Abstract
Wireless communication has played a significant role in modern healthcare systems. However, the death toll from chronic diseases, such as cancer, continues to increase. Hyperthermia combined with radiotherapy and/or chemotherapy is a promising strategy for cancer treatment, and temperature control is critical for the success of this intervention. In vivo sensors are an emerging technology in healthcare. Thermal awareness has also received attention in in vivo sensor research. In this context, we have been motivated to use in vivo sensors to regulate the temperature changes in cancer cells during combined treatment. Limitations in existing in vivo thermal-aware routing algorithms motivated us to use the in vivo “lightweight rendezvous routing” approach. However, smartphone-driven telemedicine applications are proliferating to provide remote healthcare and collaborative consultation, required in combined therapies. In this context, we have proposed a telemedicine application where a smartphone not only regulates temperature scheduling in in vivo sensors, but also communicates with local or remote clinicians to maintain collaborative efforts for combined therapies against cancer.
Key words: telemedicine, smartphone, in vivo sensor, cancer treatment, hyperthermia, radiotherapy, chemotherapy
Introduction
The World Health Organization concluded that cancer was the overall leading cause of death in 2010.1 Recent combined trials demonstrated that hyperthermia increases the sensitivity of cancer cells to chemotherapy or radiotherapy, indicating it is a promising strategy for combined therapy for cancer.2,3 Hyperthermia enhances the performance of radiotherapy and chemotherapy as long as the tissue temperature is maintained below a threshold value to avoid tissue damage due to excessive heat.3,4
In vivo sensors are an emerging technology with applications in artificial retinas, pacemakers, in vivo cardioverter defibrillators, insulin pumps, and glucose monitors.5 Thermal awareness has become an important topic in related research6–9 and has motivated our search for an in vivo temperature control solution for cancer treatment. In vivo sensors stimulate human tissues with electromagnetic induction and power dissipation. A sensor with minimal electronics for simple monitoring is rarely a problem from the thermal point of view. However, a sensor with high data rate wireless communication that can stimulate human tissues with thousands of channels at a high rate or inform us of the status of an implant is thermally significant. Such a sensor generates thermal energy and is not easy to control in an in vivo environment. In vivo thermal-aware routing algorithms like Thermal-Aware Routing (TARA),6 Least Temperature Routing (LTR),7 Least Temperature Route Routing (LTRT),8 Advanced Least Temperature Routing (ALTR),7 and Hotspot Prevention Routing (HPR)9 have been reported to generate less heat during packet transmission. However, these algorithms have drawbacks such as unwanted hotspots, latency, redundant hops, and a high routing complexity time, which makes them inapplicable for an in vivo environment with limited power. Moreover, existing communication protocols for sensor networks such as directed diffusion,10 omniscient multicast, or flooding are not applicable to this network. These limitations motivated us to develop an alternative approach, which we named event-based in vivo monitoring.
We have proposed a “lightweight rendezvous routing” (LR) algorithm to schedule temperature in an in vivo sensor network deployed for use in hyperthermia, radiotherapy, and chemotherapy. In this technique, in vivo node sets are divided into small clusters. In each cluster, nodes are subscribed to a temperature increase event. When a node's temperature is increased above a threshold value, it stops thermal service. It also immediately contacts the corresponding subscriber through a broker to initiate the thermal service. A rendezvous node—a smartphone on the body—controls all communication, provides subscriber, publisher, broker role, and cluster information to all in vivo nodes, and receives subscription or notification confirmation from other in vivo nodes. Fault tolerance is an important component in the proposed technique. All in vivo communication steps maintain that, and the rendezvous node can control the starting and stopping of thermal services of in vivo nodes in case of packet failure. The proposed technique also outperforms existing thermal-aware routing algorithms and traditional data dissemination protocols by generating smaller increases in temperature and energy in an in vivo environment, as discussed in the performance evaluation section below.
Telemedicine11 is defined as the use of telecommunication to deliver remote healthcare112 and electronic clinical consultation.13 It is aimed at improving patient satisfaction by improving access to local or remote healthcare resources, enabling knowledge sharing among physicians and reducing overall healthcare costs.12–14 Smartphone-based telemedicine is used by physicians to communicate insulin measurements to diabetic patients and their caregivers,15 by remote dental surgeons to visualize X-ray reports,16 by resident surgeons to monitor point-of-care information and to share knowledge with a local mentor,17 and by neurosurgeons to make decisions about immediate actions after a stroke.18
Therefore, telemedicine has been introduced to improve quality of care in cancer treatment.19–24 It has been proven effective in different cancer treatment methods, including chemotherapy25 or radiotherapy.26 Telemedicine applications have been launched for different cancer types, including thyroid,27 skin,28 colorectal,29 head and neck,30 and breast31 cancer. However, the outcome of a combined treatment with hyperthermia, radiotherapy, and chemotherapy requires collaborative efforts of clinicians of remote places. Considering that a local hospital has medical equipments for all three treatment modalities, local clinicians have to consult with remote experts with the support of telemedicine.
In this context, we have proposed a telemedicine application whereby a smartphone not only regulates the proposed in vivo temperature scheduling technique, but also establishes collaboration between local clinicians and remote experts of different treatment modalities. In this telemedicine application, at first the local clinician inputs cancer type and position in the local cancer treatment control unit (CTCU), and a hypervisor ascertains experts on hyperthermia, radiotherapy, and chemotherapy. It then communicates with the remote CTCU to connect to remote experts through a smartphone. Remote experts recommend drug measurement, radiation measurement, and heating temperature to the smartphone kept near the patient. That smartphone, based on the recommendation, regulates the in vivo temperature with the proposed lightweight publish-subscribe technique, for combined cancer treatment. After treatment, notification is sent to the remote expert from the local CTCU through the smartphone. Moreover, the smartphone updates the electronic health record (EHR)/patient health record (PHR), prior to and after treatment. We have implemented the smartphone application in Android SDK version 4.0 and the doctor's panel for the CTCU with a Web interface.
This article begins with a discussion of hyperthermia therapy in cancer treatment and a summary of thermal-aware routing algorithms and their limitations. Then, proposed telemedicine-based cancer treatment is discussed, including the in vivo temperature scheduling technique, the telemedicine application, and the smartphone's role in the point of care. Then, in the next section, we provide performance evaluation and implementation results. We conclude with future work and the prospect of our telemedicine approach.
Materials and Methods
Hyperthermia in Cancer Treatment
Hyperthermia is a prominent cancer therapy among surgery, radiation therapy, chemotherapy, gene therapy, and immunotherapy.2–4 In oncology, hyperthermia is an artificial way of increasing the body tissue temperature by providing external heat to destroy cancer tumor cells or to prevent their growth.
Hyperthermia is rarely used as a single cancer treatment modality and performs better when used in combination with radiotherapy, chemotherapy, gene therapy, or immunotherapy.2–4 Hyperthermia treatment at 39–43°C improves the immune response of body tissues against tumors and increases blood flow and oxygen supply to tumors, which ultimately sensitizes tumors to radiotherapy and/or chemotherapy.4 Hyperthermia involves heating the tumor with an external energy source, such as microwaves, radio frequency, ultrasound, hot water perfusion, wire implants, ferromagnetic seeds, nanoparticles, or infrared radiators. An external applicator can be placed in or near the skin to heat the body tissues (superficial heating). Needles or plastic tubes can be placed directly into the tumor, and the heat source can be inserted into plastic tubes or a radio frequency can be passed through the needles. This internal method can raise tumor temperature more than external methods, without much damage to the surrounding normal tissues. Depending on location, depth, and staging of the tumor, one of three clinical methods may be used: local, regional, and whole-body hyperthermia delivers heat to localized, deep-seated, and disseminated cancer tumors, respectively. Local hyperthermia is used for small tumors located superficially or within an accessible body cavity, such as the rectum or esophagus. Superficial, interstitial, intraluminal, or intracavital applicators with microwaves, radio waves, or ultrasound are used to deliver heat to tumors. Regional hyperthermia involves heating large volumes of tissue deep inside the body with radio frequency or microwave applicators. External applicators, thermal perfusion, and continuous hypothermic peritoneal perfusion are commonly used approaches for this treatment method. Whole-body hyperthermia treatment is used for patients with metastatic diseases like melanoma, soft tissue sarcoma, or ovarian cancer. Thermal chambers, hot water blankets, or infrared radiators are used to destroy or sensitize cancer tumors using high temperature throughout the entire body.
In Vivo Thermal-Aware Routing Algorithms and Limitations
Radiation from electromagnetic waves and power dissipation from in vivo circuitry generate heat in surrounding biological tissues.32 The specific absorption rate33-driven bio-heat equation34 is frequently used to measure the heat generated by in vivo sensors. Generally, the generated heat is kept below the safety level defined by IEEE standards.35 As a consequence, in vivo thermal awareness is now applied in commercial healthcare applications. For example, in artificial retinas, one major focus is to minimize in vivo temperature. An ingestible sensor measures the internal heat of an athlete and wirelessly sends the data to a handheld device.36,37
In Table 1, we provide a summary of all in vivo thermal factors and the situations in which an increase in the mean temperature may result from an in vivo sensor network. In Table 2, we give the thermal properties of different components, such as human tissue, chip, coil, and silicon, in implanted sensor networks.
Table 1.
Thermal Factors in an Implanted Sensor Network
| FACTOR | THERMAL INFLUENCE | THERMAL CONFRONTATION |
|---|---|---|
| Electromagnetic field induced by a telemetry system | Implanted devices use high data rate and continuous wireless communication with an external device, and thus significant power is consumed. A substantial electromagnetic field is induced in human tissues, resulting in high temperature increase in tissues. | Implanted devices with minimal functionality intermittently communicate with an external device. A minimal electromagnetic field is induced in human tissues, resulting in negligible temperature increase. |
| Power dissipated by an implanted microchip | Implanted microchips continuously (for at least a few minutes) stimulate tissues with electrodes and result in great heat dissipation in tissues. | Implanted microchips that operate intermittently do not have a thermal effect in surrounding tissues. |
| Power dissipated by an implanted telemetry coil | High current induced in the implanted coil causes significant power dissipation, resulting in a temperature increase in surrounding tissues. | Minimal current flow through an implanted coil does not have a thermal effect on surrounding tissues. |
| Power dissipated by a simulating electrode | Implanted devices with a large number of electrodes stimulate human tissues with thousands of channels at high repetition rates, resulting in an increase in temperature in those tissues. | Implanted devices with few electrodes generate the mean temperature, as the stimulatory effect on surrounding tissues is negligible in this case. |
Other thermal factors include location of an implanted device inside the body, as the tissues near (1 mm) the implanted device are exposed to more heat than relatively distant tissues (<5–10 mm). Uniformity of power dissipation, materials used in insulation, and sizes of implanted chip and device also have effects.
Table 2.
Thermal Properties of Implantable Devices5
| MATERIAL | DENSITY (KG/M3) | SPECIFIC HEAT (J/[KG·°C]) | THERMAL CONDITION (J/[M·S·°C]) | THERMAL DIFFUSIVITY (M2/S) |
|---|---|---|---|---|
| Chip | 2,300 | 700 | 60 | 3.7×10−5 |
| Coil | 2,1400 | 134 | 71.6 | 2.5×10−5 |
| Silicone | 2,700 | 1,255 | 0.18 | 0.5×10−5 |
| Fat tissue | 920 | 2,500 | 0.25 | 1.1×10−7 |
| Cortical bone | 1,850 | 1,300 | 0.4 | 1.7×10−7 |
Thermal-aware routing algorithms (Table 3) have been proposed for temperature control in an implanted sensor network. These algorithms suffer from limitations like hotspot creation, complexity overhead, or maximum heat traversal.
Table 3.
Comparison of Thermal-Aware Routing Algorithms
| THERMAL-AWARENESS ROUTING ALGORITHM | HOTSPOT CREATION | REDUNDANT HOP CREATION | AVERAGE DELAY | COMPUTATIONAL COMPLEXITY |
|---|---|---|---|---|
| TARA6 | Avoided | Possible | Comparatively high | Preferably small |
| LTR7 | Avoided | Possible in the worst case when a packet passes through every node | Comparatively high | Preferably small |
| ALTR7 | Avoided but possible for an adaptive situation | Possible | Comparatively low, includes an adaptive situation | Generally small but higher (with inclusion of SHR) in an adaptive situation |
| HPR8 | Avoided | Not possible | Comparatively low | Much higher for SHR |
| LTRT9 | Avoided | Not possible | Apparently low, but delay occurs because of propagation of routing information | Huge overhead for Dijkstra's algorithm |
| Proposed LR | Avoided | Not possible | Minimum | Preferably small |
ALTR, Advanced Least Temperature Routing; HPR, Hotspot Prevention Routing; LR, lightweight rendezvous routing; LTR, Least Temperature Routing; LTRT, Least Temperature Route Routing; SHR, self-healing routing; TARA, Thermal-Aware Routing.
TARA6 was an early attempt at in vivo thermal-aware routing that sends a packet using the withdrawal strategy. TARA defines a hotspot region that is above a threshold temperature value. When a node sends a packet to a hotspot, it withdraws from it, and the packet is sent back to the sender. After the cooling period, the packet is resent to the destination. The protocol does not consider the shortest path; it just withdraws the packet from the hotspot.
In LTR,7 the packet is generally sent to the node that has the lowest temperature. If the number of hops increases above a threshold value, the packet is discarded. If the next node has already been visited, then the second minimum temperature node is selected for packet transmission. ALTR7 is an advancement of LTR in which a packet is sent to the lowest temperature node. However, if the number of hops is greater than the threshold value, self-healing routing follows packet transmission.
HPR9 uses the shortest hop-routing algorithm for sending a packet to a destination that does not have a hotspot. If the next hop is the destination or a node with a temperature below the threshold, the packet is transmitted. However, if the next hop is a node with a temperature greater than the threshold temperature, a hotspot is assumed, and the packet is instead forwarded to the coolest neighbor that has not yet been visited. The problem with HPR is that temperature information has to be propagated to other nodes, resulting in a large overhead.
LTRT8 was created to solve the problems associated with the previous algorithms. With LTRT, the packet is sent along a path that produces the smallest change in temperature from the source to destination. The algorithm uses Dijkstra's algorithm to determine the shortest path from the source to destination and avoids hotspot formation and redundant multihops. The problem with the algorithm is that temperature information needs to be propagated to every node at a regular interval. After the shortest path is created, the function of temperature control is established. Maintaining Dijkstra's algorithm creates a large overhead for an implanted sensor network.
Proposed Telemedicine-Based Temperature Scheduling for Cancer Treatment
We have proposed telemedicine-based combined therapy for cancer, including the in vivo temperature scheduling technique, the telemedicine application, and the smartphone's role in the point of care of telemedicine.
Problem statement
For the treatment of cancer, the combined application of hyperthermia, radiotherapy, and/or chemotherapy has become the most prominent therapy.4 If the temperature is below a threshold temperature, hyperthermia enhances the performance of radiotherapy and chemotherapy. However, if it is above the threshold, human cells become sensitized, making the treatment dangerous to human health. We have been studying implanted sensor nodes that reflect temperature control in the combined therapy of hyperthermia, radiotherapy, and/or chemotherapy for cancer treatment. If the temperature of a node (publisher) is increased above the threshold, it will communicate with the broker node to disseminate temperature information to the subscriber node or nodes. If the temperature dissipation is not performed successfully, a remote gateway node should be notified by the broker node.
Proposed LR temperature scheduling algorithm for in vivo sensors: motivation
The limitations of existing in vivo thermal-aware routing algorithms motivated us to identify an alternate solution. We considered the use of event-based communication (publish-subscribe system) in small clusters of implanted sensor nodes deployed in cancer treatment. Our work is inspired by the rendezvous routing (RR) algorithm.38 However, RR has some disadvantages, and if we consider its operation in the context of an implanted sensor network, it produces a large overhead. A lightweight solution for this implanted sensor network is needed. Hence, we propose a lightweight temperature control algorithm called LR for this implanted sensor network.
Proposed LR temperature scheduling algorithm for in vivo sensors: lightweightness
The term “lightweight” is very significant in our proposed algorithm because the subscribed or published mechanism uses at most two hops on the path to a rendezvous node (the smartphone). The broker node of each cluster communicates directly with the rendezvous node, so the message must propagate among the broker nodes. For the same reason, we do not need to maintain a distributed hash table or any data structure that may produce a large overhead for constrained resources in in vivo sensor nodes. Also, the common rendezvous node is an external device, and a broker node cannot become a rendezvous node. Therefore, an implanted sensor network does not need to maintain broker metadata. These problems—namely, redundant hop traversal, broker metadata propagation, and distributed hash table maintenance—of the rendezvous algorithm motivated us to develop a lightweight event-based algorithm for use in an implanted sensor network.
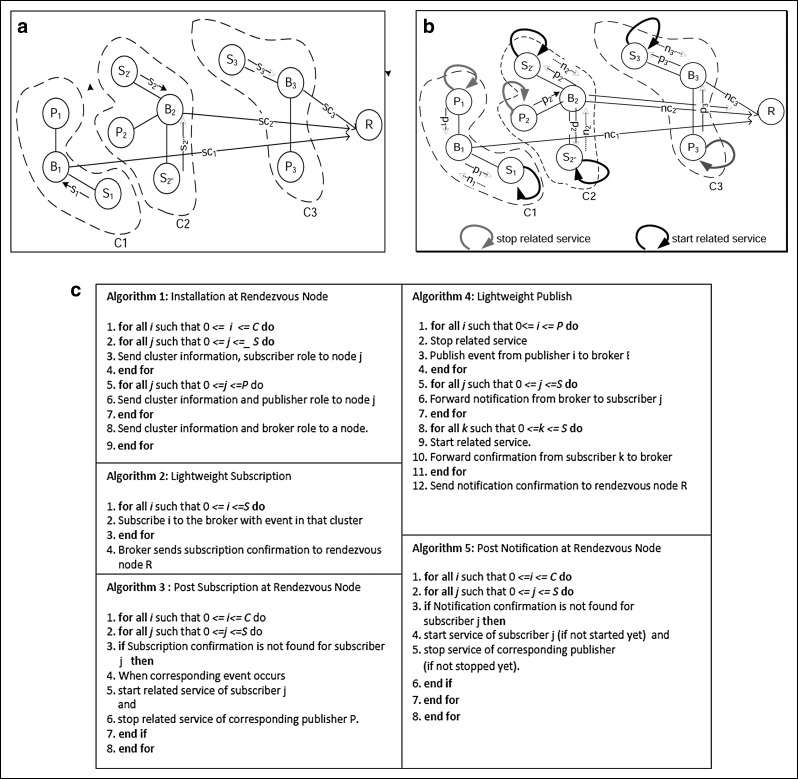
Proposed LR temperature scheduling algorithm for in vivo sensors: proposed algorithm (LR) (Fig. 1c)
Fig. 1.
(a) Lightweight subscription operating on body sensor nodes that are divided into three clusters: C1, C2, and C3. In C2, subscribers S2′ and S2″ send subscription messages s2′ and s2″, respectively, to broker B2. B2 then sends a subscription confirmation message (sc2) to rendezvous node R. The same operation is performed for clusters C1 and C3. (b) A lightweight publisher operates on body sensor nodes that are divided into three clusters C1, C2, and C3. In C2, when the event occurs, publisher P2 stops related services and sends a notification message (p2) to broker B2. B2 then forwards notification (p2) to subscribers S2′ and S2″. S2′ and S2″ immediately start related services and send confirmations (n2′ and n2″, respectively) to broker B2. B2 then sends notification confirmation (nc2) to the rendezvous node R. The same operation is performed for clusters C1 and C3. (c) Algorithms of lightweight temperature scheduling routing.
In the proposed LR algorithm, body sensor nodes are divided into some small clusters (Fig. 1a and b). A rendezvous node has the cluster information and publisher, subscriber, or broker role of each node in any cluster. Each cluster has a single broker node and some subscriber and publisher nodes. At first, the rendezvous node transmits the cluster information and subscriber, publisher, and broker role to each body sensor node (Algorithm 1). Then, using the lightweight subscription algorithm (Fig. 1a) (Algorithm 2), in each cluster, the subscriber nodes subscribe to a broker node. The broker node then sends a subscription confirmation message (list of successfully subscribed nodes) to the rendezvous node, which then determines whether each subscriber is successfully subscribed in each cluster (Algorithm 3). In the case of unsuccessful subscription (due to packet loss from subscriber to broker or broker to rendezvous node), the rendezvous node waits for the event to occur. When that event finally occurs, thermal services to subscribers and publishers are started and stopped, respectively. For lightweight publishers (Fig. 1b) (Algorithm 4), in each cluster, when an event occurs, the publisher stops the thermal service and then sends a notification message to the broker, which then forwards it to the subscribers. Subscribers immediately initiate thermal service and notify the broker, which sends notification confirmation to the rendezvous node. The rendezvous node then checks each cluster to determine whether all the subscribers are successfully notified (Algorithm 5). If notification has not occurred because of packet loss from publisher to broker, from broker to subscriber, or from broker to rendezvous node, the rendezvous node directly communicates with the subscribers and publisher to immediately start (if not started) or stop (if not stopped) thermal service.
Proposed LR temperature scheduling algorithm for in vivo sensors: implementation scenario
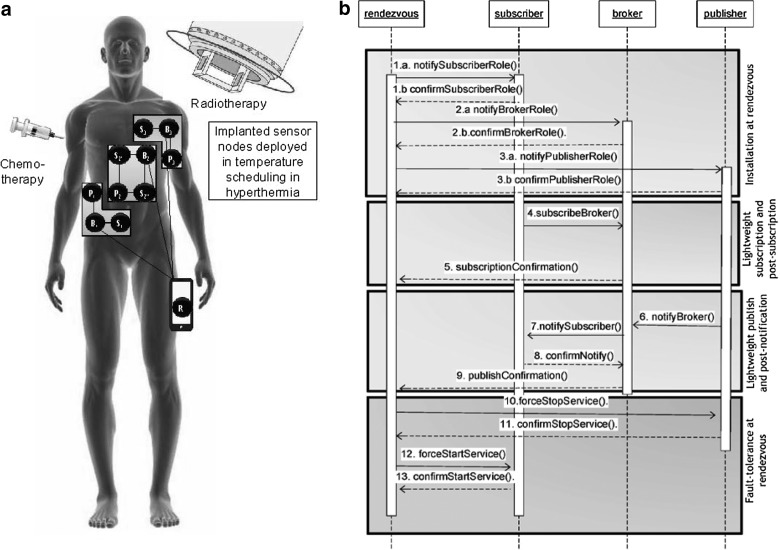
We assume that radiotherapy, chemotherapy, and hyperthermia are applied to a cancer patient. Implanted sensor nodes and a rendezvous node (the smartphone) are involved in the temperature control inside the body (Fig. 2a). A middleware implementing the proposed LR algorithm, installed in each in vivo sensor, allows the sensor to operate as a subscriber, publisher, or broker node in a cluster. Figure 2b describes the overall workflow in the proposed technique. First, the rendezvous smartphone assigns subscriber, broker, or publisher roles to the implanted sensor nodes (steps 1–3). In each cluster, subscribers are assigned to the broker node for the temperature event (steps 4 and 5). If any node (publisher) exceeds the threshold temperature, the thermal service is stopped, and the broker is contacted immediately. The broker instructs the corresponding subscriber to immediately start thermal service (steps 6–9).
Fig. 2.
(a) Sensor nodes in the body and the rendezvous node/smartphone are involved in temperature control in hyperthermia treatment combined with radiotherapy and/or chemotherapy. Middleware is implementing the proposed lightweight rendezvous routing algorithm in each in vivo sensor node. (b) Sequence diagram of the implementation of the proposed lightweight rendezvous routing in in vivo sensor nodes and the smartphone/rendezvous node.
We believe that a single point of failure could be very dangerous in such a critical healthcare scenario; therefore, fault tolerance was an important component in our implementation. All subscription and notification confirmations (steps 5 and 9) are forwarded to the rendezvous smartphone so it can control the start and stop points of thermal services of in vivo sensors (steps 10–12). Communication among in vivo sensors also involves fault tolerance (steps 1.b, 2.b, 3.b, 6, and 8).
Telemedicine-Based Application
We now present the scope, architecture, and workflow of the proposed telemedicine application.
Scope
Treatment of cancer with hyperthermia, radiotherapy, and/or chemotherapy requires expertise specific to each therapy, as well as information related to factors such as cancer type, location, and patient history. Telemedicine enables knowledge sharing among clinicians and experts for combined cancer treatment. In the proposed technique, such knowledge sharing is essential for determining the optimum hyperthermia temperature to be scheduled by the in vivo sensors, for drug measurement in chemotherapy, and to determine the radiation level for radiotherapy. Local clinicians can apply the treatment method recommended by remote experts, assuming that the hospital has the correct medical equipment. The smartphone enables the notification of local or remote clinicians and experts or nurses regarding the treatment method, regardless of whether the patient is at the point of care or outside the hospital. However, patient mobility is restricted during treatment because the patient is simultaneously receiving heat, radiation, and a drug dose. However, the use of a bedside smartphone, during the post-treatment period, enables the notification of local or remote caregivers. A smartphone can provide patient updates to the EHR/PHR before and after treatment. Local or remote expert diagnosis of a patient through EHR/PHR is also essential for health insurance calculations and billing.
Architecture
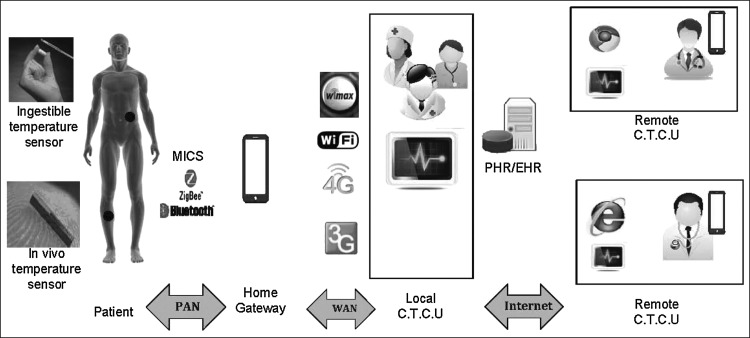
We present a telecommunication infrastructure (Fig. 3) for the proposed telemedicine application. In vivo sensors communicate with each other using the MICS frequency band to control temperature. The sensors communicate with the bedside smartphone using Zigbee or Bluetooth technology in a personal area network. Alternatively, a laptop or desktop computer with an adapter can be used instead of a smartphone. The smartphone can be used to communicate with caregivers at local hospitals using 3G/4G/Wi-Fi/Wimax in a wireless area network. Information regarding the patient's cancer type, location, and history is available to remote experts through the PHR/EHR. Remote experts can access the medical records through the Internet, analyze the data, and consult with local clinicians.
Fig. 3.
Architecture of the proposed telemedicine approach. C.T.C.U, cancer treatment control unit; PAN, personal area network; PHR/EHR, patient health record/electronic health record; WAN, wide area network.
Workflow
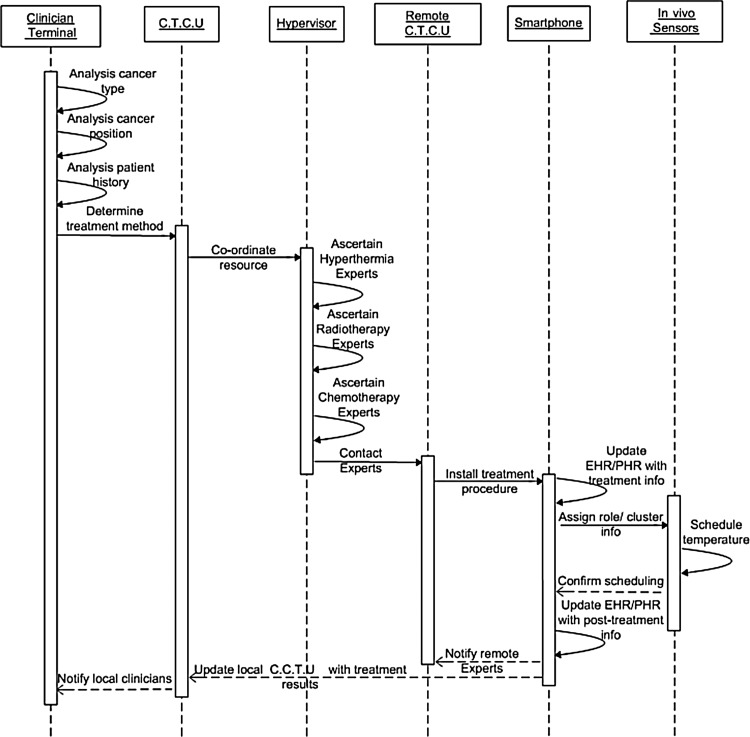
Clinicians analyze cancer type, position, and patient history and request assistance from the CTCU to determine the treatment method (Fig. 4). The CTCU, in turn, communicates with a hypervisor that coordinates resources and has access to a database of records of experts on hyperthermia, radiotherapy, or chemotherapy. Therefore, it can identify experts for specific treatment methods. With location or position information for the remote experts, the hypervisor can inform the neighboring CTCUs of treatment methods. Then, the CTCU sends a treatment procedure on the smartphone of the expert, nurse, and patient. The patient's bedside smartphone updates the EHR/PHR with the treatment information and communicates with the patient's in vivo sensors to assign role and cluster information. The in vivo sensors, in turn, schedule the temperature for combined cancer treatment and notify the smartphone of the result. The smartphone first updates the EHR/PHR and then notifies the smartphone of remote experts and the local CTCU with post-treatment information. Local clinicians are informed about treatment results from the local CTCU.
Fig. 4.
Sequence diagram of the proposed telemedicine application. C.T.C.U, cancer treatment control unit; EHR/PHR, electronic health record/patient health record.
The Smartphone's Role in the Telemedicine Approach
Use cases
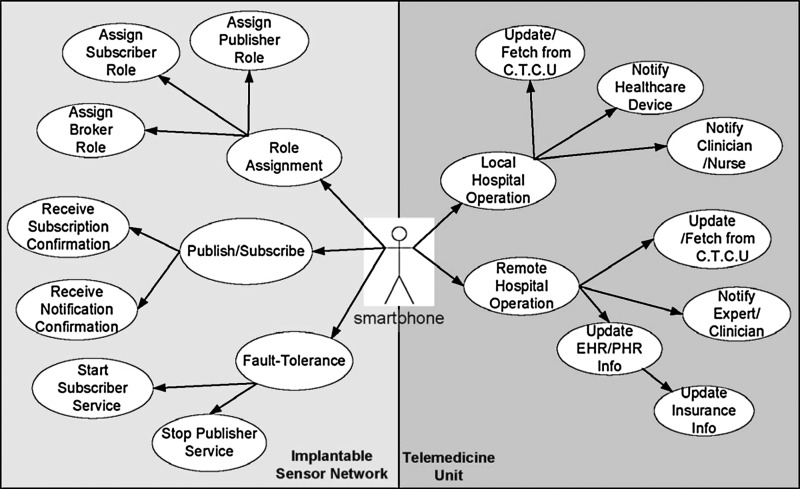
The smartphone plays a vital role in the proposed telemedicine mechanism (Fig. 5). It works as a rendezvous node for the implanted sensor network and a gateway for the telemedicine unit. The smartphone, as a rendezvous node, assigns subscriber, publisher, and broker roles to in vivo sensors and manages the published-subscribed methods to control temperature in the sensors. However, if there is ever a communication failure in the in vivo sensors, the smartphone activates fault tolerance actions by forcibly starting or stopping the sensor services. As a gateway to the telemedicine unit, the smartphone notifies local or remote clinicians, nurses, or healthcare devices of patient and treatment information. The phone also transmits the treatment procedures from the local CTCU and regularly updates the EHR/PHR for insurance facilities.
Fig. 5.
Use cases of smartphone in telemedicine application. C.T.C.U, cancer treatment control unit; EHR/PHR, electronic health record/patient health record.
Internal workflow
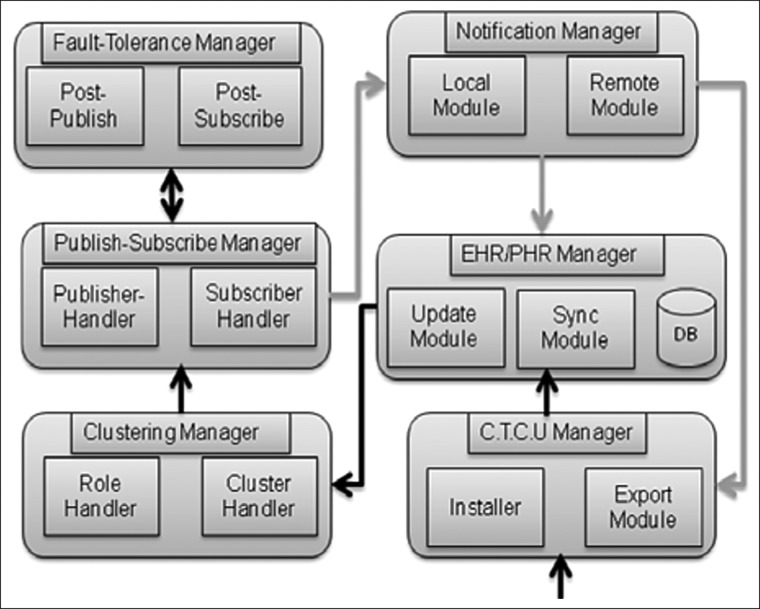
The smartphone receives the treatment procedure from the CTCU (Fig. 6). The CTCU manager inside the smartphone first installs the treatment information. Then, the EHR/PHR manager updates the EHR/PHR database with the new information and manages the local database so, in the case of network unavailability, it can store information. A synchronization module is present to synchronize the local database to a remote EHR/PHR. The clustering manager assigns subscriber/publisher/broker roles to the in vivo sensors. The publish-subscribe manager continuously coordinates publish-subscribe methods to control in vivo sensors. In a communication failure, the smartphone's fault-tolerance manager publisher or the subscription manager handles the failure.
Fig. 6.
Internal workflow of smartphone application. C.T.C.U, cancer treatment control unit; EHR/PHR, electronic health record/patient health record.
The notification manager's local module updates the EHR/PHR manager with the post-treatment information. The remote module provides the same information to a remote CTCU or an expert's smartphone.
Results
In this section, we present performance evaluation of the in vivo temperature scheduling technique and implementation results of telemedicine application.
Performance Evaluation
We have compared, in a Java simulator, the proposed technique (LR) with the conventional RR algorithm, the existing thermal-aware routing algorithms, and traditional data-dissemination techniques in a sensor network (Fig. 7). We assume that 10 nodes are deployed in a 6×6 mesh topology. We assume that 1 unit of temperature is generated for every packet transmission among nodes. When a node sends or receives a packet from a gateway node, it generates 2 units of temperature.
Fig. 7.
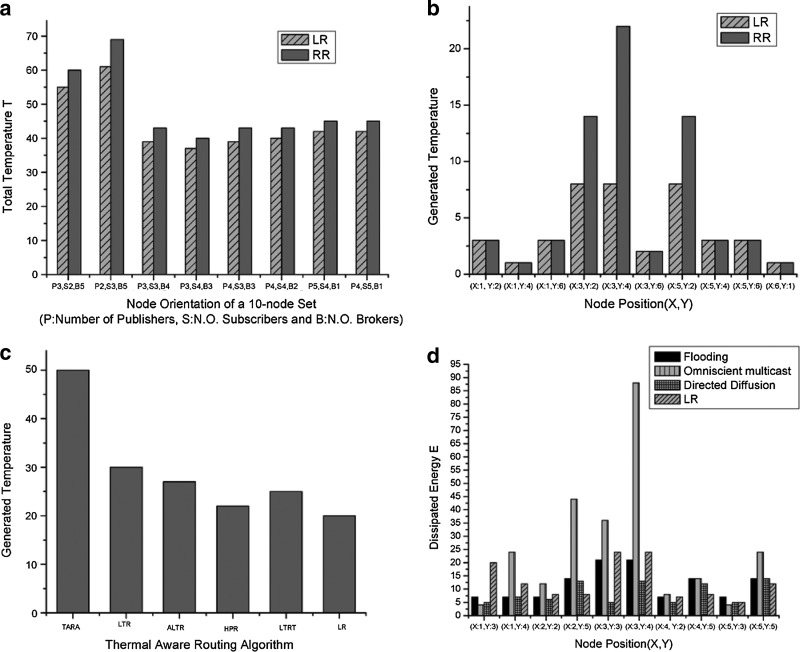
(a and b) Using lightweight rendezvous routing (LR) and rendezvous routing (RR) algorithms: (a) total temperature in eight different node orientations with a 10-node set and (b) generated temperature at different nodes (in the node orientation with three publishers [P], four subscribers [S], and three brokers [B]). (c) Performance comparison of LR with Thermal-Aware Routing (TARA), Least Temperature Routing (LTR), Advanced Least Temperature Routing (ALTR), Hotspot Prevention Routing (HPR), and Least Temperature Route Routing (LTRT). LR generates less heat than other protocols. (d) Performance comparison of dissipated energy on different nodes using flooding, omniscient multicast, directed diffusion, and LR.
LR generates less of an increase in temperature than does RR in different orientations for 10 nodes. When the node orientation has three publishers, four subscribers, and three brokers, LR generates the lowest temperature (Fig. 7a). In Figure 7b, the three broker nodes of LR generate a much lower temperature than that of RR because in LR the three broker nodes communicate directly with the rendezvous node. However, in RR a broker node communicates with other broker nodes on its way to the rendezvous node.
LR outperforms existing thermal-aware routing algorithms by generating less of an increase in temperature (Fig. 7c). TARA generates the maximum temperature, although ALTR is better than LTR because of the use of the hop count threshold. HPR is better than LTR, ALTR, and LTRT; however, LR produces the lowest temperature of all methods. If the region surrounding a node is greater than 7 units, it is assumed to be a hotspot. We assume 10 hops as the maximum hop count for LTR and ALTR. The threshold temperature of LTRT is assumed to be 6 units. TARA generates the maximum temperature as it tries to avoid hotspots and traverses more multiple hops. LTR and ALTR provide better performance than TARA by choosing the lowest temperature node. However, ALTR is better than LTR because, when 10 hops are crossed, the shortest hop routing algorithm is used. HPR is far better than the previous three algorithms because it chooses the shortest hop in accordance with the temperature parameter. LTRT is superior to TARA, LTR, or ALTR but worse than HPR because of the creation of three smaller clusters with the publish-subscribe system.
LR dissipates less energy than flooding, omniscient multicast, or directed diffusion (Fig. 7d). In the flooding scheme, sources (placed at bottom and top positions of the square) flood all events to every node in the network. The nodes placed at the middle of the square dissipate higher energy because there is relatively higher packet transmission in that area. In omniscient multicast, each source generates the shortest path multicast tree to all sinks. All multicast trees use a common interim node to create the shortest path, resulting in large energy dissipation. In directed diffusion, energy dissipation is low compared with that for flooding and multicast. Interests are propagated from sources to sinks (placed at middle positions) using interim nodes (brokers), and events are propagated in the reverse path. In LR, energy dissipation is relatively small compared with that of the previous three protocols. Nodes are divided into three clusters, and a lightweight publish-subscribe mechanism is a separate action in each of the three clusters. As brokers are involved in communication with the rendezvous node, energy dissipation is comparably higher in those broker nodes than in other nodes.
Implementation Results
Prior to implementation, we have performed extensive study on the general impact of telemedicine in cancer treatment and then cancer type-specific telemedicine applications. To analyze the implications for different kinds of therapy used alone or in combination, we have studied data from three recent clinical trials39–41 on combined therapy. The first trial39 considers radiotherapy alone and radiotherapy plus hyperthermia. The other trials40,41 consider influence of hyperthermia on chemotherapy. For example, when we use a hyperthermia range of 40–45°C, chemotherapy requires comparatively less drug (doxorubicin, 479 mM; mitoxantrone, 608 mM, etc.). The reason is that the chemotherapy drug becomes more sensitive to the body cell in high temperature. We have presented these implications through the graphical user interface of the Android application where a hyperthermia, radiotherapy, or chemotherapy expert recommends heat, radiation, or chemical measurement, according to therapies, used alone or combined. If we consider our lightweight in vivo technique, it will indicate the threshold value of temperature, by following the heating range used in hyperthermia.
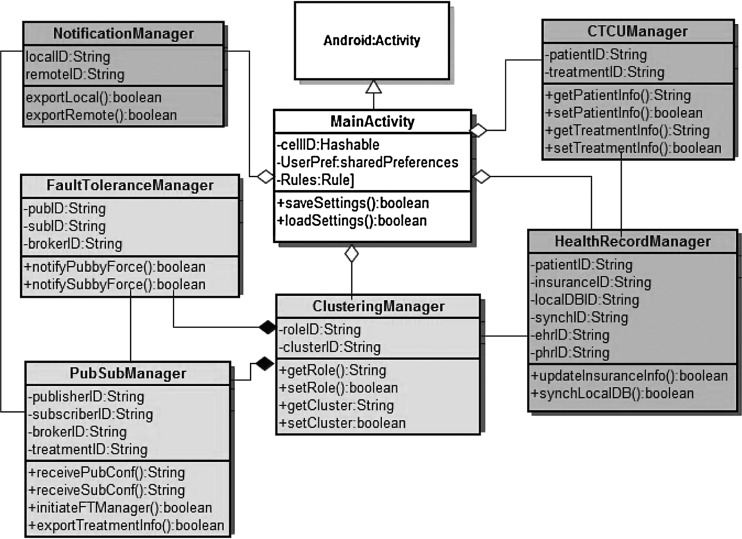
We have implemented our smartphone application (Fig. 8) in the Android platform version 4.0. The class diagram is presented in Figure 9. The smartphone works as a gateway to in vivo BSN and point of care in telemedicine architecture. Therefore its activity is an aggregation of functionalities related to in vivo body sensor networks (BSN) and telemedicine architecture. In Figure 9, light gray-colored classes represent properties of the in vivo lightweight publish-subscribe system; however, dark gray-colored classes represent properties of telemedicine functionalities.
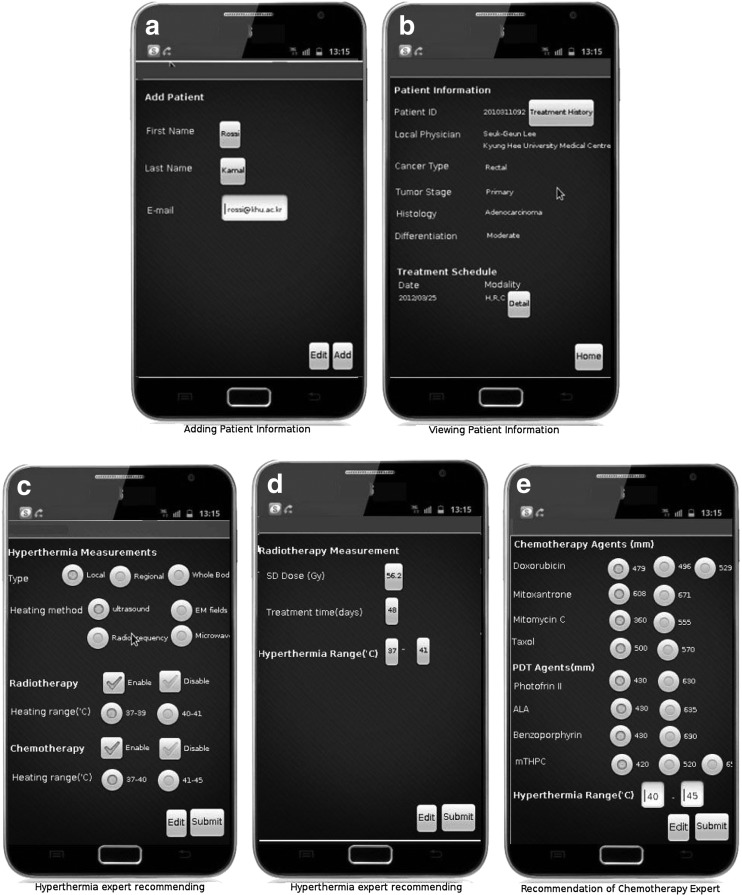
Fig. 8.
Screenshots of smartphone applications.
Fig. 9.
Class diagram of smartphone application.
Figure 8 represents the screenshots of the smartphone application. Patient information is checked by putting first name, last name, and e-mail (Fig. 8a). The detailed information is displayed with patient treatment history (Fig. 8b). The remote hyperthermia expert recommends the heating type or method and the temperature range through the smartphone graphical user interface (Fig. 8c). Similarly, a radiotherapy expert recommends the heating range and radiation measurement (Fig. 8d), and a chemotherapy expert recommends the drug measurement, along with the heating range (Fig. 8e).
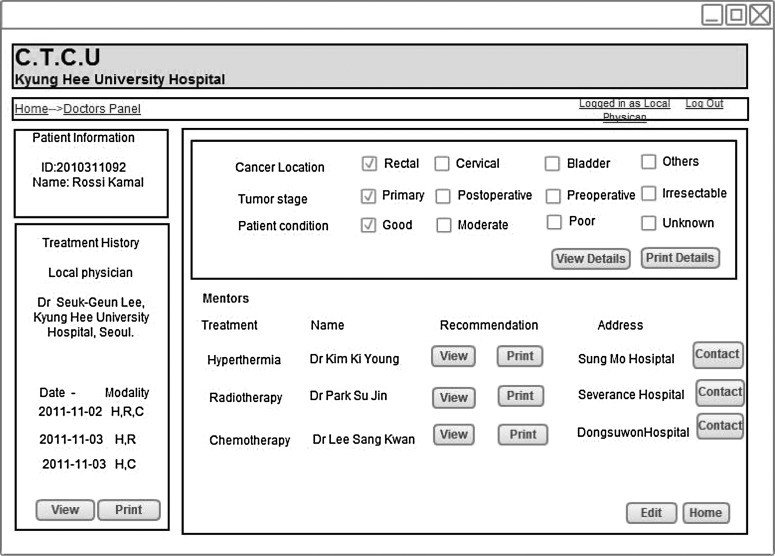
The local clinician inputs the cancer location, tumor, stage and patient's condition through the Web panel (Fig. 10). He or she can observe the patient's treatment history and can also view the recommendations from the hyperthermia, radiotherapy, or chemotherapy expert.
Fig. 10.
Doctors' Web panel in local cancer treatment control unit (C.T.C.U).
Discussion
Collaborative efforts are gaining popularity in healthcare. Telemedicine fosters such collaboration by enabling remote healthcare support to a hospital that has a scarcity of medical equipment and by delivering clinical consultancy to resident surgeons inexperienced with specific diseases or treatments. The outcome of combined cancer treatment depends on the joint efforts of experts in hyperthermia, radiotherapy, and chemotherapy. A local hospital that has equipment for these three treatment methods might lack experts for each method. Telemedicine resolves this by allowing experts to share knowledge bases across distance. In the proposed technique, local clinicians analyze the cancer type, position, etc., and receive assistance from remote experts in determining a suitable treatment method. Experts on hyperthermia, radiotherapy, or chemotherapy can suggest an optimum temperature for in vivo sensors. A patient's bedside smartphone, updated with the suggested treatment method, can communicate with in vivo sensors to control temperature for cancer treatment. The smartphone plays a vital role by notifying local or remote clinicians or healthcare devices of the treatment outcome. This smartphone-based telemedicine application can be used in the monitoring of cancer patients or treatment of other chronic diseases.
With an in vivo temperature scheduling technique, we considered the different orientations of 10 in vivo nodes. We need to consider a larger set of nodes and a body organ-specific node orientation in the future and need to determine the thermal energy source for hyperthermia treatment. It must also be determined if an implanted sensor node or external methods, including ultrasound and laser, should be used to generate temperature. If we choose the first option of an internal source, we need to determine the appropriate implanted sensor nodes. Medical stents, which are currently deployed in healthcare, can be of assistance to in vivo sensors because of the stent's wireless telemetry support. If we choose the second option of external thermal source, a rendezvous node will wirelessly communicate with the external source to accurately increase/decrease thermal energy at the organ position. After completion of the experimental steps and determination of the appropriate sensor device, we need to run a preclinical trial to test the proposed in vivo temperature control techniques in an animal model.
Acknowledgments
This research was supported by the Next-Generation Information Computing Development Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (grant 2011-0020518) and partially by the Korean Ministry of Knowledge Economy, under the Information Technology Research Center support program supervised by the National IT Industry Promotion Agency (NIPA-2012-(H0301-12-1004)).
Disclosure Statement
No competing financial interests exist.
References
- 1.American Cancer Society. Global cancer facts & figures. 2nd. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Weinberg R. The biology of cancer. New York: Garland Science; 2007. [Google Scholar]
- 3.Dollinger M. Rosenbaum E. Everyone's guide to cancer therapy: How cancer is diagnosed, treated, and managed day to day. 5th. Andrews McMeel Publishing LLC; Kansas City, MO: 2008. Cable. [Google Scholar]
- 4.Baronzio G. Hager D. Hyperthermia in cancer treatment: A primer. New York: Springer; 2006. [Google Scholar]
- 5.Lazzi G. Thermal effects of bioimplants. IEEE Eng Med Biol Mag. 2005;24(5):75–81. doi: 10.1109/memb.2005.1511503. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q. Tummala N. Gupta S. Schwieber L. TARA: Thermal-aware routing algorithm for implanted sensor networks. In: Prasanna VK, editor; Iyengar S, editor; Spirakis P, editor; Welsh M, editor. Lecture notes in computer science 3560: Distributed computing in sensor systems. Berlin: Springer; 2005. pp. 206–217. [Google Scholar]
- 7.Bag A. Bassiouni M. Energy efficient thermal aware routing algorithms for embedded biomedical sensor networks. 2006 IEEE International Conference on Mobile Adhoc and Sensor Systems (MASS); New York. IEEE; 2006. pp. 604–609. [Google Scholar]
- 8.Takahashi D. Xiao Y. Hu F, et al. Temperature-aware routing for telemedicine applications in embedded biomedical sensor networks. EURASIP J Wireless Commun Network. 2008;26:1–26. [Google Scholar]
- 9.Bag A. Bassiouni M. Hotspot preventing routing algorithm for delay-sensitive applications of in vivo biomedical sensor networks. Inf Fusion. 2008;9:389–398. [Google Scholar]
- 10.Intanagonwiwat C. Govindan R. Estrin D, et al. Directed diffusion for wireless sensor networking. IEEE/ACM Trans Network. 2003;11:2–16. [Google Scholar]
- 11.Perednia D. Allen A. Telemedicine technology and clinical applications. JAMA. 1995;273:483–488. [PubMed] [Google Scholar]
- 12.Hailey D. Roine R. Ohinmaa A. Systematic review of evidence for the benefits of telemedicine. J Telemed Telecare. 2002;8:1–7. doi: 10.1258/1357633021937604. [DOI] [PubMed] [Google Scholar]
- 13.Pattichis C. Kyriacou E. Voskarides S, et al. Wireless telemedicine systems: An overview. IEEE Antennas Propagation Mag. 2002;44:143–153. [Google Scholar]
- 14.Woodward B. Istepanaian R. Richards C. Design of a telemedicine system using a mobile phone. IEEE Trans Inf Tech Biomed. 2001;5:13–15. doi: 10.1109/4233.908361. [DOI] [PubMed] [Google Scholar]
- 15.Franc S. Daoudi A. Mounier S, et al. Telemedicine and diabetes: Achievements and prospects. Diabetes Metab. 2011;37:463–476. doi: 10.1016/j.diabet.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Aziz S. Ziccardi V. Telemedicine using smartphones for oral and maxillofacial surgery consultation, communication, and treatment planning. J Oral Maxillofac Surg. 2009;67:2505–2509. doi: 10.1016/j.joms.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Chang A. Ghose S. Littman-Quinn R, et al. Use of mobile learning by resident physicians in Botswana. Telemed J E Health. 2012;18:11–13. doi: 10.1089/tmj.2011.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takao H. Murayama Y. Ishibashi T, et al. A new support system using a mobile device (smartphone) for diagnostic image display and treatment of stroke. Stroke. 2012;43:236–239. doi: 10.1161/STROKEAHA.111.627943. [DOI] [PubMed] [Google Scholar]
- 19.London W. Morton E. Marinucci D, et al. The implementation of telemedicine within a community cancer network. JAMA. 1997;4:18–24. doi: 10.1136/jamia.1997.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olver I. Nayagam S. Evaluation of a telemedicine link between Darwin and Adelaide to facilitate cancer management. Telemed J. 2000;6:213–218. doi: 10.1089/107830200415144. [DOI] [PubMed] [Google Scholar]
- 21.Ricke J. Bartelink H. Telemedicine and its impact on cancer management. Eur J Cancer. 2000;36:826–833. doi: 10.1016/s0959-8049(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 22.Doolittle G. Allen A. Practising oncology via telemedicine. J Telemed Telecare. 1997;3:63–70. doi: 10.1258/1357633971930869. [DOI] [PubMed] [Google Scholar]
- 23.Doorenbos A. Demiris G. Towle C, et al. Developing the Native People for Cancer Control Telehealth Network. Telemed J E Health. 2011;17:30–34. doi: 10.1089/tmj.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng C. Stokes T. Wang M. caREMOTE: The design of a cancer reporting and monitoring telemedicine system for domestic care. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; New York. IEEE; 2011. pp. 3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mark L. Joanna R. Annie Y, et al. Chemotherapy side-effect management using mobile phones. 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. EMBS 2008; New York. IEEE; 2008. pp. 5152–5155. [DOI] [PubMed] [Google Scholar]
- 26.Olsen D. Bruland O. Davis B. Telemedicine in radiotherapy treatment planning: Requirements and applications. Radiother Oncol. 2000;3:255–259. doi: 10.1016/s0167-8140(99)00185-1. [DOI] [PubMed] [Google Scholar]
- 27.Gibelli G. Gibelli B. Nani G. Thyroid cancer: Possible role of telemedicine. Acta Otorhinolaryngol Ital. 2008;28:281–286. [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips C. Burke W. Allen M. Stone D. Wilson J. Reliability of telemedicine in evaluating skin tumors. Telemed J. 1998;4:5–9. doi: 10.1089/tmj.1.1998.4.5. [DOI] [PubMed] [Google Scholar]
- 29.Makin G. Breen D. Monson J. The impact of new technology on surgery for colorectal cancer. World J Gastroenterol. 2001;7:612–621. doi: 10.3748/wjg.v7.i5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Head B. Studts J. Bumpous J, et al. Development of a telehealth intervention for head and neck cancer patients. Telemed J E Health. 2009;15:44–52. doi: 10.1089/tmj.2008.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curran V. Church J. A study of rural women's satisfaction with a breast cancer self-help network. J Telemed Telecare. 1999;5:47–54. doi: 10.1258/1357633991932388. [DOI] [PubMed] [Google Scholar]
- 32.Heetderks W. RF powering of millimeter- and submillimeter-sized neural prosthetic implants. IEEE Trans Biomed Eng. 1988;35:323–327. doi: 10.1109/10.1388. [DOI] [PubMed] [Google Scholar]
- 33.Ulaby F. Fundamentals of applied electromagnetics. Upper Saddle River, NJ: Prentice-Hall; 1999. [Google Scholar]
- 34.Pennes H. Analysis of tissue and arterial blood temperature in the resting human forearm. J Appl Physiol. 1948;1:93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- 35.IEEE standard for safety levels with respect to human exposure to radio frequency electromagnetic fields, 3 kHz to 300 GHz. New York: IEEE; 1992. IEEE Std C95.1-1991. [Google Scholar]
- 36.Hunt A. Stewart I. Calibration of an ingestible temperature sensor. Physiol Meas. 2008;29(11):71–87. doi: 10.1088/0967-3334/29/11/N01. [DOI] [PubMed] [Google Scholar]
- 37.Sparling P. Snow T. Millard-Stafford M. Monitoring core temperature during exercise: ingestible sensor vs. rectal thermistor. Aviat Space Environ Med. 1993;64:760–763. [PubMed] [Google Scholar]
- 38.Pietzuch P. Bacon J. Hermes: A distributed event-based middleware architecture. Proceedings. 22nd International Conference on Distributed Computing Systems Workshops, 2002; New York. IEEE; 2002. pp. 611–618. [Google Scholar]
- 39.Zee J. González D. Rhoon G, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet. 2000;355:1119–1112. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 40.Itoh Y. Yamada Y. Kazaoka Y, et al. Combination of chemotherapy and mild hyperthermia enhances the anti-tumor effects of cisplatin and adriamycin in human bladder cancer T24 cells in vitro. Exp Ther Med. 2010;1:319–323. [Google Scholar]
- 41.Mourant J. Johnson T. Los G. Bigio I. Non-invasive measurement of chemotherapy drug concentrations in tissue: Preliminary demonstrations of in vivo measurements. Phys Med Biol. 1999;44:1397–1417. doi: 10.1088/0031-9155/44/5/322. [DOI] [PubMed] [Google Scholar]