Abstract
The antifungal spirocyclic guanidine alkaloid, ptilomycalin A, from marine sponge Monanchora arbuscula, inhibits melanogenesis of Cryptococcus neoformans in vitro through inhibition of biosynthesis of laccase in the melanin biosynthetic pathway with an IC50 of 7. 3 μM.
Keywords: antifungal, melanin inhibitor, Cryptococcus, laccase, marine natural product
Cryptococcus neoformans is an encapsulated fungal pathogen that is responsible for cryptococcosis in immunocompromised patients such as those with AIDS, organ transplant recipients and individuals receiving chemotherapy.1 Cryptococcosis is also a serious problem in immunocompetent individuals.2 New variants include C. neoformans var. gattii and C. neoformans var. grubii;3 the latter is implicated in the emergent cryptococcosis in the Pacific Northwest region of the United States, with attendant fatalities. C. neoformans var. gattii usually infects immunocompetent hosts.4 The high mortality rates associated with disseminated cryptococcosis, particular cases that penetrate the blood-brain barrier prompted our search for new therapeutic leads.
C. neoformans possesses three important virulent properties: 1) ability to grow at 37 °C,5 2) production of polysaccharide capsule,6 and 3) ability to produce melanin.5 Melanization in C. neoformans occurs when exogenous catecholamines such as dopamine, L-dopa and other neurotransmitters, are oxidatively polymerized by laccase.7 The abundance of these melanin precursors in the central nervous system may explain the unique tropism of cryptococcal cells for the brain.8
Melanin in C. neoformans is deposited in the outermost layer of the cell wall and contributes to increased cell wall thickness.9 In vitro studies demonstrated that melanin protects the fungal cells against oxygen- and nitrogen-derived oxidants produced by host effector cells.10 Melanized Cryptococcus is less susceptible to phagocytosis by macrophages.11 Additionally, recent evidence suggests that melanin decreases the susceptibility of C. neoformans to antifungal agents such as amphotericin B (AMB) and caspofungin.12 These reports highlight the need for new agents that may abrogate C. neoformans resistance. Since melanin is associated with virulence and resistance of C. neoformans against AMB and caspofungin, melanin biosynthetic pathway makes a potential target for antifungal intervention. In this report we show that the marine natural product, ptilomycalin A (1), inhibits melanization of C. neoformans and is a potent inhibitor of yeast laccase.
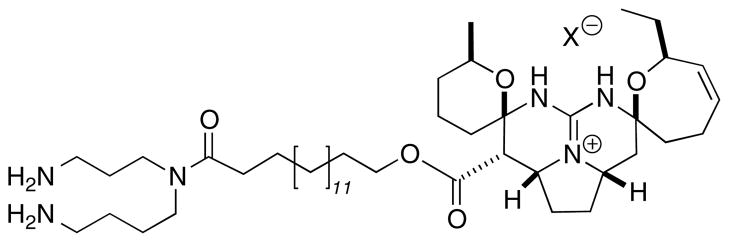
In our continuing search for mechanism-specific antifungal compounds from marine organisms,13 namely inhibitors of melanin biosynthesis, we screened 342 crude extracts against melanized clinical isolates of C. neoformans var. grubii (MAT α, H99 serotype A) and var. gattii (MAT α, serotype B). The fungal isolates were grown in defined minimal medium14 with L-dopa as the substrate for melanization (opaque, dark pigmentation of the colonies) as described previously.10 Our initial screening of marine sponges by halo assay15 resulted in the identification of one active hit: the extract of Monanchora arbuscula collected from the Bahamas.16 The crude extract induced formation of non-melanized cells at the halo surrounding the disk after 6 days of incubation. The plate was incubated further for two days however, no melanization was observed. These results, in conjunction with bioassay-guided isolation-purification17 lead to the active compound ptilomycalin A (1, Fig. 1), identified by spectroscopic analysis (MS, NMR) and comparison with literature values.18 Compound 1 was first isolated, independently, from two different sponges: Hemimycale sp. from the Red Sea,18b and Ptilocaulis spiculifer18a,c from the Caribbean, and subsequently from two seastars from New Caledonia.18d,19 We show, here, that bis-spirocyclic guanidine 1 is responsible for inhibition of melanization in C. neoformans, and, to the best of our knowledge, is one of the most potent laccase inhibitors described to date.
Figure 1.
Structure of ptilomycalin A (1).
The effect of 1 on the melanization of C. neoformans was observed through the course of our antifungal screening by the paper-disk diffusion halo assay15 using amphotericin B (AMB, Sigma) as a reference. C. neoformans were exposed to 1 or AMB (delivered from stock solutions in DMSO) and allowed to grow for 6 to 8 days in the presence of 1 mM L-dopa at 30 °C. Unlike the incubations with AMB, the halo surrounding disks containing 1 (20 μg/disk) was not clear, instead a halo of non-melanized cells were observed (Table 1). These cells were removed and washed thoroughly with defined minimal medium without L-dopa to remove traces of 1. Washed cells were inoculated into defined minimal medium agar with 1mM L-dopa and incubated for 6 to 8 days. The cells were viable and able to melanize, indicating that 1 interferes with melanization in C. neoformans. In contrast, AMB showed a very clear zone of inhibition, confirming its potent fungicidal activity (Fig. 2A). C. neoformans is known to produce melanins in the presence of other catecholamines and the pigments vary with the chemical structure of the substrate.20
Table 1.
In vitro activities of 1 and AMB against melanized C. neoformans var. grubii and var. gattii grown in the presence of catecholamines by the halo assay.
| Samplea | Substrateb | Halo diameter, mm C. neoformans | |
|---|---|---|---|
| var. grubii | var. gattii | ||
| 1 | L-dopa | 17c | 17c |
| Dopamine | 15c | 18c | |
| Epinephrine | 12c | 12c | |
|
| |||
| AMB | L-dopa | 21 | 17 |
| Dopamine | 18 | 22 | |
| Epinephrine | 18 | 18 | |
Concentration per disk was at 20 μg.
Cells were grown in the presence of 1mM catecholamine in the minimal medium for 6 to 8 days to promote melanization.
Halo surrounding the disk revealed the presence of non-melanized cells.
Figure 2.
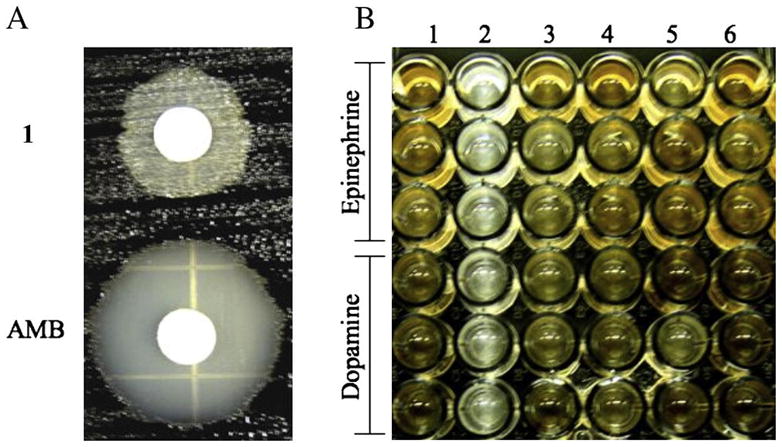
A) Halo assay of ptilomycalin A (1) and amphotericin B (AMB) against melanized C. neoformans var. grubii. Cells were grown in defined minimal media with 1mM L-dopa B) Microtiter plate assay showing the effect of (1 and 6) DMSO (2) 1 0.8 μg/mL (3) Glyphosate 200 μg/mL (4) AMB 0.0625 μg/mL or (5) Miconazole (MCZ) 0.5 μg/mL against C. neoformans var. grubii.
In order to determine whether or not melanization of C. neoformans is inhibited by 1 in the presence of other catecholamines such as epinephrine or dopamine, a halo assay was carried out. Non-melanized cells on the halo surrounding the disk containing 1 were observed in all cases (Table 1). Furthermore, these cells were viable and able to melanize when transferred to defined minimal medium agar with 1mM dopamine or epinephrine. In contrast, AMB elicited a consistent clear zone.
We further tested the effect of 1 on the melanization of C. neoformans in a liquid medium using microtiter plate assay and compared it to other antifungal agents. An overnight culture of non-melanized C. neoformans was diluted to fresh defined minimal medium to an optical density of about 0.010 to 0.020. Cells were exposed to sublethal concentrations of a weak laccase inhibitor (glyphosate, 200 μg/mL) or antifungal compounds (AMB, 0.0625 μg/mL; miconazole (MCZ), 0.5 μg/mL) or 1 (0.8 μg/mL) and allowed to grow for 10 days in defined minimal medium containing 1 mM catecholamine (epinephrine, dopamine or L-dopa) at 30 °C. Melanization and pigmentation in treated cells was observed visually after 10 days of incubation and compared to that of the control (cells, DMSO). In the presence of 1, only non-melanized cells grew, however growth of melanized cells was observed in the presence of AMB, glyphosate or MCZ (Fig. 2B).
The cells treated with 1 remained non-melanized after 21 days, indicating that melanization is inhibited or delayed. AMB and MCZ elicit antifungal activity by targeting membrane bound ergosterol21a and biosynthesis of 14α-demethylase,21b respectively,20,21 but have not been reported to inhibit melanin biosynthesis. Glyphosate is a broad-spectrum herbicide which inhibits the 5-enolpyruvylshikimate-3-phosphate synthase in the shikimate pathway for the biosynthesis of the phenylalanine and tyrosine; the precursors of melanin.22 Glyphosate has also been reported to inhibit melanization of C. neoformans;23 however, under our conditions, glyphosate showed no significant inhibitory activity on either growth or melanization of C. neoformans.
A drop test assay showed that 1 delayed the melanization of C. neoformans. A culture of C. neoformans (107 cells/mL) containing 1 concentrations of 0, 0.06, 0.2, 0.6 and 2 μM was dropped into a plate of defined minimal medium with 1mM L-dopa and incubated at 30 °C until melanization occurred. The delay in melanization was shown to be dose dependent (Table 2). With concentrations of 1 as low as 0.06 μM, melanization was delayed compared to a culture without 1. At 2 μM, 1 delayed melanization until 25 days. Taken together, the results obtained by the halo, broth and drop test assays strongly suggest that melanogenesis of C. neoformans is inhibited by 1. Since these long-term incubations eventually lead to melanization, we cannot rule out that 1 is degraded under these conditions.
Table 2.
Ptilomycalin A (1) concentration-dependence of melanization in C. neoformans var. grubii at 30 °C.
| Concentration of 1, μM | Onset of melanization at 30 °C, days |
|---|---|
| 0 | 6 |
| 0.06 | 9 |
| 0.20 | 12 |
| 0.60 | 20 |
| 2.00 | 25 |
Melanin biosynthesis in C. neoformans is catalyzed by laccase that is expressed and localized in the outer layer of the cell wall.19, 24 This enzyme is capable of oxidizing a wide variety of aromatic diphenolic and catecholamine substrates but is unable to oxidize monophenolic substrates such as tyrosine and tyramine.19 Laccase production is increased when cells are grown in the absence of glucose.19 As ptilomycalin A (1) was observed to inhibit the melanization of C. neoformans, we tested its effect on laccase activity by a colorimetric method that monitors the production of ‘dopachrome’, a chromophoric intermediate (λmax = 480 nm) formed during the oxidation of dopa and other catecholamines.25 Cells were grown in the presence of asparagine salts and absence of glucose to increase laccase production. The known non-specific laccase inhibitor, sodium azide (NaN3), was used as a positive control for the assay.26 Cells, which were permeabilized by exposure of the whole culture to toluene-ethanol (1:4), were shown to oxidize the laccase substrate, epinephrine, and used in the inhibition assay.
Purified laccase from Trametes versicolor (Sigma) was also examined in a cell-free assay (Figure 3).26,27,28 Ptilomycalin A (1) inhibits laccase activity in a dose-dependent manner (Fig. 3A; IC50 of 7.3 μM for C. neoformans and 4.7 μM for laccase of T. versicolor) when compared with NaN3 (Table 3). Similar results were observed with dopamine and L-dopa (IC50 of 4.9 and 5.8 μM, respectively). These data support the hypothesis that 1 suppresses melanogenesis of C. neoformans by inhibiting the enzyme responsible for its biosynthesis.
Figure 3.
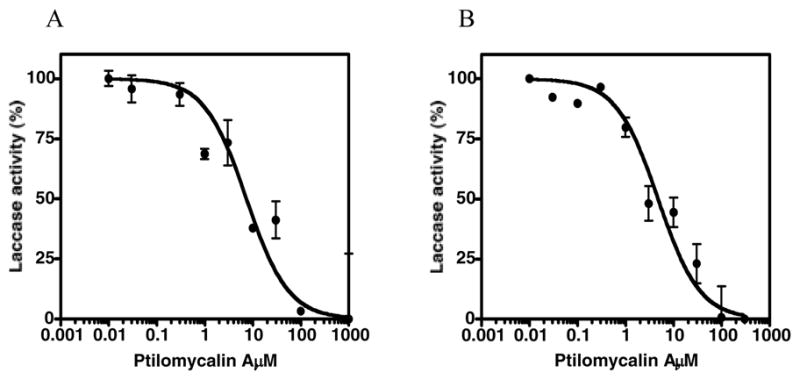
Dose-response curves for ptilomycalin A (1) against laccase from (A) C. neoformans var. grubii (whole-cell based assay) and B) Trametes versicolor (cell-free assay). Enzyme activity was measured using epinephrine as the substrate. Each value is expressed as mean ± SE of the two experiments.
Table 3.
Inhibition of laccase from Trametes versicolor (IC50s, μM)a by ptilomycalin A (1) and NaN3
| Inhibitors | Epinephrine | Substrateb Dopamine | L-dopa |
|---|---|---|---|
| 1c | 4.7(0.24) | 4.9(0.20) | 5.8 (0.04) |
| 7.30 (0.54)d | |||
| NaN3c | 263(0.04) | 257(1.09) | 279 (0.05) |
| 329(0.07)d |
Each value is the mean of two experiments, standard error is given in parenthesis.
Epinephrine conc., 100 μM; Dopamine conc., 200 μM; L-dopa conc., 300 μM.
Ptilomycalin A conc. ranges from (0 to 1 mM); NaN3 conc. ranges from (0 to 3 mM). Inhibitors were incubated with the enzyme for 30 min prior to addition of the substrate.
IC50 values of cell-based assay
We examined the interaction of ptilomycalin A (1) and AMB against C. neoformans by a halo assay (Table 4). Ptilomycalin A (1) alone at 10 μg showed the presence of non-melanized cells on the halo surrounding the disk, while AMB at 2 μg showed a clear zone of inhibition. Interestingly, when these two agents are combined in cultures, an increase in the zone of inhibition was observed but non-melanized cells were absent from the halo, indicating that 1 synergizes the fungicidal activity of AMB. Synergism of these two drugs can be understood from their known modes of action. Laccase inhibition by 1 selects for propagation of non-melanized C. neoformans cells. The more permeable, non-melanized cells are rendered more susceptible to the antifungal activity of AMB, a known membrane-disrupting agent, that forms discrete ion-permeable pores through non-covalent assembly with molecules of the fungal sterol, ergosterol.
Table 4.
In vitro activity of ptilomycalin A (1) and AMB, alone and in combination against C. neoformans by halo assay.
| Sample | Dose. per disk (μg) | Halo diameter mm C. neoformansa | |
|---|---|---|---|
| var. gattii. | var. grubii | ||
| AMB | 2 | 10.0 | 15.5 |
| 1 | 10 | 9.50 b | 13.5b |
| AMB + 1 | 2 +10 | 15.0 | 21.0 |
Cells were grown in the presence of 1mM L-dopa for 6 days.
Halo surrounding the disk revealed the presence of non-melanized cells.
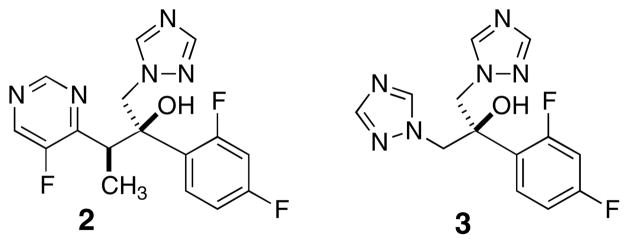
Earlier reports characterized several biological activities of ptilomycalin A (1): cancer cell cytotoxicity,18a,b antifungal activity,18b and anti-viral properties.18d,e,f Compound 1 inhibited the DNA polymerase activity of HIV reverse transcriptase (60% at 10 μM).29 The work described here reveals the first example of inhibition, by 1, of an oxidase enzyme (laccase). Laccases are proteins characterized by tetranuclear copper belonging to the ‘blue copper oxidase’ family that includes ascorbate oxidase. X-ray structures of fungal laccases from Cerrena maxima30a and Trametes hirsuta reveal similar structures; the presence of four active cores, each populated by different by numbers of Cu(II) atoms ligated to His residues. Structures of inhibitor-laccase complexes have yet to appear. Inhibition of fungal cell growth by oxidases is a well-known MOA. For example, the widely-used antifungal ‘azole’ drugs (e.g. Fluconazole™, 2, Figure 4) act by inhibition of cytochrome P450 oxidases, responsible for the biosynthesis of fungal ergosterol, and owe much of their useful therapeutic indices to their lower affinities for the analogous mammalian enzymes.
Figure 4.
Structures of fluconazole (2) and voriconazole (3).
One report31 implicates inhibition of laccase and consequent melanin formation in C. neoformans by the potent azole antifungal voriconazole (3, Figure 4), but, interestingly, not by fluconazole (2). These results may implicate the triazole group in competitive ligand exchange at one or more laccase His-Cu cores, but secondary structural features also appear to be important. We propose that, under physiological conditions, the bis-spirocyclic guanidine group of 1 interferes with the laccase Cu-His ligand field.
Structure-activity studies of ptilomycalin A (1) at the active Cu(II)-His cores may illuminate the molecular structural features required for potential laccase inhibitors that may show selectivity for fungal enzymes over their mammalian counterparts. These investigations, which include an expanded search for additional laccase inhibitors from marine organisms, are ongoing in our laboratories.
In summary, ptilomycalin A (1) from the marine sponge Monanchora arbuscula was shown to strongly suppress melanogenesis of C. neoformans in vitro by potent inhibition laccase, a copper-containing oxidase responsible for the biosynthesis of fungal melanin. Ptilomycalin A (1) acts synergistically with AMB, thus enhancing the antifungal activity of the latter. This study is the first report demonstrating the anti-Cryptococcal activity by 1 and suggests that bis-spirocyclic guanidines may be worthy of investigation as antifungal agents to suppress virulence in the CNS.
Acknowledgments
We are grateful to A. Gelli of the UC Davis School of Medicine for the clinical isolates of C. neoformans, J. Pawlik (University of North Carolina, Wilmington) and the crew of the R/V Seward Johnson for support of field collection activities, and for generous funding of this research from the NIH (AI039987).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedicated to Prof. William Fenical, Scripps Institution of Oceanography, on the occasion of his 70th birthday
References and Notes
- 1.Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, Brandt ME, Gardner T, Sattah M, de Leon GP, Baughman W, Hajjeh RA. Clin Infect Dis. 2003;36:789. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 2.Murayama S, Sakai S, Soeda H, Yabuuchi H, Masuda K, Inoue H, Watanabe H, Matsuo Y. Clin Imaging. 2004;28:191. doi: 10.1016/S0899-7071(03)00145-1. [DOI] [PubMed] [Google Scholar]
- 3.Bottone EJ, Salkin IF, Hurd NJ, Wormser GP. J Infect Dis. 1987;156:242. doi: 10.1093/infdis/156.1.242. [DOI] [PubMed] [Google Scholar]
- 4.Speed B, Dunt D. Clin Infect Dis. 1995;21:28. doi: 10.1093/clinids/21.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, Polacheck I, Popkin TJ. J Bacteriol. 1982;150:1414. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon-Chung KJ, Rhodes JC. Infect Immun. 1986;51:218. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson PR, Wakamatsu K, Ito S. J Bacteriol. 1998;180:1570. doi: 10.1128/jb.180.6.1570-1572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton AJ, Holdom MD. Med Mycol. 1999;37:375. doi: 10.1046/j.1365-280x.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 9.Feldmesser M, Kress Y, Casadevall A. Microbiology. 2001;147:2355. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Casadevall A. Infect Immun. 1994;62:3004. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Aisen P, Casadevall A. Infect Immun. 1995;63:3131. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Wang Y, Casadevall A. Antimicrob Agents Chemother. 1994;38:2648. doi: 10.1128/aac.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) van Duin D, Casadevall A, Nosanchuk JD. Antimicrob Agents Chemother. 2002;46:3394. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For example, oceanapiside A, an inhibitor of sphingolipid biosynthesis (Nicholas GN, Hong TW, Molinski TF, Lerch ML, Cancilla MT, Lebrilla CB. J Nat Prod. 1999;62:1678. doi: 10.1021/np990190v.and bengazoles C-G, bis-oxazoles with an as-yet undefined mechanism of action. Searle PA, Richter RK, Molinski TF. J Org Chem. 1996;61:4073. doi: 10.1021/jo952261a.For a review, see Molinski TF. Curr Med Chem: Anti-Infect Agents. 2004;3:197.
- 14.The defined minimal medium is made up of the following per liter 15 mM glucose, 10 mM MgSO4·7H2O, 29.4 mM KH2PO4, 13 mM glycine (Sigma), 3 μM thiamine (Sigma, T4625) and with or with out 1 mM L-dopa (Fluka).
- 15.The halo assay was performed in defined minimal media without L-dopa for non-melanized and with L-dopa for melanized C. neoformans. Each disk (6.5 mm) was delivered 15 μL (containing 300 μg) of the crude sample dissolved in DMSO. The disks were allowed to dry at RT for 3h. The dried disks were placed on the lawn of target cells and incubated for 6 to 8 days at 30°C. Zones of inhibition were measured and reported to the nearest 0.5 mm. The experiment was carried out twice.
- 16.The sponge was collected by hand using scuba from the Bahamas (accession number 02-009: Monanchora arbuscula) and was frozen immediately after collection and stored at −20°C until use.
- 17.A frozen sample of Monanchora arbuscula (collected in the Bahamas in 2002; 69 g) was lyophilized (12.4 g) and extracted with MeOH overnight at room temperature (500 mL × 3). The combined extracts were filtered and concentrated to 500 mL under reduced pressure. The crude extract (equivalent to 4.2 g) was partitioned following a modified Kupchan method using hexane (500 mL, Fraction A), two portions of CHCl3 (500 mL × 2, Fraction B and C) and n-BuOH (200 mL × 2, Fraction D) to yield 0.31, 1.21, 0.06 and 0.17 g of organic extracts (Fractions A-D, respectively) and 2.39 g of aqueous extract (Fraction E). Fraction B was fractionated using Sephadex LH-20 (MeOH) to yield eight fractions. The third Sephadex fraction (113 mg) was purified by reversed phase HPLC (Dynamax, 5μm, C8 column, 10 × 250 mm, 1:4 H2O/MeOH + 0.1% TFA) to afford ptilomycalin A (1, 24 mg, 0.19% of dry weight) that was identified by MS and NMR and comparison with literature data.18
- 18.Kashman Y, Hirsh S, McConnell OJ, Ohtani I, Kusumi T, Kakisawa HJ. J Am Chem Soc. 1989;111:8925.Hirsh S, Kashman Y. Tetrahedron. 1989;45:3897.Ohtani I, Kusumi T, Kakisawa H, Kashman Y, Hirsh S. J Am Chem Soc. 1992;114:8472.Palagiano E, De Marino S, Minale L, Riccio R, Zollo F, Iorizzi M, Carré JB, Debitus C, Lucarain L, Provost J. Tetrahedron. 1995;51:3675.Shi J-G, Sun F, Rinehart KL. 3,756,734. WO Patent. 1998Rinehart KL, Jares-Erijman EA. 5,756,734. US Patent. 1998For reviews of 1 and related compounds, see Heys L, Moore CG, Murphy PJ. Chem Soc Rev. 2000;29:57.Berlinck RGS. Nat Prod Rep. 1999;16:339.and Berlinck RGS. Nat Prod Rep. 1996;13:377. doi: 10.1039/np9961300377.
- 19.The absolute configuration of 1 was assigned by total synthesis. Coffey DS, McDonald AI, Overman LE, Rabinowitz MH, Renhowe PA. J Am Chem Soc. 2000;122:4893.
- 20.Williamson PR. J Bacteriol. 1994;176:656. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Shadkchan Y, Segal E. Antimicrob Agents Chemother. 1999;44:787. doi: 10.1093/jac/44.6.787. [DOI] [PubMed] [Google Scholar]; (b) Hitchcock CA, Dickinson K, Brown SB, Evans EG, Adams DJ. Biochem J. 1990;266:475. doi: 10.1042/bj2660475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinrücken HC, Amrhein N. Biochem Biophys Res Commun. 1980;94:1207. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]
- 23.Nosanchuk JD, Ovalle R, Casadevall A. J Infect Dis. 2001;183:1093. doi: 10.1086/319272. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Infect Immun. 2001;69:5589. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda R, Sugita T, Jacobson ES, Shinoda T. J Clin Microbiol. 2002;40:1214. doi: 10.1128/JCM.40.4.1214-1218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannes C, Majcherczyk A. J Biotechnol. 2000;78:193. doi: 10.1016/s0168-1656(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzo M, Moldes D, Rodriguez Couto S, Sanroman MA. Chemosphere. 2005;60:1124. doi: 10.1016/j.chemosphere.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Polacheck I, Hearing VJ, Kwon-Chung KJ. J Bacteriol. 1982;150:1212. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black GP, Coles SJ, Hiz A, Howard-Jones AG, Hursthouse MB, McGown AT, Loya S, Moore CG, Murphy PJ, Smith NK, Walshe NDA. Tetrahedron Lett. 2001;42:3377. [Google Scholar]
- 30.(a) Lyashenko AV, Bento I, Zaitsev VN, Zhukhlistova NE, Zhukova YN, Gabdoulkhakov AG, Morgunova EY, Voelter W, Kachalova G, Stepanova EV, Koroleva OG, Lamzin VS, Tishkov VI, Betzel C, Lindley PF, Mikhailov AB. J Biol Inorg Chem. 2006;11:963. doi: 10.1007/s00775-006-0158-x. [DOI] [PubMed] [Google Scholar]; (b) Polyakov KM, Fedorova TV, Stepanova EV, Cherkashin EA, Kurzeev SA, Strokopytov BV, Lamzin VS, Koroleva OV. Acta Crystal D. 2009;65:611. doi: 10.1107/S0907444909011950. [DOI] [PubMed] [Google Scholar]
- 31.Martinez LR, Ntiamoah P, Gácser A, Casadevall A, Nosanchuk JD. Antimicrob Agents Chemother. 2007;51:4396. doi: 10.1128/AAC.00376-07. [DOI] [PMC free article] [PubMed] [Google Scholar]