Abstract
Background
Tourette syndrome is a combined motor and vocal tic disorder that begins in childhood and takes a chronic course. It arises in about 1% of all children, with highly varying severity. Transient and usually mild tics are seen in as many as 15% of all children in elementary school. The diagnosis is often delayed by several years.
Methods
We selectively reviewed the pertinent literature, including the guidelines of the European Society for the Study of Tourette Syndrome for the diagnosis and treatment of tic disorders.
Results
Tic disorders usually take a benign course, with spontaneous improvement in adolescence in about 90% of patients. Psychoeducation is the basis of treatment in each case and almost always brings marked emotional relief. Specific treatment is needed only for more severe tics and those that cause evident psychosocial impairment. 80–90% of patients with Tourette syndrome have comorbidities (attention deficit–hyperactivity disorder, obsessive-compulsive disorder, depression, anxiety, emotional dysregulation, autoaggression), which often impair their quality of life more than the tics do and therefore become the main target of treatment. There is little evidence for the efficacy of treatment for tics. Small-scale controlled studies with a brief follow-up period have been carried out for some neuroleptic drugs. Behavior therapy should be tried before drug treatment. A further option for very severely affected adults is deep brain stimulation.
Conclusion
Because of the low level of the available evidence, no definitive recommendations can be made for the treatment of tics.
Tourette syndrome is a neuropsychiatric disorder that begins in childhood and is characterized by motor and vocal tics. It is named after Georges Albert Édouard Brutus Gilles de la Tourette (1857–1904), a pupil of Charcot at the Salpêtrière, who published the first case series of patients with this disorder. The motor and vocal tics typically fluctuate in number, frequency, intensity, and complexity over the course of the disease. Until very recently, Tourette syndrome was thought to be exceedingly rare (an “orphan disease”); epidemiologic studies have revealed, however, that tic disorders are actually quite common, and that their diagnosis is often delayed (e1). Many patients with tics suffer from stigmatization and prejudice for years until the true nature of their problem is recognized.
This review is based on pertinent publications from 2000 onward that were retrieved by a search in the PubMed, PsycINFO, and EMBASE databases with the key words “Tourette syndrome” and “tic disorder,” and on the guidelines of the European Society for the Study of Tourette Syndrome (ESSTS), published in early 2011. The ICD-10 diagnostic criteria, clinical features, diagnostic assessment, and treatment options for Tourette syndrome are presented from a combined child/adolescent psychiatric, neurological, and adult psychiatric perspective. The purpose of this review is to improve physicians’ understanding of this complex neuropsychiatric disorder and of the comorbidities that often accompany it.
Definition and clinical features
A tic is a rapid, repeated, non-rhythmic movement or sound production that occurs suddenly, serves no purpose, and is experienced as meaningless. Tics are classified by their quality (motor or vocal) and their degree of complexity (simple or complex). They can appear isolated or in combination, and they can be either transient or chronic.
Tics in children of elementary-school age are often mild and transient and cause no impairment, so they cannot be called a disease in the narrow sense of the term. Their correct diagnosis is important, however, so that the child’s parents can be told what the problem is and what to expect, preventive action can be taken against prejudice and teasing, and inadequate treatments can be avoided. Even today, the time to diagnosis is often as long as 5 to 10 years (1, e1).
Typically, tics are preceded by a unpleasant “premonitory urge,” i.e., the feeling that a certain type of tic is about to be produced; the feeling disappears as soon as the tic is actually produced (e2). Another typical feature of tics is that they can be voluntarily suppressed, though usually only briefly. Both of these phenomena – the premonitory urge and voluntary suppressibility – are age-dependent, being more prominent in adults than in children (2).
Motor tics
Simple motor tics, by definition, affect only a small number of muscle groups and involve only short-lasting, circumscribed movements. They are most often seen in the face and head, with ocular tics being particularly common. In contrast, complex motor tics are defined as those that involve multiple muscle groups and/or seem to fulfill a purpose. Copropraxia, echopraxia, and palipraxia are special types of complex motor tics (Box 1).
Box 1. Examples of simple and complex motor tics.
-
Simple motor tics
-
winking, blinking, eye-rolling, wide eye-opening\
(without moving the eyebrows)
eyebrow-raising
wrinkling or turning up the nose
puffing up the cheeks
mouth opening, pulling the corners of the mouth
lip movements
sticking out the tongue
jaw movements
frowning
grimacing
tooth-chattering
head-shaking, throwing, turning, twitching, or nodding
shoulder-shrugging
arm and hand movements
abdominal movements
trunk movements
leg and foot movements
-
-
Complex motor tics
seemingly intentional movements, facial expressions, gesticulations with the head, hands, arms, trunk, legs, feet
picking at clothes
hopping, jumping
clapping, finger-tapping
spinning
bending and bowing the trunk
wide arm movements
foot-stamping-
dystonic tics (rare) with slow turning movements
writing tics
echopraxia: non-purposeful imitation of other persons’ observed movements
copropraxia: making obscene gestures such as showing the middle finger, indecent movements of the trunk and pelvis, crotch-holding
palipraxia (rare): repetition of one’s own movements (auto-aggressive behavior)
Vocal tics
Throat-clearing and sniffling are the most common kinds of vocal tic; exclamations and shouts are much rarer. Especially in children, tics are often misdiagnosed as an airway disease such as asthma or an allergy. Coprolalia, echolalia, and palilalia are complex vocal tics (Box 2).
Box 2. Examples of simple and complex vocal tics.
-
Simple vocal tics
throat-clearing
sniffling, snorting
coughing, rasping
snuffling
blowing out the lips/tongue (making a ”raspberry”)
inhaling or exhaling noisily
squeaking, squealing, grunting
whistling, humming
shouting
saying syllables (hm, eh, ah, ha)
making animal noises or other sounds
spitting
-
Complex vocal tics
echolalia: repetition of heard sentences, words, sillables, or sounds, not for the purpose of communication; can also lead to the generation of new tics
coprolalia: saying obscene words
palilalia: involuntary repetition of one’s own spoken words
blocking of speech, stuttering
saying fragments of speech
saying other socially inappropriate words (NOSI = “non-obscene, socially inappropriate behavior,” e.g., “fat, fat, fat,” “help, help,” “yes, yes, yes”)
Coprolalia is the clinical manifestation that the public most commonly associates with Tourette syndrome, although it is present in only 19–32% of patients (3). It usually involves the uttering of short words that are considered to be foul language, often of an obscene nature (Box 2). Coprolalia is more common in more severe cases of Tourette syndrome with multiple comorbidities (3).
The ICD-10 diagnostic criteria for tic disorders
Tourette syndrome – Tourette syndrome is a combined vocal and motor tic disorder with, by definition, at least two types of motor tic and at least one type of vocal tic. The ICD-10 diagnostic criteria further require the onset of illness in childhood or adolescence, duration of at least one year (though there may be symptom-free intervals lasting several months), and fluctuation of the the tics over time. No particular degree of severity is required.
Chronic motor tic disorder – Chronic motor tic disorder differs formally from Tourette syndrome exclusively in the absence of vocal tics. The motor tics are usually milder than those seen in patients with Tourette syndrome, and the comorbidities are rarer and generally less severe.
Chronic vocal tic disorder – Chronic vocal tic disorder is rare. It is characterized by the persistent occurrence of exclusively vocal tics. Comorbidities are just as frequent as in Tourette syndrome.
Transient tic disorder – Transient tic disorder is seen only in children and is characterized by tics that disappear within one year. These are usually mild, simple motor tics that the children themselves may not even notice (Box 3).
Box 3. ICD-10 classification of primary tic disorders.
-
F95.0:
transient tic disorder (duration < 12 months)
-
F95.1:
chronic motor or vocal tic disorder (duration > 1 year, no remission for longer than 2 months,only motor or only vocal tics; first tics before age 18)
-
F95.2:
combined tic disorder, at least one vocal and at least two motor tics (Gilles de la Tourette syndrome)
-
F95.8:
other tic disorders
-
F95.9:
tic disorder, unspecified
Epidemiology and clinical course
The worldwide prevalence of Tourette syndrome is thought to be about 1% (4). It is assumed that as many as 10–15% of children in elementary school have transient simple motor tics. Boys and men are affected about four times as often as girls and women (4, e3).
Tics usually appear with gradually increasing intensity between the ages of 6 and 8 years (5, e1). There is no relation between the age of onset and the severity of the tic (e3). Motor tics appear, on the average, two to three years earlier than vocal tics. Typically, the initial manifestations of the disease are simple motor tics involving the face and head. No particular criteria (“markers”) have yet been identified that could be used to predict the future course of the disease (5). The tics are at their most severe, on average, between the ages of 10 and 12 (5, e4). About 90% of patients have spontaneous improvement after the tics reach their peak. This is reflected in the much lower prevalence of Tourette syndrome in adults (6). There is still debate, however, over whether chronic tic disorders ever fully disappear, and, if so, how often (1, e3, e5– e7). In all cases, there are spontaneous fluctuations in the localization, number, frequency, complexity, type, and severity of the tics.
Most patients say that the tics become more frequent when they are under emotional stress (e8, e9) and less common with relaxation or concentration (e10). Tics are suggestible and can sometimes be evoked by external stimuli, e.g., a discussion of tics during a medical consultation. So-called echo phenomena can involve imitation not only of the voluntary movements of other persons, but also of other patients’ tics (e11).
Diagnosis
Tic disorders are clinically diagnosed on the basis of a detailed history and a neurological and psychiatric examination. Further diagnostic evaluation is only rarely needed, e.g., when the manifestations are atypical or when a secondary tic disorder is suspected (7).
The term “tic” should be used only to denote hyperkinesias and sound productions that meet the defined diagnostic criteria. Neither conversion disorders with tic-like movements nor movement disorders of unknown origin should be designated as tics.
Tics are usually easy to distinguish from other types of hyperkinetic movement, such as:
chorea,
dyskinesia,
hemifacial spasm,
restless legs syndrome, and
focal epileptic seizures.
Differential diagnosis
The disorders that are most difficult to distinguish from tic disorders are the following:
dissociative movement disorders
compulsive behaviors
generalized hyperactivity
mannerisms
(less commonly) dystonia and myoclonus (7).
In rare cases, tics can arise as a manifestation of another disease (e.g., Wilson’s disease, neuroacanthocytosis, Fragile X syndrome, Sydenham’s chorea, Huntington’s disease); they can also be iatrogenic or substance-induced (e.g., by carbamazepine, phenytoin, lamotrigine, amphetamines, dopaminergic drugs, or cocaine). Tardive tics are a rare complication of neuroleptic use (7).
Comorbidities
80–90% of patients with Tourette syndrome have not only tics, but also psychiatric manifestations (8). The number and severity of accompanying disorders rise as the tics become more severe (5).
Among patients of all ages with Tourette syndrome, children are the most likely (50–90%) to suffer additionally from attention deficit–hyperactivity disorder (ADHD) (8, e14, e15). Other common comorbidities in childhood that often markedly impair psychosocial functioning are the following:
obsessive-compulsive behavior and anxiety (e16)
impulse-control disorders (in the terminology of the DSM IV-TR, “intermittent explosive disorder”)
emotional dysregulation
disorders of social behavior
autism spectrum disorders (ASD)
Adults often have obsessive-compulsive symptoms or behavior as well as auto-aggression, depression, and sleep disorders; addiction and autism spectrum disorders are less common (1, 5, 8).
As studies have shown, the health-related quality of life of patients with Tourette syndrome is impaired above all by the accompanying psychiatric disorders—in children, mainly obsessive-compulsive disorder and ADHD; in adults, mainly depression (9, 10).
Etiology
Structural and functional imaging have revealed abnormalities in the motor and somatosensory portions of the corticostriatal-thalamocortical circuit (11, 12, e22, e23). In addition, recent studies have shown that structures outside this circuit are also involved—in particular, the limbic system (13). A major pathophysiological role for the dopaminergic system has been presumed ever since the discovery that dopamine-receptor antagonists can alleviate tics. It seems most likely that an abnormality of presynaptic regulation exists in combination with phasic dysfunction of dopaminergic transmission (14). Further transmitter systems seem to be involved as well; impaired functioning of the serotoninergic system is postulated (15).
Genetic and environmental factors
Family studies and molecular genetic studies have revealed that tic disorders have a major genetic component (e24– e29). It is currently estimated that the first-degree relatives of a patient with Tourette syndrome have a 5% to 15% risk of developing the disease themselves and a 10% to 20% risk of developing any sort of tic (16). Many candidate genes have been identified, and the pattern of inheritance seems to be complex (Box 4) (17– 19, e29, e30). In addition to genetic factors, environmental influences seem to play a major role as well. It is presumed that a combination of genetic vulnerability and environmental influences results in the development of tic disorders (20, e24, e26, e28) (Box 5).
Box 4. Susceptibility genes associated with tic disorders.
The SLITRK1 gene: the SLIT and NTRK proteins play a role in the development of corticostriatal-thalamocortical circuitry
The L-histidine decarboxylase (HDC) gene: the enzyme HDC catalyzes the formation of histamine
The IMMP2L (mitochondrial inner membrane protease subunit 2) gene: the mutated protein leads to functional impairment of the mitochondrial cytochrome 1c protein and thereby activates apoptosis
The NLGN4X gene: the protein neuroligin-4 plays a role in synaptogenesis
The CNTNAP2 (contactin-associated protein 2) gene: the protein product of this gene influences the distribution of calcium channels in the central nervous system
Box 5. Non-genetic risk factors.
-
Prenatal:
smoking during pregnancy
psychosocial stress during pregnancy
intrauterine growth retardation, low birth weight
-
Perinatal:
premature birth
perinatal hypoxia
-
Postnatal:
infection,especially Group A β-hemolytic streptococci (GABHS) with PANDAS (“pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections”; it is unclear whether the temporal association reflects causation or coincidence) (20)
psychosocial stress (often worsens tics)
Treatment
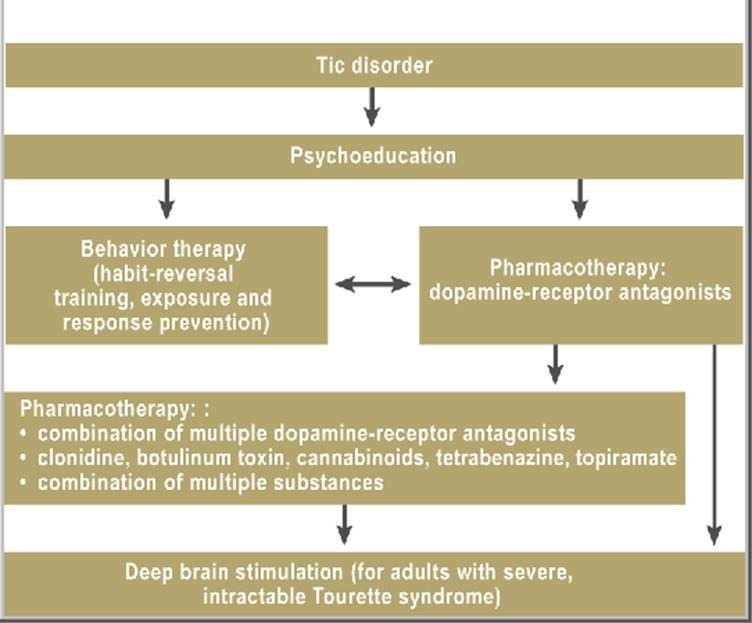
For many patients and their families, the diagnosis itself brings considerable relief. If the patient is a child, it is important that his or her teachers and other significant adults should also understand the nature of the condition. Thorough patient education should include not only information about the cause and future course of the disease, but also counseling on social issues such as the various types of compensatory aid that are available, how to request certification as a disabled or severely disabled person, the issue of driving, and the choice of an occupation (e31). Psychoeducation should always be the first step of treatment (Figure). It is not at all rare for patients to feel stress as a result of their tic disorder for no other reason than the way others respond to it (intolerance, teasing) (e31).
Figure.
Flowchart for the treatment of tics (modified from [26]).
Tics cannot be cured, nor is there any causal therapy. Even symptomatic treatment is problematic, as no form of treatment is available that can simultaneously alleviate all of the manifestations of Tourette syndrome and its comorbidities. Tics should be treated symptomatically when they produce marked physical or psychosocial impairment. None of the available treatments affect the course of tic disorders. Whenever the efficacy of a treatment is assessed, the characteristic spontaneous fluctuations of tics must be taken into account.
Although there have been many publications about treatments for chronic tic disorders, the evidence for the efficacy of any particular treatment is weak. Controlled trials are available for some typical neuroleptic drugs (haloperidol, pimozide) and some atypical ones (risperidone, ziprasidone), but all of these were carried out on small groups of subjects over periods of no more than 8 weeks (21– 23). A Cochrane review that was slated for publication could not be prepared because of the poor state of the data (Pierce & Rickards, DOI: 10.1002/14651858.CD008151). In controlled studies, behavior-therapeutic approaches have been found moderately efficacious (up to EF 0.68) against moderately severe tics (24, 25).
Pharmacotherapy
In Germany, dopamine-receptor antagonists (neuroleptic drugs) are considered the substances of first choice for the treatment of tics (26). Haloperidol is the only medication that is approved in Germany for the treatment of Tourette syndrome, and its use for this purpose is supported by the highest level of evidence (EBM level I); nevertheless, it is hardly used any more because of its known side effects. Nearly all medically treated patients now receive off-label treatment with atypical neuroleptic drugs, although there have been only a few controlled trials of their use for this indication (26). Their most common adverse effects are fatigue, increased appetite, weight gain, sexual dysfunction, and akathisia (26).
Treatment with dopamine-receptor antagonists should be started at a low dose that is then gradually increased until either a therapeutic benefit is seen or intolerable side effects arise. The dose may need to be adjusted later because of spontaneous fluctuations of the tic(s); in children, the dose may need to be increased to keep up with bodily growth. Tolerance can also develop (26). The agents of first choice for use in children are currently tiapride, risperidone, and aripiprazole; for adults, the German Neurological Association, in its guidelines, recommends tiapride, sulpiride, and risperidone (recommendation level A for each), as well as aripiprazole (recommendation level B) (Table). A retrospective open-label trial of sulpiride for the treatment of Tourette syndrome has been published, with 63 participating patients (27), but there has not yet been any study of amisulpride, which might have a more favorable side-effect profile (28).
Table. The treatment of tics.
| Drug | Dosage | Initial dose (mg) | Recommendedmaximum dose (mg) | Maximum approved dose (mg) | Remarks |
| Tiapride | (2–)3x/day | 50–100 | 600(–800) | 1200 | drug of first choice for children |
| Sulpiride | 2x/day | 50−100 | 800–1200 | 1600 | effective against depression, OCD |
| Risperidone | 2x/day | 0.5−1 | 4–8 | 16 | effective against aggression as well |
| Aripiprazole | 1x/day | 2.5 | 10–30(–45) | 30 | often better tolerated than other neuroleptic drugs |
| Pimozide | 1x/day, in the evening | 0.5−1 | 8(–12) | 16 | combination with macrolides and sertraline can lead to fatal QTc prolongation |
| Haloperidol | 2–3x/day | 0.5 | 10–15(–20) | 100 | highly effective, but more severe side effects |
| Tetrabenazine | 3x/day | 12.5 | 75 | 200 | causes depression more commonly than neuroleptic drugs, must not be combined with MAO inhibitors; a reserve medication |
| Botulinum toxin | IM | suitable only for single, selected tics | |||
| Tetrahydrocannabinol | 2–3x/day | 2.5 | 20(–30) | not approved | not suitable for children or psychotic patients |
| HRT | weekly 60–90 minute sessions for 10 weeks | ||||
| ERP | “exposure and response prevention” | ||||
| DBS | the optimal target and stimulation parameters remain unknown, as does the effect of DBS on tics and comorbid psychatric disorders and their long-term course | should be considered only in intractable, very severe cases, in adults | |||
DBS=deep brain stimulation, ERP=exposure and response prevention, HRT=habit-reversal training, OCD=obsessive-compulsive disorder, MAO=Monoamine oxidase, IM=intramuscular.The pediatric doses do not differ fundamentally from the adult ones. Doses generally do not need to be calculated by age, weight, or height; rather, the dose should be gradually increased according to clinical need and tolerability. On the other hand, treatment should be stopped even at a low dose, if necessary, if unacceptable side effects arise (fatigue, weight gain). Many children tolerate neuroleptic drugs well even at high doses, while many adults develop severe side effects even at low doses. Clearly, the degree of toleration of neuroleptic drugs seems to depend mainly on the patient’s genetic predisposition, rather than on age and weight. (Table modified from [e36].)
Unfortunately, the very poor state of the data currently allows no definitive recommendation about treatment (26). Aripiprazole seems to have a better side-effect profile than other atypical neuroleptic drugs (e32). Further alternatives include other atypical neuroleptic drugs, pimozide, combinations of the substances just named, tetrabenazine, topiramate, and (in selected cases) local botulinum-toxin injections and cannabinoids (Table). Clonidine is used preferentially in English-speaking countries (29), even though its tic-suppressing effect is rather weak in comparison to that of the neuroleptics mentioned above (e33, e34).
Behavior therapy
Habit reversal training (HRT) was recently introduced as an alternative to drug therapy for tics. In HRT, the patient prevents the occurrence of a tic by performing a previously learned alternative behavior instead. This method lessens the frequency of tics by about 30% (24, 25). Comparable results can be obtained with exposure and response prevention (ERP), a strategy for interrupting the automatism described by many patients in which a premonitory urge is necessarily followed by a tic. A European expert commission recently recommended that behavior therapy (if available) should always be tried before drug treatment (24). Behavior therapy often fails because of inadequate motivation, particularly in children.
Deep brain stimulation
Very severely affected adult patients with medically intractable Tourette syndrome may benefit from deep brain stimulation (30, 31). The initial findings of open uncontrolled trials and small controlled trials suggest that deep brain stimulation not only lessens the frequency of tics but also improves comorbid psychiatric disorders, including obsessive-compulsive symptoms, depression, anxiety, and autoaggression. The optimal target for deep brain stimulation has not yet been conclusively identified (30). The procedure is occasionally complicated by infection and rarely by intracerebral hemorrhage. The most common stimulation-induced side effects are fatigue, lack of energy, visual disorders, and dizziness (32) (Table).
The treatment of comorbidities
It is recommended that psychiatric disorders accompanying Tourette syndrome should be treated in the same way as when they occur in the absence of Tourette syndrome. Alongside drug therapy, psychotherapeutical interventions are an important component of the multimodal treatment concept, particularly for children. Selective serotonin reuptake inhibitors (SSRI) are the mainstay of drug treatment for obsessive-compulsive symptoms, anxiety, and depression; fluoxetine is the only one approved for use in children aged 8 or older. Methylphenidate is the drug of first choice for treatment of ADHD in patients with comorbid tics. Multiple independent trials and a meta-analysis have unequivocally shown that methylphenidate does not cause any lasting worsening of the tics (e33, e35). Alternative drugs include atomoxetine and clonidine, alone or in combination with methylphenidate (1, e35).
Conclusions
Many patients with chronic tic disorders and Tourette syndrome have such mild tics that they need no treatment. Psychoeducation of the patient, his or her family, and other persons who interact with the patient in everyday life is essential to prevent stigmatization. For tic disorders that are severe enough to require treatment, the available therapeutic options are still unsatisfactory. Genetic studies are expected to provide the basis for new approaches to the pharmacotherapy of tic disorders.
Key Messages.
The general knowledge of tic disorders is deficient across Europe even today, both among laypersons and among physicians. These disorders are often misdiagnosed for years before being correctly identified.
Transient tic disorders are quite common among school-age children, with a prevalence of about 10–15%. In most cases, no treatment is needed. Knowing that the disorder is benign is very reassuring for the affected children and their parents.
Tourette syndrome is characterized by a combination of at least two motor tics and at least one vocal tic. The severity of these tics varies widely; in most patients, they become much milder during adolescence.
80–90% of patients with Tourette syndrome have comorbid disorders such as attention deficit hyperactivity disorder, depression, anxiety, and obsessive-compulsive disorder, which often impair the quality of life more than the tics themselves and are accordingly the main target of treatment.
The available scientific evidence on the pharmacotherapy of Tourette syndrome is still of poor quality. The atypical neuroleptic drugs are the agents of first choice for the treatment of tics. Behavior-therapeutic approaches have also been well evaluated.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Ludolph has served as a paid consultant for, and has received reimbursement for travel costs from Shire Pharmaceuticals. She has received lecture honoraria from Janssen-Cilag, Medice Pharma, and Lilly as well as support for research from Novartis and has carried out clinical trials in cooperation with the Janssen-Cilag, Otsuka, Shire, and Boehringer Ingelheim companies.
Prof. Roessner has received payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals, lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma, and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with the Novartis, Shire, and Otsuka companies.
Prof. Münchau has served as a paid consultant Pharm Allergan. He has received reimbursement of travel costs and medical conference attendance fees from Merz Pharmaceuticals and Pharm Allergan, lecture honoraria from Merz Pharmaceuticals, Pharm Allergan, and Ipsen Pharma, and financial support for research from Merz Pharmaceuticals, Pharm Allergan, Ipsen Pharma, and Medivation Inc.
Prof. Müller-Vahl has received financial support for research from the Lundbeck company and has carried out (and is currently carrying out) clinical trials in cooperation with the Otsuka Pharma and Boehringer Ingelheim companies.
References
- 1.Müller-Vahl K. 1st edition. Berlin: Medizinisch Wissenschaftliche Verlagsgesellschaft; 2010. Tourette-Syndrom und andere Tic-Erkrankungen im Kindes- und Erwachsenenalter. [Google Scholar]
- 2.Banaschewski T, Woerner W, Rothenberger A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol. 2003;45:700–703. doi: 10.1017/s0012162203001294. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RD, Zinner SH, Müller-Vahl KR, Fast DK, Burd LJ, Kano Y, et al. Coprophenomena in Tourette syndrome. Dev Med Child Neurol. 2009;51:218–227. doi: 10.1111/j.1469-8749.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 4.Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008;65:461–472. doi: 10.1016/j.jpsychores.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436–447. doi: 10.1017/s0012162200000839. [DOI] [PubMed] [Google Scholar]
- 6.Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, Hartmann A, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part I: assessment. Eur Child Adolesc Psychiatry. 2011;20:155–171. doi: 10.1007/s00787-011-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalifa N, von Knorring AL. Psychopathology in a Swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- 9.Müller-Vahl K, Dodel I, Müller N, Münchau A, Reese JP, Balzer-Geldsetzer M, et al. Health-related quality of life in patients with Gilles de la Tourette’s syndrome. Mov Disord. 2010;25:309–314. doi: 10.1002/mds.22900. [DOI] [PubMed] [Google Scholar]
- 10.Pringsheim T, Lang A, Kurlan R, Pearce M, Sandor P. Understanding disability in Tourette syndrome. Dev Med Child Neurol. 2009;51:468–472. doi: 10.1111/j.1469-8749.2008.03168.x. [DOI] [PubMed] [Google Scholar]
- 11.Ludolph AG, Juengling FD, Libal G, Ludolph AC, Fegert JM, Kassubek J. Grey-matter abnormalities in boys with Tourette syndrome: magnetic resonance imaging study using optimised voxel-based morphometry. Br J Psychiatry. 2006;188:484–485. doi: 10.1192/bjp.bp.105.008813. [DOI] [PubMed] [Google Scholar]
- 12.Thomalla G, Siebner HR, Jonas M, Bäumer T, Biermann-Ruben K, Hummel F, et al. Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain. 2009;132:765–777. doi: 10.1093/brain/awn339. [DOI] [PubMed] [Google Scholar]
- 13.Ludolph AG, Pinkhardt EH, Tebartz van Elst L, Libal G, Ludolph AC, Fegert JM, et al. Are amygdalar volume alterations in children with Tourette syndrome due to ADHD comorbidity? Dev Med Child Neurol. 2008;50:524–529. doi: 10.1111/j.1469-8749.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeh CB, Lee CS, Ma KH, Lee MS, Chang CJ, Huang WS. Phasic dysfunction of dopamine transmission in Tourette’s syndrome evaluated with 99mTc TRODAT-1 imaging. Psychiatry Res. 2007;156:75–82. doi: 10.1016/j.pscychresns.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Müller-Vahl KR, Meyer GJ, Knapp WH, Emrich HM, Gielow P, Brücke T, et al. Serotonin transporter binding in Tourette Syndrome. Neurosci Lett. 2005;385:120–125. doi: 10.1016/j.neulet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Tourette Syndrome Association International Consortium for Genetics. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80:265–272. doi: 10.1086/511052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 18.Scharf JM, Moorjani P, Fagerness J, Platko JV, Illmann C, Galloway B, et al. Lack of association between SLITRK1var321 and Tourette syndrome in a large family-based sample. Neurology. 2008;70:1495–1496. doi: 10.1212/01.wnl.0000296833.25484.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ercan-Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O’Roak BJ, Mason CE, et al. L-histidine decarboxylase and Tourette’s syndrome. N Engl J Med. 2010;362:1901–1908. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martino D, Dale RC, Gilbert DL, Giovannoni G, Leckman JF. Immunopathogenic mechanisms in tourette syndrome: A critical review. Mov Disord. 2009;24:1267–1279. doi: 10.1002/mds.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallee FR, Kurlan R, Goetz CG, Singer H, Scahill L, Law G, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39:292–299. doi: 10.1097/00004583-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Dion Y, Annable L, Sandor P, Chouinard G. Risperidone in the treatment of tourette syndrome: a doubleblind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22:31–39. doi: 10.1097/00004714-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Scahill L, Leckman JF, Schultz RT, Katsovich L, Peterson BS. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130–1135. doi: 10.1212/01.wnl.0000055434.39968.67. [DOI] [PubMed] [Google Scholar]
- 24.Verdellen C, van de Griendt J, Hartmann A, Murphy T. European clinical guidelines for Tourette syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry. 2011;20:197–207. doi: 10.1007/s00787-011-0167-3. [DOI] [PubMed] [Google Scholar]
- 25.Piacentini J, Woods DW, Scahill L, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303:1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011;20:173–196. doi: 10.1007/s00787-011-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson MM, Schnieden V, Lees AJ. Management of Gilles de la Tourette syndrome using sulpiride. Clin Neuropharmacol. 1990;13:229–235. doi: 10.1097/00002826-199006000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Müller-Vahl KR. Die Benzamide Tiaprid, Sulpirid und Amisulprid in der Therapie des Tourette-Syndroms. Nervenarzt. 2007;78:264–271. doi: 10.1007/s00115-006-2131-x. [DOI] [PubMed] [Google Scholar]
- 29.Pringsheim T, Doja A, Gorman D, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. 2012;57:133–143. doi: 10.1177/070674371205700302. [DOI] [PubMed] [Google Scholar]
- 30.Müller-Vahl KR, Cath DC, Cavanna AE, Dehning S, Porta M, Robertson MM, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: deep brain stimulation. Eur Child Adolesc Psychiatry. 2011;20:209–217. doi: 10.1007/s00787-011-0166-4. [DOI] [PubMed] [Google Scholar]
- 31.Steeves T, McKinlay BD, Gorman D, et al. Canadian guidelines for the evidence-based treatment of tic disorders: behavioural therapy, deep brain stimulation, and transcranial magnetic stimulation. Can J Psychiatry. 2012;57:144–151. doi: 10.1177/070674371205700303. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn J, Gründler TOJ, Lenartz D, Sturm V, Klosterkötter J, Huff W. Tiefe Hirnstimulation bei psychiatrischen Erkrankungen. Dtsch Arztebl Int. 2010;107(7):105–113. doi: 10.3238/arztebl.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Mol Debes NM, Hjalgrim H, Skov L. Limited knowledge of Tourette syndrome causes delay in diagnosis. Neuropediatrics. 2008;39:101–105. doi: 10.1055/s-2008-1081457. [DOI] [PubMed] [Google Scholar]
- e2.Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette’s syndrome. Am J Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- e3.Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- e4.Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003;45:315–319. doi: 10.1017/s0012162203000598. [DOI] [PubMed] [Google Scholar]
- e5.Burd L, Kerbeshian PJ, Barth A, Klug MG, Avery PK, Benz B. Long-term follow-up of an epidemiologically defined cohort of patients with Tourette syndrome. J Child Neurol. 2001;16:431–437. doi: 10.1177/088307380101600609. [DOI] [PubMed] [Google Scholar]
- e6.Ohta M, Kano Y. Clinical characteristics of adult patients with tics and/or Tourette’s syndrome. Brain Dev. 2003;25:32–36. doi: 10.1016/s0387-7604(03)90006-4. [DOI] [PubMed] [Google Scholar]
- e7.Pappert EJ, Goetz CG, Louis ED, Blasucci L, Leurgans S. Objective assessments of longitudinal outcome in Gilles de la Tourette’s syndrome. Neurology. 2003;61:936–940. doi: 10.1212/01.wnl.0000086370.10186.7c. [DOI] [PubMed] [Google Scholar]
- e8.Hoekstra PJ, Steenhuis MP, Kallenberg CG, Minderaa RB. Association of the small life events with self reports of tic severity in pediatric and adult tic disorder patients: a prospective longitudinal study. J Clin Psychiatry. 2004;65:426–431. doi: 10.4088/jcp.v65n0320. [DOI] [PubMed] [Google Scholar]
- e9.Lin H, Yeh CB, Peterson BS, Scahill L, Grantz H, Findley DB, Katsovich L, Otka J, Lombroso PJ, King RA, Leckman JF. Assessment of symptom exacerbations in a longitudinal study of children with Tourette’s syndrome or obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2002;41:1070–1077. doi: 10.1097/00004583-200209000-00007. [DOI] [PubMed] [Google Scholar]
- e10.Sacks O. An Anthropologist on Mars: Seven Paradoxical Tales. New York: Alfred A. Knopf; 1995. A surgeon’s life. [Google Scholar]
- e11.Finis J, Moczydlowski A, Pollok B, Biermann-Ruben K, Thomalla G, Heil M, Krause H, Jonas M, Schnitzler A, Münchau A. Echoes from childhood-imitation in Gilles de la Tourette Syndrome. Mov Disord. 2012;27:562–565. doi: 10.1002/mds.24913. [DOI] [PubMed] [Google Scholar]
- e12.Singer HS. Motor stereotypies. Semin Pediatr Neurol. 2009;16:77–81. doi: 10.1016/j.spen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- e13.Singer HS. Stereotypic movement disorders. Handb Clin Neurol. 2011;100:631–639. doi: 10.1016/B978-0-444-52014-2.00045-8. [DOI] [PubMed] [Google Scholar]
- e14.Banaschewski T, Neale BM, Rothenberger A, Roessner V. Comorbidity of tic disorders & ADHD: conceptual and methodological considerations. Eur Child Adolesc Psychiatry. 2007;16:5–14. doi: 10.1007/s00787-007-1002-8. [DOI] [PubMed] [Google Scholar]
- e15.Brown L, Dure LS. The treatment of comorbid attention-deficit disorder and Tourette’s syndrome. In: Kurlan R, editor. Handbook of Tourette’s syndrome and related tic and behavioural disorders. New York: Marcel Dekker; 2005. pp. 455–465. [Google Scholar]
- e16.Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H, Lin H, Bentley MJ, Gilbert DL, Singer HS, Coffey BJ. the Tourette Syndrome Study Group, Kurlan RM, Leckman JF: Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012;21:451–457. doi: 10.1007/s00787-012-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Burd L, Kerbeshian PJ, Barth A, Klug MG, Avery PK, Benz B. Long-term follow-up of an epidemiologically defined cohort of patients with Tourette syndrome. J Child Neurol. 2001;16:431–437. doi: 10.1177/088307380101600609. [DOI] [PubMed] [Google Scholar]
- e18.Budman CL, Bockmore L, Stokes J, Sossin M. Clinical phenomenology of episodic rage in children with Tourette syndrome. J Pediatr Psychol. 2003;27:203–208. doi: 10.1016/s0022-3999(02)00584-6. [DOI] [PubMed] [Google Scholar]
- e19.Budman CL, Bruun RD, Park KS, Olson ME. Rage attacks in children and adolescents with Tourette’s disorder: a pilot study. J Clin Psychiatry. 1998;59:576–580. doi: 10.4088/jcp.v59n1103. [DOI] [PubMed] [Google Scholar]
- e20.Budman CL, Rockmore L, Stokes J, Sossin M. Clinical phenomenology of episodic rage in children with Tourette syndrome. J Psychosom Res. 2003;55:59–65. doi: 10.1016/s0022-3999(02)00584-6. [DOI] [PubMed] [Google Scholar]
- e21.Burd L, Freeman RD, Klug MG, Kerbeshian J. Tourette syndrome and learning disabilities. BMC Pediatr. 2005;5:34–40. doi: 10.1186/1471-2431-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e22.Worbe Y, Malherbe C, Hartmann A, Pélégrini-Issac M, Messé A, Vidailhet M, Lehéricy S, Benali H. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–1946. doi: 10.1093/brain/aws056. [DOI] [PubMed] [Google Scholar]
- e23.Neuner I, Ludolph AG. Neurobiology, clinical characteristics and therapy in Tourette’s syndrome. Fortschr Neurol Psychiatr. 2011;79:724–732. doi: 10.1055/s-0031-1281853. [DOI] [PubMed] [Google Scholar]
- e24.Hyde TM, Aaronson BA, Randolph C, Rickler KC, Weinberger DR. Relationship of birth weight to the phenotypic expression of Gilles de la Tourette’s syndrome in monozygotic twins. Neurology. 1992;42:652–658. doi: 10.1212/wnl.42.3.652. [DOI] [PubMed] [Google Scholar]
- e25.Nee LE, Caine ED, Polinsky RJ, Eldridge R, Ebert MH. Gilles de la Tourette syndrome: clinical and family study of 50 cases. Ann Neurol. 1980;7:41–49. doi: 10.1002/ana.410070109. [DOI] [PubMed] [Google Scholar]
- e26.Pauls DL. An update on the genetics of Gilles de la Tourette syndrome. J Psychosom Res. 2003;55:7–12. doi: 10.1016/s0022-3999(02)00586-x. [DOI] [PubMed] [Google Scholar]
- e27.Price RA, Kidd KK, cohen DJ. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42:815–820. doi: 10.1001/archpsyc.1985.01790310077011. [DOI] [PubMed] [Google Scholar]
- e28.Remschmidt H, Hebebrand J. Das Tourette-Syndrom: Eine zu selten diagnostizierte Tic-Störung? Dtsch Arztebl. 1993;90(24):A1805–A1810. [Google Scholar]
- e29.Deng H, Gao K, Jankovic J. The genetics of Tourette syndrome. Nat Rev Neurol. 2012;8:203–213. doi: 10.1038/nrneurol.2012.26. [DOI] [PubMed] [Google Scholar]
- e30.State MW. The genetics of Tourette Disorder. Curr Opon Genet Dev. 2011;21:302–309. doi: 10.1016/j.gde.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e31.Marcks BA, Berlin KS, Woods DW, Davies WH. Impact of Tourette Syndrome: a preliminary investigation of the effects of disclosure on peer perceptions and social functioning. Psychiatry. 2007;70:59–67. doi: 10.1521/psyc.2007.70.1.59. [DOI] [PubMed] [Google Scholar]
- e32.Wenzel C, Kleimann A, Bokemeyer S, Müller-Vahl KR. Aripiprazole for the treatment of tourette syndrome: a case series of 100 patients. J Clin Psychopharmacol. 2012;32:548–550. doi: 10.1097/JCP.0b013e31825ac2cb. [DOI] [PubMed] [Google Scholar]
- e33.Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- e34.Müller-Vahl KR. The treatment of Tourette’s syndrome: current opinions. Expert Opin Pharmacother. 2002;3:899–914. doi: 10.1517/14656566.3.7.899. [DOI] [PubMed] [Google Scholar]
- e35.Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:884–893. doi: 10.1097/CHI.0b013e3181b26e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e36.Shapiro A, Shapiro E, Young JG, Feinberg TE. Signs, symptoms, and clinical course. In: Shapiro A, Shapiro E, Young JG, Feinberg TE, editors. Gilles de la Tourette Syndrome. 2nd edition. New York: Raven Press; 1988. pp. 127–193. [Google Scholar]