrombectomy devices in acute stroke.3 The subsequent approval of the Penumbra system (Penumbra Inc, Alameda, California) in January 2008 resulted in rapid evolution in the treatment of acute ischemic stroke over the last 6 years. However, there is a paucity of studies addressing the rates and patterns of utilizations and associated outcomes in patients in the general population. In the current study, we studied the trend of endovascular treatment from 2004 to 2009 including further assessment within age strata to determine overall and subgroup clinical outcomes using the Nationwide Inpatient Sample (NIS) database. Our study was directed towards providing a comprehensive assessment of multimodal endovascular treatment including intra-arterial thrombolysis and mechanical thrombectomy at a national level.
2. Materials and Methods
2.1 Dataset and patient demographics
We analyzed the data from NIS data files provided by the Agency for Healthcare Research and Quality (AHRQ), the largest all-payer inpatient care database in the United States, for the years 2004-2009. NIS represents an approximately 20% stratified random sample of all patients admitted at nonfederal hospitals, including those managed by city and state governments and for-profit and not-for-profit organizations. Hospital characteristics, geographic region, ownership, location (urban or rural), teaching status, and bed size, are used for stratification in an attempt to create a sample that is maximally representative of hospitalizations in the United States. Detailed information regarding the dataset is publically available at http://www.hcup-us.ahrq.gov.
Using International Classification of Disease—Clinical Modification, 9th revision, (ICD-9 CM) primary diagnosis codes (433 to 437.1), we first identified patients with acute ischemic stroke. Data regarding patient’s demographic and clinical characteristics including age, gender, race/ethnicity, and co-morbid conditions including hypertension, diabetes mellitus, congestive heart failure, renal failure, coagulopathy, chronic lung disease, and valvular heart disease was acquired from AHRQ co-morbidity data files. In-hospital medical complications were identified by ICD-9-CM secondary diagnosis codes. For in-hospital diagnoses we used ICD-9-CM codes as follows: acquired pneumonia (486, 481, 482.8, and 482.3), urinary tract infection (599.0, 590.9), sepsis (995.91, 996.64, 038, 995.92, and 999.3), deep venous thrombosis (451.1, 451.2, 451.81, 451.9, 453.1, 453.2, 453.8, and 453.9), pulmonary embolism (415.1), and secondary intracranial hemorrhages (430, 431, and 432). We also estimated the length of stay and total hospital charges in patient groups. Discharge outcome was categorized into home discharge as minimal disability, any other discharge status as moderate to severe disability and in-hospital mortality as described in a previous publication.7 We determined the proportion of patients in the two groups admitted to the various types of hospitals based on geographic location and teaching status.
2.2 Determination of endovascular treatment
We used a combination of ICD-9 CM procedure and Medicare severity diagnosis related group (MS-DRG) codes to identify the patients who were treated with endovascular treatment. Patients undergoing mechanical thrombectomy were identified using the ICD-9 procedure code 39.74; as previously described by Brinjikji et al.(8) We also used (MS-DRG codes 543) code for identifying patients with percutaneous mechanical thrombectomy for ischemic stroke. If a patient received ICD-9 CM code 99.10 and subsequent ICD-9 CM 00.41-00.43, the patients was also labeled as receiving endovascular treatment. Most endovascular treatments are a combination of intra-arterial thrombolytics and thrombectomy(9). Therefore, identifying these patients as those treated with endovascular treatment without further stratifications provides a more accurate ascertainment.
2.3 Statistical analysis
Statistical analyses were performed by using the SAS software, version 9.1 (SAS Institute, Cary, NC) to convert raw counts generated from the NIS database into weighted counts in order to generate national estimates. Descriptive analysis was performed providing frequencies with 95% confidence intervals [CI] and means for categorical and continuous variables. Comparisons were carried out using Fisher’s exact probability test and p-values were noted. The conventional threshold of statistical significance (P < .05) was used to determine the significance of variables in our findings in light of the potential undue effect of a very large sample size. SAS procedures SURVEYMEANS, SURVEYFREQ, and SURVEYLOGISTIC were utilized for descriptive analyses and modeling procedures.
We performed two sets of analyses: The first analysis was a comparison of demographic and clinical characteristics, as well as in-hospital outcomes between the two time periods: 2004-2007 (post-MERCI) and 2008-2009 (post-Penumbra). The time periods were based on the FDA approval of the MERCI retriever (2004) and the Penumbra (2008) acute stroke treatment devices. In-hospital outcomes included hospital complications, discharge disposition, hospital charges, and length of hospitalization. We also performed multivariate stepwise logistic regression analysis to assess the outcome of patients treated with any endovascular treatment (in any modality). Logistic regression model was adjusted for age gender, cerebrovascular risk factors and hospital location and teaching status. In hospital mortality was analyzed in a dichotomized manner for all patients treated with endovascular treatment while minimal versus moderated/severe disability was assessed as a dichotomized variable for all patients discharged alive. We performed trend analysis for endovascular treatment per year from 2004-2009 in the overall adult population and within age strata (10 year strata), and plotted the in-hospital mortality every year with the endovascular treated patients.
Results
3.1. Baseline demographic and clinical characteristics
Of the 3,292,842 patients with acute ischemic stroke, 72,342 (2.2%) received intravenous tPA, and 13,799 (0.4%) underwent endovascular treatment (see figure 1 for trend). In univariate analysis, patients who underwent endovascular treatment from “post-MERCI” were younger than those treated in the “post-Penumbra” period. The mean age of 61.9 years (95% confidence interval [CI] 60.7-63.2) was lower in the post-MERCI period compared with 66.3 years (95% CI 65.3-67.2, p<.0001) in the post-Penumbra period. Patients within the post-MERCI period were less likely to have hypertension (p=0.0109), congestive heart failure (p=0.0017), and renal failure (p=0.0055), respectively. There was no statistical difference for in-hospital medical complications including rates of secondary intracranial hemorrhages between the two study periods (Table 1). Length of stay decreased (p<0.0001) and total hospital charges increased (p<0.0001) in the post-Penumbra period compared with the post-MERCI period.
Figure: 1.
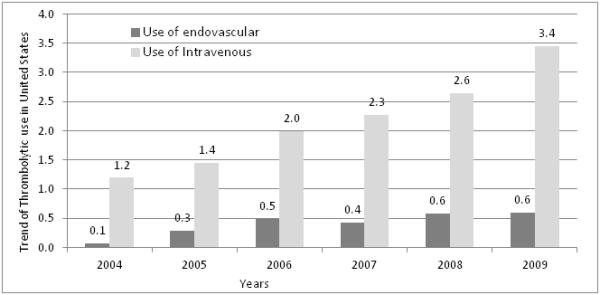
National trends in proportion of ischemic stroke patients who received intravenous thrombolytic and endovascular endovascular treatment. Dark gray bar represents patients who underwent endovascular treatment and light gray bar represents patients who underwent thrombolytic treatment
Table 1.
Baseline demographics, characteristics, and outcomes between the two time periods.
| Ischemic stroke patients treated with endovascular treatment |
|||
|---|---|---|---|
| Study period 2004- 2007 |
Study period 2008-2009 |
p-value | |
| Overall number | 7114 | 6685 | |
| Age(mean years 95% CI) | 61.9 (60.7-63.2) | 66.3 (65.3-67.2) | <.0001 |
| % women | 3075 (43.2) | 3134 (46.8) | 0.0374 |
| Race/ethnicity* | |||
| White | 3691 (72.8) | 4077 (77.2) | 0.0608 |
| Blacks | 683 (13.4) | 441 (8.3) | |
| Hispanic | 406 (8.0) | 425 (8.0) | |
| Other | 284 (5.6) | 340 (6.5) | |
| Co-morbid conditions | |||
| Hypertension | 4592 (64.5) | 4701 (70.3) | 0.0109 |
| Diabetes mellitus | 1817 (25.5) | 1641 (24.5) | 0.584 |
| CHF | 820 (11.5) | 1109 (16.5) | 0.0017 |
| Renal failure | 389 (5.4) | 577 (8.6) | 0.0055 |
| Coagulopathy | 275 (3.8) | 326 (4.8) | 0.2406 |
| Chronic lung disease | 992 (13.9) | 942 (14.0) | 0.9062 |
| Valvular heart disease | 547 (7.7) | 673 (10.1) | 0.0794 |
| Geographic region | |||
| Northeast | 1488 (20.9) | 1268 (18.9) | 0.9559 |
| North-central | 1949 (27.4) | 1729 (25.8) | |
| South | 2148 (30.1) | 2055 (30.7) | |
| West | 1530 (21.5) | 1633 (24.4) | |
| Hospital location and teaching status | |||
| Rural | 134 (1.8) | 46 (0.6) | 0.1925 |
| Urban nonteaching | 1452 (20.4) | 1168 (17.5) | |
| Urban teaching | 5524 (77.6) | 5425 (81.7) | |
|
Medical complications and in hospital procedures |
|||
| Intracranial hemorrhages | 738 (10.4) | 846 (12.6) | 0.0855 |
| Myocardial infarction | 247 (3.5) | 282 (4.2) | 0.3072 |
| Pneumonia | 552 (7.7) | 407 (6.1) | 0.1382 |
| Urinary tract infection | 1047 (14.7) | 1019 (15.2) | 0.7116 |
| Sepsis | 190 (2.6) | 160 (2.4) | 0.6695 |
| Pulmonary embolism | 86 (1.2) | 89 (1.3) | 0.7929 |
| Deep venous thrombosis | 185 (2.6) | 110 (1.6) | 0.0822 |
| Mechanical ventilation | 1217 (17.1) | 889 (13.3) | 0.0265 |
| Gastrostomy | 787 (11.1) | 972 (14.5) | 0.0122 |
| Discharge status | |||
| Length of hospital stay (mean days, 95% CI) |
11.2 (10.41-12.1) | 9.2 (8.49-9.9) | <.0001 |
| Hospital charges | 105244(96424- 114063) |
138241(122718- 153764) |
<.0001 |
| Home | 2264 (31.8) | 1791 (26.7) | 0.0344 |
| Transfer to Nursing term Facility | 3233 (45.4) | 3606 (53.9) | 0.0003 |
| Died in hospital | 1584 (22.3) | 1270 (18.9) | 0.0518 |
Race/ethnicity is not uniformly reported in NIS, Abbreviations used: confidence interval (CI).
3.2 Clinical outcomes at discharge
In-hospital mortality decreased in patients treated during the post-Penumbra era (Figure 2), while moderate to severe disability increased: (22.3% vs. 18.9%), p=0.0518 and (45.4% vs. 53.9%), p=0.0003 respectively (Table 1). In the multivariate logistic regression analysis, after adjusting for age, gender, presence of hypertension, congestive heart failure, renal failure, and secondary intracranial hemorrhages, there was no difference in the odds of minimal disability between the two time intervals: odd ratio (OR) 0.8,95% CI 0.7-1.04) p=0.1127. The odds of in-hospital mortality decreased (OR 0.7, (95% CI 0.6-0.9, p=0.0072) while moderate to severe disability increased (OR 1.4, 95% CI 1.2-1.7, p=0.0002) in patients treated during the post-Penumbra period.
Figure: 2.
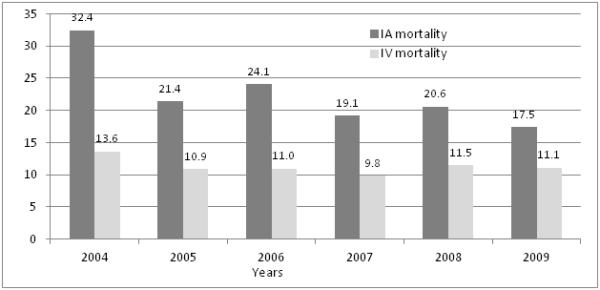
National trends in mortality among ischemic stroke patients who received intravenous thrombolytics and endovascular endovascular treatment. Dark gray bar represents patients who underwent endovascular treatment and light gray bar represents patients who underwent thrombolytic treatment
3.3 Trend of endovascular treatment use by age strata and years
Utilization of endovascular treatment increased from 0.1% to 0.6% p<0.0001 from 2004 to 2009: with a similar trend was observed for intravenous tPA over the years (Figure 1). When we compared treatment by age strata, we observed that patients <45 years had the highest rate of utilization (1.3%) while patients >85 years had the lowest rate of utilization of endovascular treatment (0.2%) (Figure 3). In hospital mortality decreased significantly over the 6 years in the endovascular treated patients (p=0.0096) (Figure 2).
Figure: 3.
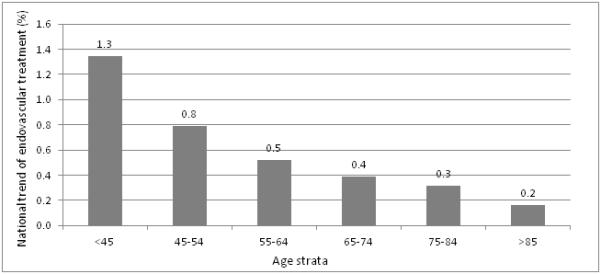
Overall utilization of endovascular treatment in various age strata over the 6 year period in United States
4. Discussion
We analyzed the in-hospital outcomes of acute ischemic stroke patients in the United States selected for endovascular treatment since FDA approval of the MERCI retriever device. We found that the utilization of endovascular treatment increased from 0.1% to 0.6% from 2004 to 2009 with prominent differences in utilization increasing within age strata. A comparison of the post-MERCI and the post-Penumbra period suggested that in-hospital mortality had decreased while moderate to severe disability had increased in patients (Table 1). The results do not reflect upon individual devices but the time period that signifies greater availability and acceptance of new endovascular devices like the MERCI and Penumbra system. Most endovascular treatments are a combination of intra-arterial thrombolytics and thrombectomy. Our study is unique in that it incorporates intra-arterial thrombolysis patients in addition to those treated with mechanical clot retrieval, which leads to a more accurate approximation of overall endovascular treatments nationally, unlike previous studies2. Such patient identification was made possible because of the availability of the new ICD-9 CM procedure and DRG codes in 2004. We identified these patients as those treated with endovascular treatment without further stratifications 6 fold to provide an accurate reflection.
The increase in the number of patients who received endovascular treatment for acute ischemic stroke from 2004 to 2009 might be explained by an increasing number of comprehensive stroke centers, increased physician acceptance, advanced imaging modalities, and better reimbursement for stroke hospitalizations subsequent to mechanical thrombectomy. We think that patient identification in expanded time windows because of higher utility of advanced imaging modalities may have contributed to the observed increase. The American Heart Association statement suggests that Computed Tomography-Perfusion CT-P may be used to select patients for intravenous thrombolysis treatment beyond a strict 3-hour time window.10 Therefore, a larger number of patients who present after 6 hours of symptom onset may be receiving endovascular treatment. However, the exact contribution of each of the factors is not measurable.
We found a prominent difference in utilization of endovascular treatment increases within age strata. The rate of utilization of endovascular treatment was much lower among older patients with ischemic stroke. Part of this difference in implementation may be due to the relatively higher rate of death and disability associated with endovascular treatment among older patients observed in clinical studies. The pooled analysis of the MERCI and Multi-MERCI trials demonstrated that increased age was associated with worse outcomes and increased risk of mortality.11, 12 Although several studies have demonstrated a higher rate of death and disability among older patients compared with younger patients undergoing endovascular treatment13, it remains unclear whether the outcome of older patients treated with endovascular treatment is better than those who do not undergo any treatment. Zacharatos et al.14 in a adjusted case control study found that after adjusting for initial National Institutes of Health Stroke Scale (NIHSS) score and age, patients aged ≥80 years with acute ischemic stroke who had endovascular treatment had an approximately 5.8 fold higher odds of achieving a favorable outcome at discharge compared with those who received non-thrombolytic medical treatment. Similarly, patients treated with IV tPA or endovascular treatment had approximately 7.2 fold higher odds of experiencing neurological improvement at 7 days or discharge, thus supporting a role for acute thrombolytic therapies in patients 80 years or older.
We found that in-hospital mortality had decreased while moderate to severe disability had increased in patients treated in the post-Penumbra period compared with post-MERCI period. The interpretation of this observation is that newer endovascular techniques and devices have increased survival but at the cost of greater moderate to severe disability. Besides the increased costs of health care and support services in patients with disability, the acceptability of such an outcome for patients and family’s needs to be considered. Moderate to severe disability despite more advanced methodology to recanalize occluded arteries may be secondary to increased rates of futile recanalizations. Hussein et al. showed that futile recanalization is a relatively common occurrence following endovascular treatment, especially among patients with severe deficits and elderly patients15. It is also possible that improvement in ancillary care such as neurocritical care and adherence to best practices have improved survival. However, it appears to be an important challenge for successful implementation of newer endovascular techniques to reduce disability in addition to mortality. These results highlight the unsolved issues regarding the best selection criteria for identifying patients who could benefit from endovascular treatment. The results also identify gaps in implementation of new devices into routine practice in a manner that is consistent with clinical benefit observed in trials.
We have used primary ICD-9-CM diagnostic and procedure codes for stroke admission and thrombolytic use. These codes have shown a true positive rate of up to 84% in previous studies for identification of stroke admission16 while a sensitivity of 55% and specificity of 98% for thrombolytic use17. The high specificity of both codes suggests patient identification is most likely accurate but there is potential for underestimation. The database lacks the standard severity scales including NIHSS and modified Rankin scale scores, and therefore limits our ability to adjust for severity of neurological deficits or recanalization. While our results are valid for overall treated population, the data does not allow us to identify subgroups defined by initial severity of deficits and time to treatment that are at highest likelihood for futile recanalization. We are unable to provide mechanistic explanation for the observed findings in the absence of procedure specific information. The discharge functional outcome cannot be measured with the available data, and the closest index is the destination of discharge. In a previous analysis, we found that discharge to nursing home had a positive predictive value of 95% for modified Rankin scale of 2-6 at 3 months (18). However, the conclusions regarding disability observed in the study sample need to be interpreted with this understanding.
“There appears to be a significant increase in the proportion of acute ischemic stroke patients receiving endovascular treatment over the last 6 years in the United States. Our results highlight the need to implement endovascular techniques in a balanced manner across various age groups that also result in the reduction of disability in addition to mortality. More advanced imaging modalities for better patient selection, improved devices and operator experience, and improvement in ancillary care will potentially continue to improve outcomes and survival in endovascular stroke patients.”
Acknowledgements
None
Funding Page:
This study was performed independently of any financial support.
Footnotes
Disclosures:
Dr. Qureshi has following disclosures: National Institute of Health U01-NS062091-01A2 (medication provided by EKR Therapeutics), American Heart Association Established Investigator Award 0840053N, and the Minnesota Medical Foundation, Minneapolis MN
All authors have read and approved submission of the manuscript.
The material in the manuscript has not been published and is not being considered for publication elsewhere in whole or in part in any language except as an abstract.
Contribution of authors:
Ameer E. Hassan DO, Saqib A. Chaudhry MD, Mikayel Grigoryan MD, Wondwossen G. Tekle MD, Adnan I. Qureshi MD equally assisted in the synthesis and discussion of ideas, and share equal responsibility for the information written in the manuscript above.
None of the authors have any conflict of interest to disclose and there are no financial conflicts to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The national institute of neurological disorders and stroke rt-pa stroke study group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Suri MF, Nasar A, He W, Kirmani JF, Divani AA, et al. Thrombolysis for ischemic stroke in the united states: Data from national hospital discharge survey 1999-2001. Neurosurgery. 2005;57:647–654. discussion 647-654. [PubMed] [Google Scholar]
- 3.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the merci trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Siddiqui AM, Suri MF, Kim SH, Ali Z, Yahia AM, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: A prospective study. Neurosurgery. 2002;51:1319–1327. doi: 10.1097/00006123-200211000-00040. discussion 1327-1319. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Chebl A, Bajzer CT, Krieger DW, Furlan AJ, Yadav JS. Multimodal therapy for the treatment of severe ischemic stroke combining gpiib/iiia antagonists and angioplasty after failure of thrombolysis. Stroke. 2005;36:2286–2288. doi: 10.1161/01.STR.0000179043.73314.4f. [DOI] [PubMed] [Google Scholar]
- 6.Bose A, Henkes H, Alfke K, Reith W, Mayer TE, Berlis A, et al. The penumbra system: A mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409–1413. doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi AI, Chaudhry SA, Hassan AE, Zacharatos H, Rodriguez GJ, Suri MF, et al. Thrombolytic treatment of patients with acute ischemic stroke related to underlying arterial dissection in the united states. Arch Neurol. 2011;68:1536–1542. doi: 10.1001/archneurol.2011.213. [DOI] [PubMed] [Google Scholar]
- 8.Brinjikji W, Rabinstein AA, Kallmes DF, Cloft HJ. Patient outcomes with endovascular embolectomy therapy for acute ischemic stroke: A study of the national inpatient sample: 2006 to 2008. Stroke. 2011;42:1648–1652. doi: 10.1161/STROKEAHA.110.607952. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Janjua N, Kirmani JF, Harris-Lane P, Suri MF, Zhou J, et al. Mechanical disruption of thrombus following intravenous tissue plasminogen activator for ischemic stroke. J Neuroimaging. 2007;17:124–130. doi: 10.1111/j.1552-6569.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 10.Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: A scientific statement from the american heart association. Stroke. 2009;40:3646–3678. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (merci) trial, part i. AJNR Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 12.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the multi merci trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 13.Brinjikji W, Rabinstein AA, Kallmes DF, Cloft HJ. Patient outcomes with endovascular embolectomy therapy for acute ischemic stroke: A study of the national inpatient sample: 2006 to 2008. Stroke. 2011;42:1648–1652. doi: 10.1161/STROKEAHA.110.607952. [DOI] [PubMed] [Google Scholar]
- 14.Zacharatos H, Hassan AE, Vazquez G, Hussein HM, Rodriguez GJ, Suri MF, et al. Comparison of acute nonthrombolytic and thrombolytic treatments in ischemic stroke patients 80 years or older. Am J Emerg Med. 2012;30:158–164. doi: 10.1016/j.ajem.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Hussein HM, Georgiadis AL, Vazquez G, Miley JT, Memon MZ, Mohammad YM, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: A multicenter study. AJNR Am J Neuroradiol. 2010;31:454–458. doi: 10.3174/ajnr.A2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The greater cincinnati/northern kentucky stroke study: Preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi AI, Harris-Lane P, Siddiqi F, Kirmani JF. International classification of diseases and current procedural terminology codes underestimated thrombolytic use for ischemic stroke. J Clin Epidemiol. 2006;59:856–858. doi: 10.1016/j.jclinepi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, MFK S. Discharge destination as a surrogate for modified rankin scale defined outcomes at 3 and 12 months among stroke survivors. ARCHIVES-PMR. 2012 doi: 10.1016/j.apmr.2012.02.032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


