Abstract
Estrogens exert a wide variety of actions on reproductive and non-reproductive functions. These effects are mediated by slow and long lasting genomic as well as rapid and transient non-genomic mechanisms. Besides the host of studies demonstrating the role of genomic actions at the physiological and behavioral level, mounting evidence highlights the functional significance of non-genomic effects. However, the source of the rapid changes in estrogen availability that are necessary to sustain their fast actions is rarely questioned. For example, the rise of plasma estrogens at pro-estrus that represents one of the fastest documented changes in plasma estrogen concentration appears too slow to explain these actions. Alternatively, estrogen can be synthesized in the brain by the enzyme aromatase providing a source of locally high concentrations of the steroid. Furthermore, recent studies demonstrate that brain aromatase can be rapidly modulated by afferent inputs, including glutamatergic afferents. A role for rapid changes in estrogen production in the central nervous system is supported by experiments showing that acute aromatase inhibition affects nociception as well as male sexual behavior and that preoptic aromatase activity is rapidly (within min) modulated following mating. Such mechanisms thus fulfill the gap existing between the fast actions of estrogen and their mode of production and open new avenues for the understanding of estrogenic effects on the brain.
Keywords: aromatase, male sexual behavior, estrogen synthesis, preoptic area, copulatory behavior
1. Introduction
Gonadal steroids produce a wide range of cellular effects that are largely, but not exclusively, mediated through intracellular receptors. These steroid hormone receptors are members of a large family of ligand-activated transcription factors that bind response elements located on the DNA and regulate the transcription of genes encoding a wide variety of proteins. These proteins include synthesis enzymes (such as tyrosine hydroxylase), transporters, receptors, signal transduction proteins (phosphatases, kinases, accessory proteins, etc) or degradation enzymes (such as monoamine oxidases), which ultimately modify neurotransmission [152,163]. These effects are relatively slow and develop with latencies ranging from one hour to several days. The role of genomic actions of steroids in adults has been extensively documented based on studies of physiology and behavior. Steroids exert profound organizational effects on the developing brain and the increase of secretion of sex steroid hormones at puberty and their maintenance throughout the reproductive life are responsible for the activation and maintenance of secondary sex characters and behaviors such as courtship, reproductive behavior, aggressive behaviors, etc [34]. Seasonal fluctuations and/or variations across the estrus cycle of circulating levels of estrogens positively correlate with variation in these reproductive responses [13,198,282]. A variety of effects of estrogens and other steroids on responses unrelated to reproduction have also been identified. For example, estrogens are known to influence cognitive functions, pain mechanisms, fine motor skills, mood, temperature regulation and sleep [163,272].
In addition, a host of studies have also identified effects of steroids that are too rapid (seconds to minutes) to be mediated through the activation of DNA transcription and protein synthesis [132,163,176]. This is particularly the case for 17β-estradiol (E2) which can rapidly (within a few seconds to several minutes, see below) activate a wide variety of intracellular signaling pathways including modulations of intracellular calcium concentrations [38,165] and protein phosphorylations [176,181,269,270,276]. These actions seem to be initiated primarily at the cellular membrane and lead to modulations of electrical activity [132,176] and neuronal activation [2,3,102,269,288] in various brain regions (for review see [152,163].
Although there is no longer any doubt about the existence of non-genomic effects, many uncertainties remain. One of the most debated questions is whether these effects are mediated by interactions of estrogens with the “classical” nuclear estrogen receptors (ERs) located in the cell nucleus or associated with the neuronal membrane and whether these effects are elicited by physiologically relevant concentrations of estrogens. In addition, the nature of the mechanism(s) able to modulate rapidly the bioavailability of estrogen (that is necessary to explain the existence of these fast effects) has rarely been questioned or even discussed, even though this represents a fundamental aspect of the rapid effects of steroids. Furthermore, while there are a plethora of data characterizing rapid, presumably non-genomic effects of estrogens based on cellular measures of action, there is still little information concerning the significance of these effects at the organismal or behavioral level of analysis. This review will try to address these last two questions. Firstly, we will examine the potential sources of estrogen for the brain that are rapidly modulated and, secondly, we will summarize what is known about functional significance of fast effects of estrogen for physiology and behavior. In addition, the question of the physiological relevance of doses that can activate the rapid effects of estrogens will also be discussed.
Physiological relevance of the fast cellular effects of steroids
Rather surprisingly, while the existence of non-genomic effects of steroids is not subject to doubt anymore, our knowledge of their implications in the regulation of physiological and behavioral processes remains quite limited. Perhaps the most progress has been made for the C21 steroids corticosterone and progesterone. In the case of corticosterone, rapid effects have been identified on various measures of sexual behavior [174] and stress [187] in amphibians and on the production of vocalizations in batrachoidid fishes ([207,208], Remage-Healey and Bass, 2006, cited in [206]). Furthermore, there is good evidence for a highly specific membrane-binding site of corticosteroids [81,82,188]. Several lines of evidence also indicate rapid, membrane-mediated effects of progesterone on sexual behavior in the ventral tegmental area of Syrian hamsters [71,95,96,196] and this steroid or its metabolites can clearly bind to membrane receptors such as the GABAA complex [156], the NMDA receptor [123,124] or the membrane progestin receptor recently identified in one piscine and several other vertebrate species [290,291]. One study reported rapid effects of testosterone on striated penile muscles related to the expression of male sexual behavior [218]. Remage-Healey and colleagues also recently demonstrated that 11-keto-testosterone (11kT, the main androgen in fishes) rapidly increases the duration of fictive vocalizations stimulated by electrical stimuli in an in vitro preparation ([208], Remage-Healey and Bass, 2006, cited in [206]). Finally, Clifton and Andrew (1981, [61]) also suggested the existence of rapid effects of testosterone on aggressive behavior in chicks presented with foreign objects. However, these effects appeared 4 hours after the injection of testosterone in animals that received an injection the previous day, so it cannot be ruled out that this effect results from the activation of genomic mechanisms.
A few rapid effects of estrogens have also been reported based on behavioral and/or physiological measurements. In rats, there is evidence that estrogens potentiate, via non-genomic mechanisms, their genomic action on lordosis behavior. For example, Kow and Pfaff (2004, [138]) showed that a pulse of E2 as short as 15 min given in the hypothalamic ventromedial nucleus (VMN) potentiates the effects of a second pulse of 1h in inducing high receptivity in ovariectomized female rats. A similar effect is obtained if purified E2 conjugated with bovine serum albumin (a large protein that prevents estrogens from crossing the plasma membrane) is used to replace E2 in the short pulse suggesting that its effect is mediated through a non-genomic mechanism. This conclusion is further supported by the fact that the effect of the short E2 pulse can be mimicked by the activation of protein kinase A or C as previously demonstrated in vitro [266]. These data thus suggest that non-genomic and genomic actions of estrogens interact to activate lordosis in a synergistic manner.
Behaviors such as tonic immobility, dorsal immobility and amphetamine-elicited rotational behavior are differentially expressed across the estrus cycle [35,36,167,244,264,265]. The striatum has been implicated in the control of these behaviors and accordingly estrogens implanted in the striatum affect their expression within 4 hours both in males and females [264,265]. Given the absence of nuclear estrogen receptors in the striatum, these effects are thought to be mediated by non-genomic mechanisms. This is consistent with the observation that E2 modulates apomorphine-induced postural deviation and amphetamine-induced rotational behavior with short latencies in the order of one hour in ovariectomized females [33,126]. In addition, estrogens rapidly affect intracellular calcium concentrations in striatal neurons in vitro through a membrane-initiated non-genomic mechanism [165]. Thus, estrogen in the striatum appears to potentiate, acutely and probably non-genomically, dopaminergic activity that would in turn enhance sensorimotor processing. This cellular effect may be part of the mechanism controlling paced mating behavior in female rats [285]. Other rapid effects of steroids on measures of cell function have been described in the hypothalamus. For example, a single injection of E2 to ovariectomized females results in CREB phosphorylation within 15 min in the medial preoptic nucleus, the medial septum and the VMN of mice [3] and within 15–30 min the preoptic area, the bed nucleus of the stria terminalis and the anteroventral periventricular nucleus in rats [102,288]. A physiological role for these cellular effects at the level of the organismal has not been reported yet, but they are likely involved in the hormonal feedback on neural systems that regulate gonadotropin secretion.
Non-genomic effects of estrogens are not limited to females. Unlike females, males of course do not exhibit a cyclical “rapid” rise of circulating concentrations of estrogens. However, they can display substantial concentrations of estrogens originating either directly from the testis or produced locally in the brain by aromatization of testosterone (Table 1; [13,106,118]). Although the existence of rapid variations in the availability of estrogen in the brain is rarely discussed (see below), fast effects of estrogens on male behaviors have been reported. For instance, Cross and Roselli (1999; [69]) showed that a subcutaneous injection of E2, but not testosterone, stimulates the expression of male sexual behavior in castrated rats within 35 min. In Japanese quail, an intraperitoneal injection of a bolus of 17β-estradiol facilitates the expression of male sexual behavior. This effect occurs within 15 min and vanishes after 30 min. In order to prime the mechanisms involved in sexual behavior, the animals used in this experiment were castrated males that were chronically treated with a sub-threshold dose of testosterone that is unable by itself to elicit sexual activity in most birds. The rapid behavioral activation observed in this experiment [64] thus potentially illustrates in males the synergistic interaction between rapid and slower genomic actions of estradiol as also demonstrated by recent work on female rats (see work of Kow and Pfaff and Vasudevan et al. described above; [138,266]). In plain midshipman fish, an intramuscular injection of E2 increases within 5 min the duration of fictive vocalizations stimulated by electrical stimuli in an in vitro preparation [208]. This effect persists for 15 to 30 min. Interestingly, 17α-estradiol or testosterone do not produce similar effects. Hayden-Hixson and colleagues also showed that a microinjection of E2 in the anterior hypothalamus stimulates agonistic behavior (flank marking) within 15 min in male hamsters [110].
Table 1. Circulating levels of testosterone and estradiol in males and females of various species.
In order to facilitate the comparison with receptors affinities, concentrations that were often expressed in ng/ml or pg/ml have all been transformed in nM or pM using the following conversion ratios: 1 ng/ml = 3.47 nM for testosterone (MW= 288.48) and 1 pg/ml = 3.67 pM (MW= 272.38) for estradiol.
| Testosterone (nM) | Estradiol (pM) | |||
|---|---|---|---|---|
| Species | Male | Female | Male | Female |
| Rat | Baseline: | Baseline: | Baseline: | Estrus to diestrus (Baseline): |
| 1.0 – 19.0 nM [98,100,105,128,210,261] | 0.4 nM [105] | <18.35 – 1101.0 pM [105, 261] | 18.4 – 135.8 pM [7,51,105, 243] | |
| Pro-estrus | ||||
| After mating: | 1.4 – 1.7 nM [105] | Pro-estrus | ||
| 5.8 – 33.9 nM [128, 200] | 146.8 – 367 pM [7,51,105,148,243, 253] | |||
| After social exposure: | ||||
| 4.2 – 10.4 nM [129] | ||||
| Mouse | Resting levels: | Day 12 of pregnancy: | Intact: | Intact (Baseline): |
| 6.9 – 41.6 nM [62, 144] | 0.7 nM [155] | 9.4 pM [94] | 41.8 – 165.2 pM [57,134] | |
| Dependent on strain and sexual activity | Aromatase overexpression: | |||
| 3.1 – 48.2 nM [32] | 20.9 – 825.8 pM [94, 144] | |||
| After social interaction: | ||||
| 27.8 – 86.8 nM [150] | ||||
| Rabbit | Baseline: | Estrus: | ||
| 0.45 – 34.7 nM [111] | 0.2 nM [203] | |||
| After social interaction: | After coitus: | |||
| 13.1 – 26.8 nM [107] | 0.9 nM [203] | |||
| Quail | Baseline: | Baseline: | Baseline: | Morning/evening |
| 1.6 – 12.2 nM [21,28,72, 75] | 1.6 nM [21, 28] | 183.5 – 367 pM [21] | 917.5 – 1101.0 pM [21, 73] | |
| Midday | ||||
| 367 pM [21, 73] | ||||
| Ring dove | 0.6 – 2.4 [88] | <34.7 [88] | ||
| Zebra finch | Intact: | Intact: | Breeding: | Breeding |
| 1.0 – 11.8 nM [6,120,268] | 1.7 – 3.5 nM [120, 268] | 319.3 pM [225] | 370.7 pM [225] | |
| CX: | OVX: | Non-breeding: | Non-breeding | |
| 1.5 – 2.6 nM [6, 268] | 1.4 nM [268] | 337.6 pM [225] | 715.7 pM [225] | |
| Rhesus monkey | Baseline: | Baseline: | ||
| 29.4 nM [212] | 91.8 – 220.2 pM [182, 280] | |||
| After prolonged housing with female: | Pre-ovulatory peak: | |||
| 52.6 nM [212] | 734 – 1284.5 pM [135, 182, 280] | |||
| Human | Young men | Baseline: | Baseline: | Pre-ovulatory peak |
| 19.1 – 26.0 [48] | 0.4 – 172.8 nM [52,127,283] | 0.9 – 157.1 pM [41,42,52] | 734 – 1570.8 pM [256, 283] | |
| Old men | After HYST or BSO | Follicular phase: | ||
| 8.7 – 17.4 nM [48, 262] | 0.001 – 3.2 nM [11,47,235–237] | 146.8 – 215.1 pM [256, 283] | ||
| Luteal phase | ||||
| 440.4 pM [256] | ||||
| Post-menopausal or after HYST or BSO | ||||
| 0.3 – 447.7 pM [11,41,42,47,235–237] | ||||
Abbreviations: CX, castrates; HYST, hysterectomy; OVX, ovariectomized; BSO, bilateral salpingo-oophorectomy; MW, molecular weight.
Several generalizations can be derived from these observations. Firstly, these behavioral effects occur with latencies ranging from 5 min to 1 hour, most effects being observed around 15–30 min. This is considerably shorter than the several days commonly required for genomic activation of behavioral responses (see below for further discussion of this issue). This rapidity of action is all the more striking that most of these effects were observed following systemic injections [33,64,69,208] so that the hormone had first to reach the target tissue and accumulate above a certain threshold before it could activate a cellular response and trigger the neuronal circuits involved in the expression of the behavior considered. Alternatively, most of the intra-tissue applications were performed via insertion of hormone in crystals [110,126,138]. It is thus likely that some time was required to dissolve hormone from these implants and produce a behaviorally active concentration of E2. Together, these data therefore suggest that the effects of E2 observed in these experiments would occur even faster if the steroid could be delivered instantaneously to its targets in effective concentrations. Secondly, some of these rapid behavioral effects appear to be transient as compared to the long lasting genomic effects. Thirdly, the rapid actions of estrogens appear to be somewhat region specific. Most of the studies available to date report fast effects of peripheral injections. But there are some examples of an estrogen implant applied only for a brief period in a specific brain area that activates the expression of complex behaviors in the absence of steroid priming i.e. in the absence of activation of specific circuits involved in the control of these behaviors [110,126,138,264,265]. Finally, fast effects of estrogen, and other steroids, have been identified in various vertebrate species (for review, see [206]). Therefore, these mechanisms of rapid action seem to be conserved across species.
Non-genomic effects on cell function
Fast effects of steroids were described for the first time in 1941 by Hans Selye, who observed a rapid anesthetic and sedative effect of progesterone [234]. In the 60s and in the beginning of the 70s, several studies suggested that estrogens modulate the electrical activity of various neuronal populations [49,279,286]. The concept of fast and non-genomic action of estrogen was first introduced in 1976 by Martin Kelly who demonstrated that E2 applied by microiontophoresis modified within a few seconds neuronal activity recorded in vivo from septal and preoptic cells [130]. It is now clear that these fast effects of estrogens are diverse and involve several signaling pathways. However, the underlying mechanisms remain largely unknown and even the definition of a non-genomic action, as opposed to a genomic action, is not straightforward. As a matter of fact, these two modes of action are often compared in term of latency of onset (fast – non-genomic versus slow – genomic), but, although it is relatively easy to differentiate latencies of a few seconds from latencies of several hours or days, it is hard to define the mode of action of effects observed at intermediate latencies of about 30 min (some genomic effects are initiated within 30–45 min). Furthermore, some non-genomic processes result in the activation of gene transcription and have for this reason been qualified as “indirect” [163].
In order to provide simple (and probably simplistic) guidelines, it is generally considered that non-genomic effects of steroids share one or several of the following criteria: (a) they are too fast to be generated by de novo protein synthesis; (b) they may be reproduced in the presence of inhibitors of RNA or protein synthesis; (c) they may be reproduced following treatment with conjugated estrogen that is unable to pass the cell membrane (e.g. E2-BSA); (d) they are observed in brain regions devoid of nuclear steroid receptors [152].
The non-genomic effects of estrogens seem to be initiated at the cell membrane. The existence of membrane estrogen receptors was first proposed in 1977 by Pietras and Szego [195]. Since then, an important controversy developed concerning the nature of this (these) receptor(s) (for review see [45,143,177,232,259,273]). In brief, one hypothesis postulates the existence of novel membrane receptors, such as GPR30 [89] and ER-X [260], that are different from the classical nuclear receptors, while the alternative hypothesis supports the involvement of the classical nuclear receptors, ERα and ERβ, that would be able to integrate the cell membrane and activate various intracellular cascades. In addition to their action on a specific membrane receptor whose nature remains to be elucidated, some steroids can act as co-agonists or allosteric antagonists of neurotransmitters’s receptors. The best known example is certainly the allosteric modulation of the GABAA receptors by neurosteroids [156]. E2 can also behave as an allosteric co-agonist of α4β2 nicotinic receptors [190] or as an allosteric antagonist of NMDA receptors [277] and serotonin receptors 5-HT3 [278]. Finally, the existence of a binding site for estrogens has also been identified on an ionic voltage-dependent channel, the high conductance calcium-dependent potassium-channel called maxi-K (hSlo, [263]).
Regardless of the nature of the membrane receptor involved, the action of estrogens initiated at the cell membrane activates a variety of intracellular responses (Fig. 1), including the modulation of intracellular calcium [44,55,122,165,175,209] and protein kinase C (PKC; [266,276]). There is also evidence that membrane effects of estrogens can activate intracellular signaling pathways involving cyclic AMP (cAMP; [103,170,254]), protein kinase A (PKA; [104,141,266]), the “mitogen activated protein kinases” (or MAP kinases; [39,122,153,205,260,269,276]), and the tyrosine kinases [39]. The activation of these intracellular signaling pathways results primarily in phosphorylations/dephosphorylations producing different kinds of physiological responses such as the decoupling of a receptor from its effector system [141,171–173] or the modulation of the catalytic activity of an enzyme [191]. Finally, as mentioned previously, the activation of these cascades of intracellular events may result in a transcriptional activation caused, for example, by the phosphorylation of CREB (cAMP response element [CRE]-binding protein) which then acts at the level of the cAMP response element notably (CRE; [2,3,46,102,288]).
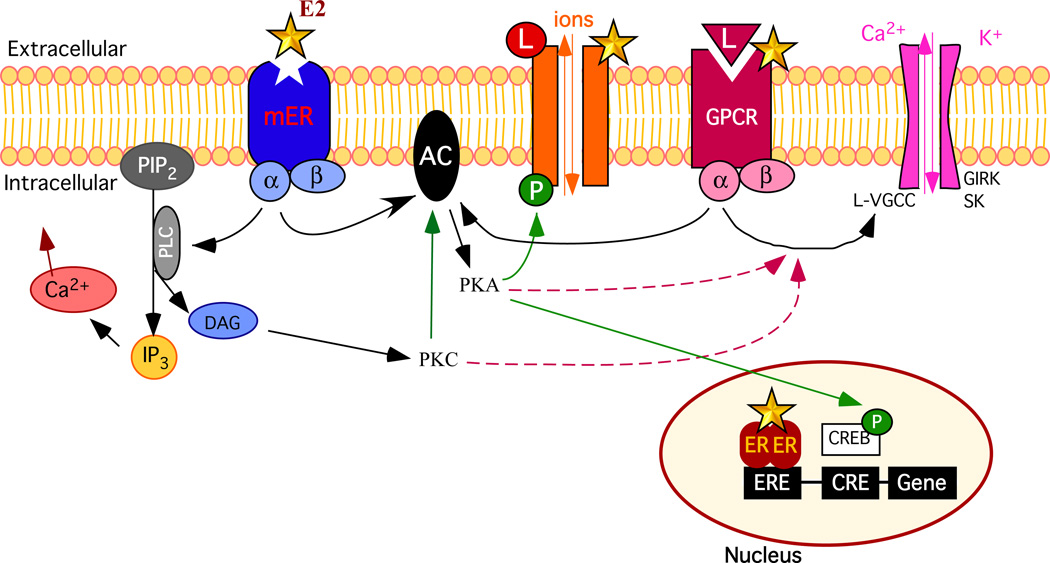
Figure 1. Schematic overview of some cellular pathways affected by estradiol.
In order to simplify this drawing only selected intracellular events are presented. Upon binding of their ligand (L), G-protein coupled receptors (GPCR, red receptor) such as metabotropic glutamate receptors (mGluR), 5-HT receptors, GABAB receptors, µ opioids receptors, α- and β-adrenergic receptors activate different intracellular signaling pathways through their G protein (made of α and β subunits). The activation of the G protein can lead to the activation of phospholipase C (PLC; not shown) that catalyzes the hydrolysis of membrane-associated phosphatidyl-inositol 4,5-biphosphate (PIP2) into 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). IP3 induces calcium release from the intracellular stores (reticulum endoplasmic). DAG activates protein kinase C (PKC). In turn, PKC can stimulate, through phosphorylation (P), the activity of adenylate cyclase (AC) to produce cAMP and activate protein kinase A (PKA). PKA can also phosphorylate various proteins such as other receptors, ion channels (such as Type-L voltage-gated Ca2+ channels [L-VGCC], G-protein-coupled, inwardly rectifying K+ channels [GIRK] or the small conductance, Ca2+-dependent K+ channel [SK]) or cAMP-responsive binding protein (CREB). Rapid non genomic signaling: 1/ Estradiol (E2, yellow star) can allosterically modulate the activity of ionotropic receptors (such as acetylcholine, kainate or NMDA receptors, orange receptors) or GPCR by directly interacting with this receptor. 2/ E2 can also activate a membrane-bound estrogen receptors (mER, blue receptor; the term is used here in a general way to cover both ERα and ERβ associated with the membrane and the more recently identified estrogen membrane receptor [GPR30, ER-X, etc] specifically named mER) that is coupled to a G protein. Thereby, E2 can modulate the activity of ionic conductance through phosphorylation of ionotropic receptors or uncoupling (dashed arrows) of GPCR from their ionic channels or intracellular effectors (not shown). It can also mobilize intracellular Ca2+ through activation of PLC or uncoupling of a GPCR from PLC (not shown). Delayed genomic signaling: E2 can bind to nuclear estrogen receptors (ER) that form dimers and bind the estrogen-responsive element (ERE) on the DNA resulting in the activation of the transcription of specific genes. In addition, rapid effects of E2 mediated through mER resulting in the activation of protein kinases can lead to phosphorylation of CREB, which can alter gene transcription through its interaction with the cAMP responsive element (CRE; indirect genomic effect).
It is also the case that purely cytoplasmic effects, i.e., effects independent of the binding to a membrane receptor, have also been identified. Among others, it has been shown that the binding of estradiol to a cytoplasmic estrogen receptor, ERα or ERβ, allows an interaction between the ER and the protein kinase Src resulting in the activation of the intracellular cascade Src/Ras/ERK [168]. Moreover, the binding of E2 to the cytoplasmic ERα activates the protein kinase Akt via a direct interaction of ER with the protein kinase PI3K ([112,238]; for more information see [80]).
As illustrated in Table 2, the latency at which non-genomic effects of estrogen are observed varies from a few seconds to 30 minutes. Estrogen modulates electrical activity of neurons within a few seconds in some experiments [165,190,277,278] but with relatively longer latencies of up to 3–5 minutes in other biological systems [103,104,137,141,202,284]. The fastest of these effects appear to be associated with the allosteric modulations of ionotropic receptors [190,277,278], but most effects on electrical activity result from a modulation of ion channel activity through G protein linkage (for review see [132,176]). Estrogen-induced changes in intracellular calcium concentrations have also been identified with latencies of a few seconds [44,54,175,209] to several minutes [55,122]. Finally, the time-course of protein phosphorylations and of changes in kinase activity following estradiol treatment ranges between 5 and 30 min [39,122,153,168,260,269,276].
Table 2. Summary of latencies and effective concentrations of estrogens that were shown to activate non-genomic effects of estrogens in vitro.
| Type of effects | Species/Tissue | Methods | Latency | [E2] | Ref | ||
|---|---|---|---|---|---|---|---|
| Electrophysiological recordings | Allo | ACh-R | Human | Expressed in Xenopus oocytes | A few sec | 3 – 15µM | [190] |
| NMDA-R | Rat – Hippocampus | Cell culture | A few sec | EC50 = 7µM | [277] | ||
| 5-HT3R | Human – Embryonic kidney | 5-HT3R–transfected (HEK)293 cells | A few sec | 300nM | [278] | ||
| Synaptic | Rat – Hippocampus | Slices | < 2 min | 10nM | [284] | ||
| Synaptic | Rat (pups) – Hippocampus | Slice culture | A few min | 1nM | [39] | ||
| G protein | Ca2+ currents | Rat – Neostriatum | Slices | A few sec | 1 – 100pM | [165] | |
| KA currents | Rat – Hippocampus | Slices | 3 min | 50 – 100nM | [103, 104] | ||
| Depol. | Guinea pig – VMH | Slices | 3 – 5 min | 10nM | [170] | ||
| µ opioid –R | Rat – Arcuate nucleus | Slices | A few min | 20nM | [141] | ||
| GABABR | C57BL/6 mice – Arcuate nucleus | Slices | 5 min | 100nM | [202] | ||
| NMDA-R Hist-R |
Rat – VMN | Slices | 10 – 15 min | 10nM | [137] | ||
| Intracellular signaling | [Ca2+]i | L-VGCC | Rat – DRG | Primary culture – Neurons | A few min | 100nM – 1µM | [55] |
| Mouse – DRG | Primary culture – Neurons | 5 min | IC50 = 27nM | [56] | |||
| PLC | Rat | Primary culture – Astrocytes | 10 – 30 sec | EC50 = 12.7nM | [54] | ||
| EGF/intracell ular stores |
Human – Breast cancer Human – Cervical carcinoma |
MCF-7 cells HeLa cells |
5 min | 10nM | [122] | ||
| PLC | Chicken, Rat and Pig – Ovary | Isolated granulosa cells | A few sec | 100nM | [175] | ||
| PI3K | Monkey – Kidney fibroblast | GPR30-GFP–transfected COS7 cells ERα-GFP–transfected COS7 cells ERβ-GFP–transfected COS7 cells |
< 2 sec < 2 sec < 2 sec |
EC50 = 0.3nM 1nM 1nM |
[44] | ||
| PLC EGF |
Monkey – Kidney fibroblast | GPR30-GFP–transfected COS7 cells ERα-GFP–transfected COS7 cells |
1 sec 1 sec |
EC50 = 0.5nM 1nM |
[209] | ||
| IP3 | Chinese – Hamster ovary | ERα-transfected-CHO-K1 cells ERβ-transfected-CHO-K1 cells |
15 sec 15 sec |
10nM 10nM |
[205] | ||
| MAP-K signaling | P-ERK | Human – Breast cancer Human – Cervical carcinoma |
MCF-7 cells HeLa cells |
5 min | 10nM | [122] | |
| P-ERK | Human – Neuroblastoma | SK – N – SH cells | 5 – 15 min | 10nM | [276] | ||
| P-ERK | Mouse – WT & ERαKO - Neocortex | Organotypic explant cultures | 30 min | 10nM | [260] | ||
| P-ERK | Chinese – Hamster ovary | ERα-transfected-CHO-K1 cells ERβ-transfected-CHO-K1 cells |
10 min 10 min |
10nM 10nM |
[205] | ||
| P-ERK | Rat – Fibroblast | ERα-transfected-Rat-2 fibroblasts ERβ-transfected-Rat-2 fibroblasts |
10 – 15 min 10 – 15 min |
0.3 – 10nM 0.3nM |
[270] | ||
| P-ERK | Murine – Hippocampus | ERα-transfected-HT-22 cells ERβ-transfected- HT-22 cells |
10 min 10 min |
10nM 10nM |
[269] | ||
| P-ERK | Human - Prostate cancer | LNCaP-FGC cells | 5 min | 10nM | [168] | ||
| P-CREB | Murine – Hippocampus | ERα-transfected-HT-22 cells ERβ-transfected- HT-22 cells |
10 min 20 min |
10nM 10nM |
[269] | ||
| cFos | Human – Neuroblastoma | SK – N – SH cells | 3h | 10nM | [276] | ||
| P-CREB (mGluR) |
Rat – Hippocampus | Cell culture – Pyramidal neuron | τ = 29 sec | EC50 = 5.5pM | [46] | ||
| cAMP | Chinese – Hamster ovary | ERα-transfected-CHO-K1 cells ERβ-transfected-CHO-K1 cells |
20 min 20 min |
10nM 10nM |
[205] | ||
| cAMP | Human – Breast cancer Human – Embryonic kidney |
GPR30–transfected SKBR3 cells GPR30–transfected (HEK)293 cells |
20 min | 10nM | [254] | ||
| eNOS | Human – Vascular endothelium | Cell culture | 10 – 15 min | EC50 = 0.1nM | [238] | ||
| G protein coupling | GTPγS-Binding | 5-HT1A R 5-HT1B R CB1-R GABAB R |
Rat – OVX | Hippocampus, Cortex, Amygdala Hypothalamus Cortex, Hippocampus Cerebellum |
2 hours | 30µg 10µg 10 – 30µg 10µg |
[171] |
| 5-HT1A R | Rat – Hippocampus | Explant culture | 60 min | 50nM | [172] | ||
| 5-HT1A R | Rat – Hippocampus Rat – Frontal cortex |
Explant culture Explant culture |
15 min 15 min |
50nM 50nM |
[173] | ||
| Gs | Human - Embryonic kidney | GPR30–transfected (HEK)293 cells | 20 min | 100nM | [254] | ||
| Gs & Gi | Chinese – Hamster ovary | ERα-transfected-CHO-K1 cells ERβ-transfected-CHO-K1 cells |
5 min 5 min |
10nM 10nM |
[205] | ||
Abbreviations : 5-HT3R, 5-HT(serotonin)3 receptor ; Allo, allosteric modulations ; ACh-R, acetylcholine receptors ; [Ca2+]i, intracellular calcium concentration ; cAMP, cyclic Adenosine 5’-monophosphate ; CB1-R, cannabinoid receptor 1 ; DRG, dorsal root ganglia ; EGF, Epidermal Growth Factor ; ERα, estrogen receptor α; ERβ, estrogen receptor β; GFP , green fluorescent protein ; GPR30, G Protein Receptor 30 ; Hist, histamine ; IP3, inositol-1,4,5,-triphosphate ; KA, Kainate ; L-VGCC, L-type voltage-gated calcium channel ; NMDA-R, NMDA receptor ; MAPK, Mitogen-activated protein kinase ; P-CREB, phosphorylation of cAMP response element-binding Protein(CREB) ; P-ERK, phosphorylation of extracellular-regulated kinases (ERK-1/ERK-2) ; PI3K, Phosphatidylinositol triphosphate kinase ; PLC, phospholipase C ; VMH, ventromedial hypothalamic nucleus ; VMN, hypothalamic ventromedial nucleus.
The broad spectrum of latencies associated with estrogen actions in vitro is fully consistent with the fact that they encompass a wide variety of potential mechanisms. However, independent of the nature of the effects described (potentiation/inhibition of neurotransmitter effects, protein phosphorylations, modulation of intracellular calcium levels, etc), it appears that most of these effects result from the activation of second messengers systems. Mounting evidence also suggests that membrane estrogen receptors are coupled to G proteins [89,131,176,211]. The broad range of latencies described is thus probably explained by this diversity of mechanisms but also to a large extent by the number of successive intracellular events required for the activation of the measured effects. In addition, it should be noted that numerous studies did not report a time-response curve for the described effects. Consequently, the reported latency may simply be the result of an arbitrary choice of the experimenters when they designed their experiment. The latency of some effects also depends on technical issues that limit the temporal resolution of the data acquisition, so that the latency reported may actually be longer than the actual velocity of the effect.
Together, the range of latencies of non-genomic effects of estrogen identified in vitro is in agreement with the time-course of the fast effects of estrogens observed on measures of physiology or behavior (5 min to 1 hour). If one extrapolates from the in vitro data, it is reasonable to speculate that the fastest effects observed with the use of organismal-level measurements (i.e. physiology or behavior) rely on modulations of ionotropic channel function, while slower effects result from a modulation of intracellular events through G protein activation that ultimately induce changes in ionic conductance, protein phosphorylation, enzymatic activity, etc. These effects then potentially modify the firing rate of neurons and neurotransmitter release that will finally lead to measurable effects on physiology and behavior [133]. The slight delay in the onset of in vivo as compared to in vitro effects is caused, in all probability, by the time needed for the steroid injected in periphery to reach an effective concentration at its target site(s). Indeed, acute intravenous injections of estradiol lead to a maximal estrogen concentration 5 min after the injection [287]. Furthermore, an early report on fast actions of estrogen showed that it takes about 15–16 min for significant changes in the firing rate of preoptic-hypothalamic neurons to be observed following a single intravenous injection of estradiol in ovariectomized females. Interestingly, this effect persisted for 20 to 30 min and then vanished [286].
In summary, it seems clear that the non-genomic effects of estrogens are quite diversified, require or not the binding to a membrane receptor and involve a variety of intracellular signaling pathways. These signaling cascades are probably not linear but act via parallel or even divergent pathways [80]. Estrogens may thus activate intracellular cascades without acting at the transcriptional level. These cascades could also amplify the genomic action of the hormone as suggested by the work of Pfaff and colleagues [266] or influence cellular function prior to the initiation of transcription.
Where does estrogen come from?
Together, these observations raise the question of the mechanisms controlling the rapid fluctuations of estrogen bioavailability that are presumably needed to sustain the fast actions of the hormone. Intuitively, it can indeed be hypothesized that, for fast non genomic actions of steroids to be biologically effective, the endogenous ligand’s availability must also change quickly. That is not to say that slow changes of steroids levels would not activate non-genomic effects. However, in this case, steroid action would result in a potential waste of the temporal-resolution provided by fast membrane responses.
What is the mechanism mediating fast behavioral effects if the concentration of the ligand (steroid) does not vary and does not vary quickly? Quite surprisingly, this aspect of brain E2 action has rarely been considered in the literature. Although fast effects of estrogens are not limited to females, the acute effects of estrogen in the brain seem to be exclusively attributed to the pre-ovulatory peak of estrogen produced cyclically in females. In fact, in most cases there is little discussion of the origin of the hormone that exerts these proposed effects. How then are these rapid effects triggered in males? From a physiological perspective, this question is fundamental. If estrogens have the ability to quickly trigger specific and transient cellular processes or specific behaviors, it is legitimate to postulate that their production and clearance should also be finely and rapidly tuned in order to respond appropriately to relevant stimuli. Likewise, if estrogens can activate a specific behavior through a local action only, the mechanism controlling their production should additionally be region-specific. This neuroanatomical specificity could be provided by the discrete distribution of the receptors and/or their intracellular effectors but the origin of the hormone should be envisaged as well.
As alluded to previously, estrogens can be synthesized directly in the brain by aromatization of androgens such as testosterone. The involvement of this local source of estrogens in the regulation of the fast effects of these steroids should thus be considered. Rapid changes in local estrogen production could simultaneously provide answers to the problem of the source of rapid changes in ligand and of its anatomical specificity. From a functional point of view, it would also probably be useful to produce estrogens locally where they are needed rather than flooding the entire organism with a compound that can have severe adverse effects (e.g. promote tumor growth, alter structure or function of reproductive organs, induce general toxicity, etc [5,27,94,99,134,154]) especially at the high doses that seem to be required to activate non-genomic responses (See below). Is it therefore possible that estrogens locally produced in the brain by aromatization of testosterone represent an endogenous stimulus triggering all or part of the fast non-genomic effects reviewed above. This organization would additionally provide anatomical specificity to the observed effects while avoiding inappropriate if not toxic effects potentially resulting from the activation of non-neural tissues.
The fast effects of estrogens: systemic or central origin of the steroid?
In both sexes, androgens such as testosterone or androstenedione can be converted into estrogens by an enzymatic process, called aromatization, which is catalyzed by the enzyme aromatase (or estrogen synthase). This enzyme is present primarily in the ovary (but also to a lesser extent in the testes), the placenta, the bones and the brain [179,224,240]. Under normal conditions, aromatase expression in the avian and mammalian central nervous system is restricted to specific neuronal populations. These neuronal groups are mainly located in the hypothalamic/preoptic (medial preoptic nucleus, ventromedial nucleus) and limbic system (bed nucleus of stria terminalis, amygdala, hippocampus, septum, etc), but more scattered populations of aromatase expressing neurons are also present in the cerebral cortex [24,160,215,216,252,271]. In zebra finches and canaries, aromatase is also detected in high concentrations in parts of the nidopallium adjacent to the song control nucleus HVC [14,166,222]. In contrast, in the brain of teleost fishes, aromatase is expressed in radial glia [93]. In addition, in birds and mammals, aromatase is observed after in vivo brain injury in the glial cells within the damaged brain areas indicating that astrocytes from most brain areas have the potential for expressing aromatase and therefore produce E2 in response to injury [10,192,193]. Because under normal physiological conditions circulating concentrations of testosterone produced by the gonads are fairly high in males and even females of many species (Table 1), estrogens may therefore be produced locally in the brain by aromatization of this androgen which freely enters the central nervous system. Furthermore, there is evidence that brain areas such as the rat hippocampus or the avian telencephalon are capable of producing estrogen de novo from cholesterol [115,197]. Several studies also suggest that androgen precursors, such as DHEA (dehydroepiandrosterone), and androgens are also produced de novo in the brain [68,147,161,164,246]. These steroids could thus also act as a substrate for aromatase.
A prominent role for brain aromatase has been established for the control of male sexual behavior in a variety of species [12,22,30,58–60,70,116,258,274,275]. Central aromatization also plays a role in the control of aggressive behaviors [227–229,247,258], neuroprotection [10], nociception [43,83–85], the development of sexual preferences [214] and synaptic plasticity [197]. Most of these studies, however, concern relatively slow, presumably genomic, effects of estrogens. Whether the more rapid, presumably non-genomic, effects of estrogens on brain and behavior relate to a central or peripheral source of the steroid should be considered independently.
Gonadal production of steroids
In both sexes, estrogens may be delivered to the brain via gonadal secretion or by local production in specific regions of the central nervous system (see Fig. 2). As discussed above, it is reasonable to hypothesize that estrogen availability has to be modulated rapidly in order to take advantage of its fast non-genomic effects. One can thus ask whether gonadal steroids vary rapidly enough to achieve this goal?
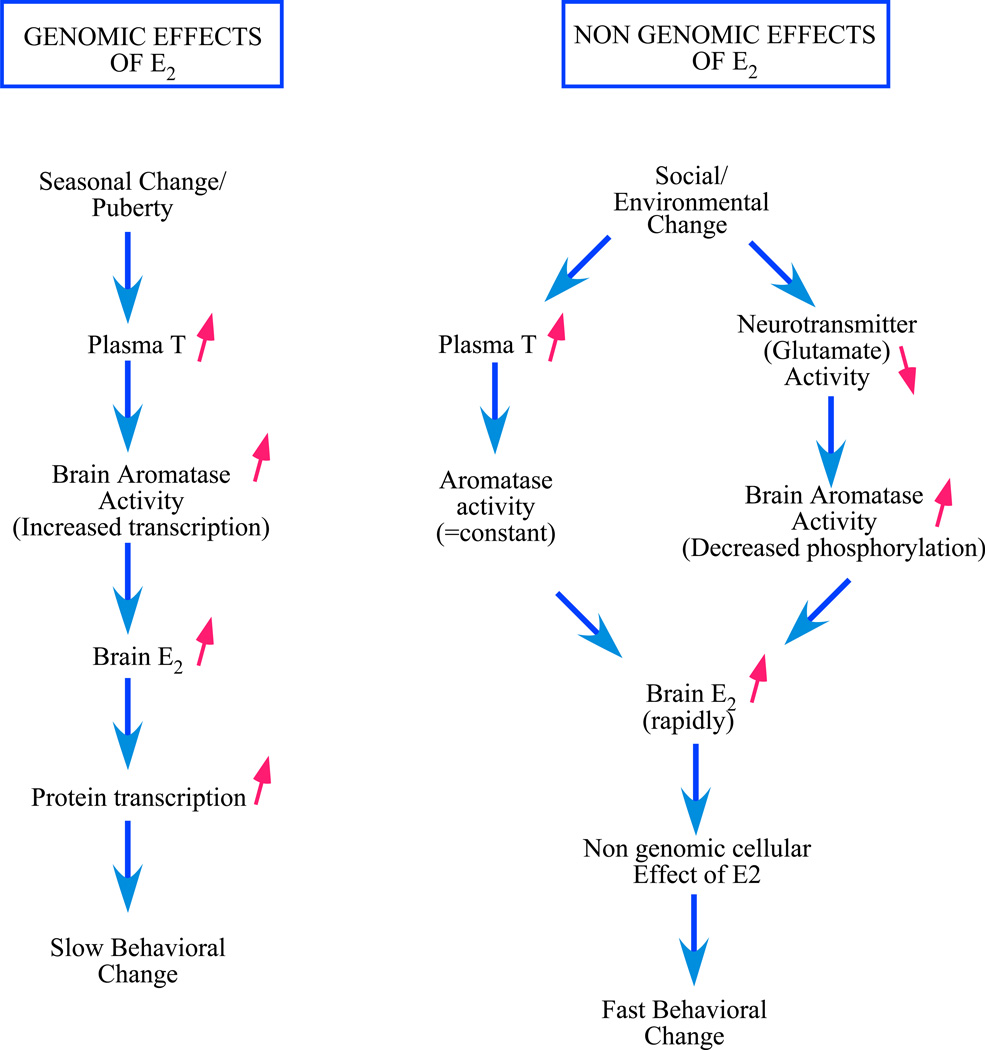
Figure 2. Potential sources of estrogens in males associated with genomic and non-genomic effects on physiology and behavior.
On the one hand, genomic effects are associated with a slow rise of testosterone concentrations in the plasma. These changes are correlated with changes in testis function at puberty and across seasons. This rise in plasma testosterone results in an increased aromatase activity in the brain (through an activation of transcription of its gene). The estrogens produced locally by the aromatization of testosterone influence the transcription of various genes ultimately resulting in long lasting behavioral changes. On the other hand, non-genomic effects are associated with rapid changes in estrogens bio-availability. Such rapid changes in brain estrogen concentrations could result from variations of plasma testosterone that is subsequently aromatized locally into estrogen by brain aromatase as well as from variations in neurotransmitters’ activity, such as glutamate or dopamine, that rapidly regulate brain aromatase activity and the subsequent production of estrogen. These fast changes in brain estrogen concentrations could in turn induce fast non-genomic actions in specific cell populations that would ultimately result in fast behavioral effects. In both cases, aromatase concentration remains constant and either the substrate of the enzyme (testosterone) rapidly becomes more concentrated resulting in an increased product formation or the substrate remains constant but the rate of conversion into estrogen (the enzymatic efficiency) is rapidly increased.
In females, the estrogen surge at pro-estrus is often implicitly considered as the source of rapid changes in estrogen brain concentration. However, to our knowledge, no study has documented increases in plasma E2 concentrations that would take place within a time frame compatible with the latency of a few seconds or minutes observed for the activation of non-genomic effects of E2. This does not mean that this ovarian source of estrogen cannot activate non-genomic effects when estrogen reaches a threshold concentration. Rather we would argue that this peripheral source of estrogen would not be able to trigger rapid on/off switches of the response and would therefore constitute a poor use of the capacity of the membrane non-genomic response mechanisms.
Available studies indicate that, in rats, the rise of estrogen plasma concentrations at pro-estrus occurs over a period of several hours slowly rising during 24 hours to “rapidly” reach a peak in about 12 hours [7,51,243,253]. Ovulation and receptivity occur about 24 hours after this peak has been reached [162]. This time frame is in agreement with the activation by estrogen of the transcription of hypothalamic progesterone receptors that appear to be required for the activation of female sexual receptivity [158,162,196]. The pre-ovulation secretion of estrogen appears even slower in primates including humans [117,280]. This rate of increase does not seem compatible with the dynamics of non-genomic effects of estrogens that occur with latencies of a few minutes to an hour (see above) although, this relatively abrupt rise of circulating levels could activate preparatory effects such as those described by Pfaff and colleagues [138,242,266,267].
It is established that social stimuli influence endocrine function in males and females of a variety of vertebrate species (see [13,108,136,180,185] for review). Most of these effects take place over a relatively long period (>days) and are therefore likely to influence genomic but not the more rapid non-genomic effects of steroids (fish: [145]; frog: [50]; birds: [119,183,184]; rat: [201]; rhesus monkey: [212]).
In addition, rapid increases (in the minute range) of circulating hormone levels triggered by environmental/social stimuli have also been reported. For instance, plasma testosterone levels may be acutely altered in males by the presentation of a female and/or copulation (mice: [32,150]; rat: [128,200]; Rabbit: [107,111,220]; Hamster : [129]; marmoset : [292]; macaques: [53]; Birds: [281]). The fastest effects reported occurred within 5–15 min (in rat: [200]; in mouse: [32]; in rabbit: [220]). A release of pituitary hormones in response to some of these stimuli has also been shown to precede the testosterone release [62,92,111,125,128,129]. The nature of the stimulus eliciting these responses has been questioned. Some studies based on repeated exposures to female odors or repeated mating suggested that mating per se was necessary [255], while other experiments indicated that the smell or sight of an estrus female or a pre-copulatory interaction with an estrus female were sufficient to increase plasma hormone levels [32,62,151,200,220,292]. It has also been suggested that these effects may be purely social, and not sexual, given that a similar increase of hormone concentrations could be evoked by exposure to a non-receptive female [97,125,129,150]. Interestingly, in male rats, LH and testosterone secretion can be conditioned so that after learning they are rapidly induced by a neutral stimulus previously associated with mating [100] or by the arena where males were previously mated [128].
Social stimuli related to aggression, stress or establishment of social rank have also been shown to affect plasma androgen levels in various species. Again, most of these effects seem to develop relatively slowly [185,223], but some occur in a rather rapid fashion. For example, Harding and Follett reported that a short aggressive interaction (about 20 min) between male blackbirds results in increased levels of LH and androgens measured immediately after the encounter [109]. Likewise, Wingfield demonstrated an increase of testosterone plasma concentrations in response to territorial intrusion in male song sparrows within 120 min [281]. In male teleost fish, watching a fight between two males raises the androgen levels (testosterone and 11-keto-testosterone, the main androgen in fishes) within 90 min [186]. In the toad fish, 11-keto-testosterone was elevated 20 min after an acoustic playback challenge [207]. In the green anole lizard, plasma testosterone is increased by 470% after agonistic encounters in winners but not in losers [101]. Finally, in male guinea pig, plasma testosterone was significantly decreased 20 min after an agonistic encounter. This finding was interpreted as the expression of a relaxation phase after a physical strain associated with the encounter [219].
The acute peripheral releases of testosterone that have been described in males occur within a few minutes (from about 5 min to two hours depending on the experiment) following the presentation of the eliciting stimuli and appear to be transient. The functional significance of these rapid endocrine changes, particularly in the context of the control of social behavior, remains however unclear. In the case of sexual encounters, the increased hormone levels have been proposed to enhance sexual arousal [108]. However, the underlying mechanism of the behavioral effects of such rapid hormonal changes has not been identified. It has been shown that an injection of testosterone given to a sexually experienced and intact male rat one hour before he was allowed to copulate with a female decreased the inter-intromission interval and facilitated ejaculation [157]. Similarly, testosterone also affects penile reflexes of rats in a rapid manner (within 6 to 30 min; [218]).
The rapid behavioral effects of changes in plasma testosterone concentrations could obviously be mediated by interactions of the androgen with its cognate receptor. However, behavioral effects of increases in plasma testosterone levels could also be related to fast effects of estrogens. Indeed, contrasting with the relatively limited number of examples of fast actions of androgen reported at the cellular level (e.g. [37,149]), rapid cellular effects of estrogens have been extensively documented. It is thus possible that a bolus of testosterone secreted by the testes following a social encounter is rapidly aromatized in the brain and the estrogen produced then contributes to the modulation of behavioral effects. Such a mechanism would provide anatomical specificity to the behavioral response since only aromatase-containing regions would react even though the entire body (and brain) would be exposed to increased testosterone concentrations. Whether the magnitude and time-course of these hormonal changes is compatible with the activation of non-genomic effects of estrogens is however unknown and more research on this topic would definitely be warranted.
Brain synthesized estrogens
Alternatively, as already discussed above, rapid changes in this brain aromatase activity could generate equally rapid changes in brain estrogen concentrations that would trigger fast behavioral responses independent of changes in plasma androgen concentrations. It has traditionally been assumed that brain aromatase activity, as opposed to aromatase expressed in ovaries or adipose tissue, is regulated by sex steroids which act as transcription factors after binding to their cognate receptor to regulate the transcription of the enzyme. Treatment of castrates with testosterone markedly increases aromatase activity in several brain regions including the preoptic/hypothalamic area in birds and mammals [213,217,231]. Testosterone similarly increases the concentration of the aromatase protein and the corresponding messenger RNA supporting the idea that testosterone induced changes in brain aromatase activity result of an increase in gene transcription [1,23]. As expected, these effects are relatively slow and take many hours if not days to be expressed [25].
Besides this genomic and slow control of brain aromatase activity, recent experiments from our group have demonstrated that aromatase activity is rapidly modulated by calcium-dependent phosphorylations independently of changes in enzyme concentration. These experiments were performed in quail, taking advantage of the high concentration of the enzyme detected in the avian as compared to the mammalian brain [226,231,251]. They showed that the enzymatic activity measured in preoptic/hypothalamic homogenates submitted to phosphorylating conditions (i.e. in the presence of increased but physiological concentrations of ATP, Mg2+ and Ca2+) is profoundly inhibited within 15 minutes [16]. This inhibition is blocked by compounds chelating divalent ions such as EGTA and EDTA or by kinases inhibitors such as inhibitors of protein kinase A (PKA) or C (PKC) indicating that it is indeed caused by phosphorylation processes [16,18]. The identification of putative phosphorylation sites for PKC on the predicted quail aromatase sequence and western-blotting experiments on quail brain aromatase partially purified by immunoprecipitation confirmed that phosphorylation sites located on the aromatase protein itself are affected by the phosphorylating conditions that influence the enzymatic activity [18,19].
Similar effects were observed in preoptic/hypothalamic explants in which the cellular integrity of the neurons and a large part of their connectivity were maintained [16]. In these explants, aromatase activity is rapidly (within 5 min) and reversibly inhibited by conditions that increase the intracellular Ca2+ concentration such as a K+-induced depolarization or the exposure to thapsigargin, a drug that mobilizes intracellular pools of Ca2+ [16]. Due to technical limitations, it was impossible to assess changes in aromatase activity after latencies shorter than 5 min, but circumstantial evidence strongly supports the notion that the changes observed after 5 min take place much more rapidly at the cellular level (see [16,17] and below for discussion).
Furthermore, application of glutamatergic agonists (AMPA, kainate and to a lesser extent NMDA, the effect was actually not statistically significant in this latter case) in these explants resulted in similar inhibitions. These AMPA/kainate effects are also reversible and are blocked by specific antagonists [17]. The implication of DA in the control of aromatase activity has also been suggested but the exact mechanism of this action remains unknown [4].
Electrophysiological studies indicated that preoptic aromatase-containing cells are sensitive to glutamate, dopamine and norepinephrine [63,66] and showed the existence in quail and rat preoptic area of a tonic electrical activity that can be blocked by glutamate and GABA antagonists [63,66,113]. A recent study also showed a modulation of estrogen synthesis within 30 min in hippocampal slices of male rats incubated in the presence of Mg2+ and NMDA. Interestingly, this effect resulted in a 2-fold increase in E2 concentration in the preparation. The authors of this study hypothesized that this effect depends on NMDA-induced Ca2+ influx and thereby depends on synaptic communication [114]. Why application of NMDA tended to decrease estrogen formation in the quail preoptic/hypothalamic explants and increase it in the rat hippocampus remains unclear at present. This difference could relate to differences in the species used, brain area investigated or specific experimental conditions (e.g., ionic, in particular Mg2+, concentration in the medium). It must also be mentioned that, in addition to these rapid effects of NMDA receptor activation on estrogen synthesis, estrogens regulate, presumably by genomic mechanisms, the concentration of NMDA receptors in the songbird hippocampus [221]. This suggests the existence of bi-directional interactions between NMDA receptors and estrogens that normally utilize two different time frames when regulating biological events. How these effects interact under intact physiological conditions remains to be investigated.
Finally, it should be noted that, in addition to its presence in cell bodies, aromatase has also been identified throughout the length of neuronal dendrites and axons, including pre-synaptic boutons, in a relatively large number of brain areas [87,91]. Aromatase activity is also enriched in synaptosomes prepared from quail or rat brain [230,250] and dense aromatase immunoreactivity is observed in presynaptic boutons at the surface of synaptic vesicles [114,178,194]. Together, these data suggest that aromatase activity can be rapidly (with latencies in the minute or possibly the second range) modulated at the pre-synaptic level by afferent inputs potentially providing a mechanism able to quickly change the local production of estrogens.
Rapid changes in estrogen availability are thus likely to occur at the synaptic level suggesting that estrogen in the brain may play a role more similar to the role of neurotransmitters or neuromodulators than previously thought. Given the lipophilic nature of estrogen, the mechanism(s) of its release and retention may be questioned. However, the definition of what should be considered a neurotransmitter has recently been extended to unconventional transmitters such as nitric oxide, a gas that is not stored in cells and is not released in an exocytotic manner [76,245]. More work will, of course, be necessary before estrogen produced in the brain can be fully accepted as a neurotransmitter (see [20] for a further discussion). Nevertheless, these rapid changes in local estrogen concentration appear perfectly suited to mediate the rapid effects of this hormone. In addition, as pointed out previously, the intervention of brain aromatase offers anatomical specificity to the behavioral effects since estrogen is synthesized and will produce its effects only locally. Its restricted localization, in particular at the level of pre-synaptic boutons, could also contribute to speed up the rise of estrogen concentration since production takes place exactly where needed (pre-synaptic level) and does not require a transport/diffusion of the hormone to distant target site. This implies however that the (unidentified) brain sites mediating rapid behavioral effects of estrogens should overlap with the brain areas expressing aromatase. An important remaining question is thus whether the estrogen release/synthesis sites are localized in proximity to estrogen receptor systems that mediate fast behavioral effects and, at the cellular level, whether these receptor systems are present in the pre/post-synaptic membrane in close association with pre-synaptic aromatase.
What is a physiological contentration of estrogen?
The fact that estrogen production is very localized in the brain suggests that high concentrations may be rapidly attained in specialized locations such as the synaptic clef in specialized synapses. These local brain concentrations may thus exceed at discrete sites the physiological concentrations of circulating estrogens. This could then explain why some (most) of the rapid effects of estrogens that have been observed in vitro can only be activated with what has been considered so far as supra-physiological concentrations.
In a recent article, Warner and Gustafsson (2006; [273]) argued that the E2 concentrations used in some studies are higher than physiologically relevant concentrations, classifying these concentrations as pharmacological and toxicological. Based on the maximal plasma levels measured in cycling females (±100 pg/ml i.e. 0.4 nM in rodents; ±350 pg/ml i.e. 1.3 nM in primates including humans, see Table 1 for details), it can be accepted that physiological concentrations of E2 range from the high picomolar (i.e. 100 pM; rat: [7,51,243]) to low nanomolar concentrations (i.e. 1–2 nM; quail: [21,74]; Rhesus monkey: [280]; Human: [117]; see Table 1 for details). While some rapid effects of E2 are elicited in vitro at such concentrations [46,165,209,266], numerous studies reported effects that could only be triggered by concentrations in the high nanomolar to micromolar range ([2,55,104,122,137,153,202,254,269,276–278,284]; see Table 2 for details). Obviously, these effects cannot be triggered by the levels of estrogen circulating in the periphery, not even by the pro-estrus surge of E2. However, are these effects necessarily of a pharmacological nature? We do not believe that this is necessarily the case because although these fast effects cannot be elicited by peripheral concentration of estrogen, they could be activated by the potentially higher concentrations produced locally by aromatase activity.
This scenario would be in agreement with the concept that “estrogen synthesized in extragonadal compartments acts predominantly at the local tissue level in a paracrine or intracrine fashion” and consequently “the total amount of estrogen synthesized by these extragonadal sites may be small, but the local tissue concentrations achieved are probably high and exert biological influence locally” [239,241]. This model is supported by reports of higher concentrations of estrogen in certain brain areas, such as the hypothalamus, preoptic area and the hippocampus, than in the plasma [40,114]. It must also be noticed that these measures of tissue concentrations are likely to be underestimations of the actual concentrations present at discrete locations close to the site of synthesis and action. Even if assays are performed in microdissected brain regions that express high level of aromatase, heterogeneity still persists in these samples and the potentially high concentration of estrogen present at specific sub-cellular sites (e.g. the synaptic cleft) is diluted in tissue containing lower concentrations of the steroid.
This model will remain hypothetical until technology allows us to determine estrogen concentrations at the sub-cellular level. The model is also currently associated with a major caveat namely that, if high concentrations of estrogens are required to activate fast physiological or behavioral responses, then (membrane?) estrogen receptor systems that are selectively sensitive to these high concentrations should be present in the brain. In contradiction with this prediction, membrane estrogen receptors have been identified during the last decade, but their affinity for estrogen is not fundamentally different from the affinity of nuclear receptors (see Table 3). Indeed, independently of their cellular localization in the nucleus or in the membrane, the so-called “classical” intra-cellular receptors (ERα and ERβ) respond to concentrations of estradiol in the high picomolar range. In addition, novel membrane receptors such as GPR30 and ER-X respond to concentrations in the low nanomolar range. This discrepancy between the doses of estrogen needed to activate fast effects and the affinity of the known estrogen membrane receptors cannot be explained at this time. For these effects of estrogen to be considered as physiological in nature, either a novel estrogen receptor with lower affinity should be identified or some physiological mechanism (e.g. a specialized regulation of the coupling between the receptor and associated G protein) should be uncovered to explain the requirement of such high dose of steroid.
Table 3. Affinity (Kd) of identified estrogen receptors as a function of their cellular localization and the system in which they have been measured.
Note that GPR30 was identified in the plasma membrane in the studies of Thomas and colleagues [254] but in the endoplasmic reticulum membrane in the studies of Revankar, Bologa, Prossnitz and colleagues [44,209]. Thomas et al. (2005) found Bmax values of 114 pM and 100 pM for SKBR3 cells and HEK-293 cells transfected with GPR30 [254].
| Nuclear | Membrane | |||
|---|---|---|---|---|
| Kd | Origin | Kd | Origin | |
| ERα | 0.1 nM | in vitro synthesized human ERα [139] | 0.30 nM | ERα-GFP–transfected COS7 cells [44] |
| 0.283 nM | ERα–transfected CHO cells [205] | 0.287 nM | CHO cells [205] | |
| ERβ | 0.6 nM | Clone 29, rat prostate cDNA library [140] | 0.38 nM | ERβ-GFP–transfected COS7 cells [44] |
| 0.6 nM | In vitro synthesized human ERβ [139] | 1.17 nM | CHO cells [205] | |
| 1.23 nM | ERβ–transfected CHO cells [205] | |||
| ER-X | 1.6 nM | |||
| GPR30 | 5.7 nM | GPR30-GFP–transfected COS7 cells [44] | ||
| 6.6 nM | GPR30-GFP–transfected COS7 cells [209] | |||
| 2.7 nM | ERα-/ERβ-/GPR30+-transfected SKBR3 cells [254] | |||
| 3.3 nM | ERα-/ERβ-/GPR30+-transfected (HEK)293 cells [254] | |||
| E2-BSA | Membrane fraction: | |||
| 4.5 nM | Hypothalamus [204] | |||
| 8.7 nM | Olfactory bulbs [204] | |||
| 30.2 nM | Cerebellum [204] | |||
Abbreviations: ERα, estrogen receptor α ; ERβ, estrogen receptor β ; GFP , green fluorescent protein ; GPR30, G Protein Receptor 30 ; (HEK)293 cells, Human Embryonic Kidney cells; CHO cells; Chinese Hamster Ovary cells; COS7 cells, Monkey Kidney fibroblast; SKBR3 cells, Human Breast Cancer cells.
Nevertheless, based on these considerations, it is enlightening to reconsider the circulating concentrations that are presumably reached following the acute systemic injections of estrogens in behavioral studies that identified rapid effects of this steroid. These may be considered as supra-physiological when compared to endogenous circulating levels in males or even females. Indeed, assuming that distribution is immediate and the distribution volume equals the entire body (1 liter /kg body weight), the average concentrations reached in these experiments in the body fluids should range between 100 and 500 ng/ml which is about 2 to 3 orders of magnitude above the endogenous concentrations of estrogens in the plasma (after an injection of 1–10µg/mouse [3] or an injection of 20µg/kg (in rat : [69]; in midshipman fish : [208]), 100µg/kg (in rat : [69,102]), 500 µg/kg (in rat : [288]; in quail : [64]) or 1 mg/kg (in midshipman fish : [208])). The goal in these experiments is however not to mimic circulating levels of estrogens but to induce in a few discrete brain locations, concentrations that are presumably produced by local aromatase activity. Although much higher than concentrations observed in the plasma, these experimentally-induced concentrations may thus appropriately mimic the natural situation in target areas.
In conclusion, the data summarized here suggest that estrogen concentrations that are higher than physiological circulating levels may be reached locally in the brain through the conversion of testosterone by the enzyme aromatase. This could explain why some of the rapid effects of estrogens observed in vitro can only be activated with what is considered as supra-physiological concentrations. The estrogen-sensitive system (receptor?) that mediates these effects of higher concentrations is however unclear at present and its identification should be a priority for future research
What terminates estrogen action in the brain?
Warner and Gustafsson also compared the effects of E2 and neurotransmitters and observed that “the changes [induced by neurostransmitters] are not only rapid, they are also transient” and “there are multiple mechanisms at the synapse to terminate neurotransmitter action and rectify intracellular ion homeostasis”. They stated that the case of E2 is different because “plasma levels do not change rapidly and transiently and no extremely rapid metabolic pathways to terminate estrogen action at the cell surface have been described” [273]. Based on the data reviewed above, we would like to argue that changes in brain estrogen production can also be rapid. Furthermore, there are multiple mechanisms that could lead to the rapid termination of E2 action based either on diffusion of the active hormone from its production/action site (thus leading to concentrations that are below the threshold for action) or on its degradation through various catabolic pathways.
Available evidence indeed is consistent with the notion that plasma concentrations of E2 do not vary rapidly enough to support the rapid non-genomic effects of this steroid but E2 can be synthesized in the brain and the rate of this local conversion of testosterone appears to be rapidly regulated. We have seen that aromatase activity can be rapidly modified within 5 min and probably even faster. Glutamatergic inputs seem able to rapidly turn on or off the local synthesis of E2 depending on the brain region considered [17,114]. Therefore, such a mechanism should provide a source of rapid and transient changes of estrogen production and thus concentration - not in the plasma, but locally in the brain – that matches quite well the time-frame of the rapid effects described for this hormone.
Moreover, inactivation mechanisms are present in the brain to terminate estrogenic signaling when the synthesis of the steroid has been switched off. Estrogenic hormones are catabolized to hormonally inactive (or less active) water-soluble metabolites that are excreted in the urine and/or feces. The metabolic elimination of estrogens relies on oxidative metabolism (largely hydroxylations by cytochromes P450 enzymes [289] and conjugations by glucuronidation [31], sulfonation [248,249] and/or O-methylation [159]. While hydroxylated products retain some estrogenic activity, conjugations appear to markedly decrease hormonal action. The metabolism of estrogens mostly takes place in the liver, but detectable levels of activity of metabolic enzymes are also expressed in other tissues including the brain. High levels of 2- and 4-hydroxylases have, for example, been identified in the brain, notably the preoptic-hypothalamic region [29,142,257,289]. The 2- and 4-hydroxyestrogens (also called catecholestrogens) are biologically active compounds but they are rapidly metabolized by the catecholamine-O-methyltransferases (COMT) into methoxyestrogens that have a weaker hormonal activity [159]. COMT is widely distributed in the brain [159] so that estrogens can be effectively transformed into less active compounds. Significant glucuronidase and sulfotransferase activities have also been identified in the brain [8,169,199]. As suggested by Song and Melner (2000), the inactivation in target tissues by these enzymes could function as a molecular switch to control local estrogen activity. In addition, aromatase itself appears to catalyze in the placenta both the conversion of testosterone into E2 and the 2-hydroxylation of estrogens into catecholestrogens. The switch between these two enzymatic activities (catalyzed by the same protein) would dependent on the availability of the substrates and the local pH [189]. If this scenario turns out to be true in the brain, this mechanism would provide a very effective way to rapidly control local concentrations of neuro-active estrogens. Together, these data demonstrate that active pathways for estrogen synthesis and catabolism are thus present in the brain and they may proceed at speeds compatible with the rapid induction and termination of estrogenic signaling that is required to explain the fast and transient effects produced by the steroid.
Fast effect of aromatase inhibition on physiology and behavior
Effects on appetitive and consummatory aspects of male sexual behavior
If rapid modulations of brain estrogen synthesis and catabolism are involved in the non-genomic control by estrogens of physiological and behavioral processes, the blockade of aromatase activity should rapidly lead to detectable changes in these responses. Accordingly, in recent experiments conducted in quail, systemic injections of a large dose of Vorozole™, a non-steroidal aromatase inhibitor, significantly reduced most aspects of male copulatory behavior in sexually active males (gonadally intact males or castrates implanted with 40 mm testosterone capsules). Maximal effects were observed after 30 or 45 minutes [67]. The acute effect of aromatase inhibition was even more pronounced on the expression of appetitive male sexual behavior. Appetitive sexual behavior was assessed by the frequency of rhythmic cloacal sphincter movements (RCSM) and by the expression of the learned social proximity response, two sexually motivated behavior that have been shown previously to be dependent on the aromatization of testosterone [15,26,79,233]. A significant inhibition of RCSM was observed 30 or 45 min after the injection of Vorozole™ and was no longer significant after 60 min. The behavior returned to a normal rate on the next day [67]. Androstatrienedione (ATD), another unrelated aromatase inhibitor with a very different chemical structure, induced a similar rapid inhibition of RCSM [67]. These behavioral inhibitions correlated with inhibitions of the aromatase activity measured in the preoptic-hypothalamus area (see [67] for further details). The fact that similar effects of aromatase inhibition were observed in gonadally intact males and in castrates chronically treated with testosterone suggests that the behavioral effect resulted from a rapid modulation of aromatase activity and thus local estrogen bioavailability rather than from a rapid change in secretion of gonadal testosterone. Together, these pharmacological data thus support a role for rapid modulations of estrogen synthesis in the regulation of both appetitive and consummatory aspects of male sexual behavior.
Conversely, in other experiments, we mimicked the putative effects of a rapid activation of brain aromatase activity by injecting a large dose of E2 to castrated male quail treated with a sub-threshold dose of testosterone, unable by itself to activate a full copulatory behavior, in order to prime the mechanisms involved in sexual behavior. As expected, a marked induction of all aspects of male copulatory behavior was observed in these experiments 15 min following the estrogen injection and this effect was no longer present at 30 min post injection [64].
We also investigated whether preoptic aromatase activity is rapidly modulated in vivo during sexual activity. Gonadally intact males were allowed to see a female or copulate with her for 1, 5 or 15 min and sacrificed immediately after the interaction. Control subjects were simply handled and returned to their home cage for the duration of the test. The visual access as well as sexual interactions with a female resulted in a 20% decrease of aromatase activity within 1 min. The enzymatic inhibition reached a maximum after 5 min and was back to normal after 15 min, [65]. As expected based on in vitro observations (see above), these data thus demonstrate that aromatase activity can be modulated very rapidly (faster than in the 5 min limit that could be tested in vitro) and that activity changes occur in vivo in physiologically relevant situations. The fact that enzymatic activity dropped following sexual interactions could however appear somewhat counterintuitive and an increase could have a priori been expected (e.g. to prepare to additional interactions with the female). It must however be pointed out that copulation in sexually experienced quail occurs with a time-course very different from what is observed in rodents. Adult male quail immediately engage in mating after the introduction of a female in their cage and usually achieve cloacal contact and sperm transfer within a few seconds. A successful copulation is thus usually achieved in less than one min. Sexual behavior occurs in bouts of activity of a few minutes separated by periods during which the bird takes care of alternative needs, such as preening its plumage, feeding, etc [121]. This drop of local estrogen synthesis thus coincides with and possibly determines the transient period of refractoriness that follows sexual activity. It is thus possible that the rapid changes of aromatase activity observed in this study do not reflect what happens in the brain during the expression of sexual behavior (potentially a very short-lived increase in enzyme activity) but are rather associated with its termination.
At the mechanistic level, recent data from Dominguez and colleagues indicate that glutamate concentration progressively increases in the rat preoptic area in the course of mating to peak at ejaculation [78]. If a similar release of glutamate takes place in the preoptic area of quail during copulation, this change in neurotransmitter activity could then represent the cellular mechanism controlling the decrease of aromatase activity that was observed in these experiments.
We cannot however exclude that the expression of the behavior is preceded in quail by an extremely fast increase in estrogen production that cannot be detected at present due to technical limitations. An in vivo "on line" measure of activity would be required since the time needed to dissect and freeze the brain prevents analysis to latencies shorter than one min. Both an increase and then a decrease of enzymatic activity would thus participate in the control of the onset and the termination of bouts of sexual activity within a very short period of time. Alternatively, aromatase in quail could be constantly active at (nearly) maximal levels thus allowing the immediate initiation of sexual behavior at any time. The expression of mating behavior would then turn off the enzyme for a short period of time resulting in a transitory cessation of the behavior. This would still be in agreement with our pharmacological data indicating that a bolus of estrogen is able to establish the local estrogen concentration required to allow the initiation of the behavior, while Vorozole™ treatment obviously mimics the natural drop of aromatase activity following mating.
Effects on nociception
Estrogen has been shown to control nociception and analgesia in both sexes in human as well as in animal models [90]. Estrogen actions on nociception and analgesia involve a genomic modulation of the signaling of opioid and adrenergic systems in the peripheral and the central the nervous system [9,77,146]. Moreover, recent studies suggest that estrogen also controls nociception in a non-genomic manner. Indeed, 17β-estradiol, as well 17β-estradiol conjugated with bovine serum albumin, inhibits ATP-induced intracellular calcium concentration within 5 min in the dorsal root ganglia (DRG) that contains a subset of nociceptive neurons [55]. This inhibition of ATP-induced Ca2+ flux by estradiol results from the blockade a voltage dependent calcium channel. The inhibition is not observed in ERαKO but persists in ERβKO mice indicating that this non-genomic modulation of DRG signaling is mediated through ERα [56]. Since ATP has been implicated in sensory transduction of noxious stimuli, these studies also provide a mechanism through which estradiol may inhibit Ca2+ fluxes associated with ATP-induced nociception.
Aromatase is also expressed and enzymatically active in pain-sensitive neurons in the dorsal horn of the spinal cord in quail [83]. Locally produced estrogens appear to modulate pain responses to noxious stimuli. Indeed, the latency of withdrawal from a 54°C hot water bath is markedly increased by castration in quail. By contrast, chronic testosterone treatment decreases this latency and it has been shown that this effect depends on its aromatization into estrogen [84]. Rapid effects on nociception of intrathecal injections of aromatase inhibitors (vorozole™ and ATD) are also observed: the foot withdrawal latency from a hot water bath is markedly increased 1 and 5 min after an intrathecal injection of the aromatase inhibitor, ATD or Vorozole™, and this effects has disappeared 30 min after the injection. These acute effects of a systemic injection of vorozole™ are inhibited by a concurrent injection of E2 [85]. Similar effects were reported recently in rats [86]. Together these data indicate that the rapid effects of locally produced estrogens on neuronal physiology and behavior do not only concern the brain but also the spinal cord. Such effects can be measured by behavioral responses in addition to the rapid electrophysiological effects previously identified in single neurons.
Conclusions
Besides their well-known long lasting action, mounting evidence indicates that estrogens also exert rapid effects on physiological and behavioral processes. These effects are activated within a few minutes and have been described in both sexes. Rapid effects of steroids, estrogens in particular, have been the subject of intense debates concerning namely the question of the existence and nature of the receptors involved. However, the fundamental issue of the source of rapid changes in estrogen concentrations required to sustain their fast effects has not received much attention. In a few rodent studies, it was assumed that the source of estrogen was the pro-estrus rise preceding receptivity and ovulation in females. Available data suggest that this relatively slow increase in plasma hormone is unable to take advantage of the rapid temporal resolution of most recently identified non-genomic effects of estrogens. In addition, fast effects of estrogens are also observed in males in which no such variation in plasma concentrations of the hormone have been described. Instead, rapid changes of testosterone levels elicited by social cues have been reported. The cerebral aromatization of the androgen may thus be one source of rapid change of local estrogen availability in males. In addition, rapid changes of aromatase activity resulting in fast regulations of estrogen availability in the brain have been identified. Such variations in brain aromatase activity provide a source of rapid and transient production of high concentrations of estrogen that seems to perfectly match the requirements for numbers of non-genomic effects. These findings thus open new avenues in the understanding of estrogen action on the brain. Of course, numerous questions still have to be addressed. So far, rapid changes of aromatase activity have been described in the preoptic/hypothalamic area and future studies should continue to investigate the mechanisms underlying these rapid activity changes and determine whether they take place in all brain regions where aromatase is present. Likewise, although these effects may theoretically occur both in males and in females, they have been studied in males exclusively to this date. Little attention has also been devoted to the mechanism(s) that control estrogen catabolism in the brain. The characterization of these rapid changes in brain estrogen production and catabolism and the analysis of their physiological and behavioral consequences will certainly have a profound impact on the planning future research programs and design of clinical interventions that involve actions of estrogen in the brain.
Acknowledgements
Research in our laboratory and preparation of this article were supported by grants NIH/NIMH R01 MH50388 to G.F.B. and FRFC 2.4562.05 to J.B. We thank all our collaborators who helped to produce the original data that are the basis of this article, in particular Drs M. Baillien and H.C. Evrard. We also thank Drs J. Bakker, M. Keller, G.E. Hoffman and M.M. McCarthy for suggesting a number of useful references and ideas that are used in this review. Finally, we thank M. Taziaux for discussion and comments on the manuscript as well as three anonymous reviewers who provided helpful comments on the earlier version of the manuscript.
References
- 1.Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 2.Abraham IM, Han S-K, Todman MG, Korach KS, Herbison AE. Estrogen receptor Beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. Journal of Neuroscience. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 4.Absil P, Baillien M, Ball GF, Panzica GC, Balthazart J. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res Rev. 2001;37:38–58. doi: 10.1016/s0165-0173(01)00122-9. [DOI] [PubMed] [Google Scholar]
- 5.Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pnieswski EE. Further evidence that androgen aromatization is essential for the activation of in male quail. Physiology and behavior. 1980;24:441–446. doi: 10.1016/0031-9384(80)90233-4. [DOI] [PubMed] [Google Scholar]
- 6.Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 7.Ahdieh HB, Siegel HI, Wade GN. The role of the uterus in regulation of heat duration in cycling rats. Horm Behav. 1985;19:292–303. doi: 10.1016/0018-506x(85)90028-5. [DOI] [PubMed] [Google Scholar]
- 8.Albert C, Barbier O, Vallée M, Beaudry G, Bélanger A, Hum DW. Distribution of uridine diphosphate-glucuronsyltransferase (UGT) expression and activity in cynomolgus monkey tissues : evidence for differential expression of steroid-conjugating UGT enzymes in steroid target tissues. Endocrinology. 2000;141:2472–2480. doi: 10.1210/endo.141.7.7583. [DOI] [PubMed] [Google Scholar]
- 9.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 10.Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- 11.Aziz A, Bergquist C, Brannstrom M, Nordholm L, Silfverstolpe G. Differences in aspects of personality and sexuality between perimenopausal women making different choices regarding prophylactic oophorectomy at elective hysterectomy. Acta Obstet Gynecol Scand. 2005;84:854–859. doi: 10.1111/j.0001-6349.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 12.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Ball GF, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 2. San Diego, CA: Academic Press; 2002. pp. 649–798. [Google Scholar]
- 14.Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. Journal of Neurobiology. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in japanese quail are differentialy regulated by subregions of the preoptic medial nucleus. Journal of Neuroscience. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J.Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 17.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Baillien M, Charlier TD, Cornil CA, Ball GF. Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level. Journal of Steroid Biochemistry & Molecular Biology. 2003;86:367–379. doi: 10.1016/s0960-0760(03)00346-7. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006 doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Balthazart J, Delville Y, Sulon Y, Hendrick JC. Plasma levels of luteinizing hormone and of five steroids in photostimulated, castrated and testosterone-treated male and female Japanese quail (Coturnix coturnix japonica) General Endocrinol (Life Sci.Adv.) 1986;5:31–36. [Google Scholar]
- 22.Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal inhibitor R76713 on testosterone-induced sexual behavior in the japanese quail (Coturnix coturnix japonica) Hormones and Behavior. 1990;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- 23.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J.Steroid Biochem.Molec.Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 24.Balthazart J, Foidart A, Baillien M, Harada N, Ball GF. Anatomical relationship between aromatase and tyrosine hydroxylase in the quail brain: double-label immunocytochemical studies. Journal of Comparative Neurology. 1998;391:214–226. [PubMed] [Google Scholar]
- 25.Balthazart J, Foidart A, Hendrick C. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiology & Behavior. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- 26.Balthazart J, Reid J, Absil P, Foidart A. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behavioral Neuroscience. 1995;109:485–501. [PubMed] [Google Scholar]
- 27.Balthazart J, Schumacher M, Malacarne G. Interaction of androgens and estrogens in the control of sexual behavior in male Japanese quail. Physiol.Behav. 1985;35:157–166. doi: 10.1016/0031-9384(85)90330-0. [DOI] [PubMed] [Google Scholar]
- 28.Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the japanese quail: behavior, morphology and intracellular metabolism of testosterone. General and Comparative Endocrinology. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- 29.Balthazart J, Stoop R, Foidart A, Granneman JCM, Lambert JGD. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain Res.Bull. 1994;35:339–345. doi: 10.1016/0361-9230(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 30.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol.Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- 31.Barbier O, Bélanger A. The cynomolgus monkey (Macaca fascicularis) is the best animal model for the study of steroid glucuronidation. Journal of Steroid Biochemistry & Molecular Biology. 2003;85:235–245. doi: 10.1016/s0960-0760(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 32.Batty J. Acute changes in plasma testosterone levels and their relation to measures of sexual behaviour in the male house mouse (Mus musculus) Anim Behav. 1978;26:349–357. doi: 10.1016/0003-3472(78)90053-2. [DOI] [PubMed] [Google Scholar]
- 33.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neuroscience Letters. 1990;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 34.Becker JB, Breedlove SM, Crews D, McCarthy MM. Behavioral Endocrinology. 2nd edn. Cambridge, Massachusetts London, england: The MIT Press; 2002. 776 pp. [Google Scholar]
- 35.Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- 36.Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- 37.Benten WPM, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Molecular Biology of the Cell. 1999;10:3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyer C, Morali G, Naftolin F, Larsson K, Perez-Palacios G. Effect of some antiestrogens and aromatase inhibitors on androgen induced sexual behavior in castrated male rats. Hormones and Behavior. 1976;7:353–363. doi: 10.1016/0018-506x(76)90040-4. [DOI] [PubMed] [Google Scholar]
- 39.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc.Natl.Acad.Sci.USA. 2000;97:3602–3067. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bixo M, Backstrom T, Winblad B, Andersson A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J Steroid Biochem Mol Biol. 1995;55:297–303. doi: 10.1016/0960-0760(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 41.Bjornerem A, Straume B, Midtby M, Fonnebo V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen GK. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab. 2004;89:6039–6047. doi: 10.1210/jc.2004-0735. [DOI] [PubMed] [Google Scholar]
- 42.Bjornerem A, Straume B, Oian P, Berntsen GK. Seasonal Variation of Estradiol, Follicle Stimulating Hormone and Dehydroepiandrosterone Sulfate in Women and Men. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-0866. [DOI] [PubMed] [Google Scholar]
- 43.Blomqvist A. Sex hormones and pain: a new role for brain aromatase? J Comp Neurol. 2000;423:549–551. [PubMed] [Google Scholar]
- 44.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 45.Boulware MI, Mermelstein PG. The influence of estradiol on nervous system function. Drug News Perspect. 2005;18:631–637. doi: 10.1358/dnp.2005.18.10.959578. [DOI] [PubMed] [Google Scholar]
- 46.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–1589. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- 48.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 49.Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rat. Brain Research. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- 50.Burmeister S, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- 51.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 52.Cashdan E. Hormones, sex, and status in women. Horm Behav. 1995;29:354–366. doi: 10.1006/hbeh.1995.1025. [DOI] [PubMed] [Google Scholar]
- 53.Cerda-Molina AL, Hernandez-Lopez L, Chavira R, Cardenas M, Paez-Ponce D, Cervantes-De la Luz H, Mondragon-Ceballos R. Endocrine changes in male stumptailed macaques (Macaca arctoides) as a response to odor stimulation with vaginal secretions. Horm Behav. 2006;49:81–87. doi: 10.1016/j.yhbeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 55.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 56.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 57.Chappell PE, Lydon JP, Conneely OM, O'Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- 58.Christensen LW, Clemens LG. Blockade of testosterone-induced mounting behavior in the male rat with intracranial application of the aromatization inhibitor, androst-1,4,6,-triene-3,17-dione. Endocrinology. 1975;97:1545–1551. doi: 10.1210/endo-97-6-1545. [DOI] [PubMed] [Google Scholar]
- 59.Christensen LW, Clemens LG. Intrahypothalamic implants of testosterone or estradiol and resumption of masculine sexual behavior in long-term castrated male rats. Endocrinology. 1974;95:984–990. doi: 10.1210/endo-95-4-984. [DOI] [PubMed] [Google Scholar]
- 60.Clancy AN, Zumpe D, Michael RP. Intracerebral infusion of an aromatase inhibitor, sexual behavior and brain estrogen receptor-like immunoreactivity in intact male rats. Neuroendocrinology. 1995;61:98–111. doi: 10.1159/000126830. [DOI] [PubMed] [Google Scholar]
- 61.Clifton PG, Andrew RJ. A comparison of the effects of testosterone on aggressive responses by the domestic chick to the human hand and to a large sphere. Animal Behaviour. 1981;29:610–620. [Google Scholar]
- 62.Coquelin A, Bronson FH. Release of luteinizing hormone in male mice during exposure to females: habituation of the response. Science. 1979;206:1099–1101. doi: 10.1126/science.573924. [DOI] [PubMed] [Google Scholar]
- 63.Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. Journal of Neuroscience. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthazart J. Sexual behavior affects preoptic aromatase activity and brain monoamines’ levels. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornil CA, Seutin V, Motte P, Balthazart J. Electrophysiological and neurochemical characterization of neurons of the medial preoptic area in Japanese quail (Coturnix japonica) Brain Research. 2004;1029:224–240. doi: 10.1016/j.brainres.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 67.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 70.Davis PG, Barfield RJ. Activation of masculine sexual behavior by intracranial estradiol benzoate implants in male rats. Neuroendocrinology. 1979;28:217–227. doi: 10.1159/000122865. [DOI] [PubMed] [Google Scholar]
- 71.DeBold JF, Frye CA. Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Hormones and Behavior. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 72.Delville Y, Hendrick JC, Sulon J, Balthazart J. Testosterone metabolism and testosterone-dependent characteristics in Japanese quail. Physiol Behav. 1984;33:817–823. doi: 10.1016/0031-9384(84)90053-2. [DOI] [PubMed] [Google Scholar]
- 73.Delville Y, Sulon J, Balthazart J. Diurnal variations of sexual receptivity in the female Japanese quail (Coturnix coturnix japonica) Horm Behav. 1986;20:13–33. doi: 10.1016/0018-506x(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 74.Delville Y, Sulon J, Balthazart J. Hormonal correlates of gonadal regression and spontaneous recovery in Japanese quail exposed to short day-lengths. Arch.Int.Physiol.Bioch. 1985;93:123–133. doi: 10.3109/13813458509079598. [DOI] [PubMed] [Google Scholar]
- 75.Delville Y, Sulon J, Hendrick JC, Balthazart J. Effect of the presence of females on the pituitary-testicular activity in male Japanese quail (Coturnix coturnix japonica) Gen.Comp.Endocrinol. 1984;55:295–305. doi: 10.1016/0016-6480(84)90115-1. [DOI] [PubMed] [Google Scholar]
- 76.Deutch AY, Roth RH. Neurotransmitters. In: Squire LR, Roberts JL, Spitzer NC, Zigmond MJ, McConnell SK, Bloom FE, editors. Fundamental Neuroscience. Academic Press; 1999. pp. 163–196. [Google Scholar]
- 77.Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 78.Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26:1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Domjan M, Hall S. Sexual dimorphism in the social proximity behavior of Japanese quail (Coturnix coturnix japonica) Journal of Comparative Psychology. 1986;100:68–71. [PubMed] [Google Scholar]
- 80.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends in Endocrinology and Metabolism. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans SJ, Murray TF, Moore FL. Partial purification and biochemical characterization of a membrane glucocorticoid receptor from an amphibian brain. Journal of Steroid Biochemistry & Molecular Biology. 2000;72:209–221. doi: 10.1016/s0960-0760(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 82.Evans SJ, Searcy BT, Moore FL. A subset of kappa opioid ligands bind to the membrane glucocorticoid receptor in an amphibian brain. Endocrinology. 2000;141:2294–2300. doi: 10.1210/endo.141.7.7587. [DOI] [PubMed] [Google Scholar]
- 83.Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. Journal of Comparative Neurology. 2000:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 84.Evrard HC, Balthazart J. Aromatization of androgens into estrogens reduces response latency to a noxious thermal stimulus in male quail. Hormones and Behavior. 2004;45:181–189. doi: 10.1016/j.yhbeh.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 85.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. Journal of Neuroscience. 2004;24:9225–9229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evrard HC, Erskine MS. Spinal estrogen synthesis in spinal dorsal horn neurons. Soc. Neurosci. 2005 Abstracts 746.16. [Google Scholar]
- 87.Evrard HC, Harada N, Balthazart J. Immunocytochemical localization of aromatase in sensory and integrating nuclei of the hindbrain in Japanese quail (Coturnix japonica) J Comp Neurol. 2004;473:194–212. doi: 10.1002/cne.20068. [DOI] [PubMed] [Google Scholar]
- 88.Feder HH, Storey A, Goodwin D, Reboulleau C, Silver R. Testosterone and "5alpha-dihydrotestosterone" levels in peripheral plasma of male and female ring doves (Streptopelia risoria) during and reproductive cycle. Biol Reprod. 1977;16:666–677. doi: 10.1095/biolreprod16.5.666. [DOI] [PubMed] [Google Scholar]
- 89.Filardo EJ, Thomas P. GPR30 : a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and Biobehavioral Reviews. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 91.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J.Chem.Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 92.Folman Y, Drori D. Effects of social isolation and of female odours on the reproductive system, kidneys and adrenals of unmated male rats. J Reprod Fertil. 1966;11:43–50. [Google Scholar]
- 93.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J.Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fowler KA, Gill K, Kirma N, Dillehay DL, Tekmal RR. Overexpression of aromatase leads to development of testicular leydig cell tumors : an in vivo model for hormone-mediated TesticularCancer. Am J Pathol. 2000;156:347–353. doi: 10.1016/S0002-9440(10)64736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frye CA, Mermelstein PG, DeBold JF. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Research. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- 96.Frye CA, Petralia SM, Rhodes ME, Stein B. Fluoxetine may influence lordosis of rats through effects on midbrain 3a,5a-THP concentrations. Annals New York Academy of Sciences. 2003;1007:37–41. doi: 10.1196/annals.1286.004. [DOI] [PubMed] [Google Scholar]
- 97.Gelez H, Fabre-Nys C. The "male effect" in sheep and goats: a review of the respective roles of the two olfactory systems. Horm Behav. 2004;46:257–271. doi: 10.1016/j.yhbeh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 98.George FW. Androgen metabolism in the prostate of the finasteride-treated, adult rat: a possible explanation for the differential action of testosterone and 5 alpha-dihydrotestosterone during development of the male urogenital tract. Endocrinology. 1997;138:871–877. doi: 10.1210/endo.138.3.5009. [DOI] [PubMed] [Google Scholar]
- 99.Gill K, Kirma N, Tekmal RR. Overexpression of aromatase in transgenic male mice results in the induction of gynecomastia and other biochemical changes in mammary glands. J Steroid Biochem Mol Biol. 2001;77:13–18. doi: 10.1016/s0960-0760(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 100.Graham JM, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–1041. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- 101.Greenberg N, Crews D. Endocrine and Behavioral-Responses to Aggression and Social-Dominance in the Green Anole Lizard, Anolis-Carolinensis. General and Comparative Endocrinology. 1990;77:246–255. doi: 10.1016/0016-6480(90)90309-a. [DOI] [PubMed] [Google Scholar]
- 102.Gu GB, Rojo AA, Zee MC, Yu JH, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. Journal of Neuroscience. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gu Q, Korach KS, Moss RL. Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 104.Gu Q, Moss RL. Novel mechanism for non-genomic action of 17β-oestradiol on kainate-induced in isolated rat CA1 hippocampal neurones. J.Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26:1013–1022. doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- 106.Hall PF. Testicular steroid synthesis : Organization and synthesis. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. Vol. 1. New York: Raven Press; 1994. pp. 1335–1362. [Google Scholar]
- 107.Haltmeyer GC, Eik-Nes KB. Plasma levels of testosterone in male rabbits following copulation. J Reprod Fertil. 1969;19:273–277. doi: 10.1530/jrf.0.0190273. [DOI] [PubMed] [Google Scholar]
- 108.Harding CF. Social modulation of circulating hormone levels in the male. J Amer.Zool. 1981;21:223–231. [Google Scholar]
- 109.Harding CF, Follett BK. Hormone changes triggered by aggression in a natural population of blackbirds. J Science. 1979;203:918–920. doi: 10.1126/science.570304. [DOI] [PubMed] [Google Scholar]
- 110.Hayden-Hixson DM, Ferris CF. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. J Physiology & Behavior. 1991;50:793–799. doi: 10.1016/0031-9384(91)90020-o. [DOI] [PubMed] [Google Scholar]
- 111.Hilliard J, Pang CN, Penardi R, Sawyer CH. Effect of coitus on serum levels of testosterone and LH in male and female rabbits. J Proc Soc Exp Biol Med. 1975;149:1010–1014. doi: 10.3181/00379727-149-38945. [DOI] [PubMed] [Google Scholar]
- 112.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshii E, Fujiwarai N, Taniguchii N, Murata Y. Estrogen Induces the Akt-dependent Activation of Endothelial Nitric-oxide Synthase in Vascular Endothelial Cells. J Journal of biological Chemistry. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- 113.Hoffman NW, Kim YI, Gorski RA, Dudek FE. Homogeneity of intracellular eletrophysiological properties in different neuronal subtypes in medial preoptic slices containing the sexually dimorphic nucleus of rat. J.Comp.Neurol. 1994;345:396–408. doi: 10.1002/cne.903450306. [DOI] [PubMed] [Google Scholar]
- 114.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nature Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- 116.Honda S, Harada N, Takagi T. Novel exon 1 of the aromatase gene specific for aromatase transcripts in human brain. Biochemical and biophysiological research communications. 1994;198:1153–1160. doi: 10.1006/bbrc.1994.1163. [DOI] [PubMed] [Google Scholar]
- 117.Hotchkiss J, Knobil E. The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. Vol. 2. New York: Raven Press; 1994. pp. 711–750. [Google Scholar]
- 118.Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 1. San Diego, CA: Academic Press; 2002. pp. 3–137. [Google Scholar]
- 119.Hutchison JB, Katongole CB. Plasma testosterone in courting and incubating male barbary doves (Streptopelia risoria) J Endocrinol. 1975;65:275–276. doi: 10.1677/joe.0.0650275. [DOI] [PubMed] [Google Scholar]
- 120.Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984;103:363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- 121.Hutchison RE. Hormonal differentiation of sexual behavior in Japanese quail. Hormones and Behavior. 1978;11:363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- 122.Improta-Brears T, whorton AR, Codazzi F, York JD, Meyer T, McDonnell DP. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. PNAS. 1999;96:4686–4691. doi: 10.1073/pnas.96.8.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++responses: structure-activity studies. J.Pharm.Exp.Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- 124.Irwin RP, Maragakis NJ, Rogawski MA, Purdy RH, Farb DH, Paul SM. Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons. Neuroscience Letters. 1992;141:30–34. doi: 10.1016/0304-3940(92)90327-4. [DOI] [PubMed] [Google Scholar]
- 125.Johnston RE, Bronson F. Endocrine control of female mouse odors that elicit luteinizing hormone surges and attraction in males. Biol Reprod. 1982;27:1174–1180. doi: 10.1095/biolreprod27.5.1174. [DOI] [PubMed] [Google Scholar]
- 126.Joyce JN, Van Hartesveldt C. Estradiol application to one striatum produces postural deviation to systemic apomorphine. Pharmacol Biochem Behav. 1984;20:575–581. doi: 10.1016/0091-3057(84)90307-1. [DOI] [PubMed] [Google Scholar]
- 127.Judd HL, Yen SS. Serum androstenedione and testosterone levels during the menstrual cycle. J Clin Endocrinol Metab. 1973;36:475–481. doi: 10.1210/jcem-36-3-475. [DOI] [PubMed] [Google Scholar]
- 128.Kamel F, Mock EJ, Wright WW, Frankel AI. Alterations in plasma concentrations of testosterone, LH, and prolactin associated with mating in the male rat. Horm Behav. 1975;6:277–288. doi: 10.1016/0018-506x(75)90014-8. [DOI] [PubMed] [Google Scholar]
- 129.Kamel F, Wright WW, Mock EJ, Frankel AI. The influence of Mating and Related Stimuli on Plasma-Levels of Luteinizing-Hormone, Follicle-Stimulating Hormone, Prolactin, and Testosterone in Male Rat. Endocrinology. 1977;101:421–429. doi: 10.1210/endo-101-2-421. [DOI] [PubMed] [Google Scholar]
- 130.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Research. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 131.Kelly MJ, Qiu J, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) Journal of Steroid Biochemistry & Molecular Biology. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- 132.Kelly MJ, Ronnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 3. 2002. pp. 361–380. [Google Scholar]
- 133.Kelly MJ, Wagner EJ. Estrogen modulation of G-protein-coupled receptors. Trends in Endocrinology and Metabolism. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 134.Kirma N, Gill K, Mandava U, Tekmal RR. Overexpression of aromatase leads to hyperplasia and changes in the expression of genes involved in apoptosis, cell cycle, growth, and tumor suppressor functions in the mammary glands of transgenic mice. Cancer Res. 2001;61:1910–1918. [PubMed] [Google Scholar]
- 135.Knobil E. On the control of gonadotropin secretion in Rhesus monkey. Recent Progress in Hormone Research. 1976;30:1–36. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- 136.Komisaruk BR, Terasawa E, Rodriguez-Sierra JF. How the brain mediates ovarian responses to environmental stimuli. Neuroanatomy and neurophysiology. In: Adler NT, editor. Neuroendocrinology of reproduction. New York and London: Plenum Press; 1981. pp. 349–376. [Google Scholar]
- 137.Kow L-M, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rats hypothalamic ventromedial nucleus. Brain Research. 2005;1043:124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 138.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101:12354–11235. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JÅ. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 140.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JÅ. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc.Natl.Acad.Sci.USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol.Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 142.Lambert JJ, Belelli D, Shepherd SE, Pistis M, Peters JA. The selective interaction of neurosteroids with the GABAa receptor. In: Baulieu EE, Robel P, Schumacher M, editors. Neurosteroids. A new regulatory function in the nervous system. Humana press; 1999. pp. 125–142. [Google Scholar]
- 143.Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 144.Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- 145.Liley NR, Olsen KH, Foote CJ, Van der Kraak GJ. Endocrine changes associated with spawning behavior in male kokanee salmon (Oncorhynchus nerka) and the effects of anosmia. Horm Behav. 1993;27:470–487. doi: 10.1006/hbeh.1993.1034. [DOI] [PubMed] [Google Scholar]
- 146.Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 147.London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- 148.Lu JK, LaPolt PS, Nass TE, Matt DW, Judd HL. Relation of circulating estradiol and progesterone to gonadotropin secretion and estrous cyclicity in aging female rats. Endocrinology. 1985;116:1953–1959. doi: 10.1210/endo-116-5-1953. [DOI] [PubMed] [Google Scholar]
- 149.Lutz LB, Jamnongjit M, Yang W-H, Jahani D, Gill A, Hammes SR. Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligands. Molecular Endocrinology. 2003;17:1106–1116. doi: 10.1210/me.2003-0032. [DOI] [PubMed] [Google Scholar]
- 150.Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- 151.Macrides F, Bartke A, Fernandez F, D'Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinology. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- 152.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system : Mechanisms and nonreproductive functions. Annual Reviews of Physiology. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 153.Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. Journal of Biochemical Chemistry. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 154.Mahendroo MS, Cala KM, Landrum DP, Russell DW. Fetal death in mice lacking 5alpha-reductase type 1 caused by estrogen excess. Mol Endocrinol. 1997;11:917–927. doi: 10.1210/mend.11.7.9933. [DOI] [PubMed] [Google Scholar]
- 155.Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 156.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptors. Science. 1986;232:1004–1008. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 157.Malmnas CO. Short-latency effect of testosterone on copulatory behaviour and ejaculation in sexually experienced intact male rats. J Reprod Fertil. 1977;51:351–354. doi: 10.1530/jrf.0.0510351. [DOI] [PubMed] [Google Scholar]
- 158.Mani SK, Blaustein JD, O'Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- 159.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacity of the new selective COMT inhibitors. Pharmacological Reviews. 1999;51:593–628. [PubMed] [Google Scholar]
- 160.Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- 161.Matsunaga M, Ukena K, Tsutsui K. Androgen synthesis in the quail brain. Brain Research. 2002;948:180–185. doi: 10.1016/s0006-8993(02)03147-5. [DOI] [PubMed] [Google Scholar]
- 162.McCarthy MM, Becker JB. Neuroendocrinology of sexual behavior in the female. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. Cambridge, Massachusetts London, England: MIT press; 2002. pp. 117–151. [Google Scholar]
- 163.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 164.Mensah-Nyagan AG, Do-Rego J-L, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation os steroid biosynthesis in the central nervous system. Pharmacological Reviews. 1999;51:63–81. [PubMed] [Google Scholar]
- 165.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J.Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- 167.Meyer ME, Van Hartesveldt C. Differential effects of intrastriatal estradiol on the dorsal immobility response in male rats. Pharmacol Biochem Behav. 1992;43:303–306. doi: 10.1016/0091-3057(92)90672-3. [DOI] [PubMed] [Google Scholar]
- 168.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-src complex triggers prostate cancer cell proliferation. EMBO.J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. Journal of Clinical Endocrinology & Metabolism. 2002;87:5760–5768. doi: 10.1210/jc.2002-020670. [DOI] [PubMed] [Google Scholar]
- 170.Minami T, Oomura Y, Nabekura J, Fukuda A. 17β-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 171.Mize AL, Alper RH. Acute and long-term effects of 17β-estradiol on Gi/o coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPγS binding. Brain Research. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- 172.Mize AL, Alper RH. Rapid uncoupling of serotonin-1A receptors in rat hippocampus by 17β-estradiol in vitro requires protein kinases A and C. Neuroendocrinology. 2002;76:339–347. doi: 10.1159/000067583. [DOI] [PubMed] [Google Scholar]
- 173.Mize AL, Young LJ, Alper RH. Uncoupling of 5-HT1A receptors in the brain by estrogens : regional variations in antagonism by ICI 182,780. Neuropharmacology. 2003;44:584–591. doi: 10.1016/s0028-3908(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 174.Moore FL, Orchinik M. Membrane receptors for corticosterone: a mechanism for rapid behavioral responses in an amphibian. Hormones and Behavior. 1994;28:512–519. doi: 10.1006/hbeh.1994.1049. [DOI] [PubMed] [Google Scholar]
- 175.Morley P, Whitfield JF, Vanderhyden BC, Tsang BK, Schwartz J-L. A new, non genomic estrogen action : the rapid release of intracellular calcium. Endocrinology. 1992;131:1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- 176.Moss RL, Gu Q, Wong M. Estrogen : nontranscriptional signaling pathway. Recent progress in hormone research. 1997;52:33–69. [PubMed] [Google Scholar]
- 177.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News in Physiological Sciences. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 178.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. Neuroendocrinol. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 179.Naftolin F, MacLusky NJ. Aromatization hypothesis revisited. In: Serio M, Motta M, Zanisi M, Martini L, editors. Sexual differentiation: basic and clinical aspects. New York: Raven Press; 1984. pp. 79–91. [Google Scholar]
- 180.Nelson RJ. An introduction to behavioral endocrinology. 2 edn. Sunderland, Massachussets: Sinauer Associates, Inc.; 2000. 0 pp. [Google Scholar]
- 181.Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD. Estradiol (E2) elicits Src phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology. 2001;142:5145–5148. doi: 10.1210/endo.142.12.8546. [DOI] [PubMed] [Google Scholar]
- 182.O'Byrne KT, Thalabard JC, Grosser PM, Wilson RC, Williams CL, Chen MD, Ladendorf D, Hotchkiss J, Knobil E. Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology. 1991;129:1207–1214. doi: 10.1210/endo-129-3-1207. [DOI] [PubMed] [Google Scholar]
- 183.O'Connell ME, Reboulleau C, Feder HH, Silver R. Social interactions and androgen levels in birds. I. Female characteristics associated with increased plasma androgen levels in the male ring dove (Streptopelia risoria) Gen Comp Endocrinol. 1981;44:454–463. doi: 10.1016/0016-6480(81)90332-4. [DOI] [PubMed] [Google Scholar]
- 184.O'Connell ME, Silver R, Feder HH, Reboulleau C. Social interactions and androgen levels in birds. II. Social factors associated with a decline in plasma androgen levels in male ring doves (Streptopelia risoria) Gen.Comp.Endocrinol. 1981;44:464–469. doi: 10.1016/0016-6480(81)90333-6. [DOI] [PubMed] [Google Scholar]
- 185.Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- 186.Oliveira RF, Lopes M, Carneiro LA, Canario AV. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. [DOI] [PubMed] [Google Scholar]
- 187.Orchinik M. Glucocorticoids, stress, and behavior: shifting the timeframe. Hormones and Behavior. 1998;34:320–327. doi: 10.1006/hbeh.1998.1488. [DOI] [PubMed] [Google Scholar]
- 188.Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- 189.Osawa Y, Higashiyama T, Shimizu Y, Yarborough C. Multiple functions of aromatase and the active site structure; aromatase is the placental estrogen 2-hydroxylase. Biochem.Molec.Biol. 1993;44:469–480. doi: 10.1016/0960-0760(93)90252-r. [DOI] [PubMed] [Google Scholar]
- 190.Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic α4β2 receptor forms a binding site required for potentiation by an estrogenic steroid. Journal of Neuroscience. 2001;21:6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J.Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- 192.Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- 193.Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J Neuroendocrinol. 2001;13:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- 194.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pietras RJ, Szego CM. Specific binding site for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 196.Pleim ET, Lisciotto CA, DeBold JF. Facilitation of sexual receptivity in hamsters by simultaneous progesterone implants into the VMH and ventral mesencephalon. Hormones and Behavior. 1990;24:139–151. doi: 10.1016/0018-506x(90)90001-e. [DOI] [PubMed] [Google Scholar]
- 197.Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 198.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms : Behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 2. San Diego, CA: Academic Press; 2002. pp. 93–156. [Google Scholar]
- 199.Purinton SC, Wood CE. Ovine fetal estrogen sulfotransferase in brain regions important for hypothalamus-pituitary-adrenal axis control. Neuroendocrinology. 2000;71:237–242. doi: 10.1159/000054541. [DOI] [PubMed] [Google Scholar]
- 200.Purvis K, Haynes NB. Short-term effects of copulation, human chorioninc gonadotrophin injection and non-tactile association with a female on testosterone levels in the male rat. Journal of Endocrinology. 1974;60:429–439. doi: 10.1677/joe.0.0600429. [DOI] [PubMed] [Google Scholar]
- 201.Purvis K, Haynes NB. The effect of female rat proximity on the reproductive system of male rats. Physiol Behav. 1972;9:401–407. doi: 10.1016/0031-9384(72)90167-9. [DOI] [PubMed] [Google Scholar]
- 202.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signalling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. Journal of Neuroscience. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ramirez VD, Soufi WL. The neuroendocrine control of the rabbit ovarian cycle. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. Vol. 2. New York: Raven Press; 1994. pp. 585–611. [Google Scholar]
- 204.Ramirez VD, Zheng J. Membrane Sex-steroid receptors in the brain. Front Neuroendocrinol. 1996;17:402–439. doi: 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- 205.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in chinese hamster ovary cells. Molecular Endocrinology. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 206.Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006 doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 207.Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Hormones and Behavior. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 208.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. Journal of Neuroscience. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signalling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 210.Rittmaster RS, Magor KE, Manning AP, Norman RW, Lazier CB. Differential effect of 5 alpha-reductase inhibition and castration on androgen-regulated gene expression in rat prostate. Mol Endocrinol. 1991;5:1023–1029. doi: 10.1210/mend-5-7-1023. [DOI] [PubMed] [Google Scholar]
- 211.Ronnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 212.Rose RM, Gordon TP, Bernstein IS. Plasma testosterone levels in the male rhesus: influences of sexual and social stimuli. Science. 1972;178:643–645. doi: 10.1126/science.178.4061.643. [DOI] [PubMed] [Google Scholar]
- 213.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Research Bulletin. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 214.Roselli CE, Larkin K, Schrunk JM, Stormshak F. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav. 2004;83:233–245. doi: 10.1016/j.physbeh.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 215.Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. Journal of Steroid Biochemistry & Molecular Biology. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 216.Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J.Steroid Biochem.Mol.Biol. 1997;61:365–374. [PubMed] [Google Scholar]
- 217.Roselli CE, Resko JA. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod. 1989;40:929–934. doi: 10.1095/biolreprod40.5.929. [DOI] [PubMed] [Google Scholar]
- 218.Sachs BD, Leipheimer RE. Rapid effect of testosterone on striated muscle activity in rats. Neuroendocrinology. 1988;48:453. doi: 10.1159/000125049. [DOI] [PubMed] [Google Scholar]
- 219.Sachser N. Short-term responses of plasma norepinephrine, epinephrine, glucocorticoid and testosterone titers to social and non-social stressors in male guinea pigs of different social status. Physiol Behav. 1987;39:11–20. doi: 10.1016/0031-9384(87)90338-6. [DOI] [PubMed] [Google Scholar]
- 220.Saginor M, Horton R. Reflex Release of Gonadotropin and Increased Plasma Testosterone Concentration in Male Rabbits during Copulation. Endocrinology. 1968;82 doi: 10.1210/endo-82-3-627. 627-&. [DOI] [PubMed] [Google Scholar]
- 221.Saldanha CJ, Schlinger BA, Micevych PE, Horvath S. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of song bird hippocampus. Journal of Comparative Neurology. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- 222.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 223.Sapolsky RM. The endocrine stress-response and social status in the wild baboon. Horm Behav. 1982;16:279–292. doi: 10.1016/0018-506x(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 224.Sasano H, Harada N. Intratumoral aromatase in human breast, endometrial and ovarian malignancies. Endocrine reviews. 1998;19:593–607. doi: 10.1210/edrv.19.5.0342. [DOI] [PubMed] [Google Scholar]
- 225.Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc.Natl.Acad.Sci.USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schlinger BA, Callard GV. A comparison of aromatase, 5 alpha-, and 5 beta- reductase activities in the brain and pituitary of male and female quail (C. c. japonica) J Exp Zool. 1987;242:171–180. doi: 10.1002/jez.1402420208. [DOI] [PubMed] [Google Scholar]
- 227.Schlinger BA, Callard GV. Aggressive behavior in birds: An experimental model for studies of brain-steroid interactions. Comp.Biochem.Physiol.[A] 1990;97A:307–316. doi: 10.1016/0300-9629(90)90616-z. [DOI] [PubMed] [Google Scholar]
- 228.Schlinger BA, Callard GV. Aromatase activity in quail brain: Correlation with aggressiveness. Endocrinology. 1989;124:437–443. doi: 10.1210/endo-124-1-437. [DOI] [PubMed] [Google Scholar]
- 229.Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen.Comp.Endocrinol. 1990;79:39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- 230.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 231.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 232.Segars JH, Driggers PH. Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends in Endocrinology and Metabolism. 2002;13:349–354. doi: 10.1016/s1043-2760(02)00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Seiwert CM, Adkins-Regan E. The foam production system of the male japanese quail: characterization of structure and function. Brain Behav.Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- 234.Selye H. Anesthetic effect of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. [Google Scholar]
- 235.Sherwin BB. Changes in sexual behavior as a function of plasma sex steroid levels in post-menopausal women. Maturitas. 1985;7:225–233. doi: 10.1016/0378-5122(85)90044-1. [DOI] [PubMed] [Google Scholar]
- 236.Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 237.Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, Waldbaum A, Bouchard C, Derzko C, Buch A, Rodenberg C, Lucas J, Davis S. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226–5233. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- 238.Simoncini T, Hafesi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 240.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase - A brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 241.Simpson ER, Davis SR. Minireview: Aromatase and the regulation of estrogen biosynthesis - Some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 242.Sinchak K, Shahedi K, Dewing P, Micevych P. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. Neuroreport. 2005;16:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- 243.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 244.Smith RL, Webster DG, Van Hartesveldt C, Meyer ME. Effects of estrus, estrogen-progesterone priming, and vaginal stimulation on tonic immobility, dorsal immobility, and lordosis in the female rat. Physiol Behav. 1985;35:577–581. doi: 10.1016/0031-9384(85)90143-x. [DOI] [PubMed] [Google Scholar]
- 245.Snyder SH, Ferris CD. Novel neurotransmitters and their neuropsychiatric relevance. Am J Psychiatry. 2000;157:1738–1751. doi: 10.1176/appi.ajp.157.11.1738. [DOI] [PubMed] [Google Scholar]
- 246.Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3β-hydrosteroid dehydrogenase/Δ5-Δ4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- 247.Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, Wingfield JC. Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrow. Journal of Comparative Physiology A. 2000;186:759–769. doi: 10.1007/s003590000129. [DOI] [PubMed] [Google Scholar]
- 248.Song W-C. Biochemistry and reproductive endocrinology of estrogen sulfotransferase. Annals New York Academy of Sciences. 2001;948:43–50. doi: 10.1111/j.1749-6632.2001.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 249.Song W-C, Melner MH. Steroid tranformation enzymes as critical regulators of steroid action in vivo. Endocrinology. 2000;141:1587–1589. doi: 10.1210/endo.141.5.7526. [DOI] [PubMed] [Google Scholar]
- 250.Steimer T. Aromatase activity in rat brain synaptosomes. Is an enzyme associated with the neuronal cell membrane involved in mediating non-genomic effects of androgens. Eur.J.Neurosci. Suppl. 1988;1988:9. [Google Scholar]
- 251.Steimer T, Hutchison JB. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature. 1981;292:345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- 252.Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- 253.Takeo Y. Influence of continuous illumination on estrous cycle of rats: time course of changes in levels of gonadotropins and ovarian steroids until occurrence of persistent estrus. Neuroendocrinology. 1984;39:97–104. doi: 10.1159/000123964. [DOI] [PubMed] [Google Scholar]
- 254.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 255.Thomas TR, Neiman CN. Aspects of copulatory behavior preventing atrophy in male rats' reproductive system. Endocrinology. 1968;83:633–635. doi: 10.1210/endo-83-3-633. [DOI] [PubMed] [Google Scholar]
- 256.Thorneycroft IH, Mishell DR, Jr, Stone SC, Kharma KM, Nakamura RM. The relation of serum 17-hydroxyprogesterone and estradiol-17-beta levels during the human menstrual cycle. Am J Obstet Gynecol. 1971;111:947–951. doi: 10.1016/0002-9378(71)90951-3. [DOI] [PubMed] [Google Scholar]
- 257.Timmers RJ, Granneman JC, Lambert JG, van Oordt PG. Estrogen-2-hydroxylase in the brain of the male African catfish, Clarias gariepinus. Gen Comp Endocrinol. 1988;72:190–203. doi: 10.1016/0016-6480(88)90202-x. [DOI] [PubMed] [Google Scholar]
- 258.Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- 259.Toran-Allerand CD. A plethora of estrogen receptors in the brain : where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 260.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Sander Connolly EJ, Nethrapalli IS, Tinnikov AA. ER-X : a novel membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Torres JM, Ortega E. Steroid 5alpha-reductase isozymes in the adult female rat brain: central role of dihydrotestosterone. J Mol Endocrinol. 2006;36:239–245. doi: 10.1677/jme.1.01907. [DOI] [PubMed] [Google Scholar]
- 262.Travison TG, Morley JE, Araujo AB, O'Donnell A B, McKinlay JB. The Relationship between Libido and Testosterone Levels in Aging Men. J Clin Endocrinol Metab. 2006;91:2509–2513. doi: 10.1210/jc.2005-2508. [DOI] [PubMed] [Google Scholar]
- 263.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 264.Van Hartesveldt C, Cottrell GA, Meyer ME. Effects of intrastriatal hormones on the dorsal immobility response in male rats. Pharmacol Biochem Behav. 1990;35:307–310. doi: 10.1016/0091-3057(90)90160-j. [DOI] [PubMed] [Google Scholar]
- 265.Van Hartesveldt C, Cottrell GA, Meyer ME. The effects of intrastriatal hormones on the dorsal immobility response in gonadectomized male and female rats. Pharmacol Biochem Behav. 1989;34:459–463. doi: 10.1016/0091-3057(89)90541-8. [DOI] [PubMed] [Google Scholar]
- 266.Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 268.Vockel A, Pröve E, Balthazart J. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J.Neurobiol. 1990;21:808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- 269.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5'-monophosphatase response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 270.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)a and ERb exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 271.Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase mRNA in the rat brain : indications of regional regulation. J.Steroid Biochem.Mol.Biol. 1997;61:307–314. [PubMed] [Google Scholar]
- 272.Wang L, Andersson S, Warner M, Gustafsson JA. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc Natl Acad Sci U S A. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Warner M, Gustafsson JA. Nongenomic effects of estrogen: why all the uncertainty? Steroids. 2006;71:91–95. doi: 10.1016/j.steroids.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 274.Watson JT, Adkins-Regan E. Activation of sexual behavior by implantation of testosterone propionate and estradiol benzoate into the preoptic area of the male Japanese quail (Coturnix japonica) Horm.Behav. 1989;23:251–268. doi: 10.1016/0018-506x(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 275.Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm.Behav. 1989;23:432–447. doi: 10.1016/0018-506x(89)90055-x. [DOI] [PubMed] [Google Scholar]
- 276.Watters JJ, campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells : effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 277.Weaver CEJ, Park-Chung M, Gibbs TT, Farb DH. 17β-estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Research. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- 278.Wetzel CHR, Hermann B, Behl C, Pestel E, Rammes G, Zieglgänsberger W, Holsboer F, Rupprecht R. Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor. Molecular Endocrinology. 1998;12:1441–1451. doi: 10.1210/mend.12.9.0163. [DOI] [PubMed] [Google Scholar]
- 279.Whitehead SA, Ruf KB. Responses of antidromicallly identified preoptic neurons in the rat to neurotransmitters and to estrogen. Brain Research. 1974;79:185–198. doi: 10.1016/0006-8993(74)90410-7. [DOI] [PubMed] [Google Scholar]
- 280.Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- 281.Wingfield JC. Short-term changes in plasma levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Horm Behav. 1985;19:174–187. doi: 10.1016/0018-506x(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 282.Wingfield JC, Silverin B. Ecophysiological studies of hormone-behavior relations in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 2. San Diego, CA: Academic Press; 2002. pp. 587–648. [Google Scholar]
- 283.Wong IL, Morris RS, Lobo RA. Effect of finasteride on the ovulatory function of normal women. Fertil Steril. 2003;79:1455–1457. doi: 10.1016/s0015-0282(03)00393-5. [DOI] [PubMed] [Google Scholar]
- 284.Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. Journal of Neuroscience. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- 286.Yagi K. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Research. 1973;53:343–352. doi: 10.1016/0006-8993(73)90219-9. [DOI] [PubMed] [Google Scholar]
- 287.Yamaji T, Dierschke DJ, Bhattacharya AN, Knobil E. The negative feedback control by estradiol and progesterone of LH secretion in the ovariectomized rhesus monkey. Endocrinology. 1972;90:771–777. doi: 10.1210/endo-90-3-771. [DOI] [PubMed] [Google Scholar]
- 288.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 289.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells : review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 290.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. PNAS. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membbrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. PNAS. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Horm Behav. 2005;47:56–64. doi: 10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]