Abstract
Human postmortem studies have reported decreases with age in high affinity nicotine binding in brain. We investigated the effect of age on β2-containing nicotinic acetylcholine receptor (β2-nAChR) availability in eight brain regions of living human subjects using the ligand [123I]5-IA-85380 ([123I]5-IA) and single photon emission computed tomography (SPECT). Healthy, nonsmokers (N=47) ranging in age from 18-85 were administered [123I]5-IA using a bolus plus constant infusion paradigm and imaged 6-8 h later under equilibrium conditions. The effect of age on regional β2-nAChR availability (VT, regional brain activity/free plasma parent, a measure proportional to the binding potential) was analyzed using linear regression and Pearson’s correlation (r). Age and regional β2-nAChR availability were inversely correlated in seven of the eight brain regions analyzed, with decline ranging from 32% (thalamus) to 18% (occipital cortex) over the adult lifespan, or up to 5% per decade. These results in living human subjects corroborate postmortem reports of decline in high affinity nicotine binding with age and may aid in elucidating the role of β2-nAChRs in cognitive aging.
Keywords: nicotinic receptors, aging, [123I]5-IA-85380, SPECT
1. Introduction
Neuronal nicotinic acetylcholine receptors (nAChR) subserve important neurophysiological processes including learning and memory [18,19,34] and are significantly reduced in the neurodegenerative process associated with various types of dementia [33]. Postmortem studies of patients with Alzheimer’s disease reveal substantial (25-70%) decreases in regional nAChR expression at different stages of the disease that correlate with decline in cognitive function (see [5] for a review). Human postmortem studies in normal aging, however, have been more variable (see [13] for a review), reporting decreases, increases, or no change with age in high affinity nicotine binding in brain depending on the brain region studied [4,6,7,12,13,21,25,26,27,32].
With the advent of functional brain imaging methodologies—specifically, Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT)—it has become possible to study the effects of aging and dementia on nAChRs in living subjects. Several studies have corroborated postmortem reports of reductions in nAChRs in Alzheimer’s disease using PET [28,29] or SPECT [30], which correlate with impaired cognitive function [16]. To our knowledge, no studies have yet investigated in vivo healthy aging effects on high affinity nAChRs.
Earlier attempts to image the nAChR were hampered by high nonspecific binding, rapid metabolism and washout, and high levels of radiotracer toxicity, thus prohibiting investigation in living human subjects. Iodine-123-5-IA-85380 ([123I]5-IA; [123I]-5-iodo-3-[2(S)-2-azetidinylmethoxy] pyridine) demonstrates many of the properties necessary for in vivo imaging of β2-nAChR with SPECT including high affinity (KD=11 pM), rapid entry into brain, low nonspecific binding, and minimal toxicity [9,10]. The pharmacological specificity of [123I]5-IA for β2-nAChRs has been confirmed by the lack of binding in brain of β2 (-/-) knockout mice [24]. In human brain the highest uptake of [123I]5-IA is in the thalamus, with moderate to low levels in the striatum, hippocampus, cerebellum and cortex [11]. [123I]5-IA has been shown to be suitable for quantification of β2-nAChRs in human brain using kinetic modeling [20]. We have also recently demonstrated the feasibility and reproducibility of equilibrium imaging using a bolus plus constant infusion paradigm with [123I]5-IA [35].
The aim of the current study was to use the equilibrium model to examine the effect of age on β2-nAChR availability in healthy, human non-smokers using [123I]5-IA and SPECT. In the course of the analysis, we investigated any possible age-related bias in the assumptions of stable brain and plasma activities required for the equilibrium model. Preliminary experience with this study was presented at a meeting entitled “Imaging and the Aging Brain,” sponsored by the New York Academy of Sciences May 16-17, 2006 [23].
2. Methods
2.1. Study population
The study population consisted of 47 healthy volunteers (20 male; 27 female) who were nonsmokers ranging in age from 18 to 85 years (mean ± SD = 41.1 ± 20.6). The ethnic distribution of the sample was as follows: 34 Caucasian, 7 African-American, 5 Hispanic, and 1 Asian. Subjects underwent physical and neurological examination by a research physician, electrocardiogram (ECG), routine laboratory sampling of blood and urine, and were screened by trained research assistants to exclude psychiatric illness, alcohol or substance abuse. Based upon responses to a smoking history questionnaire, 41 of 47 subjects were never-smokers (<100 cigarettes in their lifetime). Six subjects (aged 61, 64, 77, 82, 84, and 85 years) were former smokers but had not smoked for ≥30 years of participation. Nonsmoking status was confirmed in most subjects (n=37) by plasma cotinine levels <15 ng/mL, urine cotinine levels <100 ng/mL and carbon monoxide levels <11 ppm on the day of intake and day of scan. Female subjects of childbearing potential were required to have a negative pregnancy test at screening and on the day of the SPECT scan immediately prior to tracer injection.
All subjects who were ultimately enrolled in the study had no significant medical, neurological, or psychiatric illness. A subset of subjects ≥60 years of age (n=10) were required also to have no evidence of significant cognitive impairment, as indicated by Folstein Mini-Mental State Examination (MMSE) [8] score of >26, Clinical Dementia Rating Scale [14] score of zero, and a delayed recall score on a complex verbal memory task (NYU Paragraph) falling within established limits of normal performance in older adults based upon level of education [17]. No subjects were taking cholinesterase inhibitors, anticholinergics, antidepressants, antipsychotics, anticonvulsants, or anxiolytics. Subjects were excluded if they had a pacemaker or other ferrous material in the body that would preclude a magnetic resonance image (MRI) scan for purposes of co-registration. All subjects signed informed consent, as approved by the Yale University School of Medicine Human Investigation Committee, the Yale Radiation Safety Committee, and/or the Veterans Affairs Connecticut Healthcare System, West Haven campus Human Subjects Subcommittee, for relevant procedures conducted at each site.
2.2. [123I]5-IA SPECT imaging
Subjects were imaged with [123I]5-IA and SPECT using a bolus plus constant infusion paradigm as described previously [35]. In brief, subjects were pretreated with stable supersaturated potassium iodide (SSKI, 800 mg) and two antecubital venous catheters were placed prior to radiotracer injection. [123I]5-IA was synthesized as previously described [43] and administered by a bolus (4.2 ± 0.5 mCi) followed by continuous infusion (0.6 ± 0.1 mCi/h) at a constant rate using a computer-controlled pump (IMED pump, Gemini PC-1, San Diego CA USA) for a bolus to infusion ratio of 7.0 h for 8 h. Thus, the total administered dose was 9.5 ± 1.1 mCi. Vital signs including blood pressure, heart and respiration rates were measured between 30-60 min pre and post [123I]5-IA administration. Prior to scanning, five external fiducial markers containing 1-5 μCi of [123I] were placed on the head along the canthomeatal line. Using this paradigm [123I]5-IA reaches equilibrium binding in the brain by 6 to 8 h [35].
Three consecutive 30-min emission scans and one 15-min simultaneous transmission and emission protocol scan (STEP) were acquired between 6 and 8 h of infusion on a Picker PRISM 3000XP (n=37) or 3000XP2 (n=10), three-headed camera (Philips Medical System, The Netherlands) equipped with low energy, ultra-high resolution fanbeam collimators (photopeak window 159 keV ± 10%; matrix 128 × 128). The difference in sensitivity between the two cameras has been found to be <2.5% (0.1 mCi 123I source at the center of rotation of each camera). To control for day-to-day variation in camera sensitivity a 57Co-distributed source was measured with each experiment. The axial resolution (full width at half maximum) is 12.2 mm, measured with a 123I line source in water in a cylindrical phantom. Plasma samples were collected immediately before and after the emission scans to quantify total parent tracer concentration and the free fraction (not protein bound) of parent tracer in plasma (f1) [43].
To guide the placement of brain regions on the [123I]5-IA SPECT scans, sagittal MR images were obtained on a separate day on a 1.5 Tesla GE Signa camera with a spoiled GRASS (gradient recall acquisition in the steady state) sequences with TE=5 ms, TR=24 ms, NEX=1, matrix = 256×192, field of view = 34 cm.
2.3. Image analyses
SPECT emission images were filtered using a three-dimensional Butterworth filter (order = 10, cutoff frequency = 0.24 cycles/pixel) and reconstructed using a filtered back-projection algorithm with a ramp filter on a 128 × 128 matrix to obtain 50 slices with a pixel size of 2.06 × 2.06 × 3.56 mm in the x- y- and z- axes. A non-uniform attenuation correction was performed using the attenuation map of the head derived from the transmission scan in a modified Chang’s algorithm [2]. The MRI was co-registered to the transmission and emission images using the co-registration function in SPM2. The MRI was reoriented to the inter-commissural plane and the reorientation matrix was applied to each emission image. The co-registered and reoriented MRI was used to guide the placement of standardized two-dimensional ROI templates (Figure 1) on the right and left hemispheres [35]. No attempt was made to correct for scatter or partial volume effects. The mean of two raters (KPC and EMM) who conducted the image analysis is reported. Both raters were blinded to subject identity.
Figure 1.
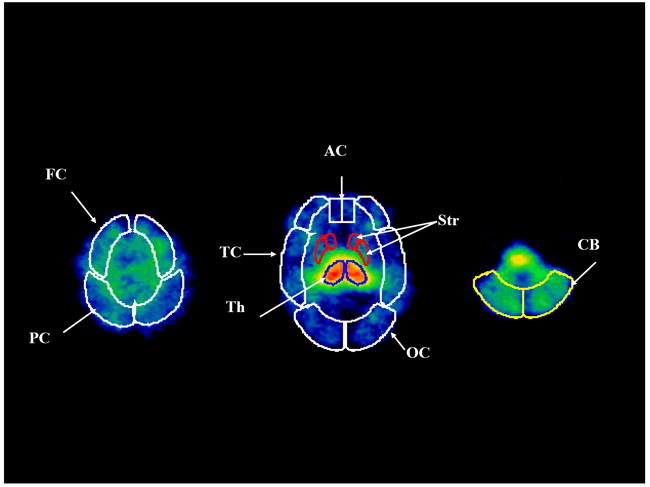
Two-dimensional standardized regions of interest are placed on three representative transverse images of [123I]5-IA uptake. FC = frontal cortex, PC = parietal cortex, TC = temporal cortex, AC = anterior cingulate, OC = occipital cortex, Th = thalamus, Str = striatum, CB = cerebellum.
2.4. Statistical analyses
The equilibrium model was applied to the present healthy subject sample to study the effects of age on regional β2-nAChR availability. We have previously validated two equilibrium outcome measures for [123I]5-IA: VT is the tissue equilibrium volume of distribution equal to the sum of V2 (nondisplaceable) and V3 (receptor bound) [35] and is calculated as the ratio of regional brain concentration to free parent plasma concentration of radiotracer (total plasma parent concentration * f1). VT’ is a similar distribution volume calculated as the ratio of regional brain concentration to total parent plasma concentration. In the equilibrium model the venous concentration of tracer is assumed to approximate the arterial concentration at late time points and is taken as a measure of plasma tracer concentration [41], an assumption that has been verified by other investigators for [123I]5IA following 2 h post injection [20]. Although VT compared to VT’ has demonstrated somewhat lower test-retest reliability, owing to the poor reliability of f1 [35], it possesses the advantage of not assuming inter-subject uniformity of f1 (See Discussion for additional analysis of these outcome measures). Given our recent observation of a significantly higher f1 in women versus men [3], and the different age distributions of males vs females in our subject sample, we opted to analyze VT as the primary outcome measure in this study. However, we also performed a secondary exploratory analysis of VT’.
The [123I]5-IA equilibrium analysis relies on several assumptions [35], most importantly, the stability over time of regional brain activity and plasma parent tracer concentration between 6 and 8 h when steady state is apparent. The present study therefore included a revalidation of those assumptions in a subject sample that represented the full adult lifespan. To examine the stability of these parameters between 6 and 8 h, a linear regression slope (rate of change per h for brain and plasma parent activity, calculated by dividing the slope by the mean activity from 6 to 8 h [40,41] was computed for each parameter in all 47 subjects. To detect any systematic age-related bias in equilibrium assumptions, the relationship between the regression slopes and age was examined for each parameter by Pearson’s product moment correlation coefficient (r).
The effect of age on regional β2-nAChR availability (VT, average values between 6 and 8 h) in eight brain regions (thalamus, frontal cortex, temporal cortex, parietal cortex, occipital cortex, anterior cingulate, striatum, cerebellum) was then analyzed using linear regression and Pearson’s r. In a secondary exploratory analysis, the effect of age on regional VT’ was analyzed using partial correlations, controlling for the contribution of sex.
3. Results
3.1. Effect of age on equilibrium assumptions
In the present sample of healthy nonsmokers, subject age was unrelated to the rate of change (%/h) at equilibrium (6-8 h post-injection of [123I]5-IA) in plasma parent activity or brain activity in any of the eight regions studied. The relationship between the regression slopes for regional tracer uptake in thalamus (as a representative region) and for plasma parent activity vs subject age is displayed in Figure 2. The rates of change (%/h) in both parameters were unrelated to age, suggesting that any potential age-related differences in tracer delivery or clearance does not alter the previously described equilibrium assumptions [35], which remain valid across the adult lifespan.
Figure 2.
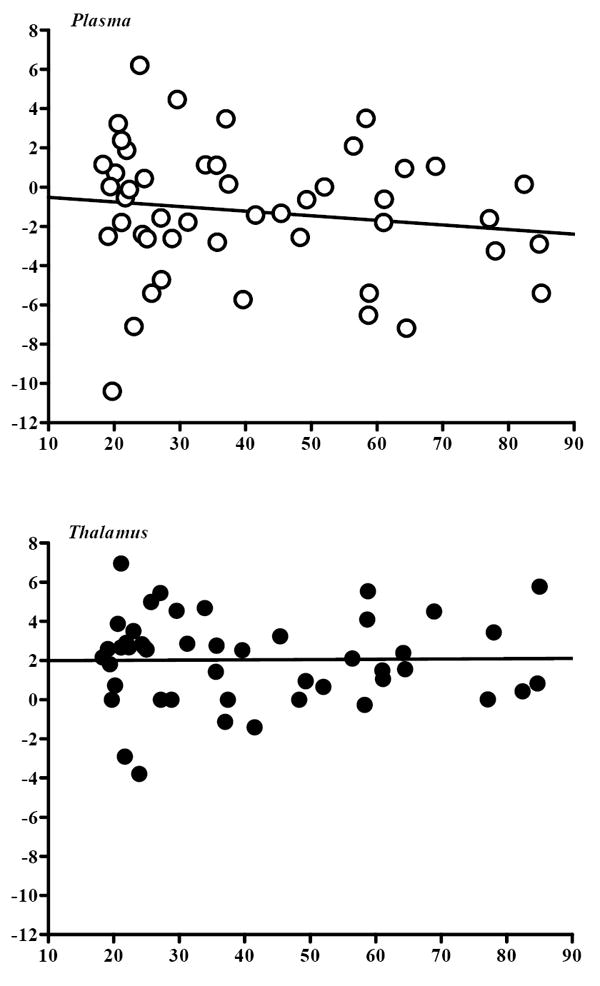
Rate of change (%/h) at equilibrium (6-8 h post-injection of [123I]5-IA) in plasma parent activity and thalamic activity, expressed as a function of subject age. Each dot denotes an individual subject (n=47). Values are obtained by linear regression and expressed as the percentage of mean regional uptake between 6 and 8 h post injection. The rate of change in each parameter was unrelated to subject age (plasma: r=.16, P=.29; thalamus: r=.04, P=.79).
3.2. Effect of age on plasma measures
Total plasma parent concentration increased significantly with age (r=.37, P=.010), suggesting that [123I]5-IA metabolism is altered with advancing age and underscoring the appropriateness of the present outcome measures (VT and VT’) that account for individual differences in tracer metabolism. There was no significant relationship between age and either f1 (r=−.13, P=.39) or the free parent concentration (r=.29, P=.086), supporting the absence of an age-bias in the assumptions underlying the secondary outcome measure VT’. As in our recently reported sample of young adults (<45 years) [3], in this sample representing the adult lifespan women had significantly greater f1 (t=-2.34, d.f.=45, P=.023), total parent (t=-2.44, d.f.=45, P=.018) and plasma free parent (t=-2.72, d.f.=45, P=.009) than men. These sex differences again highlighted the necessity of controlling for the contribution of sex in the secondary aging analysis of VT’.
3.3. Primary analysis: Effect of age on regional β2-nAChR availability (VT)
Regional β2-nAChR availability (VT) showed a significant inverse correlation with age in seven of the eight brain regions analyzed (Figure 3; Table 1). Pearson’s product moment values ranged from r=−.28 to r=−.53 in these regions, with P-values ranging from .042 to <.001 (Table 1). Linear regression analysis revealed that VT declined across the age range 18 to 85 years and was greatest in thalamus (32.0%), followed by frontal (25.7%), parietal (25.4%), and anterior cingulate (23.6%) cortices. The decreases in regional VT ranged from 2.4% to 4.8% per decade of life. The addition of sex did not significantly improve the prediction of VT in any brain region after controlling for the contribution of age (data not shown, but males and females denoted separately in Figure 3). If a Bonferroni correction were applied to these data for eight correlations (α =.05/ 8=.00625), then four of the eight regions (thalamus and frontal, parietal, and anterior cingulate cortices) would still retain significance.
Figure 3.
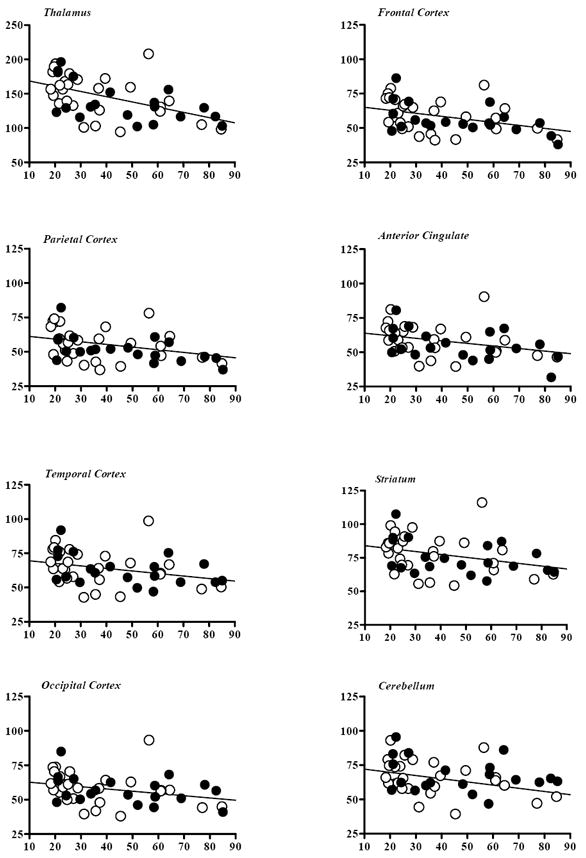
Regional β2-nAChR availability (VT) versus age in eight brain regions, as measured by [123I]5-IA and SPECT in 47 healthy subjects. Closed circles represent males and open circles females. Statistically significant decline with age was observed in all regions except cerebellum, as detailed in Table 1.
Table 1.
Effect of age on β2-nAChR availability (VT) in selected brain regions.
| Brain Region | %Declinea | %Decline/Decadeb | r | P |
|---|---|---|---|---|
| Thalamus | 32.0% | 4.8% | -.53 | <.001 |
| Frontal Cortex | 25.7% | 3.8% | -.44 | .002 |
| Parietal Cortex | 25.4% | 3.8% | -.42 | .003 |
| Anterior Cingulate | 23.6% | 3.5% | -.39 | .006 |
| Temporal Cortex | 19.1% | 2.8% | -.33 | .025 |
| Striatum | 18.3% | 2.7% | -.33 | .023 |
| Occipital Cortex | 17.6% | 2.6% | -.30 | .042 |
| Cerebellum | 16.0% | 2.4% | -.28 | .058 |
%Decline was calculated from the linear regression equation as the difference between the values at age 18 and 85 divided by the value at age 18.
%Decline/Decade was calculated as %Change divided by 6.7 decades.
3.4. Secondary analysis: Effect of age on regional β2-nAChR availability (VT’)
Regional β2-nAChR availability (VT’) showed a significant inverse correlation with age in all eight brain regions analyzed (Table 2). Partial correlations controlling for the contribution of sex were significant for each region and ranged from r=−.35 to r=−.60 with P-values ranging from .016 to <.001. Linear regression revealed that the decline in VT’ was again greatest in thalamus (36.7%), followed by frontal (29.6%), parietal (29.2%), and anterior cingulate (28.0%) cortices. The decreases in regional VT’ ranged from 3.1 % to 5.5 % per decade of life. If a Bonferroni correction were applied to these data for eight correlations, then all regions except cerebellum would still retain significance.
Table 2.
Effect of age on β2-nAChR availability (VT’) in selected brain regions.
| Brain Region | %Declinea | %Decline/Decadeb | rc | P |
|---|---|---|---|---|
| Thalamus | 36.7% | 5.5% | -.60 | < .001 |
| Frontal Cortex | 29.6% | 4.4% | -.55 | < .001 |
| Parietal Cortex | 29.2% | 4.3% | -.53 | <.001 |
| Anterior Cingulate | 28.0% | 4.2% | -.48 | .001 |
| Temporal Cortex | 23.7% | 3.5% | -.43 | .003 |
| Striatum | 23.6% | 3.5% | -.44 | .002 |
| Occipital Cortex | 22.7% | 3.4% | -.40 | .005 |
| Cerebellum | 20.5% | 3.1% | -.35 | .016 |
%Decline was calculated from the linear regression equation as the difference between the values at age 18 and 85 divided by the value at age 18.
%Decline/Decade was calculated as %Decline divided by 6.7 decades.
r-values represent partial correlations controlling for the contribution of sex.
4. Discussion
These data demonstrate a significant age-dependent decline in central nicotinic receptor availability as measured in vivo by [123I]5-IA and SPECT. Age and regional β2-nAChR availability (VT) were found to be inversely correlated in seven of the eight brain regions analyzed. Over the age range studied (18 to 85 years) the outcome measure VT declined by up to 32%, or by up to 5% per decade. Thalamus, with the highest density of β2-nAChRs, had the greatest reductions, followed by lower-density cortical regions.
4.1. Comparison to postmortem studies
Although significant reductions in high affinity nicotine binding have been found using PET in pathological states (e.g., Alzheimer’s disease; see [5] for a review), no previous in vivo studies to our knowledge have examined healthy aging effects on nAChRs. Human postmortem studies of aging (see [13] for a review) have generally shown a loss of high affinity nicotine binding, although the regional pattern has been quite variable. An age-related decline in high affinity binding in frontal cortex has been observed in most [4,7,13,21,27] but not all [12] studies. Studies of temporal cortex [21], entorhinal cortex [6,13], and hippocampus [6,13,25,26,32] have all shown aging effects. Among subcortical regions, no age-related changes in nAChRs have been observed in thalamus [12,26,27] or cerebellum [21], and only after the seventh decade in the striatum [13]. Studies of mRNA expression of nAChR subunits in human brain have shown regional differences in age-related reductions in α4 and β2 subunit mRNA expression in frontal cortex, hippocampus and putamen [38,39]. Whereas α4 and β2 subunit mRNA expression have been found to decrease in cerebral cortex during aging, β2 subunit expression decreases but α4 expression is unaltered in hippocampus [38].
The major divergence between our results and those of the postmortem literature lies in the thalamus. In contrast to in vitro binding studies using the ligands [3H]acetylcholine, [3H]tubocurarine, or [3H]nicotine that have reported no loss [12,26] or an increase [27] with age in nAChRs in this region, we observed a loss of nAChRs in thalamus that was greater in magnitude (32%) and significance (r=-.53, P<.001) than in the five cortical regions investigated. The three postmortem studies of thalamus are limited by potential biases, including those introduced by subject selection, cause of death, and postmortem interval. In their initial study with [3H]tubocurarine Nordberg and Winblad [26] studied only 9 subjects from age 67-87. The subsequent studies from this group [12,27] with [3H]nicotine examined larger samples and broader age ranges ([3H]nicotine N=23, 7-83; and N=20, 32-80; [3H]acetylcholine N=18, 7-83). However, they may still be limited by variable postmortem interval (3-67 h) [27] and inclusion of hanging suicide and drowning victims [12]. Most importantly, none of these studies excluded or controlled for cigarette smoking, which is known to increase the density of nicotine receptor binding [1,31,36] and may introduce age-biases.
Although our study possesses advantages of sample size and more controlled subject selection (including exclusion of tobacco smokers), we must acknowledge other limitations characteristic of neuroimaging investigations. Photon scatter effects are unlikely to confound our results in thalamus, since other brain regions (with positive aging effects) are of much lower activity than thalamus (However, we cannot exclude the possibility of photon scatter from the high-activity thalamus to the lower-activity striatal and cortical ROIs). Attenuation correction artifacts are also improbable, since we employed measured attenuation correction. However, partial volume effects are likely to compromise our results, since thalamic volumes have been reported to decline significantly with age [15,37,42]. Future neuroimaging studies that employ formal partial volume correction [22] will be necessary to evaluate the contribution of atrophy to aging effects on high affinity nicotinic binding in thalamus as well as other brain regions.
Due to limitations in spatial resolution with SPECT, we did not attempt to measure [123I]5-IA availability in medial temporal lobe structures, including the hippocampal formation and entorhinal cortex. Postmortem studies have consistently revealed aging effects in entorhinal cortex [6,13], hippocampus [6,13,25,26,32], and even hippocampal subregions [6]. Although in vivo studies clearly lack the anatomic resolution to replicate several of these findings, MR-guided region delineation in coronal plane may furnish useful data for larger medial temporal lobe structures.
4.2. Choice of Outcome Measure
The two equilibrium outcome measures that we previously validated for [123I]5-IA are VT (ratio of total brain uptake to free parent plasma concentration of radiotracer) and VT’ (ratio of total brain uptake to total parent plasma concentration) [35]. Both are proportional to the binding potential (BP = Bmax/KD), under the assumption that nondisplaceable uptake (V2) is uniform across individuals. In addition, VT’ assumes the inter-individual uniformity of plasma protein binding (f1). VT’ compared to VT has demonstrated better test-retest variability (mean, 7.0%-8.9% vs 12.9%-14.6%) and reliability assessed by the intraclass correlation coefficient (ICC = .30-.64 vs .28-.60) due to the poor reliability of the free fraction f1 (ICC = .35) [35]. Given our recent observation of a significantly higher free fraction f1 in women vs men [3], and the somewhat different age distributions of males vs females in our subject sample, we opted to analyze VT as the primary outcome measure in this study. Nonetheless, when we conducted a secondary exploratory analysis of aging effects on VT’ (while controlling for sex), we observed very similar results. The more statistically robust effects of age on VT’ are likely attributable to the greater reliability of the measurement of VT’ that we have previously described [35]. The fact that we observed f1 to be unrelated to age in the present study may support the use of VT’ as an alternative outcome measure for aging studies of [123I]5-IA, provided that sex effects are controlled for in the analysis.
In conclusion, this is the first in vivo investigation of healthy aging effects on high affinity nicotinic binding and demonstrates an age-related decline in β2-nAChR in both cortical and subcortical regions. Given the well-documented importance of nicotinic mechanisms for learning and memory [18,19], the present findings may aid in elucidating the role of nAChRs in the cognitive decline associated with normal aging.
Acknowledgments
The authors wish to thank Louis Amici, Jane Bartosik, Gina Morano, Andrea Perez, and Stacey Ross for their excellent technical assistance. This research was supported in part by the Office of Academic Affiliations, VA Special Mental Illness Research Education and Clinical Centers Fellowship Program in Advanced Psychiatry and Psychology, Department of Veteran Affairs (EMM), American Health Assistance Foundation A2004-216 (CHvD), R01DA015577 (JKS) and Transdisciplinary Tobacco Research Center P50AA15632 (JKS).
Footnotes
Disclosure Statement None of the authors has an actual or potential conflict of interest with this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benwell MEM, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding site in human brain. J Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 2.Chang L. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;NS-25:638–543. [Google Scholar]
- 3.Cosgrove KP, Mitsis EM, Frohlich E, Bois F, Tamagnan GD, Krantzler E, Perry E, Maciejewski PK, Epperson CN, Allen S, O’Malley S, Mazure CM, Seibyl JP, van Dyck CH, Staley JK. [123I]5-IA-85380 SPECT imaging of nicotinic acetylcholine receptor availability in nonsmokers: effects of sex and menstrual cycle phase. J Nucl Med. 2007;48(10):1633–1640. doi: 10.2967/jnumed.107.042317. [DOI] [PubMed] [Google Scholar]
- 4.Court J, Piggott M, Perry E, Barlow R, Perry R. Age-associated decline in high affinity nicotine binding in human brain frontal cortex does not correlate with the changes in choline acetyltransferase activity. Neurosci Res Comm. 1992;10:125–133. [Google Scholar]
- 5.Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer’s disease. Biol Psychiatry. 2001;49(3):175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- 6.Court JA, Lloyd S, Johnson M, Griffiths M, Birdsall NJ, Piggott MA, Oakley AE, Ince PG, Perry EK, Perry RH. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Develop Brain Res. 1997;101(1-2):93–105. doi: 10.1016/s0165-3806(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 7.Flynn DD, Mash DC. Characterization of L-[3H]nicotine binding in human cerebral cortex: comparison between Alzheimer’s disease and the normal. J Neurochem. 1986;47(6):1948–1954. doi: 10.1111/j.1471-4159.1986.tb13113.x. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Fostein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Fujita M, Tamagnan G, Zoghbi SS, Al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB. Measurement of a4b2 nicotinic acetylcholine receptors with [123I]5-I-A-85830 SPECT. J Nucl Med. 2000;41(9):1552–1560. [PubMed] [Google Scholar]
- 10.Fujita M, Seibyl JP, Vaupel DB, Tamagnan G, Early M, Zoghbi SS, Baldwin RM, Horti AG, Koren AO, Mukhin AG, Khan S, Bozkurt A, Kimes AS, London ED, Innis RB. Whole-body biodistribution, radiation absorbed dose, and brain SPET imaging with [123I]5-I-A-85380 in healthy human subjects. Eur J Nucl Med Mol Imaging. 2002;29(2):183–190. doi: 10.1007/s00259-001-0695-z. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M, Ichise M, van Dyck CH, Zoghbi SS, Tamagnan G, Mukhin AG, Bozkurt A, Seneca N, Tipre D, DeNucci C, Iida H, Vaupel DB, Horti AG, Koren AO, Kimes AS, London ED, Seibyl JP, Baldwin RM, Innis RB. Quantification of nicotinic acetylcholine receptors in human brain using [123I]5-I-A-85380 SPET. Eur J Nucl Med Mol Imaging. 2003;30(12):1620–1629. doi: 10.1007/s00259-003-1320-0. [DOI] [PubMed] [Google Scholar]
- 12.Hellström-Lindahl E, Winblad B, Nordberg A. Muscarinic and nicotinic receptor changes in the cortex and thalamus of brains of chronic alcoholics. Brain Res. 1993;620(1):42–48. doi: 10.1016/0006-8993(93)90268-r. [DOI] [PubMed] [Google Scholar]
- 13.Hellström-Lindahl E, Court JA. Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behav Brain Res. 2000;113(1-2):159–168. doi: 10.1016/s0166-4328(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 15.Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991;29(1):55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- 16.Kadir A, Almkvist O, Wall A, Langstrom B, Nordberg A. PET imaging of cortical 11C-nicotine binding correlates with the cognitive function of attention in Alzheimer’s disease. Psychopharmacology (Berl) 2006;188(4):509–520. doi: 10.1007/s00213-006-0447-7. [DOI] [PubMed] [Google Scholar]
- 17.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatric Psychiatry Neurol. 1999;12(4):168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 18.Levin E. Nicotinic systems and cognitive function. Psychopharmacology. 1992;108(4):417–431. doi: 10.1007/BF02247415. [DOI] [PubMed] [Google Scholar]
- 19.Levin E. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53(4):633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 20.Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Fukuyama H, Saga T, Saji H. Quantification of human nicotinic acetylcholine receptors with 123I-5IA SPECT. J Nucl Med. 2004;45(9):1458–1470. [PubMed] [Google Scholar]
- 21.Marutle A, Warpman U, Bogdanovic N, Nordberg A. Regional distribution of subtypes of nicotinic receptors in human brain and effect of aging studied by (+/-)-[3H]epibatidine. Brain Res. 1998;801(1-2):143–149. doi: 10.1016/s0006-8993(98)00558-7. [DOI] [PubMed] [Google Scholar]
- 22.Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, Lin MP, Price JC. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40(12):2053–2065. [PubMed] [Google Scholar]
- 23.Mitsis EM, Cosgrove KP, Staley JK, Frohlich E, Bois F, Tamagnan GD, Estok K, Seibyl JP, van Dyck CH. [123I]5-IA-85380 SPECT imaging of β2-nicotinic acetylcholine receptor availability in the aging human brain. Ann NY Acad Sci. 2007;1097:168–170. doi: 10.1196/annals.1379.015. [DOI] [PubMed] [Google Scholar]
- 24.Muhkin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-Iodo-A-85830, an α4β2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57(3):642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- 25.Nordberg A, Adolfsson R, Marcusson K, Winblad B. Cholinergic receptors in the hippocampus in normal aging and dementia of Alzheimer type. In: Giacobini E, Filogam G, Giacobini F, Vernadahis A, editors. The Aging Brain: Cellular and Molecular Mechanisms of Aging in the Nervous System Aging. Vol. 20. New York: Raven Press; 1982. pp. 231–245. [Google Scholar]
- 26.Nordberg A, Winblad B. Brain nicotinic and muscarinic receptors in normal aging and dementia. In: Fisher A, Hanin I, Lachman C, editors. Alzheimer’s and Parkinson’s Disease: Strategies for Research and Development. New York: Plenum; 1986. pp. 95–108. [Google Scholar]
- 27.Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J Neurosci Res. 1992;31(1):103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- 28.Nordberg A, Lundqvist H, Hartvig P, Lilja A, Langstrom B. Kinetic analysis of regional (S)(-)11C-nicotine binding in normal and Alzheimer brains--in vivo assessment using positron emission tomography. Alzheimer Dis Assoc Disord. 1995;9(1):21–27. doi: 10.1097/00002093-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Nordberg A, Lundqvist H, Hartvig P, Andersson J, Johansson M, Hellstrom-Lindahl E, Langstrom B. Imaging of nicotinic and muscarinic receptors in Alzheimer’s disease: Effect of tacrine treatment. Dement Geriatr Cogn Disord. 1997;8(2):78–84. doi: 10.1159/000106611. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien JT, Colloby SJ, Pakrasi S, Perry EK, Pimlott SL, Wyper DJ, McKeith IG, Williams ED. Alpha4 beta2 nicotinic receptor status in Alzheimer’s disease using 123I-5IA-85380 SPECT. J Neurol Neurosurg Psychiatry. 2007;78(4):356–362. doi: 10.1136/jnnp.2006.108209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: Membrane binding and autoradiography studies. J Pharmacol Exp Therap. 1999;289(3):1545–1552. [PubMed] [Google Scholar]
- 32.Perry EK, Perry RH, Smith CJ, Purohit D, Bonham J, Dick DJ, Candy JM, Edwardson JA, Fairbairn A. Cholinergic receptors in cognitive disorders. Can J Neurol Sci. 1986;13(4 Suppl):521–527. doi: 10.1017/s0317167100037240. [DOI] [PubMed] [Google Scholar]
- 33.Perry EK, Morris CM, Court JA, Cheng A, Fairbairn AF, McKeith IG, Irving D, Brown A, Perry RH. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: possible index of early neuropathology. Neuroscience. 1995;64(2):385–395. doi: 10.1016/0306-4522(94)00410-7. [DOI] [PubMed] [Google Scholar]
- 34.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49(3):258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 35.Staley JK, van Dyck CH, Weinzimmer D, Brenner E, Baldwin RM, Tamagnan GD, Riccardi P, Mitsis EM, Seibyl JP. 123I-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: feasibility and reproducibility. J Nucl Med. 2005;46(9):1466–1472. [PubMed] [Google Scholar]
- 36.Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26(34):8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan EV, Rosenbloom M, Serventi K, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25(2):185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 38.Tohgi H, Utsugisawa K, Yoshimura M, Nagane Y, Mihara M. Age-related changes in nicotinic acetylcholine receptor subunits alpha4 and beta2 messenger RNA expression in postmortem human frontal cortex and hippocampus. Neuroscience Lett. 1998;245(3):139–142. doi: 10.1016/s0304-3940(98)00205-5. [DOI] [PubMed] [Google Scholar]
- 39.Tohgi H, Utsugisawa K, Yoshimura M, Nagane Y, Mihara M. Alterations with aging and ischemia in nicotinic acetylcholine receptor subunits alpha4 and beta2 messenger RNA expression in postmortem human putamen. Implications for susceptibility to parkinsonism. Brain Res. 1998;791(1-2):186–190. doi: 10.1016/s0006-8993(98)00093-6. [DOI] [PubMed] [Google Scholar]
- 40.van Dyck CH, Soares JC, Tan PZ, Staley JK, Baldwin RM, Amici LA, Fu X, Garg PK, Seibyl JP, Charney DS, Innis RB. Equilibrium modeling of 5-HT(2A) receptors with [18F]deuteroaltanserin and PET: feasibility of a constant infusion paradigm. Nucl Med Biol. 2000;27(8):715–722. doi: 10.1016/s0969-8051(00)00160-8. [DOI] [PubMed] [Google Scholar]
- 41.van Dyck CH, Tan PZ, Baldwin RM, A LA, Garg PK, Ng CK, Soufer R, Charney DS, Innis RB. PET quantification of 5-HT2A receptors in the human brain: a constant infusion paradigm with [18F]altanserin. J Nucl Med. 2000;41(2):234–241. [PubMed] [Google Scholar]
- 42.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Zoghbi S, Tamagnan G, Baldwin RM, Tikriti MA, Amici L, Seibyl J, Innis R. Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in nonhuman primates. Nucl Med Biol. 2001;28(1):91–96. doi: 10.1016/S0969-8051(00)00188-8. [DOI] [PubMed] [Google Scholar]


