Abstract
Background
The aim of this study is to determine whether B12 replacement would ameliorate cognitive and psychiatric symptoms in elderly subjects with dementia and low serum B12 levels.
Methods
A test group (n=28) of nursing home residents with low serum B12 levels (<250 pg/mL) and a matched comparison group (n=28) with normal serum B12 levels (>300 pg/mL) were evaluated by blinded raters while the test group received intramuscular (IM) B12 replacement therapy. All subjects were assessed at baseline, 8 weeks, and 16 weeks with the Dementia Rating Scale, Brief Psychiatric Rating Scale, and Geriatric Depression Scale.
Results
Although B12 replacement produced significant improvement in hematologic and metabolic parameters, it yielded no significant effect on cognitive or psychiatric variables. A few subjects evidenced notable individual treatment responses; however, these were not statistically more frequent than in the normal B12 group.
Conclusions
These results suggest that B12 replacement is unlikely to benefit cognitive or psychiatric symptoms in the vast majority of elderly dementia patients with low serum B12 levels.
Keywords: vitamin B12, cobalamin, dementia, methylmalonic acid, homocysteine
Introduction
Serum vitamin B12 (cobalamin) testing has long been – and continues to be – recommended as part of the routine screening of patients with dementia (American Psychiatric Association, 2007). This recommendation reflects the widespread assumption that B12 deficiency is a reversible cause of dementia, or at least is commonly associated with cognitive impairment that may be partially correctable. However, surprisingly little research has systematically examined the cognitive and behavioral response to treatment of low serum B12 levels in the setting of dementia. A full diagnostic work-up is frequently not undertaken until a dementing illness is well established; and when B12 deficiency is diagnosed in a demented patient, it is usually not the only (or even primary) etiological process involved. Furthermore, B12 deficiency is often incidentally detected during the course of a dementia of other etiology (e.g. Alzheimer’s disease). In such circumstances there is little prognostic data available to guide clinicians regarding the likely response to B12 replacement therapy.
Further diagnostic challenges are posed by patients who present with subtle B12 deficiency (Carmel et al., 1987) or B12-related neuropsychiatric syndromes without anemia or macrocytosis (Lindenbaum et al., 1988). These diagnostic uncertainties prompted the quest for biochemical markers of tissue B12 deficiency and the development of assays for two metabolites that accumulate if B12 is lacking: methylmalonic acid (MMA) and homocysteine (Hcys) (Stabler et al., 1986; 1988). The measurement of serum MMA and Hcys in combination with serum B12 levels has enlarged the understanding of B12 deficiency considerably beyond the classically described megaloblastic anemia and subacute combined systems disease. For example, if defined by an elevated serum concentration of MMA, then B12 deficiency is a relatively common condition affecting at least 15% of the elderly (Pennypacker et al., 1992; Lindenbaum et al., 1994).
Apart from case reports, few studies have reported outcome data on the cognitive and behavioral response to treatment of low serum B12 levels in the setting of dementia. There have been particularly few placebo-controlled trials of B12 replacement in dementia (de la Fournière et al., 1997; Hvas et al., 2004), presumably because placebo controls have been deemed unethical by many investigators (Martin et al., 1992; Teunisse et al., 1996). Previous prospective studies have thus largely involved open-label designs (Martin et al., 1992; Carmel et al., 1995; Cunha et al., 1995), in some cases with comparison to neuropsychological test performance by a reference group (Teunisse et al., 1996) or matched controls (Eastley et al., 2000) with dementia and normal B12 levels (for a review of these studies, see Discussion). However, an intermediate solution not previously reported is to use a virtual control group of patients with normal levels for blinding of outcome raters.
In the present project we examined whether B12 replacement would ameliorate cognitive and behavioral measures in one particular population: nursing home residents with low serum B12 levels and relatively established cases of dementia. A test group of elderly nursing home residents with dementia and low B12 levels and a matched comparison group with normal B12 levels were both rated on cognitive and psychiatric scales in a single-blind manner (rater blind) while the test group received standard intramuscular (IM) B12 replacement therapy. In addition, we used assays of MMA and Hcys to measure tissue B12 deficiency and to relate this to clinical response to B12 replacement. This study was conducted nearly 20 years ago but has not previously been published. Given the up-to-date methodology employed, the unique protocol design, and the current interest in B-vitamins and dementia, this study provides an important extension of contemporary knowledge.
Methods
Human subjects
The study sample consisted of 28 elderly (age ≥65 years) subjects with dementia and low serum B12 levels (<250 pg/mL) and 28 subjects with dementia and normal B12 levels (>300 pg/mL), who resided in a nursing facility with both skilled and intermediate care units (the Jewish Home for the Elderly, Fairfield CT, U.S.A.). Subjects were enrolled between October 1988 and August 1989 (and completed the study by December 1989). B12 levels were obtained as part of routine annual medical examination, which included medical history, physical and neurological examinations, serum chemistries (including lactate dehydrogenase, LDH), thyroid function studies, complete blood count (CBC) (including hemoglobin, hematocrit, red blood cell (RBC), erythrocyte mean corpuscular volume (MCV), white blood cell (WBC) and differential, and platelets), B12, folate (RBC or serum), urinalysis, electrocardiogram, and chest X-ray. All residents with B12 levels <250 pg/mL were invited to participate as members of the test group. From residents with normal B12 levels (>300 pg/mL), comparison subjects were selected to participate in parallel with low B12 subjects. Normal B12 subjects were group-matched with low B12 subjects for age, gender, and severity of dementia – as measured by the Mini-mental State Examination (MMSE; Folstein et al., 1975), which was also performed as part of routine annual assessment. Cutoffs of 250 pg/mL and 300 pg/mL were adopted because of previous reports that some patients with levels up to 300 pg/mL may show B12-responsive neuropsychiatric disorders (Lindenbaum et al., 1988).
Eligibility criteria for both subject groups included DSM-IIIR criteria (American Psychiatric Association, 1987) for dementia and a score of ≤ 24 on MMSE (Folstein et al., 1975). Subjects were excluded who were currently receiving B12 replacement therapy; had severe sensory impairment (e.g. blindness) which precluded use of standard cognitive and psychiatric rating scales; or had a neurological disease likely to produce marked cognitive or behavioral change during the study (e.g. brain tumor). However, eligibility criteria were otherwise very liberal in order to study effects of B12 replacement across a broad spectrum of clinical dementia in the nursing home setting. Thus subjects were included across the full severity range of dementia (MMSE=0–24), with stable neurological diseases, or receiving central nervous system (CNS) active medications. Psychotropic medication use was common in both the low B12 (antipsychotics 7, benzodiazepines 7, antidepressants 3) and normal B12 (antipsychotics 11, benzodiazepines 5, antidepressants 4) groups. All subjects who met eligibility criteria for low or normal B12 groups agreed to participate and were enrolled during a 10-month period. All subjects (or their responsible next of kin) provided informed consent, and were studied under a protocol approved by the Yale Human Investigation Committee.
Subjects were initially evaluated by a geriatric psychiatry fellow (CHvD) to establish the diagnosis, classification, and characteristics of dementia (American Psychiatric Association, 1987). This evaluation included a review of symptoms, neurological examination, interview of family members, and detailed chart review. In addition to annual medical examinations (see above), a VDRL (test for syphilis) had been performed on all subjects at the time of nursing home admission. Twenty-seven of the 56 subjects had had a previous documented head CT (computed tomography scan) or MRI (magnetic resonance imaging) performed since onset of dementia. Subjects were classified according to the probable etiology of dementia, assuming that B12 deficiency was not exclusively causal. Classification of dementia in the low B12 group included: primary degenerative dementia of the Alzheimer type (DAT) (American Psychiatric Association, 1987) (18), multi-infarct dementia (MID) (American Psychiatric Association, 1987) (3), DAT plus MID (patients who met some criteria for both etiologies, e.g. a progressive dementia with a recent stroke) (3), DAT plus Parkinson’s disease (PD) (3), and DAT plus Herpes encephalitis (1). Classification of dementia in the normal B12 group included: DAT (22), MID (2), DAT plus MID (2), DAT plus PD (1), and DAT plus dementia due to head trauma (1).
Procedure
All low B12 subjects were tested for tissue deficiency with serum MMA (Stabler et al., 1986), and serum Hcys (Stabler et al., 1988) and administered baseline cognitive and psychiatric ratings (see below). They then received replacement B12 therapy in a standardized manner: 1,000 μg IM daily for 1 week, then 1,000 μg IM weekly for the duration of the study (16 weeks total).
All subjects (low and normal B12 groups) were rated by a second examiner (JML, RMR, APS; geriatric psychiatrist or psychiatry resident), blind to B12 status, at 0 weeks (prior to initiation of B12 treatment for the low B12 group), 8 weeks, and 16 weeks with the following scales: (1) Dementia Rating Scale (DRS, scoring range 0–144) (Mattis, 1976) which has been found to be sensitive to change in patients with moderate to severe dementia (Salmon et al., 1990); (2) Brief Psychiatric Rating Scale (BPRS, 16-item, scoring range 16–112) (Overall and Gorham, 1962); (3) Geriatric Depression Scale–abbreviated version (GDS, scoring range 0–15) (Brink et al., 1982; Sheikh and Yesavage, 1986). Some subjects with severe dementia were considered unratable using the BPRS and/or GDS and their data were not included in the analysis of those variables.
After 8 weeks, CBC, LDH, MMA and Hcys were repeated for low B12 subjects to measure hematologic and metabolic response to treatment.
Laboratory studies
All laboratory studies were performed by Metpath-Columbia Laboratory (Bridgeport, CT and Teterboro, NJ). Serum B12 and RBC folate assays utilized competitive protein binding. MMA and Hcys assays employed capillary gas chromatography-mass spectrometry. Initially, they were performed (under contractual agreement with Metpath) at the laboratories of Robert H. Allen and Sally P. Stabler (University of Colorado) where they were developed before this technology was transferred to Metpath’s Teterboro, NJ laboratory. Peripheral blood smears were read by a blinded technician in the laboratory of John E. Lindenbaum at the College of Physicians and Surgeons of Columbia University.
Statistical analysis
Baseline differences between low B12 and normal B12 groups were analyzed by two sample Student t-tests. Effects of B12 treatment on hematologic and metabolic parameters in the low B12 group were analyzed by paired t-tests at 0 and 8 weeks of treatment. However, the data for MMA were not normally distributed (Lilliefors test) and were analyzed instead by Wilcoxon matched-pairs test.
The primary efficacy variable in this study was change from baseline to week 16 in DRS score, and the secondary efficacy variables were change from baseline to week 16 in BPRS and GDS scores. All three variables were analyzed using the last observation carried forward (LOCF) approach. For each of these variables, a comparison between the two groups was performed using an analysis of covariance model (ANCOVA) with group as factor and baseline score as covariate. Change in DRS scores was not normally distributed (Lilliefors test) and were re-analyzed post-hoc by Mann-Whitney U test. The number of subjects who showed a significant individual cognitive improvement (defined as ≥12 points on DRS – an improvement equivalent to the average deterioration in score over one year in DAT patients (Salmon et al., 1990)) was compared between groups by χ2. Correlations between variables were analyzed using Pearson’s product moment correlation (r) or Spearman’s rank correlation coefficient (ρ) depending on the normality of data.
All statistical analyses employed two-tailed tests of significance, using a significance level of α 0.05. All statistical analyses were performed using=SPSS (SPSS Inc., Chicago, IL) or SYSTAT (SYSTAT Inc., Evanston, IL) software.
Results
Subject characteristics
Demographic, clinical, and baseline neuropsychological characteristics of low B12 and normal B12 dementia subjects are displayed in Table 1. The two groups did not differ significantly in age, gender, education, duration of dementia, or Hachinski ischemia score (Hachinski et al., 1975). They also did not differ in any neuropsychological measure at baseline (MMSE, DRS, GDS, or BPRS), although there was a nonsignificant trend for low B12 subjects to have higher BPRS scores than normal B12 subjects (t=1.80, df=46, p=0.079). Twenty-five of 28 low B12 and 23 of 28 normal B12 subjects were considered ratable on the BPRS. BPRS scores for these subjects were similar to those reported in other dementia samples (Gottlieb et al., 1988). Twenty-two low B12 and 21 normal B12 subjects were considered ratable on the GDS. GDS scores consistent with probable depression (>5) were common in both low (10/22) and normal (6/21) B12 subjects.
Table 1.
Subject characteristics
| LOW B12 (N = 28) |
NORMAL B12 (N = 28) |
|||||
|---|---|---|---|---|---|---|
| VARIABLE | MEAN | SD | MEAN | SD | ||
| Demographics | ||||||
| Age | 86.8 | ± | 6.9 | 87.0 | ± | 5.8 |
| Gender | 25F, 3M | 25F, 3M | ||||
| Education (years) | 10.7 | ± | 3.1 | 10.7 | ± | 2.8 |
| Dementia Characteristics | ||||||
| Duration (years) | 4.8 | ± | 3.3 | 5.5 | ± | 4.2 |
| Hachinski Score | 3.9 | ± | 3.0 | 3.9 | ± | 2.9 |
| Neuropsychological | ||||||
| MMSE | 11.9 | ± | 8.9 | 11.6 | ± | 8.0 |
| DRS | 64.4 | ± | 39.6 | 63.8 | ± | 45.4 |
| GDS | 5.2 | ± | 3.3 | 4.5 | ± | 3.5 |
| BPRS | 27.7 | ± | 7.6 | 24.4 | ± | 5.9 |
| Hematological | ||||||
| Serum B12 (pg/mL) | 186 | ± | 43* | 521 | ± | 226 |
| RBC Folate (ng/mL) | 364 | ± | 157† | 524 | ± | 161 |
| Serum LDH (U/L) | 148 | ± | 38 | 165 | ± | 39 |
| WBC (×1000/mm3) | 6.8 | ± | 1.8 | 7.4 | ± | 2.1 |
| RBC (×1000/mm3) | 4.0 | ± | 0.4 | 4.3 | ± | 1.2 |
| Hemoglobin (g/dL) | 12.1 | ± | 1.4 | 12.4 | ± | 1.5 |
| Hematocrit (%) | 36.3 | ± | 4.4 | 37.2 | ± | 4.7 |
| MCV (fL) | 91.9 | ± | 5.5 | 91.9 | ± | 6.3 |
| Platelets (×1000/mm3) | 255 | ± | 56 | 258 | 90 | |
Note: MMSE, Mini-Mental State Examination; DRS, Dementia Rating Scale; GDS, Geriatric Depression Scale; BPRS, Brief Psychiatric Rating Scale; LDH, lactate dehydrogenase; MCV; erythrocyte mean corpuscular volume.
Differs from Normal B12 group, P<0.001, t-test;
Differs from Normal B12 group, p=0.001, t-test. RBC folate levels were available for 24 subjects in each group.
Baseline hematologic findings
Baseline hematological characteristics of low B12 and normal B12 subjects are also displayed in Table 1. RBC folate levels were available for 24 low B12 and 24 normal B12 subjects (remaining subjects instead had serum folate levels). Low B12 subjects also had significantly lower RBC folate levels (364±157 ng/mL) than normal B12 subjects (524±161 ng/mL; t=3.48, df=46, p=0.001), although no folate level in either group was abnormal (RBC folate <200 μg/L or serum folate <3 μg/L). Low and normal B12 groups showed no significant differences in LDH, WBC, RBC, hemoglobin, hematocrit, MCV, or platelets. Seventeen low B12 subjects were anemic (hemoglobin <12.0 g/dL for females or <14.0 g/dL for males), compared to 13 normal B12 subjects. Only two low B12 subjects had a MCV greater than 100 fL (the same number of normal B12 subjects with MCV greater than 100 fL), consistent with previous reports (Thompson et al., 1987; Lindenbaum et al., 1988) that MCV is frequently normal in clinically important B12 deficiency.
Peripheral blood smears were available from 25 low B12 and 23 normal B12 subjects at screening. Hypersegmentation of neutrophils (1 six-lobed or >5 five-lobed granulocytes per 100 polymorphonuclear neutrophils) was noted on blinded review of smears from only 3/25 low B12 and 5/23 normal B12 subjects. Macroovalocytes were present in only 3/25 low B12 and 3/23 normal B12 subjects. Intrinsic factor antibodies were obtained in 17 low B12 subjects and were positive in only one case. Schilling tests were not performed in any subject. Twenty-two low B12 subjects had abnormally elevated MMA levels (>270 nmol/L), and 13 had abnormally elevated Hcys levels (>16.0 μmol/L) (Table 2).
Table 2.
Effect of B12 Replacement on Laboratory Variables in Low B12 Dementia Subjects
| BASELINE |
8 WEEKS |
||||||
|---|---|---|---|---|---|---|---|
| MEAN | SD | MEAN | SD | P | |||
| Methylmalonic Acid (nmol/L) | 592 | ± | 626 | 179 | ± | 66 | <0.001 |
| Homocysteine (μmol/L) | 17.8 | ± | 8.2 | 12.3 | ± | 5.6 | <0.001 |
| Serum LDH(U/L) | 148 | ± | 38 | 145 | ± | 46 | 0.514 |
| WBC (×1000/mm3) | 6.82 | ± | 1.79 | 6.98 | ± | 1.75 | 0.625 |
| RBC (×1000/mm3) | 3.95 | ± | 0.45 | 4.09 | ± | 0.51 | 0.032 |
| Hemoglobin (g/dL) | 12.1 | ± | 1.4 | 12.5 | ± | 1.7 | 0.043 |
| Hematocrit (%) | 36.3 | ± | 4.4 | 37.1 | ± | 5.0 | 0.148 |
| MCV (fL) | 91.9 | ± | 5.5 | 90.6 | ± | 4.7 | 0.035 |
| Platelets(×1000/mm3) | 255 | ± | 56 | 261 | ± | 58 | 0.569 |
Note: LDH, lactate dehydrogenase; WBC, white blood cell count; RBC, red blood cell count; MCV, erythrocyte mean corpuscular volume.
P-values are from paired t-tests for all variables except Methylmalonic Acid, which is from Wilcoxon matched-pairs test.
Responses to B12 therapy
LABORATORY EFFECTS
The effect of IM B12 replacement (after 8 weeks of treatment) on pertinent laboratory values in low B12 subjects is displayed in Table 2. B12 replacement resulted in small but statistically significant increases in RBC number (t=2.26, df=27, p=0.032, paired t-test) and hemoglobin (t=2.13, p=0.043) as well as a decrease in MCV (t=2.36, p=0.025). Predicted sharp declines with treatment were seen in serum MMA (from 592±626 to 179±66 nmol/L; Z=−4.62, p <0.001, Wilcoxon matched-pairs test) and total Hcys (from 17.8±8.2 to 12.3±5.6 μmol/L; t=6.11, p<0.001). Whereas 22 low B12 subjects had abnormally elevated MMA levels (>270 nmol/L), and 13 had abnormally elevated Hcys levels (>16.0 μmol/L) pre-treatment, only 3 MMA levels and 7 Hcys levels remained elevated after 8 weeks of B12 replacement. Baseline MMA 0.30, (r=−0.26, n=28, p=0.18) and Hcys (r=0.07, p=0.71) levels and the degree of MMA (r=−0.30, p=0.12) and Hcys (r=−0.13, p=0.52) response with treatment were all uncorrelated with B12 levels.
Residual elevations in MMA and Hcys post-treatment appeared to be related in part to variations in renal function, as described in previous reports (Rasmussen et al., 1990; Allen et al., 1993; Savage et al., 1994). Post-treatment Hcys levels were significantly correlated with blood urea nitrogen (BUN) (r=0.69, n=28, P <0.001) and serum creatinine (r=0.50, p=0.006). Post-treatment MMA levels were also significantly correlated with BUN (r=0.52, p=0.005) but not serum creatinine (r=0.31, p=0.11). Mean values of renal function tests for low B12 subjects were BUN=24±11 (range 12–57) mg/dL and serum creatinine=1.2±0.6 (range 0.7–3.8) mg/dL.
Cognitive and psychiatric effects
Outcome on cognitive and psychiatric rating scales through 16 weeks is displayed in Figures 1, 2 and 3. Two subjects (both normal B12) did not complete 16-week ratings, and their data were included in the analysis using 8-week ratings, per the LOCF analysis plan. One of these subjects died suddenly of unknown cause, and the other suffered a major stroke. Although subjects were not blinded with regard to treatment, none of the low B12 subjects was aware of receiving B12 replacement at the end of 16-weeks, and only one subject recalled that she had received IM injections.
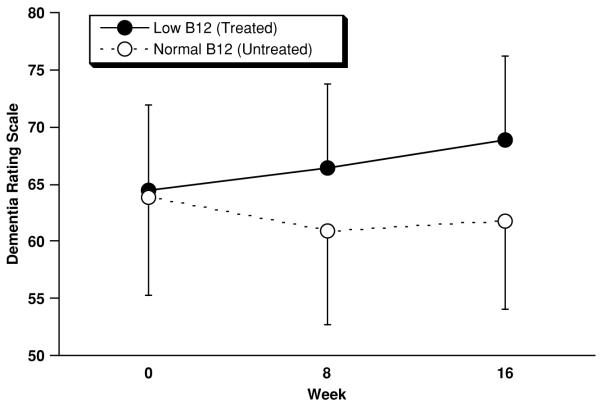
Figure 1.
Outcome on the Dementia Rating Scale (DRS) for low B12 (n=28) and normal B12 (n=28) subjects. Displayed values represent mean±SEM (SEM used for legibility). From 0 to 16 weeks low B12 subjects improved by 4.4±17.6 (SD) points, and normal B12 subjects declined by −2.0±9.9 points. However, this difference was not significant (F=3.24; df=1,53; p=0.077, ANCOVA) (Z=−0.97, p=0.33, Mann-Whitney U test).
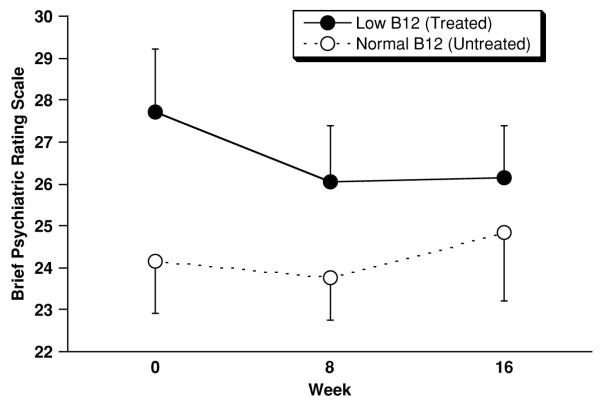
Figure 2.
Outcome on the Brief Psychiatric Rating Scale (BPRS) for low B12 (n=25) and normal B12 (n=23) subjects. Displayed values represent mean±SEM (SEM used for legibility). From 0 to 16 weeks low B12 subjects improved (declined) by −1.4±6.6 (SD) points, and normal B12 subjects worsened (increased) by 0.5±5.6 points (F=0.12; df=1,45; p=0.73, ANCOVA).
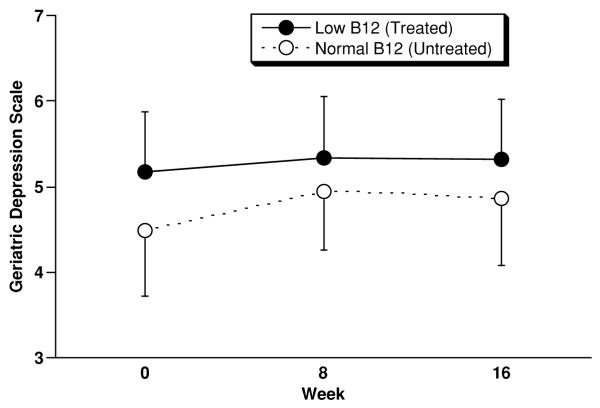
Figure 3.
Outcome on the Geriatric Depression Scale – abbreviated version (GDS) for low B12 (n=22) and normal B12 (n=21) subjects. Displayed values represent mean±SEM (SEM used for legibility). From 0 to 16 weeks low B12 subjects improved by 0.1±2.8 (SD) points, and normal B12 subjects improved by 0.3±2.5 points (F=0.00; df=1,40; p=0.97, ANCOVA).
On the DRS (Figure 1) low B12 subjects improved by 4.4±17.6 points from baseline to week 16, and normal B12 subjects declined by −2.0±9.9 points. This difference trended in favor of treated low B12 subjects according to the planned ANCOVA (F=3.24; df=1,53; p=0.077, ANCOVA). However, change in DRS scores was not normally distributed (Lilliefors Test Statistic=0.16, df=56, p=0.002) was therefore re-analyzed post-hoc and by Mann-Whitney U test and found not to be significant (Z=−0.97, p=0.33, Mann-Whitney U test). On the BPRS (Figure 2) low B12 subjects (n=25) improved (declined) by −1.4±6.6 points, and normal B12 subjects (n=23) worsened (increased) by 0.5±5.6 points (F=0.12; df=1,45; p=0.73, ANCOVA). On the GDS (Figure 3) low B12 subjects (n=22) improved by 0.1±2.8 points, and normal B12 (n=21) subjects improved by points 0.3±2.5 (F=0.00; df=1,40; p=0.97, ANCOVA). Although baseline score was the only covariate specified in the statistical plan, none of these results was altered by the addition of age, sex, and RBC folate level to the ANCOVA models.
Post-hoc analyses were performed to relate evidence for tissue deficiency (MMA or Hcys) to cognitive response to B12 replacement. First, since normal MMA and Hcys levels may exclude clinical deficiency in subjects with low B12 levels, a secondary analysis was done, considering only those low B12 subjects with an elevation (2SD) in either MMA or Hcys (n=24). Using this more stringent criterion of deficiency, the magnitude of improvement on the DRS in low B12 subjects improved slightly (to 4.8±18.9 points), although the comparison to normal B12 subjects was still nonsignificant (F=2.45; df=1,49; p=0.12, ANCOVA) (Z=−0.94, p=0.35, Mann-Whitney U test). Second, when the relationship between change in cognition and metabolites was examined, the magnitude of improvement on the DRS paradoxically showed an inverse correlation with the magnitude of reduction in both MMA (ρ =−0.42, n=28, p=0.025) and Hcys (ρ =−0.51, p=0.006).
The number of subjects who showed a significant cognitive improvement (≥12 points on the DRS) during the study was numerically, but not statistically, greater for treated low B12 subjects (n=6) than comparison subjects (n=2) (χ2 =1.31, df=1, p=0.25). Six treated low B12 subjects who met this criterion gained 71, 38, 21, 16, 15, and 13 points on the DRS, compared to two normal B12 subjects who gained 13 and 12 points on the DRS. The subject who gained 71 points was described at baseline as emotionally withdrawn with psychomotor retardation, although she was considered ratable on DRS. Her DRS improvement was accompanied by a drop (improvement) in BPRS score of 17 points. Similarly, the subject who gained 38 points on the DRS had a reduction (improvement) in GDS score of 6 points. Therefore these largest DRS increases may have been related to improvement in noncognitive behavioral symptoms, whether or not these were B12 related.
Discussion
This study examined whether B12 replacement would ameliorate cognitive and psychiatric variables in elderly nursing home residents with dementia and low serum B12 levels. Low B12 subjects demonstrated tissue deficiency, as B12 replacement was associated with robust reductions in serum metabolites (MMA and Hcys) as well as small but statistically significant changes in hematologic parameters. However, B12 treatment failed to effect statistically significant improvements in cognitive (DRS) or psychiatric (BPRS, GDS) scores. Although a few subjects evidenced notable individual treatment responses, these were not statistically more frequent than in normal B12 subjects.
This study possessed significant limitations associated with the use of a comparison group of untreated subjects with normal B12 levels, rather than a randomized, placebo-controlled design. This virtual control group was successful in maintaining blinded ratings of outcome measures. And although dementia subjects themselves were not technically blinded with regard to treatment status (and no placebo injections were administered to normal B12 subjects), this likely had negligible effect, as none of the low B12 subjects recalled receiving B12 replacement at end of study. The major limitation of this design likely involved the inherent differences between subjects as a function of B12 status, despite careful demographic matching and post-hoc examination of other subject variables, which may have been associated with different outcome at 16 weeks.
The ethics of placebo controls remains a significant problem for the study of cognitive and psychiatric effects of B12 replacement in subjects with low serum B12 levels. Although placebo-controlled trials of B12 and other B-vitamins are widely accepted in dementia patients without evidence of deficiency, no consensus exists regarding the ethics of placebo controls in patients with low levels. As detailed in the following section, other investigators have handled this problem by conducting open-label studies (Martin et al., 1992; Carmel et al., 1995; Cunha et al., 1995), sometimes with comparison to historical controls (Teunisse et al., 1996; Eastley et al., 2000) without low B12 levels, and only rarely with full placebo-controlled designs (de la Fournière et al., 1997; Hvas et al., 2004). In elderly samples with no dementia, placebo-controlled trials of B12 have sometimes handled the ethical dilemma by excluding subjects with any evidence of anemia or neurological symptoms (Hughes et al., 1970; Seal et al., 2002). However, this strategy is difficult to implement in practice, since minor hematological and neurological symptoms are prevalent in the elderly, and not necessarily related to low B12 levels. Furthermore, this practice may exclude the very subjects most likely to demonstrate behavioral improvement with treatment.
Previous treatment studies of low B12 in dementia
Apart from case reports, remarkably few studies have reported outcome data for treatment of dementia associated with low serum B12 levels. Martin et al. (1992) studied cognitive function before and after at least 6 months of open-label B12 treatment in 18 older subjects with low B12 levels (<150 pmol/L) and cognitive dysfunction. Eleven of 18 subjects improved on DRS scores, two subjects by 31 and 28 points. The only clinical characteristic found to predict improvement was short duration (<12 months) of cognitive dysfunction. Carmel et al. (1995) studied metabolic, neurologic, and electrophysiologic response to open-label B12 replacement in 16 patients with low B12 levels (<190 pg/mL), including 13 with dementia. They found that B12 improved several abnormalities but not cognitive function in the 13 patients with dementia. Cunha et al. (1995) investigated 19 patients with dementia and B12 deficiency (<200 pg/mL) and observed that 16 of 19 patients showed persistent decline with B12 treatment at follow-up (3–24 months). All patients who demonstrated improvement on MMSE score had mild dementia with a history of less than two years.
Two previous studies have examined effects of open-label B12 treatment on neuropsychological function in dementia patients – with comparison to a reference group (Teunisse et al., 1996) or matched controls (Eastley et al., 2000) with dementia and normal B12 levels. Teunisse et al. (1996) gave open-label IM B12 replacement to 26 dementia patients with low B12 levels (<200 pg/mL). After 6 months of treatment they found no cases of dementia reversal and no difference in cognitive change scores in comparison to a reference group of 69 patients with Alzheimer’s disease. Eastley et al. studied 66 patients who presented with dementia and 22 with cognitive impairment who were seen for a second assessment after treatment. Changes in neuropsychological test scores were compared with those of patients with normal serum B12, matched by age and diagnosis. B12-treated dementia patients showed no significant improvement and no less neuropsychological deterioration than their matched group. However, cognitively impaired patients improved significantly compared to matched patients on a verbal fluency test. In these two studies, psychometricians were not blinded to the B12 treatment status of patients.
Placebo-controlled trials of B12 replacement in dementia associated with low serum B12 levels are exceedingly few. In their Cochrane Review of all randomized, double-blind, placebo-controlled trials of B12 on cognitive function, Malouf and Areosa Sastre (2003) included only three trials (de la Fournière et al., 1997; Seal et al., 2002; Hvas et al., 2004). de la Fournière et al. (1997) studied 11 subjects with a diagnosis of Alzheimer’s disease with low serum B12 levels (<240 pg/ml). Subjects were randomly assigned to receive injections of B12 1,000 μg or placebo daily for 5 days and then one injection monthly for 5 months. Treatment and placebo groups did not differ significantly in cognitive outcome as measured by ADAS-Cog scores. Seal et al. (2002) aimed to determine the minimum dose of oral B12 required to maintain normal serum levels. They studied 31 geriatric inpatients (one-third of whom had dementia) with low serum B12 levels (100–150 pmol/L) and no history of anemia or neurological disorder other than stroke. They compared treatment groups randomly assigned to receive 50 μg or 10 μg of oral B12 or placebo daily for one month and found that only the 50 μg dose produced a significant increase in serum B12. No significant changes were observed in MMSE scores. Hvas et al. (2004) examined effects of B12 treatment (1,000 μg IM weekly for 4 weeks) on cognitive function and depression in a randomized placebo-controlled study of 140 subjects with elevated serum MMA (0.40–2.00 mmol/l). At baseline 40 (29%) subjects had cognitive impairment (MMSE <25), and 18 (13%) had symptoms of depression. At 3-month follow-up, no improvement was found in cognitive or depression scores. The available randomized clinical trials of B12 in dementia samples are thus remarkably limited, with only one small dedicated study (de la Fournière et al., 1997) and another study with a large subsample with dementia (Hvas et al., 2004). We would concur with the conclusions of Malouf and Areosa Sastre (2003) that these trials do not provide statistically significant evidence of treatment effect for B12 supplementation compared with placebo, on cognitive function.
Limitations of present study
In addition to the absence of a placebo-controlled design, this study had a number of additional limitations that warrant comment. First, the cutoff selected for B12 deficiency (<250 pg/mL) was somewhat higher than in most other studies. A lower cutoff may have yielded a greater degree of tissue deficiency and more significant effects of B12 replacement. Nonetheless, when a more restrictive definition of tissue deficiency was used – i.e. when only subjects with an elevation (2SD) in either MMA or Hcys were included – the results were still nonsignificant. Second, the follow-up period (16 weeks) may have been too brief to observe full effects of B12 replacement on cognitive and psychiatric variables. The limited hematological response (in hemoglobin and MCV) in the treated low B12 subjects may be due in part to the still shorter laboratory retest period (8 weeks, as compared to average RBC life span of 120 days). However, we were concerned that a longer follow-up period would result in more study non-completers and more confounding intercurrent illnesses in this fragile population. Third, the long duration of dementia in this nursing home population may have mitigated against observing cognitive and behavioral effects of B12 replacement. Some previous investigators have reported a time dependency of cognitive recovery with B12 replacement (Martin et al., 1992; Cunha et al., 1995). Martin et al. observed that only patients symptomatic for less than a year showed improvement with therapy (Martin et al., 1992), whereas Cunha et al. (Cunha et al., 1995) reported improvement only in patients with mild dementia of less than two years duration. Our low B12 sample contained only nine subjects with mild dementia (MMSE ≥20) and only six with duration ≤2years. The six treatment responders had a mean MMSE score of 10.5 and mean duration of 3.3 years and thus did not differ markedly from the overall sample. Nonetheless, the preponderance of studies in this field would suggest that future research of cognitive and psychiatric effects of B12 should focus on patients with mild symptoms of short duration.
Conclusion
These results suggest that B12 replacement is unlikely to benefit cognitive or psychiatric symptoms in the vast majority of elderly dementia patients with low serum B12 levels. However, future research is warranted for patients with mild symptoms of short duration.
Acknowledgments
The authors thank Marvin Garrell, MD, for his inspiration; the staff of the Jewish Home for the Elderly, Fairfield CT for untiring assistance; and John E. Lindenbaum, MD (1933–1997, pioneer investigator of vitamin B12 metabolism and deficiency) for helpful advice in study design and interpretation, as well as the review of peripheral blood smears as explained in the text. Dr. Lyness is currently supported by NIMH K24 MH071509.
Footnotes
Conflict of interest None.
Description of authors’ roles CHvD designed the study, evaluated subject eligibility, performed data analyses, and wrote the paper. JML, RMR, and APS performed psychometric assessments and assisted with the paper.
References
- Allen RH, Stabler SP, Savage DG, Lindenbaum J. Elevation of 2-methylcitric acid I and II in the serum, urine and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42:978–988. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd edn, revised American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. American Journal of Psychiatry. 2007 Dec;164(suppl.):1–56. [PubMed] [Google Scholar]
- Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, Rose TL. Screening tests for geriatric depression. Clinical Gerontologist. 1982;1:37–43. [Google Scholar]
- Carmel R, Sinow RM, Karnaze DS. Atypical cobalamin deficiency. Subtle biochemical evidence of deficiency is commonly demonstrable in patients without megaloblastic anemia and is often associated with protein-bound cobalamin malabsorption. Journal of Laboratory and Clinical Medicine. 1987;109:454–463. [PubMed] [Google Scholar]
- Carmel R, et al. The frequently low cobalamin levels in dementia usually signify treatable metabolic, neurologic and electrophysiologic abnormalities. European Journal of Haematology. 1995;54:245–253. doi: 10.1111/j.1600-0609.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Cunha UG, Rocha FL, Peixoto JM, Motta MF, Barbosa MT. Vitamin B12 deficiency and dementia. International Psychogeriatrics. 1995;7:85–88. doi: 10.1017/s1041610295001876. [DOI] [PubMed] [Google Scholar]
- de la Fournière F, et al. Déficience en vitamine B12 et état démentiel: étude épidémiologique multicentrique et thérapeutique. Essai préliminaire. La Semaine des hôpitaux de Paris. 1997;73:133–140. [Google Scholar]
- Eastley R, Wilcock GK, Bucks RS. Vitamin B12 deficiency in dementia and cognitive impairment: the effects of treatment on neuropsychological function. International Journal of Geriatric Psychiatry. 2000;15:226–233. doi: 10.1002/(sici)1099-1166(200003)15:3<226::aid-gps98>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Fostein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Gur RE, Gur RC. Reliability of psychiatric scales in patients with dementia of the Alzheimer type. American Journal of Psychiatry. 1988;145:857–860. doi: 10.1176/ajp.145.7.857. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, et al. Cerebral blood flow in dementia. Archives of Neurology. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- Hughes D, Elwood PC, Shinton NK, Wrighton RJ. Clinical trial of the effect of vitamin B12 in elderly subjects with low serum B12 levels. BMJ. 1970;2:458–460. doi: 10.1136/bmj.2.5707.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvas AM, Juul S, Lauritzen L, Nexo E, Ellegaard J. No effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled study. Journal of Affective Disorders. 2004;81:269–73. doi: 10.1016/S0165-0327(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. New England Journal of Medicine. 1988;318:1720–1728. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J, Rosenberg IH, Wilson PWF, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Amereican Journal of Clinical Nutrition. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- Malouf R, Sastre A. Areosa. Vitamin B12 for cognition. Cochrane Database Systematic Review. 2003 doi: 10.1002/14651858.CD004326. CD004394. [DOI] [PubMed] [Google Scholar]
- Martin DC, Francis J, Protetch J, Huff FJ. Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. Journal of the American Geriatrics Society. 1992;40:168–172. doi: 10.1111/j.1532-5415.1992.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Karasu TB, editors. Geriatric Psychiatry. Grune & Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pennypacker LC, et al. High prevalence of cobalamin deficiency in elderly outpatients. Journal of the American Geriatrics Society. 1992;40:1197–1204. [PubMed] [Google Scholar]
- Rasmussen K, Vyberg B, Pedersen KO, Brochone-Mortensen J. MMA in renal insufficiency: evidence of accumulation and implications for diagnosis of cobalamin deficiency. Clinical Chemistry. 1990;36:1523–1524. [PubMed] [Google Scholar]
- Salmon DP, Thal LJ, Butters N, Heindel WC. Longitudinal evaluation of dementia of the Alzheimer type: a comparison of 3 standardized mental status examinations. Neurology. 1990;40:1225–1230. doi: 10.1212/wnl.40.8.1225. [DOI] [PubMed] [Google Scholar]
- Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum MMA and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. American Journal of Medicine. 1994;96:239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Seal EC, Metz J, Flicker L, Melny J. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. Journal of the American Geriatrics Society. 2002;50:146–151. doi: 10.1046/j.1532-5415.2002.50020.x. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of ashorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. Haworth Press; New York: 1986. pp. 165–173. [Google Scholar]
- Stabler SP, Marcell PD, Podell ER, Allen RH, Lindenbaum J. Assay of methylmalonic acid in the serum of patients with cobalamin deficiency using capillary gas chromatography-mass spectrometry. Journal of Clinical Investigations. 1986;77:1606–1612. doi: 10.1172/JCI112476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. Journal of Clinical Investigations. 1988;81:466–74. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunisse S, Bollen AE, van Gool WA, Walstra GJM. Dementia and subnormal levels of vitamin B12: effects of replacement therapy on dementia. Journal of Neurology. 1996;243:522–529. doi: 10.1007/BF00886874. [DOI] [PubMed] [Google Scholar]
- Thompson WG, Babitz L, Cassino C, Freedman M, Lipkin M. Evaluation of current criteria used to measure vitamin B12 levels. American Journal of Medicine. 1987;82:291–294. doi: 10.1016/0002-9343(87)90070-2. [DOI] [PubMed] [Google Scholar]