Summary
Opium is one of the world’s oldest drugs, and its derivatives morphine and codeine are among the most used clinical drugs to relieve severe pain. These prototypical opioids produce analgesia as well as many of their undesirable side effects (sedation, apnea and dependence) by binding to and activating the G-protein-coupled μ-opioid receptor (μOR) in the central nervous system. Here we describe the 2.8 Å crystal structure of the μOR in complex with an irreversible morphinan antagonist. Compared to the buried binding pocket observed in most GPCRs published to date, the morphinan ligand binds deeply within a large solvent-exposed pocket. Of particular interest, the μOR crystallizes as a two-fold symmetric dimer through a four-helix bundle motif formed by transmembrane segments 5 and 6. These high-resolution insights into opioid receptor structure will enable the application of structure-based approaches to develop better drugs for the management of pain and addiction.
Opium extracts from the plant Papaver somniferum have been used for therapeutic and recreational purposes for thousands of years. Opioid alkaloids and related pharmaceuticals are the most effective analgesics for the treatment of acute and chronic pain. They also represent one of the largest components of the illicit drug market world-wide, generating revenue of approximately $70 billion in 2009, much of which supports crime, wars and terrorism (UNODC World Drug Report 2011). Intravenous use of opioid drugs is a leading cause of death by overdose in Europe and North America, and a major contributing factor to the worldwide AIDS epidemic.
Morphine and codeine are the main active opioid alkaloids in opium. In humans, they act on the central nervous system to produce a wide range of effects including analgesia, euphoria, sedation, respiratory depression, and cough suppression, and have peripheral effects such as constipation1. Gene disruption studies in mice show that the target for the majority of the effects of opioid alkaloids, whether beneficial or adverse, is the μ-opioid receptor (μOR)2. The μOR is a rhodopsin-like family A a G protein-coupled receptor having two closely related family members known as the δ and κ opioid receptors3. Hence, the μOR constitutes the main opioid target for the management of pain, acute pulmonary edema, cough, diarrhea and shivering1. However, opioid drugs are highly addictive, with the acetylated form of morphine, heroin, being the best-known example. Because of this, the clinical efficacy of opioid drugs is often limited by the development of tolerance and dependence.
While both beneficial and adverse effects are attributable to activation of the μOR, they appear to be mediated by different down-stream signaling and regulatory pathways. The μOR couples predominantly to Gi, the inhibitory G protein for adenylyl cyclase. μOR signaling through Gi is responsible for its analgesic properties4. Following activation, the μOR undergoes phosphorylation and subsequently couples to arrestins, which have both regulatory and signaling functions5. Studies suggest that ligands with the greatest addictive potential, such as morphine, promote interactions with Gi more strongly than they promote interactions with arrestins6. These studies suggest that it may be possible to develop safer and more effective therapeutics targeting the μOR.
To better understand the structural basis for μOR function we pursued a crystallographic study of this receptor using the T4 lysozyme (T4L) fusion protein strategy developed by Rosenbaum et al.7 (Supplementary Fig. 1). Using the in meso crystallization method, we obtained crystals and collected diffraction data from 25 crystals of Mus musculus μOR-T4L protein bound to the irreversible morphinan antagonist β-funaltrexamine (β-FNA). The structure was solved by molecular replacement from a 2.8 Å data set.
Transmembrane architecture
The lattice for the μOR receptor shows alternating aqueous and lipidic layers with receptors arranged in parallel dimers tightly associated through transmembrane (TM) helices 5 and 6. More limited parallel interdimeric contacts through TM1, TM2 and helix eight are observed between adjacent dimers (Supplementary Fig. 2).
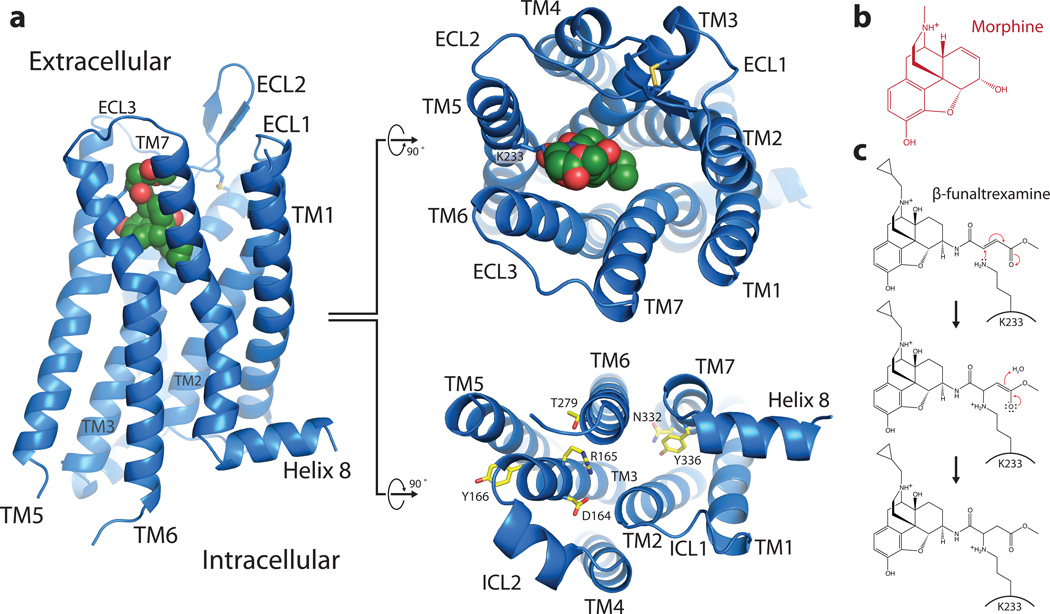
As in other GPCRs, the structure of the μOR consists of seven TM alpha-helices that are connected by three extracellular loops (ECLs 1–3) and three intracellular loops (ICLs 1–3) (Fig. 1a). TM3 is connected to the ECL2 by a conserved disulfide bridge between C1403.25 (superscripts indicate Ballesteros-Weinstein numbers8) and C217. The morphinan ligand β-FNA (Fig. 1b, 1c) makes contacts with TMs 3, 5, 6, and 7 (Fig. 1a), and the electron density observed in the structure confirms previous data identifying the K2335.39 side chain as the site of covalent attachment9 (Fig. 1c, Supplementary Fig. 3).
Figure 1. Overall view of μOR receptor structure.
a, Views from within the membrane plane (left), extracellular side (top, center panel) and intracellular side (bottom, center panel) show the typical seven-pass transmembrane GPCR architecture of the μOR. The ligand, β-FNA, is shown in green spheres. b, The chemical structure of morphine. c, The chemical structure of β-FNA and the chemical reaction with the side chain of K2335.39 in the receptor are shown. β-FNA is a semisynthetic opioid antagonist derived from morphine, shown at right.
The intracellular face of the μOR closely resembles rhodopsin with respect to the relative positions of TM3, TM5 and TM6 (Supplementary Fig. 4). Nevertheless, like the β2-adrenergic receptor (β2AR), there is no ionic bridge between the DRY sequence in TM3 and the cytoplasmic end of TM6. As with the β2AR, R1653.50 forms a salt-bridge with the adjacent D1643.49 of the DRY sequence. D1643.49 also engages in a polar interaction with R179 in ICL2, a feature that is similar to an interaction between observed between D1303.49 and S143 in ICL2 of the β2AR (Supplementary Fig. 4). In μOR, it has been shown that the mutation of T2796.34 to a lysine results in a constitutively active receptor10. This may be explained by a polar interaction observed in the crystal structure of μOR between T2796.34 and R1653.50 (Supplementary Fig. 4). This interaction may stabilize the receptor in an inactive state.
An exposed ligand binding pocket
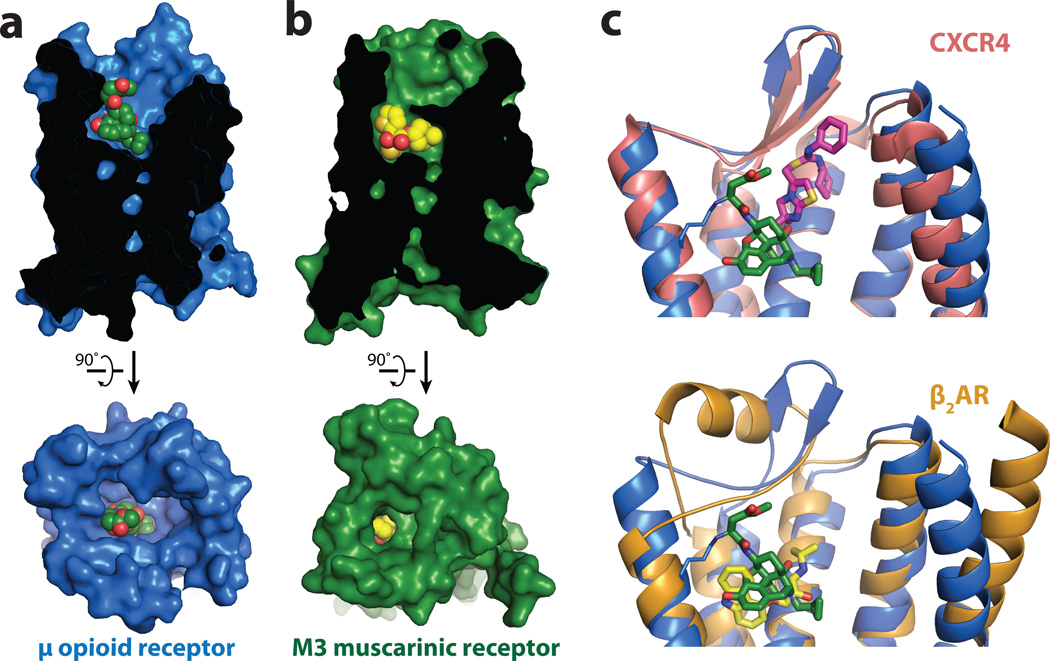
In most available GPCR structures, the ligand is partially buried within the helical bundle by more superficial residues in TM segments and ECL2. The most extreme examples are the M2 and M3 muscarinic receptors11,12, in which the ligand is covered with a layer of tyrosines (Fig. 2). This provides a structural basis for the very slow dissociation kinetics of muscarinic antagonists. For example, the dissociation half-life of the clinically used drug tiotropium at the M3R is 34.7 h while its dissociation constant (Kd) is 40 pM13. By contrast, the binding pocket for β-FNA in the μOR is largely exposed to the extracellular surface (Fig. 2a). This may explain why extremely potent opioids such as buprenorphine (Ki 740 pM), diprenorphine (Ki 72 pM), alvimopan (Ki 350 pM), and etorphine (Ki 230 pM) present rapid dissociation half-lives of 44 min, 36 min, 30 min14, and less than one minute15, respectively. Therefore, although the affinity of high affinity opioid ligands is comparable to tiotropium, the dissociation kinetics are considerably different. This feature of opioid ligands may explain why heroin overdoses are rapidly reversible by naloxone16. In addition, the extremely high potency and fast kinetics of etorphine agonism and diprenorphine antagonism allows for a system that is capable of rapid anesthesia and prompt reversal in veterinary use. As a result, etorphine is a preferred anesthetic (dose in the range of 5 to 20 μg/kg) for valuable racehorses and for captive and free ranging mammals17.
Figure 2. Comparison of ligand binding pockets.
a, The binding pocket for μOR is wide and open above the ligand, in stark contrast to the deeply buried binding pocket of the muscarinic receptors, as exemplified by the M3R shown in b. c, The small molecule antagonist IT1t (magenta) occupies a binding pocket closer to the extracellular surface of CXCR4 than β-FNA in μOR. β-FNA is positioned more similarly to the distantly related aminergic receptors as shown in c (bottom panel) for the binding site of carazolol (yellow) in the β2-adrenergic receptor (β2AR).
The μOR belongs to a subgroup of peptide GPCRs, and the closest published structure is that of the CXCR4 chemokine receptor18 (RMSD value of 1.35 Å). In the μOR the morphinan ligand β-FNA binds much more deeply than the small molecule CXCR4 antagonist IT1t and occupies a similar position as agonists and antagonists for the β2-AR (RMSD value of 1.52 Å) and other monoamine receptors (Fig. 2c).
Binding pocket and opioid specificity
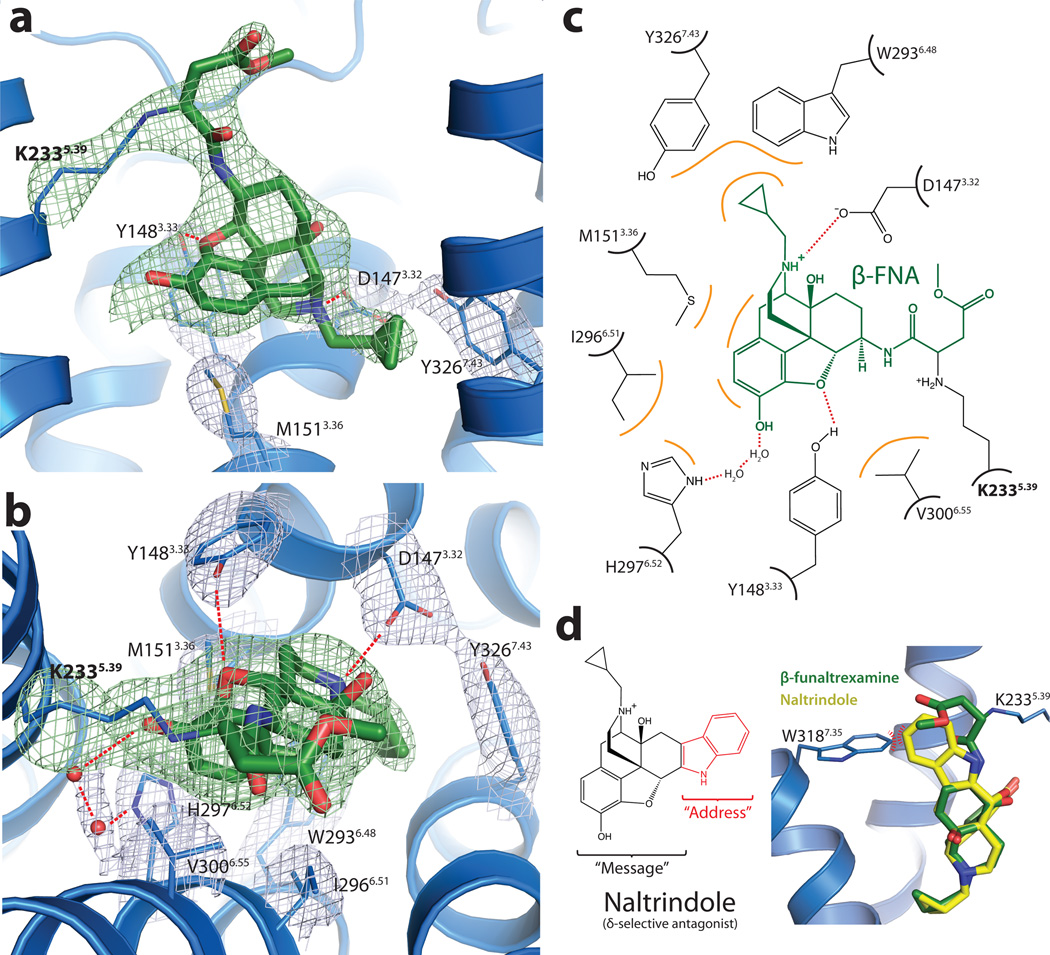
There are 14 residues within 4 Å of β-FNA. Nine of these have more direct interactions with the ligand (Fig. 3a, b and c), and are conserved in κOR and δOR. D1473.32 engages in a charge-charge interaction with the amine moiety of the ligand and hydrogen bonds with Y3267.43 (both residues are strictly conserved in all the opioid receptor subtypes). While D1473.32 occupies the same position as the counter ion in aminergic receptors, a sequence comparison shows that it is not conserved in other peptide receptors. H2976.52 interacts with the aromatic ring of the morphinan group, but does not directly hydrogen-bond with β-FNA as has been previously suggested19. However, the electron density suggests the presence of two water molecules that are well positioned to form a hydrogen bonding network between H2976.52 and the phenolic hydroxyl of the morphinan group (Fig. 3b and 3c).
Figure 3. Structural basis for morphinan ligand binding to the μOR.
a, Side view of the ligand binding pocket with polar interactions shown. TM6 is excluded from this view. The electron density used to position interacting side chains is shown in light blue colored mesh depicting the 2Fo-Fc electron density contoured at 1.3 σ. Green mesh depicts an omit map of β-FNA and K2335.39 side chain atoms contoured at 3.0 σ. b, Binding pocket viewed from the extracellular surface. Water molecules are shown as red spheres, with the accompanying electron density shown in light blue mesh. c, The binding site is diagrammed, showing the chemical structure of β-FNA (green) covalently bound to the receptor through K2335.39 (bold). Hydrophobic interactions are shown in orange and polar contacts with red dotted lines. V3006.55 and I2966.51 form extensive hydrophobic contacts with the back face of the ligand (not shown). Two water molecules are positioned between H2976.52 and the phenolic group of β-FNA d, The δOR selective ligand naltrindole includes an indole group that would clash with W3187.35 in μOR, but not with the leucine found in the equivalent position in δOR. The indole has been described as an "address" to target the ligand to δOR, while its efficacy ("message") is determined by the morphinan group on the left 40.
A direct comparison with the δOR sequence also shows that of the 14 residues within 4Å of the ligand, 11 are identical between μOR and δOR. The three differences are at μOR positions E229ECL2, K3036.58, and W3187.35, which are Asp, Trp, and Leu in the δOR, respectively. The substitution of leucine in δOR for W3187.35 is highlighted in Fig. 3d. W3187.35 was shown to be responsible for the binding selectivity of naltrindole, a δOR selective antagonist and of DPDPE ([D-Pen2,D-Pen5]Enkephalin), a δOR selective peptide agonist20. In particular, the point mutation W318L dramatically increases the affinity of both these ligands at the μOR. Positioning naltrindole (represented in Fig. 3d) into the μOR binding pocket by superimposition of its morphinan group on that of β-FNA shows that naltrindole would clash with the W318 side chain in μOR (Fig. 3d), while the leucine in this position of δOR would likely accommodate naltrindole without requiring structural rearrangement.
Endomorphins 1 and 2 are small peptides isolated from brain that were shown to have the highest affinity (low nM range) and the highest selectivity profile for the μOR receptor21. For instance, endomorphin 1 exhibits 4,000 and 15,000 fold selectivity for μOR over δOR and κOR, respectively21. Although little is known about the determinants of endomorphin binding, mutagenesis studies suggest that the μOR-selective synthetic peptide agonist DAMGO ([D-Ala2, N- MePhe4 ,Gly-ol5 ]enkephalin) occupies a space that overlaps with the β-FNA binding pocket but also extends beyond this site22. Sites of mutations that impair DAMGO binding include H2976.52 positioned near the bottom of the β-FNA pocket as well as K3036.58, W3187.35 and H3197.36 positioned above the β-FNA binding pocket (Supplementary Fig. 5). Given the residues involved in DAMGO binding to μOR, opioid peptides likely make both polar and non-polar contacts within the μOR binding pocket. This feature of opioid peptide binding is also reflected in the lack of a highly charged surface within the μOR binding pocket compared with that of the CXCR4 receptor18.
Oligomeric arrangement of μOR
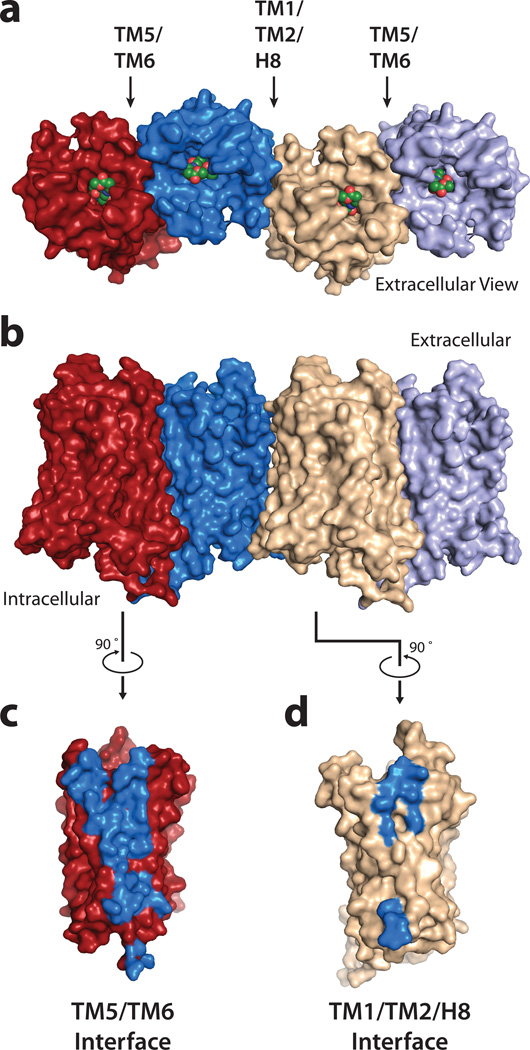
The structure of μOR shows receptor molecules intimately associated into pairs along the crystallographic two-fold axis through two different interfaces (Fig. 4a, b). The first interface is a more limited parallel association mediated by TM1, TM2 and helix eight with a buried surface area of 615 Å2 (Fig. 4d, Supplementary Fig. 6). The second and more prominent interface observed in the μOR crystal structure is comprised of TMs 5 and 6 (Fig. 4c). In this case, within each μOR-μOR pair, the buried surface area for a single protomer is 1492 Å2. This represents 92 % of the total buried surface between μOR-T4L molecules, indicating that the comparatively small 114 Å2 buried surface contributed by T4L is unlikely to drive the contact (Supplementary Fig. 7). This suggests that the pairwise association of receptor monomers may represent a physiological opioid receptor dimer or higher order oligomer, the existence of which is supported by previous biochemical, pharmacological and cell biological studies23.
Figure 4. μOR oligomeric arrangement.
a, b μOR crystallized as intimately associated pairs, with two different interfaces as defined in the text. The interface defined by TMs 5 and 6 (c) is much more extensive than for the one defined by TM1-TM2-H8 (d).
Recent computational and biochemical studies have suggested the potential role of TM4 and TM5 in the interaction between δOR receptors24. More generally, oligomers have been observed for a large number of GPCRs (recently reviewed in 25). Some of these studies have shown that TM5 and TM6 peptides can disrupt dimers of the β2AR and V2 vasopressin receptor26,27, and recent crosslinking experiments with the M3 muscarinic receptor suggest a direct dimeric contact mediated by TM5 of each monomer28. The potential involvement of the alternative TM1-TM2-H8 interface in GPCR oligomerization has previously been suggested by several different biochemical studies (reviewed in 25) and, more recently, by the structure of opsin (3CAP)29. In the case of opioid receptors, it has been shown that a μOR-TM1 domain fused to a polybasic TAT sequence could disrupt the μOR/δOR interaction in the mouse spinal cord, resulting in an enhancement of morphine analgesia and a reduction in morphine tolerance30.
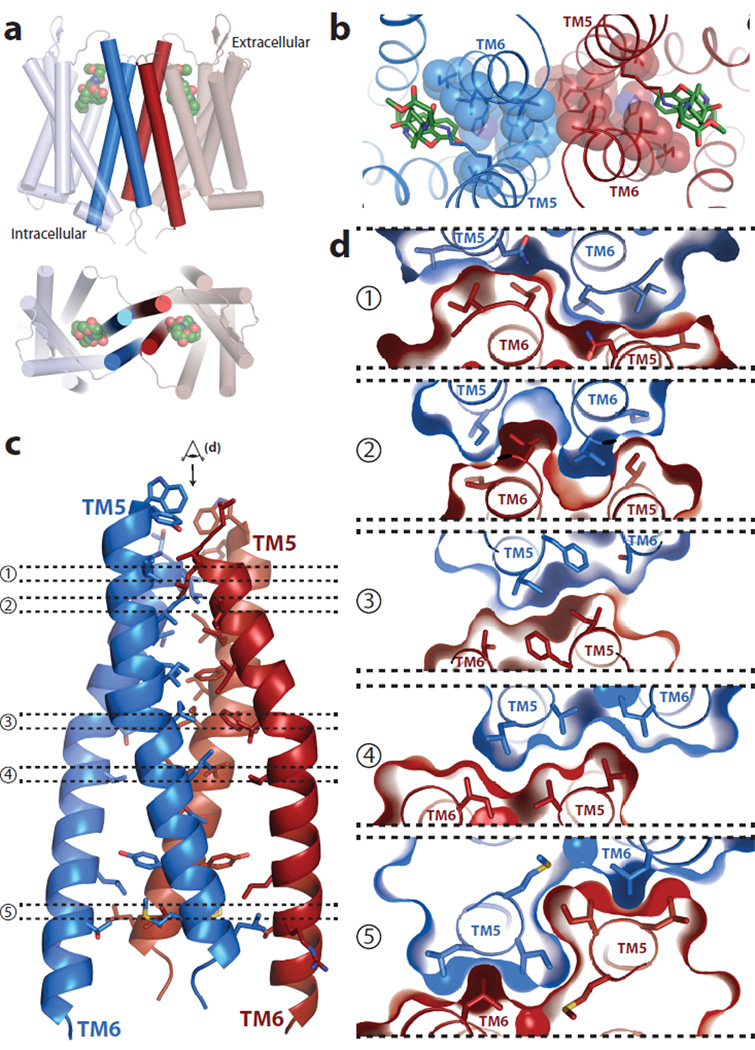
The more prominent interface observed in the μOR crystal structure is comprised of TMs 5 and 6 of each protomer arranged in a four-helix bundle motif (Fig. 5a). This interface is formed by an extensive network of interactions involving 28 residues in TM5 and TM6 (Fig. 5c, Supplementary Fig. 8). These surface packing interactions are highly complementary and are maintained all along the receptor membrane plane from the extracellular to the intracellular side of the μOR (Fig. 5 c, d). The T2796.34 residue described earlier as playing a role in maintaining the receptor in an inactive state is also part of the dimer interface, with the methyl of the threonine contacting I2565.62 of the adjacent protomer. It is thus tempting to speculate that dimerization of the μOR could have a role in regulating receptor signaling.
Figure 5. The four-helix bundle interface.
a, Schematic showing the four-helix bundle architecture of the TM5-TM6 interface b, Viewed from the extracellular surface, the binding pocket shows tight association between the ligand (green sticks) and residues that are involved directly or indirectly in forming the dimeric interface (blue spheres). c, The four-helix bundle is expanded and shown in detail with interacting residues within 4.2 Å shown as sticks. d, Tomographic representation along the dimer interface viewed from the extracellular side (as indicated in panel c) showing the high surface complementarity within the four-helix bundle interface.
The observed dimer is of interest because of existing evidence for both homo- and heterodimers (or oligomers) involving the μOR31. It has been suggested that opioid agonists such as DAMGO and methadone reduce tolerance to morphine in vivo by facilitating morphine-induced endocytosis through μOR oligomerization32,33. These studies implicate allosteric interactions between a protomer bound to DAMGO or methadone and an adjacent protomer bound to morphine. Co-expressing μOR and δOR in cells results in pharmacologic profiles distinct from either receptor expressed alone34. Of interest, morphine is more efficacious in cells expressing both μOR and δOR in the presence of a δOR-selective antagonist, suggesting an allosteric interaction between μOR and δOR protomers35. Hetero-oligomerization between μOR and non-opioid receptors has also been reported23. For example, the α2a adrenergic receptor was shown to modulate receptor μOR structure and signaling36.
Consistent with a role for oligomerization in μOR function, we observed that the amino acids involved in the dimer interface display a high degree of homology with the δOR (Supplementary Fig. 9, 10). Replacing the residues of μOR with the corresponding residues from δOR would not be predicted to interfere with dimer formation (Supplementary Fig. 9, 10). This analysis also suggests that a μOR-δOR dimer could share the same interface. Interestingly, in the μOR TM5/TM6 dimer, the two binding sites are coupled through a network of packing interactions at the dimeric interface (Fig. 5b). This network could provide a structural explanation for the distinct pharmacological profiles obtained for μOR heterodimers and for the allosteric effects of one protomer on the pharmacological properties of the other. This dimeric interface thus provides potential insights into the mechanism of allosteric regulation of one GPCR protomer by the other.
Parallel dimers have also been observed in other GPCR crystal structures, most notably of CXCR4-T4L18. Interestingly, the CXCR4 dimer is also related by a two-fold rotational symmetry axis with a receptor arrangement similar but not identical to that seen in μOR (Supplementary Fig. 8). However, for the five different CXCR4-T4L crystal structures, the largest calculated contact area between the two CXCR4 protomers is smaller (1077 Å2 for 3OE0) than in the μOR structure (Supplementary Fig. 7) and it presents a comparatively less extensive network of interactions (Supplementary Fig. 8).
The dimeric arrangement o f μOR across the TM5-TM6 interface observed in the crystal structure would likely preclude either protomer from coupling to G proteins. This is based on structural changes in TM5 and TM6 observed in the recent crystal structure of the β2AR-Gs complex37. This is also consistent with the observation that inverse agonists stabilize β2AR oligomers, while the G protein Gs reduced the extent of oligomerization38. However, we were able to model an active structure of μOR in complex with G protein based on the crystal structure of the β2AR-Gs complex. Here, we observed that a tetramer formed by the association of two dimers through a TM5/TM6 interface would accommodate two G proteins in interaction with the two distal protomers (Supplementary Fig. 11). This model of an activated μOR-G protein oligomeric complex is highly speculative but is compatible with results from a recent biophysical study suggesting that the G-protein Gi remains associated with a μOR tetramer stabilized by the agonist morphine 39.
The μOR is perhaps the most economically important GPCR in terms of the combined legal and illicit drug market. While there are a number of effective drugs targeting the μOR on the market, the ideal agonist has yet to be developed. The structure of the μOR presented here provides the first high-resolution insight into a peptide receptor that can also be activated by small molecule agonist ligands, some of which are the oldest used drugs in human history. This structure will enable the application of structure-based approaches to complement more conventional drug discovery programs. In addition, it may provide novel insight into the role of oligomerization in GPCR function.
METHODS
Expression and purification
Previously crystallized GPCRs show little density for the poorly ordered amino and carboxy terminal domains. Although these domains are not critical for maintaining high ligand affinity, these flexible regions may inhibit crystallogenesis7. We therefore removed these regions in the receptor construct used for crystallography. Specifically, a TEV protease recognition site was introduced after reside G51 in the amino-terminus and the carboxy terminus was truncated after Q360. The short third intracellular loop of μOR, consisting of residues 264–269 was replaced with T4 lysozyme residues 1–161 in a manner described previously7. In order to facilitate receptor purification, a FLAG M1 tag was added to the amino-terminus and an octa-histidine tag was appended to the carboxy terminus. Finally, a proline residue was introduced N-terminal to the octahistidine tag to allow efficient removal of C-terminal histidines by carboxypeptidase A. For these studies, we utilized the Mus musculus μOR sequence because it expressed at higher levels. The mouse and human μOR share 94% sequence identity and there are only four residues in the resolved part of the structure that differ between mouse and human μOR. These include residues 66, 137, 187, and 306, which are all in the extracellular or intracellular loops of μOR and do not make contacts in the ligand-binding pocket. The final crystallization construct (μOR-T4L) is shown in a representative snake diagram in Supplementary Fig. 1a.
We compared the pharmacological properties of μOR-T4L to those of the wild-type receptor (Supplementary Fig. 1b; see below for methods details). Both constructs showed identical affinity for the radiolabeled antagonist [3H]-diprenorphine ([3H]DPN).
The μOR-T4L construct was expressed in Sf9 cells using the baculovirus system. Culture media was supplemented with 10 μM naloxone to stabilize the receptor during expression. Cells were infected at a density of 4×106 cells per mL and culture flasks were shaken at 27 °C for 48 hr. After harvesting, cells were lysed by osmotic shock in a buffer comprised of 10mM Tris-HCl pH 7.5, 1mM EDTA, 100 µM TCEP, 1 µM naloxone, and 2 mg/ml iodoacetamide to block reactive cysteines. Extraction of μOR-T4L from Sf9 membranes was done with a Dounce homogenizer in a solubilization buffer comprised of 0.5% dodecyl maltoside (DDM), 0.3% 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 0.03% cholesterol hemisuccinate (CHS), 20 mM HEPES pH 7.5, 0.5 M NaCl, 30% v/v glycerol, 2 mg/ml iodoacetamide, 100 μM TCEP, and 1 µM naloxone. After centrifugation, nickel-NTA agarose was added to the supernatant, stirred for two hours, and then washed in batch with 100 × g spins for 5 min each with a washing buffer of 0.1% DDM, 0.03% CHAPS, 0.01% CHS, 20 mM HEPES pH 7.5 and 0.5 M NaCl. The resin was poured into a glass column and bound receptor was eluted in washing buffer supplemented with 300 mM imidazole.
We utilized anti-FLAG M1 affinity resin to further purify μOR-T4L and to exchange the ligand to the covalent antagonist β-funaltrexamine (β-FNA). Nickel-resin eluate was loaded onto anti-FLAG M1 resin and washed extensively in the presence of 10 µM β-FNA. The detergent DDM was then gradually exchanged over 1 hr into a buffer with 0.01% lauryl maltose neopentyl glycol (MNG) and the NaCl concentration was lowered to 100 mM. Receptor was eluted from the anti-FLAG M1 affinity resin with 0.2 mg/mL FLAG peptide and 5 mM EDTA in the presence of 1 µM β-FNA. To remove the amino terminus of μOR-T4L, TEV protease was added at 1:3 w/w (TEV:μOR-T4L) and incubated at room temperature for 1 hr. Receptor was then treated with carboxypeptidase A (1:100 w/w) and incubated overnight at 4°C to remove the octa-histidine tag. The final purification step separated TEV and carboxypeptidase A from receptor by size exclusion chromatography (SEC) on a Sephadex S200 column (GE Healthcare) in a buffer of 0.01% MNG, 0.001% CHS, 100 mM NaCl, 20 mM HEPES pH 7.5, and 1 µM β-FNA. After size exclusion, β-FNA was added to a final concentration of 10 µM. The resulting receptor preparation was pure and monodisperse (Supplementary Fig. 12).
Crystallization and data collection
Purified μOR-T4L receptor was concentrated to 30 mg/mL using a Vivaspin sample concentrator with a 50 kDa molecular weight cut off (GE Healthcare) and crystallization was performed using the in meso method41. Concentrated μOR-T4L was reconstituted into 10:1 monoolein:cholesterol (Sigma) in a ratio of 1:1.5 parts by weight receptor:lipid mixture. Reconstitution was done by the two-syringe method41. The resulting mesophase was dispensed onto glass plates in 80 nL drops and overlaid with 700 nL precipitant solution by a Gryphon LCP robot (Art Robbins Instruments). Crystals grew in precipitant solution consisting of 30 – 38% PEG 400, 100 mM HEPES pH 7.0, 7.5% DMSO, and 300 mM lithium sulfate. Crystals were observed after 24 hours and grew to full size after 5 days. Typical crystals prior to harvesting are shown in Supplementary Fig. 2. Diffraction data were collected at Advanced Photon Source GM/CA-CAT beamline 23ID-D using a beam size of 10 µm. Due to radiation damage, the diffraction quality decayed during exposure. Wedges of 10–20 degrees were collected and merged from 25 crystals using HKL200042. Diffraction quality ranged from 2.4–3.5 Å in most cases. The structure of the μOR was solved by molecular replacement in Phaser43 using the CXCR4 receptor as a search model. We improved the initial model by iteratively building regions of the receptor in Coot44 and refining in Phenix45. We utilized translation libration screw-motion (TLS) refinement with groups generated within Phenix. Electron density suggested the presence of a cholesterol molecule and a monoolein lipid within the lipidic layer. These were subsequently incorporated into the model. To assess the overall quality of the final structure, we used MolProbity46. The resulting statistics for data collection and refinement are shown in Supplementary table 1. Figures were prepared in PyMOL47.
Saturation binding experiments
Membrane homogenates were prepared from Sf9 cells expressing either wild-type μOR or μOR-T4L. Membranes containing μOR or μOR-T4L were incubated with the opioid antagonist, [3H]DPN, for 1 h at 22 °C in 0.5 mL of binding buffer containing 75 mM Tris-HCl pH 7.4, 1 mM EDTA, 5 mM MgCl2, 100 mM NaCl. To determine the affinity for diprenorphine, we utilized [3H]DPN concentrations ranging from 0.1 to 13.5 nM. High concentrations of un-labeled naloxone (1 µM) were used to determine non-specific binding. To separate unbound [3H]-ligand, binding reactions were rapidly filtered over GF/C Brandel filters. The filters were then washed three times with 5 mL ice-cold binding buffer. Radioactivity was assayed by liquid scintillation counting. The resulting data were analyzed using Prism 5.0 (GraphPad Software Inc., San Diego, CA). [3H]-diprenorphine ([3H]DPN; specific activity: 55.0 Ci/mmol) was obtained from PerkinElmer Life Sciences (Waltham, MA).
Supplementary Material
Acknowledgements
We acknowledge support from INSERM (S.G.), from the Stanford Medical Scientist Training Program (A.M.), from the National Science Foundation (A.C.K.), from the Lundbeck Foundation (J.M.M), from the National Institutes of Health Grants NS028471 (B.K.K) and DA031418 (B.K.K and R.K.S), and from the Mathers Foundation (B.K.K. and W.I.W.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions A.M., A.C.K., and S.G. designed experiments, performed research and analyzed data. T.S.K. and F.S.T expressed and purified receptor. J.M.M. performed preliminary biochemical experiments with wild-type μOR. R.K.S. contributed to the effort of μOR crystallization and writing of the manuscript. W.I.W supervised diffraction data analysis and model refinement. L.P. built the tetramer model and helped with the analysis of the dimer interfaces. A.M., A.C.K., S.G., and B.K.K prepared the manuscript. S.G. and B.K.K supervised the research.
Coordinates and structure factors for μOR-T4L are deposited in the Protein Data Bank (accession code 4DKL).
The authors declare no competing financial interests.
REFERENCES
- 1.Katzung BG. Basic and clinical pharmacology. 10th edn. LANGE Mac Graw Hill Medical; 2007. [Google Scholar]
- 2.Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 3.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 4.Raffa RB, Martinez RP, Connelly CD. G-protein antisense oligodeoxyribonucleotides and mu-opioid supraspinal antinociception. Eur J Pharmacol. 1994;258:R5–R7. doi: 10.1016/0014-2999(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 5.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinari P, et al. Morphine-like opiates selectively antagonize receptor-arrestin interactions. J Biol Chem. 2010;285:12522–12535. doi: 10.1074/jbc.M109.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 8.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure function relations in G protein-coupled receptors. Vol. 25. Academic press; 1995. pp. 366–428. [Google Scholar]
- 9.Chen C, et al. Determination of the amino acid residue involved in [3H]beta-funaltrexamine covalent binding in the cloned rat mu-opioid receptor. J Biol Chem. 1996;271:21422–21429. doi: 10.1074/jbc.271.35.21422. [DOI] [PubMed] [Google Scholar]
- 10.Huang P, et al. Functional role of a conserved motif in TM6 of the rat mu opioid receptor: constitutively active and inactive receptors result from substitutions of Thr6.34(279) with Lys and Asp. Biochemistry. 2001;40:13501–13509. doi: 10.1021/bi010917q. [DOI] [PubMed] [Google Scholar]
- 11.Haga K, et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2011 doi: 10.1038/nature10753. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse AC, et al. Structure and Dynamics of the M3 Muscarinic Acetylcholine Receptor. Nature. 2012 doi: 10.1038/nature10867. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disse B, et al. Ba 679 BR, a novel long-acting anticholinergic bronchodilator. Life Sci. 1993;52:537–544. doi: 10.1016/0024-3205(93)90312-q. [DOI] [PubMed] [Google Scholar]
- 14.Cassel JA, Daubert JD, DeHaven RN. [(3)H]Alvimopan binding to the micro opioid receptor: comparative binding kinetics of opioid antagonists. Eur J Pharmacol. 2005;520:29–36. doi: 10.1016/j.ejphar.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Kurowski M, Rosenbaum JS, Perry DC, Sadee W. [3H]-etorphine and [3H]-diprenorphine receptor binding in vitro and in vivo: differential effect of Na+ and guanylyl imidodiphosphate. Brain Res. 1982;249:345–352. doi: 10.1016/0006-8993(82)90068-3. [DOI] [PubMed] [Google Scholar]
- 16.Sporer KA. Acute heroin overdose. Ann Intern Med. 1999;130:584–590. doi: 10.7326/0003-4819-130-7-199904060-00019. [DOI] [PubMed] [Google Scholar]
- 17.Alford BT, Burkhart RL, Johnson WP. Etorphine and diprenorphine as immobilizing and reversing agents in captive and free-ranging mammals. J Am Vet Med Assoc. 1974;164:702–705. [PubMed] [Google Scholar]
- 18.Wu B, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science (New York, N Y) 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansour A, et al. Key residues defining the mu-opioid receptor binding pocket: a site-directed mutagenesis study. J Neurochem. 1997;68:344–353. doi: 10.1046/j.1471-4159.1997.68010344.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonner G, Meng F, Akil H. Selectivity of mu-opioid receptor determined by interfacial residues near third extracellular loop. Eur J Pharmacol. 2000;403:37–44. doi: 10.1016/s0014-2999(00)00578-1. [DOI] [PubMed] [Google Scholar]
- 21.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 22.Seki T, et al. DAMGO recognizes four residues in the third extracellular loop to discriminate between mu- and kappa-opioid receptors. Eur J Pharmacol. 1998;350:301–310. doi: 10.1016/s0014-2999(98)00240-4. [DOI] [PubMed] [Google Scholar]
- 23.Rozenfeld R, Gomes I, Devi L. Ch. 15. In: Pasternak Gavril W., editor. The opiate Receptors. Vol. 23. Humana Press; 2011. pp. 407–437. [Google Scholar]
- 24.Johnston JM, et al. Making structural sense of dimerization interfaces of delta opioid receptor homodimers. Biochemistry. 2011;50:1682–1690. doi: 10.1021/bi101474v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanelli F, De Benedetti PG. Update 1 of: computational modeling approaches to structure-function analysis of G protein-coupled receptors. Chem Rev. 2011;111:PR438–PR535. doi: 10.1021/cr100437t. [DOI] [PubMed] [Google Scholar]
- 26.Hebert TE, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 27.Granier S, et al. A cyclic peptide mimicking the third intracellular loop of the V2 vasopressin receptor inhibits signaling through its interaction with receptor dimer and G protein. J Biol Chem. 2004;279:50904–50914. doi: 10.1074/jbc.M405089200. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, et al. Structural aspects of M3 muscarinic acetylcholine receptor dimer formation and activation. Faseb J. 2011 doi: 10.1096/fj.11-191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 30.He SQ, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 33.He L, Whistler JL. An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol. 2005;15:1028–1033. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 34.George SR, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 35.Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA. G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol. 2011;79:1044–1052. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilardaga JP, et al. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen SG, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fung JJ, et al. Ligand-regulated oligomerization of beta(2)-adrenoceptors in a model lipid bilayer. Embo J. 2009;28:3315–3328. doi: 10.1038/emboj.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golebiewska U, Johnston JM, Devi L, Filizola M, Scarlata S. Differential response to morphine of the oligomeric state of mu-opioid in the presence of delta-opioid receptors. Biochemistry. 2011;50:2829–2837. doi: 10.1021/bi101701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Portoghese PS, Sultana M, Takemori AE. Design of peptidomimetic delta opioid receptor antagonists using the message-address concept. J Med Chem. 1990;33:1714–1720. doi: 10.1021/jm00168a028. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 41.Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat Protoc. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otwinowski Z, Minor W. Methods Enzymol. 1997;Vol. 276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 43.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 45.Afonine PV, Grosse-Kunstleve RW, Adams PD. A robust bulk-solvent correction and anisotropic scaling procedure. Acta Crystallogr D Biol Crystallogr. 2005;61:850–855. doi: 10.1107/S0907444905007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrodinger L. The PyMOL Molecular Graphics System, Version 1.3r1. 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.