Abstract
To assess the effect of edible mushroom extracts on the induction of T-helper 1 (Th1) immunity, we examined differences in interferon-gamma (IFN-γ) and interleukin (IL)-4 production in mice induced by hot-water extracts of 15 species of edible mushroom. Extracts from Agaricus bisporus, Flammulina velutipes, Hypsizigus marmoreus, Lentinula edodes, and Lyophyllum decastes induced both IFN-γ and IL-4 production in mice, whereas extracts from Pleurotus ostreatus only induced IL-4. In contrast, extracts from Agaricus blazei, Grifola frondosa, Morchella esculenta, Pholiota nameko, Pleurotus citrinopileatus, and Pleurotus eryngii induced only IFN-γ production. In particular, the extract from P. eryngii induced high levels of IFN-γ and reduced levels of IL-4. We further investigated the use of a trial immunogen using the P. eryngii extract as a Th1 immunostimulator. An oil-in-water emulsion of the hot-water extract from P. eryngii (immunostimulator) and ovalbumin (OVA; antigen) was used as a trial immunogen. This immunogen induced strong OVA-specific IgG2a antibody production in mice compared with the negative controls. In addition, OVA-specific IgG1 antibody levels were lower than those for the negative controls. Marked increases in serum IFN-γ levels and high-level production of IFN-γ in the culture supernatant from the CD4+ spleen cells in the trial immunogen group mice were observed. Our results suggested that the hot-water extract from P. eryngii induced Th1 immunity by acting as an immunostimulator.
Key Words: edible mushroom, immunostimulator, mushroom extract, Pleurotus eryngii, Th1 immunity
Mushrooms (Basidiomycota) have continued to attract a great deal of interest in many areas of food and biopharmaceutical research, and are well known for their nutritional and medicinal values.1,2 Several major substances with immunomodulatory and/or antitumor activity have been isolated from mushrooms. These include mainly polysaccharides, such as β-glucans, polysaccharopeptides, polysaccharide-protein conjugates, and proteins. In particular, β-glucans have a wide range of biological activities.3
Innate and adaptive immunity in mammals is complex, and important homeostasis mechanisms involve a variety of hematopoietic stem cells, including B cells, T cells, natural killer cells, and antigen presenting cells such as macrophages and dendritic cells.4 These cells are mediated and regulated by several cytokines or other bioactive factors, with T cells playing a major role.5 Mosmann et al. previously reported the importance of T helper (Th) 1 and Th2 cells in a host's immune response to infection.6 Cells designated as Th1 are characterized by interleukin (IL)-2, IL-12, and interferon-gamma (IFN-γ) production, which also activates macrophages.5,7 In contrast, Th2 cells are characterized by IL-4, IL-5, IL-6, IL-10, and IL-13 synthesis, which further promotes humoral immunity.8–10
Currently, there are major infectious diseases of humans and other animals that have no known cures, although the development of vaccines for many infectious diseases is increasingly being investigated.11 It has been reported that the Th1 immune system is more effective than the Th2 immune system in combating disease-causing intracellular pathogens. Therefore, the development of an immunostimulator in a vaccine adjuvant for the induction of Th1 immunity has great potential for preventing infectious diseases. Given that safety is a paramount consideration in the development of such adjuvants, extracts from edible mushrooms that can also have immunostimulatory effects may provide a safe solution for vaccine development.1 However, to our knowledge, a few reports have evaluated the polarization of immune responses in mice inoculated with different extracts from the many edible mushrooms available. The aim of this study, therefore, was to evaluate the immune responses of mice administered hot-water extracts from one of the 15 different species of edible mushroom. In addition, we determined whether selected extracts could induce Th1 immunity and act as potential immunostimulators for vaccine development.
Fifteen different species of edible mushrooms were used to produce the following extracts: Agaricus bisporus (Tsukuritake), Agaricus blazei (Himematsutake), Auricularia auricula (Kikurage), Flammulina velutipes (Enokitake), Grifola frondosa (Maitake), Hericium erinaceum (Yamabushitake), Hypsizigus marmoreus (Bunashimeji), Lentinula edodes (Shiitake), Lyophyllum decastes (Hatakeshimeji), Morchella esculenta (Amigasatake), Pholiota nameko (Nameko), Pleurotus citrinopileatus (Tamogitake), Pleurotus eryngii (Eringi), Pleurotus ostreatus (Hiratake), and Sparassis crispa (Hanabiratake). Each mushroom was dried using gentle airflow at 25°C for 24 h, before being resuspended in sterile Milli-Q water (Millipore, MA, USA; 20 mg/mL), and homogenized using an ART-MICCRA D-8 homogenizer (setting C) for 5 min. The homogenate was then boiled for 2 h, and any solid particles and aggregates were removed by centrifugation at 12,000 g for 20 min. The supernatant was filtered using a 0.45-μm pore-size Millex-HV filter (Millipore), and subsequently used as a hot-water mushroom extract.
Animals: Five-week-old female ddY mice were purchased from the Saitama Experimental Animals Supply Co., Ltd (Saitama, Japan). Experimental protocol was approved by the Animal Care and Use Committee of Nippon Veterinary and the Life Science University, Japan.
Ten mice were inoculated intraperitoneally with 0.5 mL of the hot-water mushroom extract prepared as described earlier. A control group of mice were similarly inoculated with 0.5 mL of sterilized Milli Q water. Seven days after inoculation, whole blood was collected from the infra-axillary vein of each mouse.12 Serum was then separated from each blood sample and stored at −80°C until use.
Cytokines IFN-γ and IL-4 were measured as markers of Th1 and Th2 cells, respectively, in the serum samples from mice inoculated with the individual mushroom extracts. Assays were performed using mouse IFN-γ and IL-4 ELISA kits (Pierce Endogen or Thermo Scientific, Rockford, IL, USA) according to each manufacturer's instructions.
Trial and control immunogens in oil-in-water (O/W) emulsions included ovalbumin (OVA; Kanto Chemical Co., Ltd., Tokyo, Japan) as an antigen, as well as the following: 10 g of squalane (Wake Pure Chemical Industries, Ltd., Osaka, Japan), 4 g of rheodol (HLB 7.1; Kao Co. Ltd., Tokyo, Japan), 2 g of glycerol (Wake Pure Chemical Industries, Ltd.), 1 mL of ovalbumin (0.1 mg/mL), and P. eryngii hot-water extract (13 mL) as the trial immunogen or distilled water (13 mL) as the negative control.
Both immunogens were inoculated intramuscularly (0.2 mL/mouse) into 10 mice. Blood was collected from the tail vein of each mouse weekly from 0 to 10 weeks postinoculation. OVA-specific IgG1 and IgG2a antibody levels were measured using enzyme-linked immunosorbent assays (ELISA), using OVA as an antigen and anti-mouse IgG1 and IgG2a rat monoclonal antibodies (Zymed Laboratories, Inc., South San Francisco, CA, USA) as conjugates, using the previously described method of Hohdatsu et al.13 In addition, equivalent amounts of the serum obtained from individual mice were pooled on a weekly basis. IFN-γ and IL-4 serum levels were measured by ELISA (Thermo Scientific).
At 4 weeks postimmunization, spleens from the trial immunogen and control immunogen-inoculated mice were removed. Single-cell CD4+ cell suspensions were prepared from whole spleen cells by using Dynabeads Mouse CD4 (L3T4) magnetic beads (Invitrogen Dynal AS, Oslo, Norway). Cytokine levels were measured in CD4+ spleen cells seeded at 2×105 cells per well in Hybridoma-SFM medium (Invitrogen-Gibco BRL, Gaithersburg, MD) containing 5% FCS, with or without 10 μg/mL OVA in 24-well microtiter plates. Microtiter plates were maintained at 37°C for 48 h in a 5% CO2 atmosphere. After stimulation, the IFN-γ and IL-4 levels in the culture supernatants were measured using IFN-γ and IL-4 ELISA kits (Thermo Scientific) according to the manufacturer's instructions.
Statistical differences between the groups were determined using a Student's t-test. P-values of <.01 and <.05 were regarded as significant.
Table 1 shows serum IFN-γ and IL-4 levels on day 7 postinoculation with the 15 different mushroom extracts. A. bisporus, F. velutipes, H. marmoreus, L. edodes, and Ly. decastes extracts induced both IFN-γ and IL-4 production. Extracts from P. ostreatus only induced IL-4. In contrast, extracts of A. blazei, G. frondosa, M. esculenta, Ph. nameko, P. citrinopileatus, and P. eryngii induced only IFN-γ production in the mice. P. eryngii extract induced high levels of IFN-γ and reduced levels of IL-4.
Table 1.
Interferon-γ and Interleukin-4 Levels in Mice Inoculated with One of 15 Different Mushroom Extracts
| Mushroom | IFN-γ (pg/mL) | IL-4 (pg/mL) |
|---|---|---|
| Agaricus bisporus (Mushroom) | 691.8±84.6* (593–853) | 479.4±28.7* (445–522) |
| Agaricus blazei (Himematsutake) | 463.1±64.2** (371–556) | 74.1±46.2*** (19–159) |
| Auricularia auricula (Kikurage) | 222.0±72.7 (111–333) | 276.1±81.8 (165–383) |
| Flammulina velutipes (Enokitake) | 940.3±130.6* (705–1076) | 838.8±115.9* (708–1046) |
| Grifola frondosa (Maitake) | 568.9±119.1* (429–782) | 167.1±12.4 (148–631) |
| Hericium erinaceum (Yamabushitake) | 420.1±165.2 (185–631) | 265.3±45.9 (171–319) |
| Hypsizigus marmoreus (Bunashimeji) | 370.9±49.0** (296–445) | 749.3±115.8* (616–999) |
| Lentinula edodes (Shiitake) | 841.1±178.0* (593–1188) | 569.2±66.3* (466–651) |
| Lyophyllum decastes (Hatakeshimeji) | 426.1±13.1** (408–445) | 434.1±60.0* (374–543) |
| Morchella esculenta (Amigasatake) | 346.1±23.4** (296–371) | 254.1±23.4 (74–288) |
| Pholiota nameko (Nameko) | 605.9±95.1* (408–705) | 151.9±61.2 (81–278) |
| Pleurotus citrinopileatus (Tamogitake) | 427.4±21.4** (371–445) | 389.1±138.9 (249–621) |
| Pleurotus eryngii (Eringi) | 966.3±329.4* (593–1586) | 93.2±49.5*** (21–162) |
| Pleurotus ostreatus (Hiratake) | 296.8±96.5 (111–408) | 737.4±96.5* (606–921) |
| Sparassis crispa (Hanabiratake) | 352.3±98.6 (222–482) | 355.3±95.0 (216–476) |
| Control | 219.2±21.9 (185–250) | 277.1±21.2 (251–318) |
Data are mean±SD (range), n=10.
Statistical significance is indicated: *P<.01, **P<.05, ***P<.05 reduced.
IFN, interferon; IL, interleukin.
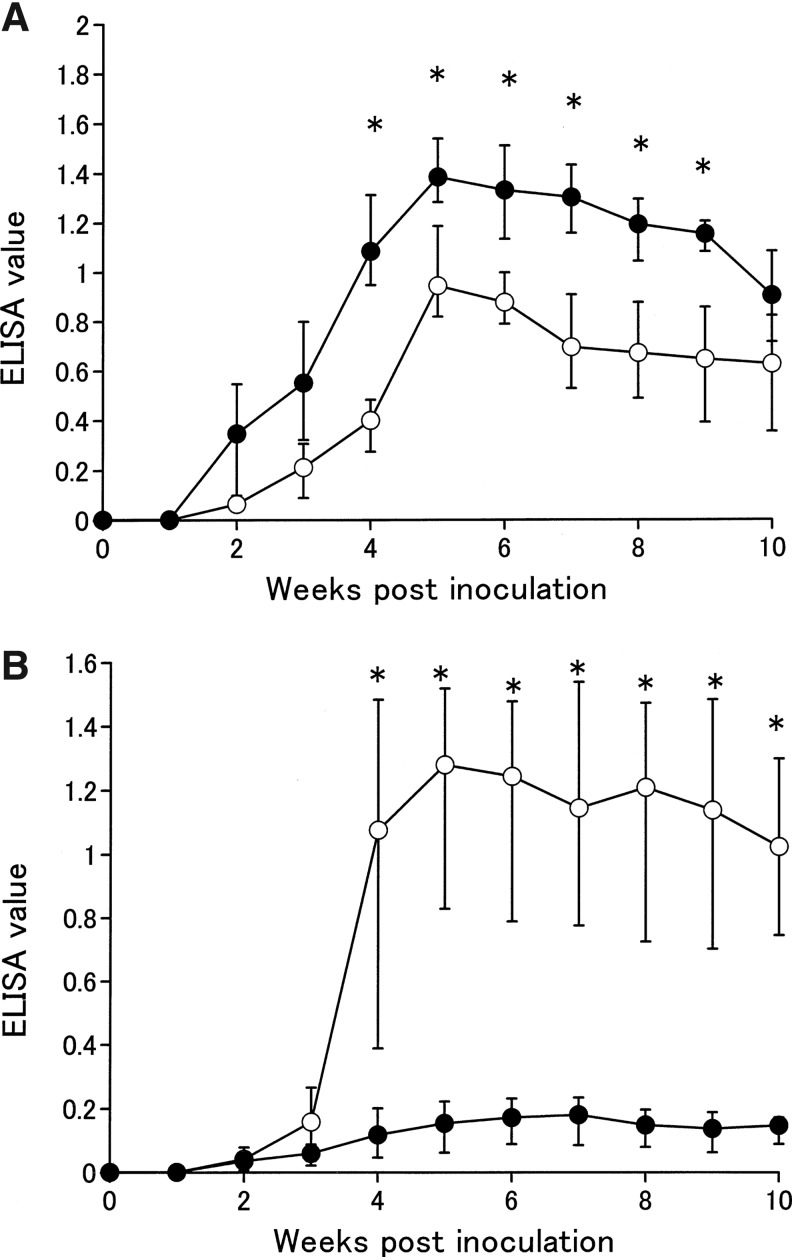
We also investigated Th1 immunostimulators using a trial immunogen from the P. eryngii extract as a potential vaccine adjuvant. Immune responses in the mice inoculated with either the trial immunogen (P. eryngii extract+OVA) or control immunogen (OVA) are shown in Figure 1. Serum IgG1 antibody titers against OVA in mice inoculated with either trial or control immunogens increased after 2 weeks, peaked at 5 weeks, and then maintained high-level antibody titers until 10 weeks postinoculation. However, antibody titers in mice inoculated with the trial immunogen were significantly reduced 4–9 weeks postinoculation when compared with those inoculated with the control immunogen (P<.01). In contrast, the serum IgG2a antibody titer did not increase in mice inoculated with the control immunogen. Furthermore, IgG2a antibodies in mice inoculated with the trial immunogen began to increase during the second week and were maintained at a high level of antibodies until 10 weeks postinoculation. Antibody levels in the mice inoculated with the trial immunogen were also higher than those inoculated with the control immunogen at 4–10 weeks postinoculation (P<.01).
FIG. 1.
Immune response against ovalbumin (OVA) in mice inoculated with either the trial immunogen or the control immunogen. Antibody titers were measured by enzyme-linked immunosorbent assays (ELISA) using anti-mouse IgG1 and IgG2a rat monoclonal antibodies. Antibody titer corresponds to the average values obtained from 10 mice. (A) Mouse IgG1 antibody response. (B) Mouse IgG2a antibody response. Symbols indicate the trial (open circle) and control (filled circle) immunogen groups. *P<.01.
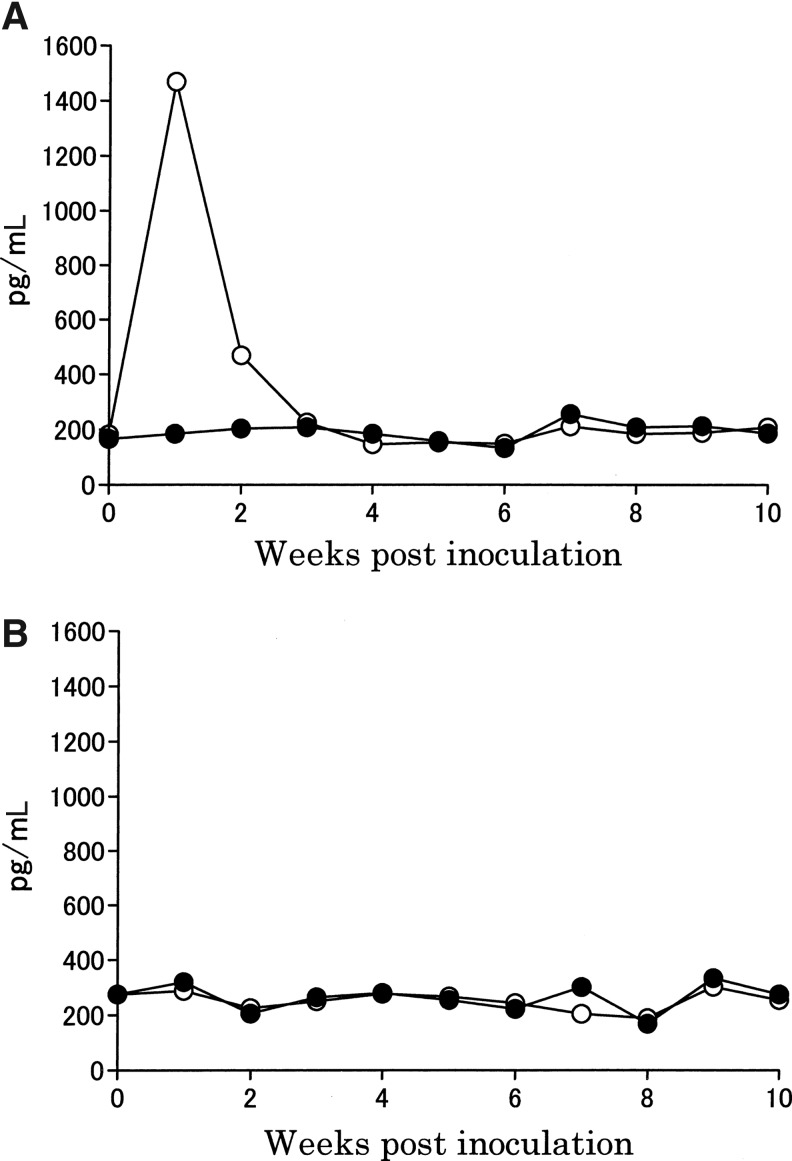
A dramatic increase in IFN-γ level in the pooled sera from the mice inoculated with the trial immunogen was observed at 1 week postinoculation, after which the levels fell to those comparable with the control immunogen at 3 weeks postinoculation (Fig. 2A). In contrast, IL-4 levels in the pooled serum from mice inoculated with either trial or the control immunogens did not increase (Fig. 2B).
FIG. 2.
Production of interferon (IFN)-γ and interleukin (IL)-4 in pooled sera from mice inoculated with the trial immunogen or the control immunogen. Cytokine values were measured using pooled sera from ten mice. (A) IFN-γ levels measured by ELISA. (B) IL-4 levels measured by ELISA. Results are expressed as the mean duplicate values of the pooled sera from ten mice per group using ELISA. Symbols indicate the trial (open circle) and control (filled circle) immunogen groups.
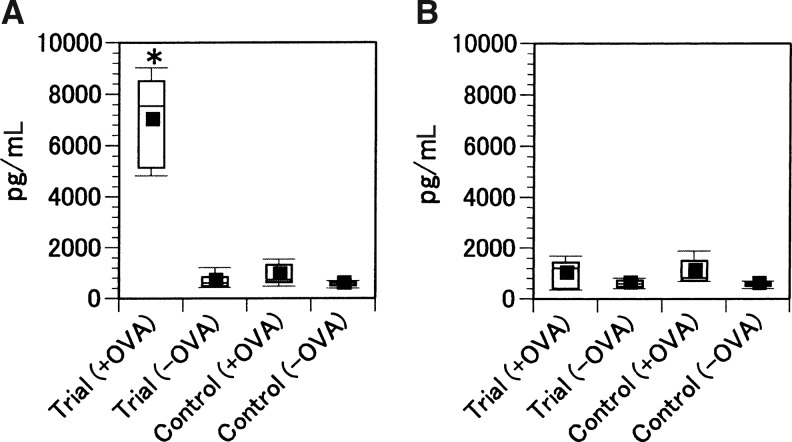
IFN-γ and IL-4 productions were assessed by ELISA using supernatants from CD4+ spleen cells from the mice inoculated with the trial or the control immunogens in the presence or absence of OVA (Fig. 3). IFN-γ levels were significantly higher in CD4+ spleen cells from mice inoculated with the trial immunogen after OVA stimulation compared with those from the mice inoculated with the control immunogen or the no-treatment group (P<.01). No significant IL-4 production was observed in any of the groups.
FIG. 3.
Production of IFN-γ and IL-4 in culture supernatants of CD4+ splenocytes from mice inoculated with either the trial immunogen or the control immunogen. Splenocytes (2×105 cells/well) from the mice inoculated with either the trial or the control immunogen were cultured for 48 h in the presence of OVA (10 μg/mL). The IFN-γ (A) and IL-4 (B) levels were measured by ELISA. The results are representative of ten similar experiments. *P<.01.
Many diseases caused by viruses and bacteria have been prevented through the use of vaccines, which mainly induce a humoral immune response (Th2). However, such prophylaxes for the intracellular pathogens, such as Herpes simplex virus, Mycobacterium tuberculosis, Treponema pallidum, Listeria monocytogenes, Leishmania major, and Toxoplasma gondii, have not yet been developed for clinical use.14–19 Given that Th1 immunity is thought to be effective against pathogens, targeting this pathway may help combat infectious diseases. The healing and immunostimulating properties of mushrooms have been known for thousands of years.3 Mushrooms contain biologically active polysaccharides that are mostly classified as β-glucans.3 Therefore, we assessed mushroom extracts that induce Th1 immunity for use as immunostimulants for uses as an adjuvant for novel vaccines.
We examined in vivo IFN-γ and IL-4 production in experimental mice after inoculation with the hot-water extracts from the 15 different edible mushroom species. We found that the IFN-γ and IL-4 levels in the sera of mice inoculated with the extracts showed marked variation. This result suggests that a diverse range of β-glucan properties exist among the mushrooms that were tested, as previously reported by Suzuki et al.20 The extracts from A. blazei, G. frondosa, M. esculenta, Ph. nameko, P. citrinopileatus, and P. eryngii induced high levels of IFN-γ production in mice. This result is in accordance with the previously reported modulation of the Th1/Th2 balance induced by the A. blazei and G. frondosa extracts.10,21–24 Interestingly, the extract from P. eryngii induced the highest level of IFN-γ production. Therefore, we selected the P. eryngii extract as an immunostimulator for the induction of Th1 immunity as well as a vaccine adjuvant candidate.
To validate the results observed during the in vivo screening, an O/W emulsion with the P. eryngii hot-water extract (the immunostimulator) and OVA (the antigen) was used as a trial immunogen. In general, IgG2a antibodies are detected as a measure of Th1 polarization.25,26 We detected OVA-specific IgG2a antibodies in mice inoculated with the trial immunogen, which suggests that the P. eryngii extract was capable of strongly inducing strong OVA-specific IgG2a antibody production in the experimental mice. OVA-specific IgG1 antibody levels were lower than those observed using the control immunogen. In addition, a remarkable increase in serum IFN-γ level and a high level production of IFN-γ in the culture supernatant of the CD4+ spleen cells from the trial immunogen group of mice were observed. These findings suggest that the P. eryngii extract induced Th1 immunity by acting as an immunostimulator.
Although our investigation targeted mushroom extracts that facilitated the production of a Th1-type immune response in mice, we do not know what kind of receptor is recognized by the P. eryngii extract. However, we hypothesize that the extract may contain substances that are potential immunostimulants, such as glucans, which are capable of inducing Th1 immunity. Glucans are a heterogeneous group of glucose polymers that consist of a backbone of β(1,3)-linked β-D-glucopyranosyl units with β(1,6)-linked side chains of varying distribution and length. Mushroom β-glucans generally have short β(1,6)-linked branches emerging from the β(1,3) backbone.3,27 Differences in the tertiary structure of these molecules may give rise to differences in their mode of action with the immune system, including antitumor functions. However, the mechanisms underlying their mode of action remain unknown.
Many receptors that function as pattern-recognition and opsonin receptors which recognize foreign substances have been reported in several organisms.28 Previous studies have reported that innate immune cells recognize certain mushroom extracts via TLR2 and/or TLR4 receptors, while others are recognized by the mannose receptor, dectin-1, and lactosylceramide.2,3 These mushoroom extract components, therefore, have potential use as immunostimulators. In this study, such in vivo interactions between a particular mushroom extract and its receptor(s) was found to induce either Th1 or Th2 immunity. Mushroom extracts from P. ostreatus induced only IL-4 production, while those from A. bisporus, F. velutipes, H. marmoreus, L. edodes, and Ly. decastes induced both IFN-γ and IL-4 production, and may be also effective as immunostimulators. However, further investigation of these functions is required, given that we only evaluated the mushroom extracts using in vivo screening.
In conclusion, screening cytokine profiles in vivo using mushrooms as immunostimulators is a useful method for examining complex immunological biological reactions. The hot-water extract from P. eryngii selected by our screening method has potential as a potent Th1 immunostimulator, and, thus, warrants further study.
Acknowledgment
This work was supported in part by a Grant-in-Aid for Scientific Research to K.I. (No. 22580342) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lull C. Wichers HJ. Savelkoul HF. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005;2:63–80. doi: 10.1155/MI.2005.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchers AT. Krishnamurthy A. Keen CL. Meyers FJ. Gershwin ME. The immunobiology of mushrooms. Exp Biol Med (Maywood) 2008;233:259–276. doi: 10.3181/0708-MR-227. [DOI] [PubMed] [Google Scholar]
- 3.Akramiene D. Kondrotas A. Didziapetriene J. Kevelaitis E. Effects of β-glucans on the immune system. Medicina (Kaunas) 2007;43:597–606. [PubMed] [Google Scholar]
- 4.Banchereau J. Briere F. Caux C. Davoust J. Lebecque S. Liu YJ. Pulendran B. Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR. Cherwinski H. Bond MW. Giedlin MA. Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 7.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR. T lymphocyte subsets, cytokines, and effector functions. Ann NY Acad Sci. 1992;664:89–92. doi: 10.1111/j.1749-6632.1992.tb39751.x. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR. Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 10.Kodama N. Harada N. Nanba H. A polysaccharide, extract from Grifola frondosa, induces Th-1 dominant responses in carcinoma-bearing BALB/c mice. Jpn J Pharmacol. 2002;90:357–360. doi: 10.1254/jjp.90.357. [DOI] [PubMed] [Google Scholar]
- 11.Pulendran B. Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Ike K. Uchida Y. Nakamura T. Imai S. Induction of interferon-gamma (IFN-γ) and T helper 1 (Th1) immune response by bitter gourd extract. J Vet Med Sci. 2005;67:521–524. doi: 10.1292/jvms.67.521. [DOI] [PubMed] [Google Scholar]
- 13.Hohdatsu T. Eiguchi Y. Ide S. Baba K. Yamagishi H. Kume T. Matumoto M. Evaluation of an enzyme-linked immunosorbent assay for the detection of transmissible gastroenteritis virus antibodies. Vet Microbiol. 1987;13:93–97. doi: 10.1016/0378-1135(87)90103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasukawa M. Inatsuki A. Horiuchi T. Kobayashi Y. Functional heterogeneity among herpes simplex virus-specific human CD4+ T cells. J Immunol. 1991;146:1341–1347. [PubMed] [Google Scholar]
- 15.Markova N. Kussovski V. Drandarska I. Nikolaeva S. Georgieva N. Radoucheva T. Protective activity of Lentinan in experimental tuberculosis. Int Immunopharmacol. 2003;3:1557–1562. doi: 10.1016/S1567-5769(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald TJ. The Th1/Th2-like switch in syphilitic infection: is it detrimental? Infect Immun. 1992;60:3475–3479. doi: 10.1128/iai.60.9.3475-3479.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh CS. Macatonia SE. Tripp CS. Wolf SF. O'Garra A. Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 18.Scott P. Pearce E. Cheever AW. Coffman RL. Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 19.Gazzinelli RT. Hakim FT. Hieny S. Shearer GM. Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 20.Suzuki Y. Adachi Y. Ohno N. Yadomae T. Th1/Th2-Balancing immunomodulating activity of gel-forming (1→3)-β-glucans from fungi. Biol Pharm Bull. 2001;24:811–819. doi: 10.1248/bpb.24.811. [DOI] [PubMed] [Google Scholar]
- 21.Inoue A. Kodama N. Nanba H. Effect of maitake (Grifola frondosa) D-fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol Pharm Bull. 2002;25:536–540. doi: 10.1248/bpb.25.536. [DOI] [PubMed] [Google Scholar]
- 22.Yuminamochi E. Koike T. Takeda K. Horiuchi I. Okumura K. Interleukin-12- and interferon-γ-mediated natural killer cell activation by Agaricus blazei Murill. Immunology. 2007;121:197–206. doi: 10.1111/j.1365-2567.2006.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takimoto H. Kato H. Kaneko M. Kumazawa Y. Amelioration of skewed Th1/Th2 balance in tumor-bearing and asthma-induced mice by oral administration of Agaricus blazei extracts. Immunopharmacol Immunotoxicol. 2008;30:747–760. doi: 10.1080/08923970802279092. [DOI] [PubMed] [Google Scholar]
- 24.Hetland G. Johnson E. Lyberg T. Bernardshaw S. Tryggestad AMA. Grinde B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer. Scand J Immunol. 2008;68:363–370. doi: 10.1111/j.1365-3083.2008.02156.x. [DOI] [PubMed] [Google Scholar]
- 25.Bobek P. Galbavy S. Hypocholesterolemic and antiatherogenic effect of oyster mushroom (Pleurotus ostreatus) in rabbits. Nahrung. 1999;43:339–342. doi: 10.1002/(SICI)1521-3803(19991001)43:5<339::AID-FOOD339>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Smith JE. Rowan NJ. Sullivan R. Medicinal mushrooms: a rapidly developing area of biotechnology for cancer therapy and other bioactivities. Biotech Lett. 2002;24:1839–1845. [Google Scholar]
- 27.Yu S. Weaver V. Martin K. Cantorna MT. The effects of whole mushrooms during inflammation. BMC Immunol. 2009;10:12. doi: 10.1186/1471-2172-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]