Abstract
Chemotherapy, especially if prolonged, disrupts attention, working memory and speed of processing in humans. Most cancer drugs that cross the blood–brain barrier also decrease adult neurogenesis. Because new neurons are generated in the hippocampus, this decrease may contribute to the deficits in working memory and related thought processes. The neurophysiological mechanisms that underlie these deficits are generally unknown. A possible mediator is hippocampal oscillatory activity within the theta range (3–12 Hz). Theta activity predicts and promotes efficient learning in healthy animals and humans. Here, we hypothesized that chemotherapy disrupts learning via decreases in hippocampal adult neurogenesis and theta activity. Temozolomide was administered to adult male Sprague–Dawley rats in a cyclic manner for several weeks. Treatment was followed by training with different types of eyeblink classical conditioning, a form of associative learning. Chemotherapy reduced both neurogenesis and endogenous theta activity, as well as disrupted learning and related theta-band responses to the conditioned stimulus. The detrimental effects of temozolomide only occurred after several weeks of treatment, and only on a task that requires the association of events across a temporal gap and not during training with temporally overlapping stimuli. Chemotherapy did not disrupt the memory for previously learned associations, a memory independent of (new neurons in) the hippocampus. In conclusion, prolonged systemic chemotherapy is associated with a decrease in hippocampal adult neurogenesis and theta activity that may explain the selective deficits in processes of learning that describe the ‘chemobrain’.
Keywords: cancer therapy, memory, neurogenesis, rat, theta
Introduction
Cancer is traditionally treated with chemotherapy and/or radiation therapy, both of which suppress tumor growth by decreasing cell proliferation and causing cell death. Cognitive impairment is reported by as many as 70% of patients experiencing cancer therapy (Dietrich et al., 2008). Furthermore, up to 50% of patients report significant and measurable declines in attention, learning, memory, and overall processing speed (Vardy and Tannock, 2007). These deficits are described as reminiscent of a ‘fog’ or slowing down of cognitive processing, and are collectively referred to as ‘chemobrain’.
Cancer treatment not only affects cancer cells but also disrupts the proliferation of healthy cells, such as those that give rise to new neurons in the adult hippocampus (Monje and Dietrich, 2012). Adult neurogenesis, in turn, influences cognition: reducing (Clelland et al., 2009; Garthe et al., 2009; Goodman et al., 2010; Shors et al., 2001; Shors et al., 2002) or enhancing (Creer et al., 2010; Sahay et al., 2011) neurogenesis, respectively, impairs or promotes performance, especially in tasks that depend on the hippocampus and/or are difficult to master. Thus, it seems likely that the cognitive deficits associated with cancer treatment are at least exacerbated by the loss of newly generated neurons in the hippocampus. A relatively clear picture emerges, relating chemotherapy-induced cognitive decline to neurogenesis and neurogenesis to learning. However, despite some recent advances (Lacefield et al., 2012), it is not known how disruption of neurogenesis alters learning-related synchronized neural activity such as 3–12-Hz oscillations in the hippocampus (theta, see Buzsáki, 2002). Proportionately more hippocampal theta activity predicts faster and better learning (Berry and Thompson, 1978; Nokia et al., 2009; Nokia et al., 2012), and learning itself induces theta activity during training in animals (Hoffmann and Berry, 2009; Wikgren et al., 2010) and humans (for reviews see Duzel et al., 2010; Jutras and Buffalo, 2010). It is suggested that synchronized oscillatory activity facilitates communication between anatomically distant, but functionally related, structures during learning. Thus, a disruption in theta activity in response to chemotherapy may prevent interregional communication, leading to deficits in learning. The hippocampus is necessary for many aspects of learning, but typically not for long-term memory storage (Takehara et al., 2003). Therefore, we hypothesized that chemotherapy would disrupt learning but not memory.
To test these hypotheses, adult male Sprague–Dawley rats were treated with temozolomide (TMZ) and then trained on classical eyeblink conditioning, while hippocampal local-field potentials were recorded. Dividing cells were labeled at different time points to allow examination of the various potential effects of TMZ on adult neurogenesis. TMZ is a chemotherapeutic agent used in a cyclic manner for several months to treat central nervous system tumors (gliomas) in both children and adults (Lashkari et al., 2011). Trace and very long delay (VLD) conditioning both require an intact hippocampus for learning (Beylin et al., 2001), whereas standard delay conditioning does not (Schmaltz and Theios, 1972). Also, in a previous study by Shors et al. (2001), antimitotic treatment had no effect on delay conditioning, whereas it severely impaired trace conditioning. Therefore, it was hypothesized that only trace and VLD conditioning would be impaired after chemotherapy. As chemotherapy is assumed to exert its negative effects on cognition by disrupting neurogenesis, and the memory for a previously acquired trace-conditioned response is independent of the hippocampus (Takehara et al., 2003), i.e. is stored by mature neurons, it was also hypothesized that chemotherapy would leave the retrieval of trace memories intact.
Materials and methods
Subjects
A total of 53 self-bred (Department of Psychology, Rutgers University) adult male Sprague–Dawley rats were used as subjects. They were 60–75 days old and weighed, on average, 366 g (standard error of the mean, ±4 g) at the beginning of the experiment. Each rat was weighed weekly (Fig. S1). All rats were single-housed during the experiment, and food and water were available ad libitum. Lights were on for 12 h a day, starting at 07:00 h. All experimental procedures were carried out during the light portion of the day. The experiments were designed to fully comply with the rules and regulations set forth by the PHS Policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health guide for the care and use of laboratory animals, and were approved by the Rutgers University Animal Care and Facilities Committee (P. I. Tracey, J. Shors, protocol no. 98-018).
Surgery
Surgery was performed prior to all other experimental treatment. Rats were anesthetized with intraperitoneal sodium pentobarbital (60 mg/kg; Nembutal, 50 mg/mL; Lundbeck, Deerfield, IL, USA). Atropine (0.54 mg/mL; Vedco, St Joseph, MO, USA) was also injected intraperitoneally to keep the rat’s airways clear during surgery. The rat was secured to a stereotaxic device (David Kopf Instruments) with blunt ear bars. A local analgesic [bupivicaine (Marcaine), 2.5 mg/mL; Hospira, Lake Forest, IL, USA] was injected subcutaneously into the site of the incision. Four screws were implanted in the skull, one in each of the four quadrants delineated by skull sutures. The skull screws were connected in pairs to serve as reference and ground for the neural recordings. Two electrodes made of Formvar-insulated nichrome wire (bare diameter, 50 μm; A–M Systems, Carlsboro, WA, USA) were lowered into the right hippocampus, aiming at the dentate gyrus (3.5–4.2 mm posterior to bregma, 1.5–2.0 mm lateral to bregma, and 3.4–3.8 mm below bregma). Two bipolar electrodes made of stainless steel wire insulated with Teflon (bare diameter, 127 μm; A–M Systems) were implanted through the upper right eyelid. The whole construction was cemented in place with dental acrylic mass anchored to the skull via the skull screws. Upon awakening, the rats were given a 1-mL oral dose of acetaminophen (Children’s Acetaminophen, 32 mg/mL; Rite Aid), returned to their home cages, and monitored daily for 5 days or until they had fully recovered.
Chemotherapy administration and cell labeling
In humans, TMZ (75–200 mg/m2, i.e. ~2–5 mg/kg) has been effectively used to treat brain tumors for over 10 years (Lashkari et al., 2011). In the experiments reported here, TMZ (Sigma) was injected once a day for 3 days, and this was followed by 4 days of recovery, for up to 6 weeks (Fig. 1). Cyclic treatment was chosen because it is the most commonly used protocol in humans (200 mg/m2 per day for 5 consecutive days every 4 weeks) (Lashkari et al., 2011). Also, this treatment protocol effectively reduced neurogenesis in mice (Garthe et al., 2009). TMZ was made into a 2.5 mg/mL solution with sterile water, and injected intraperitoneally at a dose of 25 mg/kg. The dose is similar to the most commonly used dose of ~200 mg/m2 in humans (see Oncology Tools: Dose Calculator at http://www.accessdata.fda.gov/scripts/cder/onctools/animalquery.cfm; human weight, 65 kg; human height, 170 cm; rat weight, 0.3 kg). For saline injections, the volume was matched to that of the TMZ injections. Weekly weighing indicated no weight loss in TMZ-treated rats (Fig. S1).
Fig. 1.
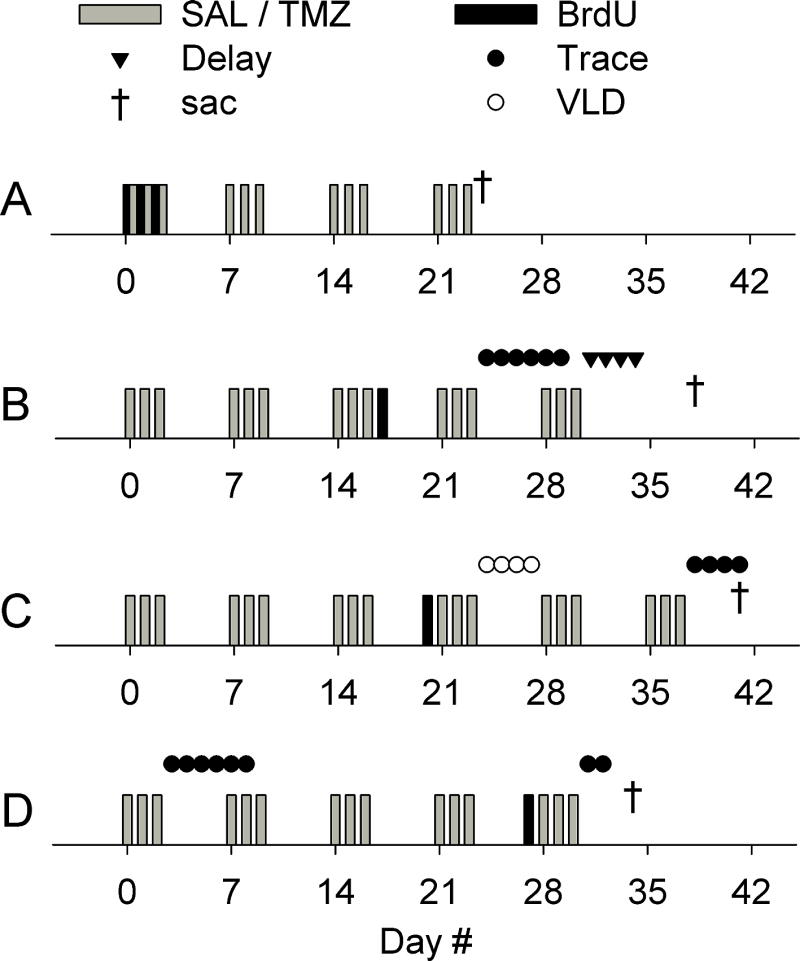
Experimental timelines. (A) In the first experiment, the effects of chemotherapy on neurogenesis alone were studied. TMZ (25 mg/kg) or saline (SAL) was injected for 4 weeks (once per day for 3 consecutive days per week). BrdU (200 mg/kg) was injected during the first treatment cycle, once per day, at least 2 h before the TMZ injection. Rats were killed after the fourth treatment cycle, and the number of BrdU-positive cells in the dentate gyrus was assessed with immunohistochemistry. (B) In the second experiment, the effects of chemotherapy on hippocampus-dependent trace eyeblink conditioning, hippocampus-independent delay eyeblink conditioning and hippocampal theta activity were studied. In this and all following experiments (see C and D), recording electrodes were implanted into the dentate gyrus in an operation prior to any other experimental treatment. Rats were injected with either saline or TMZ for a total of 5 weeks, and trained on trace eyeblink conditioning (Trace) followed by delay eyeblink conditioning (Delay). In this and all following experiments, hippocampal local-field potentials were recorded before and during conditioning. A single BrdU injection was used to confirm the effects of TMZ on the number of new cells in the granule cell layer. The injection was given 7 days prior to conditioning, and rats were killed 3 weeks after the BrdU injection. (C) In the third experiment, the effects of chemotherapy on another hippocampus-dependent task, VLD eyeblink conditioning, and on hippocampal theta activity were studied. Rats were injected with TMZ or saline for a total of 6 weeks, and trained on VLD eyeblink conditioning followed by trace eyeblink conditioning. BrdU was injected after three treatment cycles, 4 days after the most recent TMZ/saline injection, and only 4 days before starting conditioning, to determine whether the timing of the labeling in relation to the most recent treatment cycle and in relation to conditioning would affect the difference in BrdU-positive cell counts between treatment groups. As before, rats were killed 3 weeks after the BrdU injection. (D) In the last experiment, we tested whether possible learning deficits observed during trace eyeblink conditioning were caused by non-specific side effects. The effects of chemotherapy on the retention of a previously acquired memory and on hippocampal theta activity were also assessed. Initial training on trace eyeblink conditioning took place after the first treatment cycle, and was followed by retention testing 3 weeks (and three treatment cycles) later. The effect of long-term chemotherapy on the size of the proliferating cell population was also examined. For this, BrdU was injected after a total of four cycles of drug treatment, and rats were killed 7 days later.
Bromodeoxyuridine (BrdU) (Sigma) was injected intraperitoneally at a dose of 200 mg/kg (concentration, 15 mg/mL) to mark the dividing cells in the dentate gyrus. In the first experiment (Fig. 1A), the overall effect of TMZ on adult hippocampal neurogenesis was examined in naïve adult rats. To evaluate the effect of chemotherapy on a larger population of cells generated during and surviving past the drug treatment, we injected BrdU multiple times during the first treatment cycle (three daily injections): BrdU was injected first, and this was followed by a TMZ injection at least 2 h later. Each BrdU injection labeled the population of cells that were in S-phase during the 2 h for which BrdU remains systemic. In all further experiments, BrdU was injected only once, to enable more straightforward determination of the age of the labeled cell population. In the next two experiments (Fig. 1B and 1C), BrdU was injected at different time points with regard to both drug treatment and learning/training, to verify the expected reduction in the number of BrdU-labeled cells caused by TMZ, and to examine possible changes in this reduction. The rats in the first three experiments (Fig. 1A–C) were all killed 21 days after the (last) BrdU injection. In the last experiment (Fig. 1D), we assessed the effects of long-term chemotherapy on the size of the proliferating cell population. For this, BrdU was injected after a total of four cycles of drug treatment, and rats were killed 7 days later.
It is acknowledged that the repeated injections might act as a stressor, and thus affect the outcome of the experiments. However, the number of injections was the same for rats treated with saline and for those treated with TMZ. In addition, in male rats, stress facilitates rather than impairs learning [see, for example, Maeng et al. (2010)].
Conditioning procedure
To assess learning and memory, we used different variations of classical eyeblink conditioning, a type of learning for which the neural basis is well known, and learning does not require physical activity or exploration. In eyeblink conditioning, a neutral conditioned stimulus (CS) is repeatedly paired with aversive stimulation of the eyelid [unconditioned stimulus (US)]. As a result, the subject learns to blink the eyelid shut in response to the CS. In the trace variant of this task, the CS precedes the US, but the two stimuli do not overlap. In the VLD and delay variants, the CS onset precedes the US, and the two stimuli overlap and coterminate.
To study the effects of chemotherapy on hippocampus-dependent associative learning, we trained TMZ/saline-treated rats in trace eyeblink conditioning (Fig. 1B). The same rats were then trained in standard delay eyeblink conditioning, a hippocampus-independent task, to ensure that possible learning deficits observed during trace conditioning were not caused by an overall inability to learn an eyeblink conditioned response. In the next experiment (Fig. 1C), we trained TMZ/saline-treated rats in VLD eyeblink conditioning, a task that is also dependent on an intact hippocampus. We then trained the same rats in trace eyeblink conditioning, to examine whether learning VLD conditioning would facilitate learning this more complex hippocampus-dependent task. In the last experiment (Fig. 1D), rats were trained in trace eyeblink conditioning until they acquired a robust conditioned response, and then tested for the memory of the conditioned response 3 weeks later. This experiment was conducted to control for the effects of acute, non-specific side effects of TMZ and to further assess the effects of chemotherapy on retention of trace memories.
Each rat undergoing eyeblink conditioning was acclimated to the conditioning chamber by being placed inside for 1 h with the headstage secured. On the next day, training was begun by giving 10 presentations of the white noise (83 dB, 250 ms) to determine whether the rats showed any sensitized responses to the noise. Eyeblink conditioning was then started. White noise was used as a CS, and a 100-ms periorbital shock (0.65 mA) as a US. A trace conditioning trial consisted of a 250-ms CS followed by a 500-ms stimulus-free time interval that separated the CS from the presentation of the US. A delay conditioning trial consisted of an 850-ms CS that overlapped and coterminated with the US. Finally, VLD conditioning consisted of a 1500-ms CS that overlapped and coterminated with the US. Trials were presented with an intertrial interval of 25 ± 5 s.
The number of trials per day and the number of days of training for each variation of eyeblink conditioning were determined on the basis of the difficulty of the task evaluated in light of previous experience in our laboratory. Trace conditioning is harder to learn than VLD conditioning (Nokia et al., 2012), whereas VLD conditioning is harder to learn than delay conditioning. Thus, for trace conditioning, 200 trials/day for up to 6 days were given, for VLD conditioning, 200 trials/day for 4 days were given, and for delay conditioning, 100 trials/day for 4 days were given (Fig. 1).
Recording and data analysis
During training, electromyographic (EMG) signals from the upper eyelid and local-field potentials from the hippocampus were recorded. The EMG signal was bandpass filtered between 300 Hz and 500 Hz (1700 Differential AC amplifier; A–M Systems). The local-field potentials were filtered between 1 Hz and 500 Hz (PGA16; MultiChannel Systems, Reutlingen, Germany). All signals were sampled at a rate of 2000 Hz and recorded continuously (Digidata1440 and AxoScope; Molecular Devices, Sunnyvale, CA, USA). Matlab (MathWorks, Natick, MA, USA) was used for data analyses. To determine learned responding from the EMG signals, the signal amplitude was derived with Hilbert transformation. Next the mean and the standard deviation (SD) of the signal during a 250-ms period immediately preceding the onset of the CS were obtained. For each trial, the threshold for a learned response was set at mean + (4 × SD). Responses had to occur during the last 250 ms of the trace period, and the EMG signal had to stay above the predetermined threshold for at least 10 ms for a blink to be classified as a learned response. The learning criterion was set at >60% learned responses during at least one 100-trial block. When the effects of chemotherapy on retention of trace memories (Fig. 1D) were studied, an even more stringent criterion was used during initial training: Rats had to express >60% learned responses during two of three consecutive 100-trial blocks before their ability to remember the conditioned response after administration of TMZ was tested. The highest percentage of learned responses reached during a 100-trial block was used as an indicator of how well a rat had learned (peak performance).
To assess the effects of chemotherapy on hippocampal theta activity, the relative power of theta activity during a 5-min stimulus-free period immediately preceding the first eyeblink conditioning session (spontaneous) and that induced by the CS during eyeblink conditioning were derived. To examine spontaneous theta activity, the 5-min recording was divided into 50 artefact-free 3-s sweeps that were used for analysis. To examine induced theta activity, a 500-ms time period starting 250 ms after the onset of the CS was selected for analysis from each conditioning trial, thus avoiding the effect of immediate event-related potentials. Sweeps with artefacts most commonly caused by rapid large-scale movements were automatically rejected from the analysis by simple amplitude thresholding with Matlab. Next, to determine the relative power of hippocampal theta activity [theta/(delta + theta)], a fast Fourier transform was used to analyze the frequency composition of the signal. From the result, the relative power of hippocampal theta activity was determined as the ratio between the power of the signal at 4.5–10.3 Hz and the power of the signal at 1.5–10.3 Hz (theta ratio).
Naturally, induced theta ratios were analyzed separately for each experiment (Fig. 1B–D). However, regarding the effects of TMZ on spontaneous theta activity, data from two experiments (Fig. 1B and 1C) were combined to form one group, because the rats in both experiments had been subjected to identical experimental procedures (4 weeks of TMZ/saline) until the first eyeblink conditioning session. Data from the last experiment (Fig. 1D) were used to examine the effects of only 1 week of TMZ/saline treatment on spontaneous theta activity.
Histology
Rats were killed 1 week after the BrdU injection, when the effects of chemotherapy on the retention of a trace memory were assessed (Fig. 1D). In all other experiments (Fig. 1A–C), rats were killed 3 weeks after the BrdU injection(s). Rats were overdosed with an intraperitoneal injection of sodium pentobarbital (Sleepaway, 26 mg/mL; Fort Dodge Animal Health, Fort Dodge, IA, USA), and intracardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. Then, the brain was extracted and postfixed for 24 h, and coronal sections (40 μm) were cut through the entire dentate gyrus of the left hemisphere with a vibratome. Every 12th section was collected and mounted on a slide. BrdU peroxidase staining was performed as described previously [for a detailed protocol, see Anderson et al. (2011)]. A Cresyl Violet counterstain was used, as follows: rinse with dH2O; soak in 0.1% Cresyl Violet for 4–10 min; rinse with dH2O; rinse with 70% EtOH supplemented with a few drops of acetic acid; rinse with 95% EtOH followed by 100% EtOH; soak in xylene for 4 min; soak in clean xylene for >1 min; and coverslip.
From the stained slides, estimates of total numbers of BrdU-labeled cells were obtained with a modified unbiased stereology protocol (Waddell and Shors, 2008; West et al., 1991). In essence, the numbers of BrdU-labeled cells in the granule cell layer and the hilus were counted at ×100 on a Nikon Eclipse 80i light microscope from every 12th unilateral section throughout the dentate gyrus (one slide per rat, a total of 10 slices, 6.3–1.8 mm posterior to bregma) (Paxinos, 1998). The experimenters were unaware of the experimental conditions when counting the cells. The number of cells was multiplied by 24 to obtain an estimate of the total number of BrdU-labeled cells in the hippocampus. Numerous studies from our group and others have shown that up to 80% of cells labeled with BrdU in the granule cell layer mature into neurons when assessed with markers such as doublecortin (Sisti et al., 2007; Waddell and Shors, 2008), NeuN (Leuner et al., 2007; Leuner et al., 2010), or TuJ1 (Cameron and McKay, 2001; Leuner et al., 2007; Leuner et al., 2010).
The right hemisphere was used to assess the location of the electrode tip. The tissue was sectioned (40 μm), and slices were mounted on slides and stained with Cresyl Violet. The location of the electrode tip was verified under the same light microscope at ×40. Electrode locations are shown in Fig. S2.
Statistical analyses
PASW (SPSS, Chicago, IL, USA) was used for statistical analyses. Repeated measures ANOVAs and t-tests were used to analyze differences between groups and changes across time. Whenever an interaction was detected, separate ANOVAs for treatment groups were conducted.
Results
TMZ attenuates neurogenesis in the granule cell layer
Results for the effects of chemotherapy on neurogenesis in adult male rats are summarized in Fig. 2. Three rats were excluded from the analysis because of complications in sectioning the brain or staining the slides.
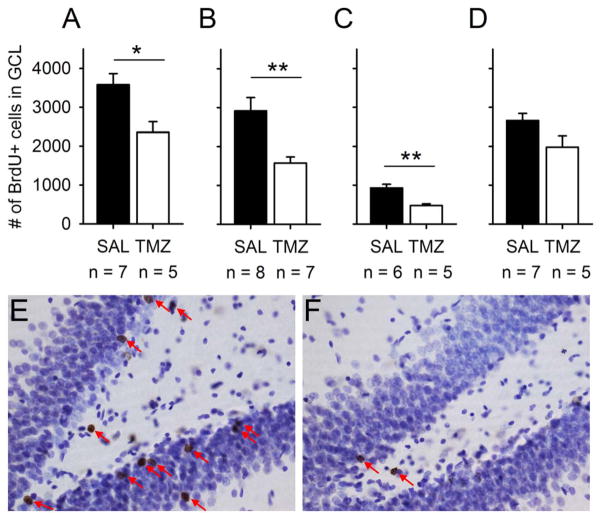
Fig. 2.
TMZ reduced the number of new cells generated in the granule cell layer (GCL) of the dentate gyrus. A–D refer to the same experiments as A–D in Fig. 1. (A) In the first experiment, the effects of chemotherapy on neurogenesis alone were studied. BrdU was injected during the first treatment cycle. TMZ reduced the number of BrdU-positive cells in the GCL by ~34%. (B) A single BrdU injection was used to confirm the effects of TMZ on the number of new cells in the GCL. The injection was given 7 days prior to conditioning, and rats were killed 3 weeks after the BrdU injection. TMZ in combination with conditioning led to an approximately 50% difference in neurogenesis between groups. (C) BrdU was injected after three treatment cycles, 4 days after the most recent TMZ or saline (SAL) injection, and only 4 days before starting conditioning, to determine whether the timing of the labeling in relation to the most recent treatment cycle and in relation to conditioning would affect the difference in BrdU-positive cell counts between treatment groups. As before, animals were killed 3 weeks after the BrdU injection. Again, TMZ in combination with conditioning led to an approximately 50% difference in neurogenesis between groups. Note that fewer new cells were present in both saline-treated and TMZ-treated rats than in the previous experiment, possibly owing to the age of the labeled cell population during learning (Anderson et al., 2011). (D) In the last experiment, the effect of long-term chemotherapy on the size of the proliferating cell population was assessed. BrdU was injected after four treatment cycles, 4 days prior to re-training on trace conditioning, and 1 week prior to killing the rats. Note that the effect of TMZ is smaller than in previous experiments. It seems that TMZ both decreases the proliferating population of cells and increases the number of post-mitotic cells that die. Cell death resulting from TMZ treatment is most obvious when rats are killed 21 days or more after a BrdU injection. (E and F) Photomicrographs of BrdU-positive cells in a rat treated with saline (E) and in a rat treated with TMZ (F) for a total of 5 weeks are shown. In both rats, BrdU was injected after the third treatment cycle, 7 days prior to conditioning, and both rats were killed 3 weeks after the BrdU injection. In A–D, vertical lines depict standard error of the mean, and asterisks refer to the statistical significance of independent samples t-tests: *P < 0.05, **P < 0.01.
To first assess the effects of chemotherapy on neurogenesis in the rat dentate gyrus (Figs 1A and 2A), TMZ (25 mg/kg) or saline was injected systemically in a cyclic manner for 4 weeks. To label dividing cells generated during treatment, BrdU was injected (200 mg/kg; once daily for a total of three times) during the first cycle. Chemotherapy reduced the number of new cells surviving at the end of treatment in the granule cell layer by ~34% (independent samples t-test: t10 = 2.98, P = 0.014; Fig. 2A).
In the next experiment (Figs 1B and 2B), rats were injected with TMZ or saline for 4 weeks, and then trained on trace conditioning followed by delay conditioning. A single BrdU injection was used to confirm that TMZ decreases the number of new cells in the granule cell layer. The injection was given after 3 weeks of treatment with either TMZ or saline, and 7 days prior to conditioning. From previous studies, it is known that new cells that are ~1 week old at the start of training are more likely to survive if an animal learns (Anderson et al., 2011). Thus, the number of BrdU-labeled cells in this experiment reflects the combined effect of drug treatment and conditioning on neurogenesis. TMZ-treated rats (most of which did not learn) possessed fewer new cells in the granule cell layer than rats injected with saline (and most of which learned) (t13 = 3.40, P = 0.005). The combined effect of drug treatment and conditioning on the number of new cells in the hippocampus was ~50% (Fig. 2B).
In the next experiment (Figs 1C and 2C), rats were injected with TMZ or saline for 4 weeks, and then trained in VLD conditioning followed by trace conditioning. Again, only one cell population was labeled with BrdU, to confirm that TMZ reduces neurogenesis. However, this time BrdU was injected 4 days after the last treatment injection, only 4 days before starting conditioning, to determine whether the timing of the labeling in relation to the most recent treatment cycle and in relation to conditioning would affect the difference in cell counts between treatment groups. Again, TMZ-treated rats (which, in this experiment, learned as well as saline-treated rats) had significantly fewer new cells in the granule cell layer than rats injected with saline (t9 = 3.96, P = 0.003; Figs 1C and 2C). Moreover, the difference between TMZ-treated and saline-treated rats was again ~50%. Note that fewer new cells were present in both saline-treated and TMZ-treated rats than in the previous experiment (Fig. 2B vs. Fig. 2C). It is known that new cells that are younger than ~1 week when training is started are actually more likely to die in response to learning (Anderson et al., 2011), so training may have decreased the number of BrdU-labeled cells from the number normally found in animals killed 21 days after a single BrdU injection. Thus, the overall number of BrdU-labeled cells in this experiment reflects the combined effect of drug treatment and learning on neurogenesis.
In the last experiment, rats were injected with TMZ/saline and then trained in trace conditioning, with retention testing 3 weeks later (Fig. 1D). To examine how TMZ affects the proliferating population of cells in the dentate gyrus, rats were treated with four cycles of TMZ before the BrdU injection, and were killed only 1 week later. Note that, in this experiment, the cells labeled with BrdU did not have the same length of time to die as in the previous experiments (Figs 1A–C and 2A–C). Although TMZ-treated rats had fewer new cells in the granule cell layer than saline-treated rats (Fig. 2D), the difference was not statistically significant [t10 = 2.09, not significant (NS)]. This verifies that, in rats, the dramatic effects of TMZ are not solely attributable to a decrease in the proliferating population of cells (i.e. the number of cells available for BrdU to label) in the granule cell layer.
There was no effect of chemotherapy on cell genesis in the hilus in any of the experiments [t9–13 = 0.11–0.96, NS (data not shown)]. In summary, TMZ reduced the number of new adult-born cells by up to 50% in adult male rats, but the decrease was only evident within the granule cell layer.
Chemotherapy retards learning of a trace-conditioned response but not VLD conditioning or retention of a previously learned response
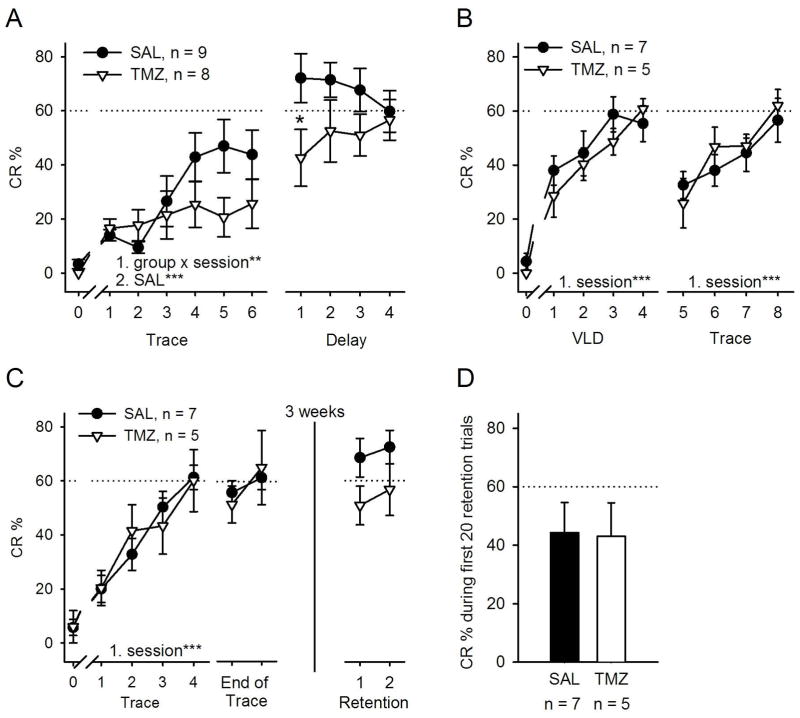
The outline of the experiments including behavioral assessment is shown in Fig. 1B–D. First, we examined the effect of prolonged chemotherapy on hippocampus-dependent associative learning, namely trace eyeblink conditioning. As a result of conditioning, the percentage of conditioned responses increased (i.e. learning occurred) only in the saline-treated group, and not in the group treated with TMZ for 4 weeks (repeated measures ANOVA: interaction of group and session, F5,75 = 3.63, P = 0.005; main effect of session in the saline-treated group, F5,40 = 8.61, P < 0.001) (Fig. 3A). After trace conditioning, the same rats were given another cycle of either saline or chemotherapy and then trained on a hippocampus-independent task, namely delay eyeblink conditioning. Both saline-treated and chemotherapy-treated rats learned delay conditioning to a comparable level (interaction of group and session, F3,45 = 2.28, NS; main effect of group, F1,15 = 2.65, NS; main effect of session, F3,45 = 0.31, NS; Fig. 3A). However, on the first day of delay conditioning (Fig. 3A, right panel), saline-treated rats outperformed chemotherapy-treated rats (independent samples t-test: t15 = 2.14, P = 0.050).
Fig. 3.
Chemotherapy disrupted acquisition of the trace eyeblink conditioned response (CR) (A, left) but left delay conditioning (A, right), VLD (B) eyeblink conditioning and retention of a previously learned response intact (C and D). A) Four weekly cycles of chemotherapy (TMZ) disrupted the subsequent acquisition of the CR during trace eyeblink conditioning but not performance during subsequent training with delay eyeblink conditioning. (B) Four weekly cycles of antimitotic treatment did not disrupt acquisition during VLD conditioning. Subsequent training with trace conditioning was also not disrupted, perhaps because the rats had already acquired the basic association during VLD training. (C) One cycle of chemotherapy (three daily injections) did not disrupt trace conditioning, and four weekly cycles of chemotherapy left retention of the already acquired CR largely intact. The apparent difference between the groups (C, right) was not statistically significant. (D) There was no difference in the responses during the first 20 trials of the retention test. All subplots: vertical lines depict standard error of the mean. Statistically significant results of repeated measures ANOVAs (1.) and independent samples t-tests are indicated. If an interaction was detected, a separate ANOVA for each group was conducted (2.). Asterisks refer to statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001. SAL, saline.
Next, we assessed the effects of chemotherapy on another hippocampus-dependent learning task known as VLD conditioning (Beylin et al., 2001). Rats were first subjected to 4 weeks of chemotherapy or saline injections, and then trained on VLD eyeblink conditioning. Both groups learned this task equally well (main effect of session, F3,30 = 7.71, P = 0.001; main effect of group, F1,10 = 0.50, NS; interaction, F3,30 = 0.79, NS; Fig. 3B, left). To determine whether learning VLD conditioning would facilitate learning the trace variant of the task, an additional two cycles of chemotherapy or saline treatment were administered, followed by trace conditioning (Fig. 1C). Previous learning of VLD conditioning did indeed facilitate trace conditioning, and both groups acquired the trace learned response equally well (main effect of session, F3,30 = 11.53, P < 0.001; main effect of group, F1,10 = 0.11, NS; interaction, F3,30 = 0.84, NS; Fig. 3B, right). To summarize, chemotherapy selectively hindered one type of hippocampus-dependent learning (trace eyeblink conditioning) while sparing performance in another, highly similar task (VLD eyeblink conditioning).
In the last experiment, we tested whether the learning deficit observed during trace eyeblink conditioning was a result of non-specific side effects. Rats were subjected to just one cycle of chemotherapy or saline, and then trained on trace eyeblink conditioning as they went through an additional cycle of treatment (Fig. 1D). Both groups readily acquired the conditioned response (main effect of session, F1,30 = 21.42, P < 0.001; main effect of group, F1,10 = 0.00, NS; interaction, F3,30 = 0.78, NS; Fig. 3C, left), indicating that the impairments in learning seen in the first experiment were in fact attributable to prolonged effects of chemotherapy. To assess the effects of chemotherapy on memory retention, chemotherapy or saline treatment was continued for another 3 weeks. Finally, retention of the previously acquired learned response was tested. There was no difference in overall responding between the groups (t10 = 0.08, NS; Fig. 3D). However, as shown, the effect was close to the 0.05 level of significance, suggesting some minimal effect on performance during retraining (main effect of session, F1,10 = 0.45, NS; main effect of group, F1,10 = 4.61, NS/P = 0.057; interaction, F1,10 = 0.02, NS; Fig. 3C, right). To summarize, long-term but not short-term chemotherapy severely impaired, and in most rats prevented, acquisition of the trace-conditioned response during eyeblink conditioning but did not significantly affect retention of the response.
Long-term antimitotic treatment attenuates hippocampal theta activity
To determine whether chemotherapy disrupts learning via changes in hippocampal oscillatory activity related to efficient learning (Nokia et al., 2009; Nokia et al., 2012), local-field potentials were recorded before and during eyeblink conditioning (Fig. 1B–D). Only rats with at least one recording electrode in the dentate gyrus were included in the analyses. One electrode per rat was selected, on the basis of tip location and signal quality (Fig. S2). The relative powers of hippocampal theta activity(theta ratio) during a 5-min stimulus-free period preceding the first eyeblink conditioning session (spontaneous) and in response to the white noise-conditioned stimulus (induced) were determined, and compared between groups and across training sessions.
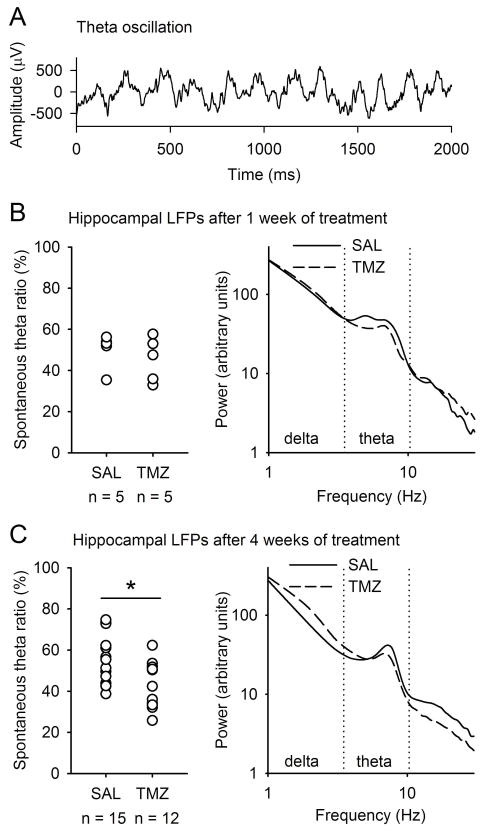
The results for spontaneous hippocampal theta activity are summarized in Fig. 4. One week of TMZ treatment did not reduce theta activity to a statistically significant degree (independent samples t-test: t8 = 0.78, NS; Fig. 4B); however, 4 weeks of chemotherapy did do so (t25 = 2.34, P = 0.027; Fig. 4C). To summarize, long-term but not short-term chemotherapy disrupts endogenous theta-band activity in the hippocampus.
Fig. 4.
Several weeks of chemotherapy attenuated spontaneous hippocampal theta (4–10 Hz) activity. (A) Local-field potentials (LFPs) recorded from the dentate gyrus typically oscillate at the theta-band frequency. (B) One week of chemotherapy (TMZ) did not reduce spontaneous hippocampal theta activity. (C) Several weeks of chemotherapy attenuated the relative power of hippocampal theta activity. The asterisk refers to independent samples t-test with a P-value of <0.05. In the right panels of both B and C, group averages of power distributions (fast Fourier transform) demonstrate that the power at the theta-band was reduced in response to chemotherapy. Absolute power values for each subject were divided by the mean power of all frequencies. For statistical analyses, the relative power of theta activity was calculated (theta/theta + delta). SAL, saline.
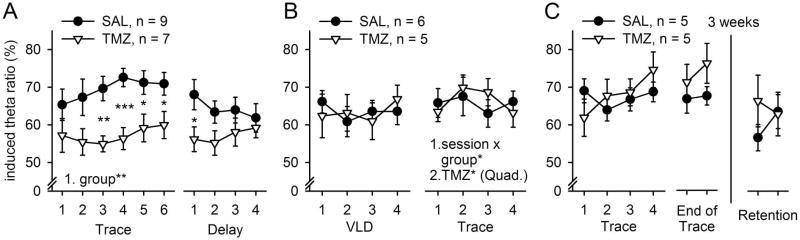
The results for hippocampal theta-band responses to the CS are summarized in Fig. 5. Several weeks of chemotherapy attenuated hippocampal theta-band responses to the CS during trace eyeblink conditioning (repeated measures ANOVA: main effect of group, F1,14 = 8.86, P = 0.010; main effect of session, F5,70 = 1.41, NS; interaction of session and group, F5,70 = 0.78, NS; Fig. 5A). The difference between groups developed early in training, before notable differences in behavior could be detected (compare Figs 3A and 5A). Theta-band responses to the CS were greater in the saline-treated group than in the TMZ-treated group, starting from the third training session and extending until the end of training on trace conditioning (t14 = 2.34–4.30, P = 0.035–0.001). Overall, hippocampal theta-band responses during subsequent delay conditioning were similar in both groups (main effect of group, F1,14 = 2.62, NS; main effect of session, F3,42 = 0.80, NS; interaction of session and group, F3,42 = 2.23, NS). However, during the first session of delay eyeblink conditioning, theta-band responses were more prevalent in the saline-treated group than in the TMZ-treated group (t14 = 2.19, P = 0.046). To summarize, chemotherapy disrupted both hippocampal theta-band responses and learning during trace conditioning. During subsequent delay conditioning, the effects were still evident, but limited to the beginning of training. Chemotherapy had no effects on hippocampal theta-band responses elicited by the CS during VLD conditioning (main effect of group, F1,9 = 0.00, NS; main effect of session, F3,27 = 1.04, NS; interaction of session and group, F3,27 = 1.34, NS; Fig. 5B). However, subtle effects of chemotherapy on hippocampal theta-band responses were evident during subsequent trace conditioning (interaction of group and session, F3,27 = 3.28, P = 0.036): in saline-treated rats, the CS induced a stable theta-band response across trace conditioning (repeated measures ANOVA: main effect of session, F3,15 = 1.55, NS). In contrast, in rats subjected to chemotherapy, hippocampal theta-band responses changed across trace conditioning (F3,12 = 4.41, P = 0.026). A quadratic trend was statistically significant (F1,4 = 32.18, P = 0.005), indicating first an increase and then a decrease across training in hippocampal responding. Note that both groups learned trace conditioning equally well at the behavioral level if they were previously trained with VLD conditioning.
Fig. 5.
Several weeks of chemotherapy attenuated stimulus-induced hippocampal theta-band (4–10 Hz) responses elicited during trace eyeblink conditioning. (A) Several weeks of chemotherapy attenuated hippocampal theta-band responses to the CS during trace conditioning when it was presented as a novel task. Note that the difference between groups developed early in training, before notable differences in behavior could be detected (compare Figs 3A and 5A). (B) Chemotherapy did not affect theta-band responses induced by the CS when a VLD protocol was used. When trace conditioning was conducted after VLD conditioning (B, right), subtle changes in induced theta activity were observed in the group subjected to chemotherapy. (C) Only one cycle of chemotherapy did not have an effect on hippocampal theta-band responses, and neither did following cycles of antimitotic treatment affect theta activity elicited by the CS during subsequent retention testing. Vertical lines depict standard error of the mean. Statistically significant results of repeated measures ANOVAs (1.) and independent samples t-tests are indicated. If an interaction was detected, a separate ANOVA for each group was conducted (2.). Asterisks refer to statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Chemotherapy did not alter oscillatory responses within the theta range in response to the CS when rats were exposed to only one cycle of treatment (main effect of group, F1,8 = 0.07, NS; main effect of session, F3,24 = 2.01, NS; interaction of session and group, F3,24 = 2.02, NS; Fig. 5C) or after a total of six cycles of treatment, when retention of trace memory was tested (main effect of group, F1,8 = 0.45, NS; main effect of session, F1,8 = 0.28, NS; interaction of session and group, F1,8 = 2.48, NS).
To summarize, the effects of chemotherapy on hippocampal theta-band responses parallel the effects of chemotherapy on learning: long-term chemotherapy disrupts hippocampal theta-band responses to the CS during trace eyeblink conditioning, but not those elicited during delay or VLD conditioning or during retention of an already acquired trace memory.
Discussion
A significant number of patients treated with chemotherapy report cognitive side effects (Vardy and Tannock, 2007). To test whether chemotherapy might impair cognition via disruptions in hippocampal neurogenesis and oscillatory activity, adult male rats were treated with either TMZ or saline, and then trained on eyeblink classical conditioning, while hippocampal local-field potentials were recorded. Several weeks of chemotherapy reduced neurogenesis, attenuated theta-band (4–10 Hz) oscillatory activity, and hindered learning. The effects of chemotherapy on learning and induced theta activity were specific to a task in which an association had to be made between temporally related but separate events [trace conditioning; see also Shors et al. (2001)]. As expected, chemotherapy did not affect the expression of an already acquired trace memory. Taken together, these findings show that chemotherapy disrupts both the structural and functional integrity of the hippocampus, and results in highly specific learning deficits.
Chemotherapy decreases neurogenesis in the granule cell layer
For some time, it has been suggested that the cognitive effects of chemotherapy are induced or at least exacerbated by disruptions in adult neurogenesis within the hippocampus (Monje and Dietrich, 2012; Monje et al., 2007). Consistent with this, several weeks of cyclic TMZ treatment reduced the number of new cells in the granule cell layer of the hippocampus by ~34% in adult male rats. Combined with the effects of conditioning (Anderson et al., 2011), the maximum difference in the number of new cells between saline-treated and TMZ-treated rats was ~50%. The effect is smaller and slower to manifest than that obtained in mice (Garthe et al., 2009), probably reflecting species differences in overall vulnerability to toxic substances. It is also possible that some of the cells labeled with BrdU were, in fact, undergoing DNA repair or apoptosis, and the effect would have been larger had we waited longer before killing the rats or used a different marker to label the cells. It seems that TMZ both decreases the proliferating population of cells (see Garthe et al., 2009) and increases the number of post-mitotic cells that die. According to our current results, cell death resulting from TMZ treatment is most obvious when animals are killed 21 days or more after a BrdU injection.
Interestingly, TMZ reduced the number of surviving new cells selectively in the granule cell layer but not in the hilus of adult male rats. The reason for this anatomically specific effect of TMZ is unknown. It seems unlikely that TMZ would penetrate different regions of the dentate gyrus differently. However, if there are differences in vascularization between the hilus and the granule cell layer, then this might be one explanation. Then again, the effects of chemotherapy on neurogenesis are not limited to drugs designed to cross the blood–brain barrier: chemotherapeutic agents targeted to treat cancer outside the central nervous system also reduce hippocampal neurogenesis (Briones and Woods, 2011; Christie et al., 2012; Mustafa et al., 2008). It is also possible that cancer treatment might affect the differentiation or migration of immature cells that are present at the time of treatment. It is known that the majority of cells labeled with BrdU in the granule cell layer differentiate into neurons [see, for example, Leuner et al. (2007)], whereas proportionately more of those in the hilus differentiate into glia (Scharfman et al., 2007). Thus, it seems that TMZ preferentially affected neurogenesis, and not the generation of glia. In fact, systemically administered chemotherapeutic drugs that do not cross the blood–brain barrier as readily as TMZ lead to fewer new hippocampal cells maturing into neurons and to abnormal dendritic morphology in those that do (Christie et al., 2012). Also, cells surviving radiation therapy preferentially differentiate into glial cells instead of neurons (Monje et al., 2002). It could also be that cells that become neurons (in the granule cell layer) instead of becoming glia (in the hilus) are more sensitive to cancer therapy, because of possible differences in DNA repair mechanisms between immature neurons and glia (Bauer et al., 2012).
Although it is targeted to affect proliferating cells, TMZ might also have (indirect) adverse effects on mature, older neurons and/or glia, thus further affecting the integrity of the hippocampal network. Consistent with this, white and gray matter loss have been reported in humans years after termination of chemotherapy [for review, see Dietrich et al. (2008)]. However, according to our current results, chemotherapy disrupts learning in a very selective manner, sparing learning that relies solely on mature neurons in the cerebellum (Thompson and Steinmetz, 2009) [see also Shors et al. (2001)] and sparing memories stored by mature neurons in the neocortex (Takehara et al., 2003). In addition, the adverse effects of cancer treatment on cognition are ameliorated by factors promoting neurogenesis in animal models (El Beltagy et al., 2010; Fardell et al., 2012; Lyons et al., 2011; Winocur et al., 2011). Thus, it seems plausible that disruptions in hippocampal neurogenesis contribute to the deficits in learning and working memory processes that are reported by humans treated systemically for cancer.
Chemotherapy disrupts acquisition of an association between discontiguous events
Chemotherapy affects various learning tasks in a selective manner, impairing performance on some tasks while sparing performance on other tasks (Briones and Woods, 2011; Christie et al., 2012; Mustafa et al., 2008) [see also Shors et al. (2001)]. Consistent with these observations, TMZ affected some but not all forms of classical eyeblink conditioning. Specifically, TMZ severely impaired hippocampus-dependent trace eyeblink conditioning. More interestingly, TMZ did not alter learning of another hippocampus-dependent task, VLD conditioning. The major difference between the two tasks lies within their temporal organization: In trace conditioning, two stimuli occur in contingency but are separated by a stimulus-free gap, whereas in VLD conditioning the stimuli to be paired are presented partially overlapping. Acquiring the learned response during trace conditioning requires more training trials than training with VLD conditioning (Nokia et al., 2012), and learning becomes even more difficult as the length of the temporal gap increases (Waddell et al., 2011). Thus, trace conditioning is both dependent on the hippocampus and difficult to master. Each of these factors seems to predict which cognitive tasks are disrupted by chemotherapy (Vardy and Tannock 2007) and/or reduced neurogenesis (Shors et al., 2001; Shors et al., 2002).
According to our current results, chemotherapy did not affect the retention or expression of a memory that was acquired early in treatment. These data are consistent with those suggesting that, over time, the memory for a learned response acquired during trace eyeblink conditioning becomes independent of the hippocampus, and instead relies on neocortical structures for long-term storage (Takehara et al., 2003). Others have reported that the new hippocampal neurons that, when still immature, encode a memory during the initial learning experience are needed for the retrieval of that memory later on, when the cells have matured (Arruda-Carvalho et al., 2011). However, it may be that only certain types of long-term memory are dependent on new hippocampal neurons, and others, such as those obtained during trace eyeblink conditioning, are not.
Chemotherapy attenuates learning-related hippocampal theta-band activity
Chemotherapy disrupts a limited set of cognitive functions, and the subjective experience of decline often surpasses that measured by neuropsychological tests (Vardy and Tannock, 2007). The symptoms of ‘chemobrain’ consist of deficits in attention, learning, working memory, and executive function, as well as an overall reduction in processing speed. In congruence with this, prolonged TMZ treatment reduced endogenous hippocampal theta activity in rats, presumably reflecting a decrease in ‘attention’ or alertness. Previous studies have indicated that the higher the proportion of theta activity before training, the better and faster one will learn (Berry and Thompson, 1978; Guderian et al., 2009; Nokia et al., 2009; Nokia et al., 2012).
Prolonged TMZ treatment disrupted hippocampal theta-band responses induced by the CS during trace eyeblink conditioning, a task that the chemotherapy-treated animals were unable to learn. In both animals (Hoffmann and Berry, 2009; Nokia et al., 2009) and humans (Lega et al., 2012), hippocampal theta-band responses have been associated with successful encoding of episodic memories. Furthermore, synchronous oscillatory activity in the theta-band is suggested to mediate information flow between functionally related brain regions during learning and memory retrieval (Hoffmann and Berry, 2009; Wikgren et al., 2010) [see also Duzel et al. (2010), Jutras and Buffalo (2010), and Sauseng et al. (2010)]. It is worth noting that the deficits in stimulus-induced theta-band responses in our current study tended to precede the behavioral impairment in learning, suggesting that the reductions in theta activity may relate to deficits in acquiring the learned response.
How TMZ, a systemically administered drug, is able to affect hippocampal theta-band responses is unclear, but could well be through disruptions in neurogenesis (see above). As granule cells in the dentate gyrus are at the forefront of processing signals entering the hippocampal tri-synaptic loop, and processing within the dentate gyrus is based on sparse networks of cells, it seems plausible that even small disruptions in the structure and functioning of the dentate gyrus could lead to deficits in encoding incoming information. At the network level, this would be reflected in, for example, attenuated theta-band responses, as was the case in our current experiment.
Conclusion
Chemotherapy preferentially interferes with complex, hippocampus-dependent learning that requires associations to be formed between related events that do not overlap in time. These deficits are accompanied by decreases in hippocampal theta activity and neurogenesis. Thus, ‘chemobrain’ may be mediated by disruptions in the very neuronal mechanisms that support learning.
Supplementary Material
Fig. S1. Temozolomide treatment using a dose of 25 mg/kg did not cause weight loss but hindered normal weight gain.
Fig. S2. Recording electrodes were placed in the dentate gyrus.
Acknowledgments
The authors would like to thank Monica Choksi and Prateek Agarwal for assisting in gathering the data. This work was supported by the National Institutes of Health (grant nos. MH-59970 and ARRA-3R01MH059970-10S1) and the National Science Foundation (grant nos. IOB-0444364 and IOS-0914386) to T. J. Shors. This work was also supported by grants from the Academy of Finland (grant no. 137783), Emil Aaltonen Foundation, and Jenny and Antti Wihuri Foundation to M. S. Nokia.
Abbreviations
- BrdU
bromodeoxyuridine
- CS
conditioned stimulus
- EMG
electromyographic
- NS
not significant
- SD
standard deviation
- TMZ
temozolomide
- US
unconditioned stimulus
- VLD
very long delay
References
- Anderson ML, Sisti HM, Curlik DM, 2nd, Shors TJ. Associative learning increases adult neurogenesis during a critical period. Eur J Neurosci. 2011;33:175–181. doi: 10.1111/j.1460-9568.2010.07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Goldstein M, Heylmann D, Kaina B. Human monocytes undergo excessive apoptosis following temozolomide activating the ATM/ATR pathway while dendritic cells and macrophages are resistant. PLoS One. 2012;7:e39956. doi: 10.1371/journal.pone.0039956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–1300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–65. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- Duzel E, Penny WD, Burgess N. Brain oscillations and memory. Curr Opin Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- El Beltagy M, Mustafa S, Umka J, Lyons L, Salman A, Chur-yoe GT, Bhalla N, Bennett G, Wigmore PM. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav Brain Res. 2010;208:112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl) 2012;220:183–193. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci USA. 2009;106:5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LC, Berry SD. Cerebellar theta oscillations are synchronized during hippocampal theta-contingent trace conditioning. Proc Natl Acad Sci USA. 2009;106:21371–21376. doi: 10.1073/pnas.0908403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Curr Opin Neurobiol. 2010;20:150–155. doi: 10.1016/j.conb.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashkari HP, Saso S, Moreno L, Athanasiou T, Zacharoulis S. Using different schedules of temozolomide to treat low grade gliomas: systematic review of their efficacy and toxicity. J Neurooncol. 2011;105:135–147. doi: 10.1007/s11060-011-0657-7. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–61. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010;5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- Lyons L, El Beltagy M, Umka J, Markwick R, Startin C, Bennett G, Wigmore P. Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology (Berl) 2011;215:105–115. doi: 10.1007/s00213-010-2122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J Neurosci. 2010;30:16188–16196. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28:323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Sisti HM, Choksi MR, Shors TJ. Learning to learn: theta oscillations predict new learning, which enhances related learning and neurogenesis. PLoS One. 2012;7:e31375. doi: 10.1371/journal.pone.0031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Penttonen M, Korhonen T, Wikgren J. Hippocampal theta-band activity and trace eyeblink conditioning in rabbits. Behav Neurosci. 2009;123:631–640. doi: 10.1037/a0015334. [DOI] [PubMed] [Google Scholar]
- Paxinos G, WC . The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34:1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman J, McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007;29:14–27. doi: 10.1159/000096208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (oryctolagus cuniculus) J Comp Physiol Psychol. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63:183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Waddell J, Anderson ML, Shors TJ. Changing the rate and hippocampal dependence of trace eyeblink conditioning: slow learning enhances survival of new neurons. Neurobiol Learn Mem. 2011;95:159–165. doi: 10.1016/j.nlm.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur J Neurosci. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Nokia MS, Penttonen M. Hippocampo-cerebellar theta band phase synchrony in rabbits. Neuroscience. 2010;165:1538–1545. doi: 10.1016/j.neuroscience.2009.11.044. [DOI] [PubMed] [Google Scholar]
- Winocur G, Binns MA, Tannock I. Donepezil reduces cognitive impairment associated with anti-cancer drugs in a mouse model. Neuropharmacology. 2011;61:1222–1228. doi: 10.1016/j.neuropharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Temozolomide treatment using a dose of 25 mg/kg did not cause weight loss but hindered normal weight gain.
Fig. S2. Recording electrodes were placed in the dentate gyrus.