Abstract
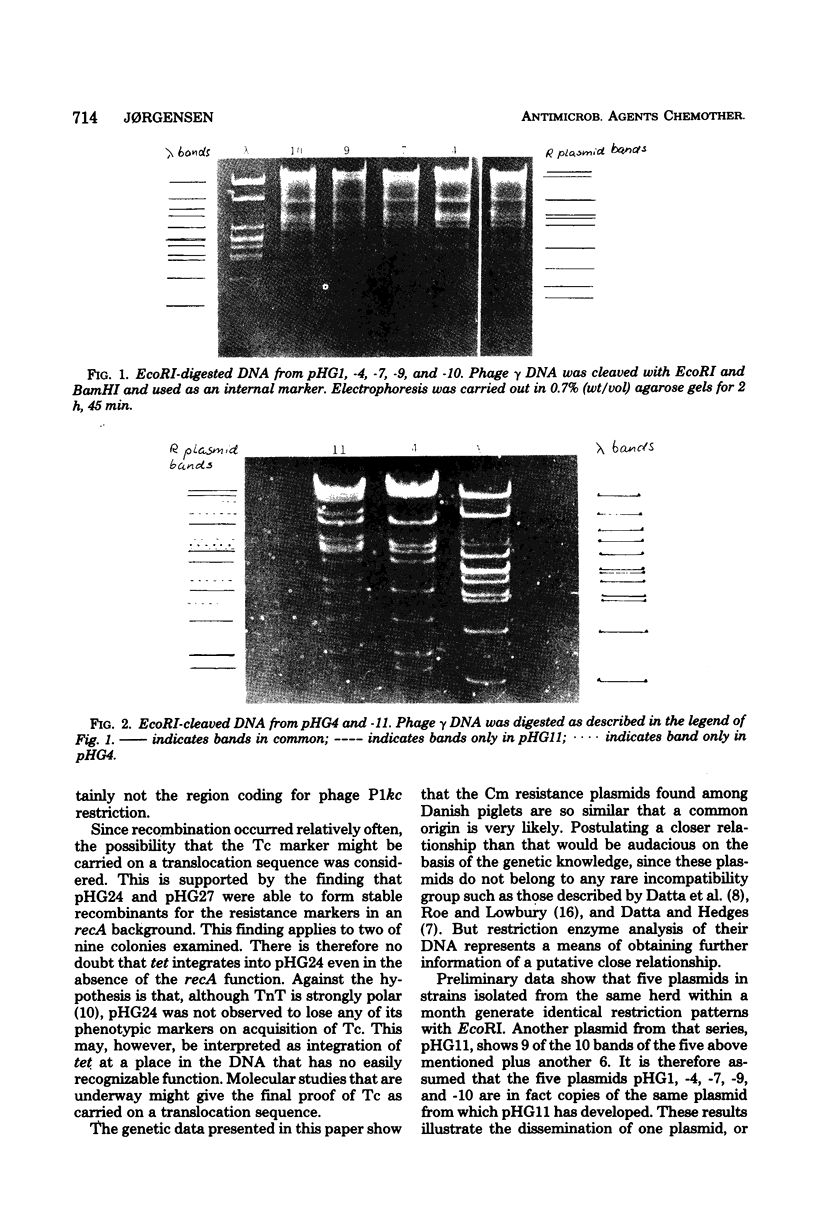
The plasmids in 19 chloramphenicol-resistant Escherichia coli strains of three pig pathogenic antigen types were studied in conjugation and transduction experiments. The plasmids had identical resistance patterns: streptomycin, spectinomycin, sulfonamides, and chloramphenicol (Sm, Sp, Su, Cm) and belonged to IncFII. One plasmid carried ampicillin resistance in addition. Restriction enzyme analysis of the deoxyribonucleic acid from five of the plasmids originating from the same herd showed that their digestion patterns with EcoRI were indistinguishable. EcoRI cleaved the deoxyribonucleic acid of a sixth plasmid from the same herd and displayed nine of the ten bands of the other five plasmids plus an additional six. It appears that the five plasmids with identical restriction patterns have a common origin and may be copies of the same plasmid from which the sixth may have developed. Four strains carried two plasmids each. In two of these strains, a plasmid with a tetracycline marker (Tc), or possibly the tetracycline marker alone, recombined frequently with the Sm Sp Su Cm plasmid without destroying any known function of the latter. The possibility that Tc is carried on a translocation sequence is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Chabbert Y. A. Stable coexistence of three resistance factors (fi-) in Salmonella panama and Escherichia coli K12. J Gen Microbiol. 1969 Sep;58(1):107–113. doi: 10.1099/00221287-58-1-107. [DOI] [PubMed] [Google Scholar]
- Chabbert Y. A., Gerbaud G. R. Surveillance épidémiologique des plasmides responsables de la résistance au chloramphénicol de Salmonella typhi. Ann Microbiol (Paris) 1974 Feb-Mar;125A(2):153–166. [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Trimethoprim resistance conferred by W plasmids in Enterobacteriaceae. J Gen Microbiol. 1972 Sep;72(2):349–355. doi: 10.1099/00221287-72-2-349. [DOI] [PubMed] [Google Scholar]
- Jorgensen S. T., Poulsen A. L. Antibiotic resistance and Hly plasmids in serotypes of Escherichia coli associated with porcine enteric disease. Antimicrob Agents Chemother. 1976 Jan;9(1):6–10. doi: 10.1128/aac.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Larsen J. L., Nielsen N. C. Indflydelse af restriktiv antibiotika-anvendelse på Escherichia coli floraens resistensforhold i svinebasaetninger. Nord Vet Med. 1975 Jul-Aug;27(7-8):353–364. [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Petrocheilou V., Grinsted J., Richmond M. H. R-plasmid transfer in vivo in the absence of antibiotic selection pressure. Antimicrob Agents Chemother. 1976 Oct;10(4):753–761. doi: 10.1128/aac.10.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocheilou V., Richmond M. H., Bennett P. M. Spread of a single plasmid clone to an untreated individual from a person receiving prolonged tetracycline therapy. Antimicrob Agents Chemother. 1977 Aug;12(2):219–225. doi: 10.1128/aac.12.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe E., Lowbury E. J. Changes in antibiotic sensitivity patterns of Gram-negative bacilli in burns. J Clin Pathol. 1972 Feb;25(2):176–178. doi: 10.1136/jcp.25.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchitz J. L., Gerbaud G. R. Classification de plasmides conférant la résistance a la gentamicine. Ann Inst Pasteur (Paris) 1972 Sep;123(3):333–339. [PubMed] [Google Scholar]




