Abstract
Mental set switching is a complex executive function that is required when the focus of attention must be altered in order to adapt to a frequently-changing environment. While there is generally acceptance that switching is subserved by a fronto-parietal network, there is a considerable lack of consistency across studies as to other brain regions involved in executing mental set switches. This functional magnetic resonance imaging study sought to determine whether paradigmatic design aspects such as stimulus complexity, motor response complexity, and stimulus ordering could account for the differences in reporting of brain regions associated with mental set switching across previous studies. Several brain regions, including the striatum and anterior cingulate, previously associated with mental set switching were found to be related more to resolving intra-stimulus interference conferred by increased stimulus complexity and increased motor response complexity than to executing the mental set switch. In considering stimulus ordering, defined as the number of non-switch trials preceding a switch trial, brain activity was not observed in the fronto-parietal regions typically associated with switching but rather in regions in the anterior prefrontal cortex, sensorimotor cortex, and secondary visual cortices. Our results indicate that these important paradigm design aspects that are theoretically unrelated to set switching per se should be balanced and controlled for in future experiments, so as not to obscure clear identification of brain regions truly engaged in mental set switching.
Keywords: fMRI, paradigm, set switching, set shifting, striatum, anterior cingulate cortex
INTRODUCTION
1.1 Mental set switching
Mental set switching is a complex executive function that is required when the focus of attention must be altered in order to adapt to a frequently-changing environment. In its broadest sense, set switching can be said to be necessary for nearly any type of cognitive set switch, whether it be shifting attention from one stimulus attribute to another (e.g., Hampshire and Owen, 20061 Loose et al., 2006), altering stimulus-response mappings in contexts with different contingencies (e.g., Rubia et al., 2006), or switching from performing one task to another (e.g., Dreher and Grafman, 2003). Despite the diversity of switch types, the underlying behavior being measured is the ability to update the executive control parameters representing a given ‘task set’ to accommodate a new task set (Logan and Gordon, 2001; Rogers and Monsell, 1995). Although the unique neural correlates of set switching are still a matter of active research, meta-analyses of relevant functional neuroimaging literature have confirmed the importance of brain activity within frontal and parietal cortex regions when a mental set switch is required (Buchsbaum et al., 2005; Wager et al., 2004). However, across the growing body of fMRI and PET studies published to date, it is clear more than these regions are commonly engaged when set switching is required. For example, in his meta-analytic synthesis of studies prior to 2004, Wager et al. (2004) noted evidence across studies for set shifting engagement of occipital cortices, sensorimotor regions, temporal cortex, insulae, anterior cingulate, and thalamus (Wager et al., 2004). Many of these same regions were also noted in a second, later meta-analysis (Buchsbaum et al., 2005). Additionally, some brain regions, such as the striatum (Casey et al., 2004; Casey et al., 2002; Luna et al., 2001; Sohn et al., 2000) and anterior cingulate cortex (Braver et al., 2003; Derrfuss et al., 2004; Rubia et al., 2006; Smith et al., 2004), are frequently implicated in small subsets of individual set switching studies.
1.2 Inconsistencies in reported neural correlates of mental set switching
Wager et al. (2004) attempted to account for the inconsistencies across studies by examining whether brain activity elicited across set shifting neuroimaging studies was related to inherent properties of brain organization, (i.e., whether different brain regions were functionally specialized for shifting attention for location, objects, attributes, rules, and tasks). However, they did not find any brain region active during a specific switch type that was not also active during all other switch types (Wager, et al. 2004). Gender has not routinely been considered as a factor that dictates neural activity differences in set switching tasks. Numerous studies have shown that there are no differences in behavioral performance on cognitive switching tasks between adult males and females (e.g., Kalkut et al., 2009). Indeed, there is only limited evidence that females engage fronto-striatal regions more than males, while males engage parietal regions more than females during performance of a Meiran-type stimulus-response set switching task (Christakou et al., 2009). Because that study included both adolescents and adults, it is not clear whether the observed gender-related differences were dominated by changes in neural activity during adolescence (Christakou et al., 2009). Another aspect of the previous body of set shifting literature that has not yet been considered when attempting to understand the Inconsistencies across studies is the design differences in the fMRI paradigms themselves. Different paradigm types can impose different types of cognitive demands, each of which could conceivably elicit different profiles of brain activity. For instance, set switching paradigms unavoidably rely on the integrity of other cognitive systems including working memory, inhibition, and attention (Miyake et al., 2000). It is possible that failure to effectively balance these paradigm design considerations (or even other cognitive demands) between switch and non-switch conditions might obscure evidence for regional activation (e.g., Rubia et al., 2006 employed a paradigm in which switch trials were preceded by no fewer than three non-switch trials, potentially biasing results towards any effects of stimulus ordering; Derfuss et al., 2004 reported that switch trials could be preceded by upwards of five non-switch trials, but gave no indication as to whether this ordering had been balanced across the run), leading to variable findings across studies and possibly inaccurate conclusions about which brain regions are functionally specialized for effecting a switch of mental set.
1.3 Summary of various paradigms employed in functional neuroimaging studies of set switching
A review of the set switching literature reveals that studies measuring the same switch type can have very different degrees of stimulus complexity and motor response options. Complex stimuli can be considered those stimuli that differ on multiple dimensions, thus increasing interference in determining the correct target. For example, studies employing tasks requiring both attribute and rule switches, such as card sorting tasks, can use simple stimuli requiring a forced choice option (e.g., Asari et al., 2005) or more complex stimuli which require the participant to choose from several possible options (e.g., Hampshire and Owen, 2006; Monchi et al., 2006; Nagahama et al., 1999). Likewise, experiments can employ response options that require a participant to either continually switch between stimulus-response set mappings (e.g., Crone et al., 2006; Derrfuss et al., 2004; Dove et al., 2000; Rubia et al., 2006; Wilkinson et al., 2001) or select from upwards of four different responses (e.g., Monchi et al., 2006). Paradigms employing these more complex motor response options also increase stimulus-response interference when selecting motor program for the correct response (e.g., Hazeltine et al., 2000; Jiang and Kanwisher, 2003). Another aspect of paradigm design that can influence the observed neural correlates of set switching lies in the ordering and timing of stimuli. It has been shown that varying the inter-stimulus interval or response-to-cue interval had a significant effect on the behavioral performance of an attribute set switching task (Badre and Wagner, 2006; Cepeda et al., 2001; Loose et al., 2006). Additionally, for those mental set switching experiments that employ a cue notifying of an impending shift, varying the cue-to-target interval (“task set reconfiguration” manipulation; (Rogers and Monsell, 1995) has also been shown to have a significant measureable effect on behavioral performance (Cepeda et al., 2001; Meiran, 1996, 2000). In line with these previous results regarding stimulus timing in set switching tasks, one might reasonably expect that stimulus ordering (i.e., having more or fewer non-switch trials preceding a switch trial) would alter both behavioral performance and the activity in those brain regions engaged in switching. Other evidence for stimulus order on brain function come from fMRI studies examining task automaticity and habituation, which have shown a role for the frontal cortices and inferior parietal lobes in reorienting attention after error perception in a Go/No-go task (Hester et al., 2005) and after a target switch in an over-practiced visual search task (Kubler et al., 2006).
1.4 Summary and hypotheses
We present results from an attribute set switching task that manipulated stimulus complexity, motor response complexity (i.e., number of possible responses), and the number of non-switch trials preceding a given switch or non-switch trial in a pseudo-random factor throughout each task session. The paradigm was constructed not only to balance several other design factors relevant to shifting, but also to determine the effect of trial type (i.e., switch or non-switch) on these paradigmatic design considerations. As our paradigm was designed to be able to quantify these three design aspects for each trial, we employed a GLM approach including measures for stimulus complexity, motor response complexity, and the number of preceding non-switch trials as parametric modulation terms. The aims of this study were twofold. First, we wanted to identify the neural correlates of set-shifting unconfounded by otherwise controllable experimental parameters, and second, we wanted to characterize the neural correlates of these paradigm design aspects and determine if the discrepancies found in the set-shifting literature are related to them. In line with the previously cited meta-analyses, we anticipate that set-shifting would engage predominantly frontal and parietal regions. We hypothesized that increasing stimulus complexity would result in increased hemodynamic activity in both frontal and motor planning regions to assist in overcoming the increased interference posed by stimuli differing on multiple dimensions. As the anterior cingulate cortex has previously been linked to interference resolution, as in Stroop tasks (Nee et al., 2007), we anticipated that this region would be engaged more for overcoming stimulus complexity interference than switching. We predicted that increasing the number of possible motor response options would result in increased hemodynamic activity in both motor planning and motor performance regions. Both the caudate and putamen have been linked to motor planning and performance (Witt et al., 2008), thus we hypothesized that these regions would be enlisted to overcome motor response complexity. A recent review of the role of interference in set switching found that both stimulus- and response-based interferences, conferred by moderately and maximally complex stimuli, had dissociable and measurable effects on set switching behavioral performance, with greater effects noted for switch trials compared to non-switch trials (Kiesel et al., 2010). Thus we anticipated that any observed activity in these frontal and motor regions would be greater during switch trials, due to the need to overcome both inter- and intra-stimulus interference, as evidenced by increased behavioral switch costs. As there is not a large body of work published on task automaticity during executive or cognitive functions, we speculated that switch trials following long trains of non-switch trials might engage brain regions associated with reorienting attention after periods of task automaticity or habituation. These included regions in the superior, medial, middle, and inferior frontal cortices as well as the inferior parietal lobes and supramarginal gyri (Hester et al., 2005; Kubler et al., 2006). As with our hypotheses for stimulus and motor response complexity, we anticipated that the effects of long trains of non-switch trials preceding a switch trial would be more pronounced during switch trial due to need to reorient attention to the new mental set.
METHODS
2.1 Participants
A total of 83 right-handed adult participants (46 females) were recruited for several studies at the Olin Neuropsychiatry Research Center through community advertisements. Participants ranged in age from 18 to 31 years (mean (SD) = 22 (2.7) years) and were screened to ensure that they were medically healthy and had no past head injury, neurologic condition, learning disability, or other neurodevelopmental conditions. The absence of current or lifetime psychiatric and substance abuse disorders was determined using the screening module of the Structured Clinical Interview for Diagnosis (SCID-IV; (First et al., 1996). All participants underwent written informed consent using procedures approved by the Hartford Hospital IRB.
2.2 FMRI Stimulus Delivery/Response Recording
The fMRI set switching task was implemented using E-Prime software (Psychology Software Tools, Inc.). Visual stimuli were presented using a projection system (5000 ANSI lumens) and displayed on a high resolution screen located just behind the participant’s head. The participant viewed the screen using a mirror attached to the head coil. Corrective lenses were provided as needed. An MR-compatible fiber optic response device (Lightwave Medical, Inc., Vancouver, B.C.) was used to acquire behavioral responses.
2.3 Set switching Paradigm
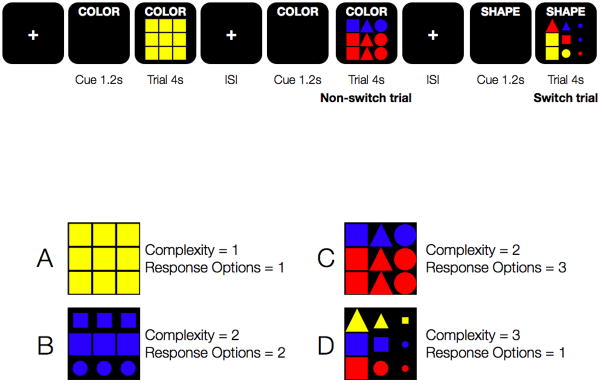
The set switching paradigm (Figure 1) was designed as a rapid, event-related fMRI task, where each of two unique runs consisted of 81 trials, 41 switch trials (counting the first trial as a switch) and 40 non-switch trials. Trials were pseudo-randomly ordered, where any given switch trial could be preceded by between 1 and 6 non-switch trials (mean = 2; median = 1 preceding non-switch trials) and stimulus complexity and response options varied in no discernable pattern. The number of non-switch trials preceding a given switch trial was distributed such that 50% of switch trials were preceded by 1 non-switch trial, 25% by 2 non-switch trials, and 25% by 3 or more non-switch trials. Each trial was 4 seconds long, preceded by a 1.2 second cue (Cepeda et al., 2001). The cue informed participants whether they would be required to count the number of different colors, sizes, or shapes on the stimulus, and this cue remained on the screen throughout the trial to remove extraneous working memory demands. The cue duration was selected based on previous evidence that 1.2 seconds was sufficient to equalize impact on behavioral switch costs due to proactive task set reconfiguration (Cepeda et al., 2001). The response type was held constant on all trials (i.e., participants always pressed button 1, 2, or 3 if there were 1, 2, or 3 dimensions counted). The task itself (counting) remained the same on all trials, but participants had to shift among attribute sets to count the relevant stimulus attribute. The stimuli were 3×3 grids of different colors (red, yellow, or blue), sizes (small, medium, or large), and shapes (triangles, circles, or squares) that ranged from very simple (all large, yellow squares, i.e., differing on only one dimension) to complex (differing on three dimensions). Importantly, the task was counterbalanced across all factors, such that the frequency of each color, size, and shape (including combinations and mappings to task) was equiprobable, ensuring that there were no frequency or familiarity confounds. Additionally, no stimulus was repeated. The interstimulus interval (ISI) was varied between 0.5 and 9.5 seconds, with an average (SD) of 2.4 (2) seconds. Each run lasted 8 minutes and 41 seconds.
Figure 1. Partial schematic of the paradigm design and examples of stimuli and associated parametric terms.
A partial schematic of the paradigm is depicted in the upper portion of the figure, and example stimuli are shown in the lower portion of the figure. Participants were given a verbal cue 1.2 seconds prior to stimulus onset as to which stimulus attribute to count (color, shape, size), and this cue remained on the screen throughout the entire trial. Participants then had 4 seconds to respond with the appropriate button press (1,2,3). ISI stands in for the variable interstimulus interval. Each stimulus was assigned an integer value (1,2,3) based on the number of dimensions, corresponding to its complexity. Stimulus A was assigned a stimulus complexity value of 1, since it did not differ on any dimensions. Stimuli B and C each contained two dimensions (B: size and shape; C: shape and color) and were assigned a stimulus complexity value of 2. Stimulus D had three dimensions (shape, size, color) and was assigned a stimulus complexity value of 3. To determine the response complexity, the number of potential valid responses were counted for each stimuli, not assuming a specific cue. For stimulus A, there was only one potential response option, as there was only one color, one shape, and one size presented. The only valid button press was 1, and stimulus A was assigned a response complexity value of 1. For stimulus B, there were two potential response options, as there was one color, two sizes, and two shapes. Valid button presses were 1 and 2, and stimulus B was, thus, assigned a response complexity value of 2. For stimulus C, there were three potential response options: one size, two colors, and three shapes (valid button presses were 1, 2, and 3). A response complexity value of 3 was assigned to stimulus 3. Finally, for stimulus D, there was again only one potential response option, as there were three sizes, three shapes, and three colors (valid button press was 3).
2.4 Imaging Parameters
MR images were acquired on a 3T Siemens Allegra (Siemens Medical Solutions, Erlangen, Germany) located at the Olin Neuropsychiatry Research Center at the Institute of Living/Hartford Hospital in Hartford, CT. The functional imaging volumes were collected in axial orientation to the anterior commissure-posterior commissure line using a single-shot-gradient-echo echo-planar sequence (TR/TE = 1,500/28 msec; flip angle = 65; FOV = 24 cm; matrix = 64; 3.4×3.4 mm in-plane resolution; slice thickness = 5 mm; 30 slices) with whole brain coverage. The two runs each consisted of 347 time points, with an initial 9 second rest session to allow for T1 effects to stabilize. These initial six images were not included in subsequent analyses.
High resolution T1- and T2-weighted anatomical images were also acquired on all participants to ensure that all were free from obvious brain structure abnormality that might influence both task performance results and interpretation of functional imaging results.
2.5 Behavioral Data Analyses
The reaction time data for correct trials were analyzed using a series of multivariate, repeated measures ANOVAs in SPSS (IBM Corporation, Somers, NY). Trial type (switch, non-switch) and each paradigm design aspect (stimulus complexity, number of potential response options, and number of preceding non-switch trials) were used as within-subjects factors. Evidence for significant multivariate effects was examined at each level of each paradigm design aspect (i.e., 1, 2, 3 for stimulus complexity and number of potential response options and 1–6 for the number of preceding non-switch trials; Figure 1). Two additional series of multivariate, repeated measures ANOVAs were also conducted using age binned to the nearest integer and gender as between-subjects factors. Again, evidence for significant multivariate behavioral task effects was examined for each between-subject factor, each within-subject factor, and all possible within- and between-subjects interactions in a mixed-effects regression model.
2.6 Image Processing
Functional images were reconstructed offline. Each run was corrected for slice-timing acquisition differences and separately realigned using INRIalign (Freire and Mangin, 2001; Freire et al., 2002) as implemented in SPM5 (Wellcome Department of Cognitive Neurology, London, UK). A mean functional image volume was constructed for each participant for each run from the realigned image volumes and used to determine the parameters for spatial normalization into standardized Montreal Neurological Institute (MNI) space. These normalization parameters were then applied to the corresponding functional image volumes, and the normalized images were smoothed with an 8 mm FWHM Gaussian kernel. All participants’ data were individually inspected to ensure that no participant had translational or rotational head motion greater than the acquired voxel size.
2.7 FMRI Statistics
The regressors from each participant’s fMRI model were derived by extracting the stimulus onset timing for all trials and modeled using a synthetic hemodynamic response function. The functional imaging data of each participant were modeled individually in SPM5 and included regressors for switch trials and non-switch trials. Stimulus complexity, response-mapping, and the number of preceding non-switch trials were included into each participant model as parametric interaction terms, in that order. Stimulus complexity was parameterized as the number of attribute dimensions on a given stimulus (i.e., 1, 2, or 3). Similarly, response-mapping was parameterized as the number of potential response options (i.e., 1, 2, or 3), based on the number of potential valid response options assuming that the cue was ignored. Example stimuli are shown in lower portion of Figure 1. While stimulus complexity and motor response complexity often corresponded in this paradigm, the actual correlation between the two regressor terms was only r = 0.26 for each run. Finally, the number of preceding non-switch trials was parameterized as the integer number of non-switch trials preceding a given trial. As SPM5 utilizes serial orthogonalization, individual participant models were rerun several times in different ways to ensure that the order in which the parametric interaction terms were included in the statistical parametric model did not affect the results. The six motion-corrected parameter estimates (x, y, and z displacements and pitch, roll, and yaw rotations) were included as covariates of no interest to statistically control signal change related to head motion. A high-pass filter (cutoff period = 128 s) was incorporated into the model to remove low-frequency signals. Contrasts corresponding to the main effect of switch trials, non-switch trials and the parametric main effects of stimulus complexity, response-mapping, and number of preceding non-switch trials were specified for each participant. All contrast images written by SPM5 represented brain activity relative to an ‘implicit baseline’ of unmodeled variance. In addition, linear combinations of these contrasts were specified to examine the difference between elicited BOLD signal changes on switch versus non-switch trials for each of these factors.
The main effects of switching were estimated using a 1-sample t-test of each participant’s contrast directly comparing switch trials to non-switch trials. The neural correlates of each paradigm design aspect were also measured via three individual 1-sample t-tests of each participant’s contrasts estimating the parametric effects of stimulus complexity, number of potential response options, or number of preceding non-switch trials, respectively, across both trial types. To determine the parametric effects of each paradigm design aspect on a given trial type (switch or non-switch), individual 1-sample t-tests were performed on every participant’s contrast terms comparing each paradigm design aspect for switch trials to that for non-switch trials. These tests allowed us to identify which of these brain regions with activity related to a given paradigm design aspect -- irrespective of parametric level (e.g., increased versus decreased stimulus complexity) -- also differed in activation in some way between switch trials compared with non-switch trials and vice versa. By choosing to use an arguably simpler parametric analysis over a more complicated four-way GLM-interaction, we lost the ability to fully describe the interaction between switching and paradigm design aspect, perhaps presenting an avenue for future work to fully describe these relationships. All group effect maps underwent whole-brain correction for multiple comparisons using False Discovery Rate (FDR; Genovese et al., 2002).
RESULTS
3.1 Behavioral Data
All participants were able to perform the set-switching task with high accuracy and consistency. The average response accuracies were 92.2% and 89.4% for non-switch and switch trials, respectively. Overall there was an average of <1% missed (no response) trials. No single participant had less than 75% accuracy or failed to respond to more than 10% of trials, regardless of trial type.
Significant multivariate effects on reaction time were observed for Trial type (switch versus non-switch trials), confirming the presence of an overall switch cost of 96 msec (F(1,82) = 198.271, p < 1.36 × 10−23). Significant multivariate effects were also observed for each of the three factors under consideration, stimulus complexity (F(2,81) = 181.381, p < 1.76 × 10−22), number of response options (F(2,81) = 190.506, p < 2.37 × 10−37), and the number of preceding non-switch trials (F(3,80) = 117.072, p < 3.55 × 10−29), indicating that these paradigmatic design factors have an overall effect on performance, regardless of trial type. Finally, significant multivariate Design Factor x Trial Type (switch versus non-switch trials) interactions were observed between each of the three factors: stimulus complexity (F(2,81) = 11.129, p < 5.4×10−5), number of response options (F(2,81) = 47.510, p < 2.23 × 10−14), and number of preceding non-switch trials (F(3,80) = 8.498, p < 5.75×10−5), indicating that each of these factors, beyond affecting overall reaction times, had an effect on switch costs (Figure 2).
Figure 2. Behavioral data from Design Factor x Trial Type interaction.
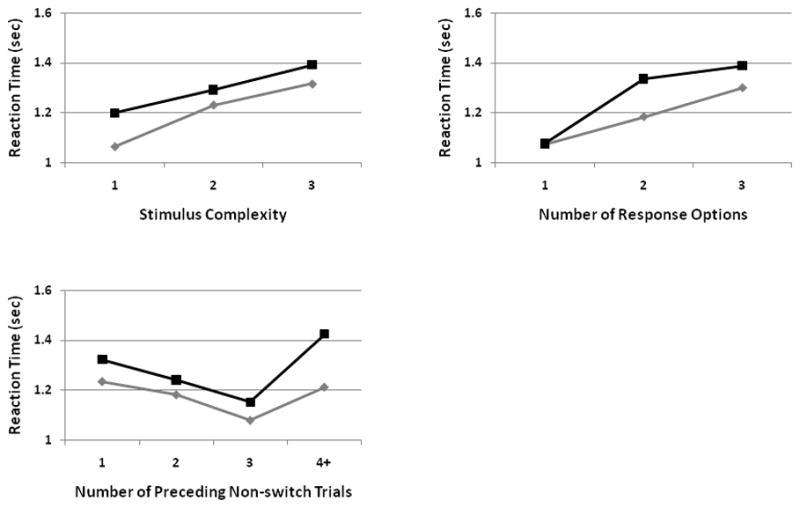
Graphs of the Design Factor x Trial Type interaction for the three design factors, where switch trial reaction times are shown in BLACK and non-switch trial reaction times in GRAY. For stimulus and response complexity, an overall slowing with increased complexity was observed. Of note, is that for trials with only one potential response option, there was an extinguishing of switch costs. In considering the number of preceding non-switch trials, there is an overall improvement in behavioral performance up to three preceding non-switch trials. For switch trials preceded by four or more non-switch trials, continued improvement is not observed, and switch costs actually revert back to the original levels of switch trials preceded by only one non-switch trial.
A second set of multivariate analyses confirmed that the significant effects of stimulus complexity, number of response options, and number of preceding non-switch trials described above were not the result of either differences due to gender or age. There were no overall multivariate age effects for switch costs, stimulus complexity, number of response options, and number of preceding non-switch trials, nor were there any significant multivariate Age x Design Factor x Trial Type interactions. Similarly, we did not observe any significant multivariate effects between gender and switch costs, stimulus complexity, number of response options, and number of preceding non-switch trials. However, significant Gender x Design Factor x Trial Type interactions were observed for stimulus complexity (F(2,80) = 3.378, p < 0.0391) and number of response options (F(2,80) = 3.189, p < 0.047), but these interactions are likely explained by slightly better overall performance in men compared with women.
3.2 Functional imaging results
3.2.1 Main effects of switching
The comparison of switch versus non-switch trials (main effects of switching) is summarized here to provide a context of key findings related to “switching” for this paradigm (Figure 3, Table 1). Switch trials resulted in greater activity in the left middle frontal gyrus (BA 6/9/46), left inferior frontal gyrus (BA 44/45), left superior and inferior parietal lobes (BA 7/40), medial and superior frontal gyrus (BA 6/8), right middle frontal gyrus (BA 6), right superior parietal lobe (BA 7), bilateral insula, bilateral thalamus, as well as several occipital and temporal regions. Non-switch trials were predominantly characterized by a large cluster of activation in the medial cuneus (BA 18) and a small but significant cluster of activity in the medial frontal gyrus (BA 10).
Figure 3. Main effects of switching.
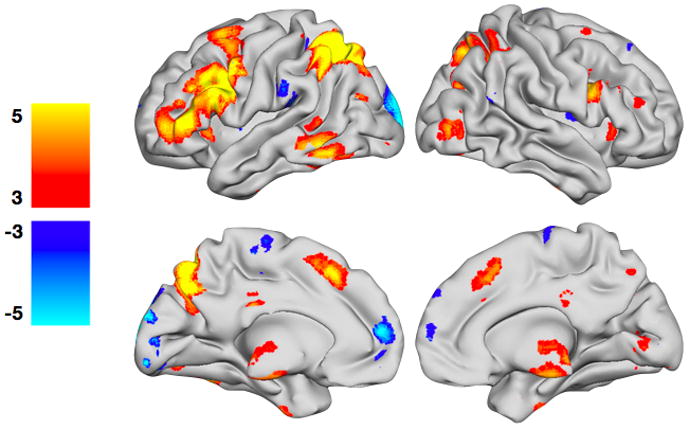
Regions with greater activity during switch trials are shown in YELLOW/ORANGE, and regions with greater activity during non-switch trials are shown in BLUE/GREEN. The t-maps have been thresholded at p < 0.05, corrected for multiple comparisons using False Discovery Rate (FDR). Color bars are expressed in terms of t-scores, and images are displayed in normal convention (left = left).
Table 1. Peak stereotactic coordinates for the main effects of switching (switch trials > non-switch trials).
Coordinates (MNI) of those regions with greater activity during switch trials are listed in the upper portion of the table, while those with greater activity during non-switch trials are listed in the lower portion. Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via False Discovery Rate (FDR) at p < 0.05.
| Region | X | Y | Z | T |
|---|---|---|---|---|
| TABLE 1 | ||||
| Main effects of switch trials greater than non-switch trials | ||||
| Frontal | ||||
| Right medial frontal gyrus/superior frontal gyrus/cingulate (BA 6/8/32) | 10 | 20 | 44 | 3.88 |
| Left medial frontal gyrus/superior frontal gyrus (BA 6/8) | −6 | 20 | 50 | 4.43 |
| Left middle frontal gyrus (BA 6) | −30 | 8 | 60 | 5.01 |
| Right middle frontal gyrus (BA 6) | 30 | 8 | 62 | 3.37 |
| 26 | 0 | 56 | 3.00 | |
| Left middle frontal gyrus (BA 9/46) | −44 | 24 | 26 | 6.38 |
| Left inferior frontal gyrus | −42 | 6 | 30 | 8.95 |
| Left inferior frontal gyrus (BA 44/45) | −50 | 36 | 8 | 5.12 |
| Right inferior frontal gyrus (BA 9) | 44 | 10 | 30 | 4.94 |
| Left inferior frontal gyrus/insula (BA 47) | −34 | 20 | −4 | 4.01 |
| Right inferior frontal gyrus/insula | 34 | 22 | −2 | 3.32 |
| Parietal | ||||
| Left superior parietal lobe (BA 7) | −24 | −64 | 48 | 7.36 |
| Right superior parietal lobe | 26 | −72 | 54 | 4.66 |
| Left inferior parietal lobe (BA 40) | −46 | −58 | 52 | 5.39 |
| Left precuneus | −6 | −74 | 42 | 5.95 |
| Right superior parietal lobe/precuneus (BA 7) | 32 | −68 | 38 | 4.38 |
| Left cingulate (BA 23/31) | 0 | −30 | 32 | 3.89 |
| Occipital | ||||
| Right cuneus | 6 | −80 | 2 | 3.50 |
| Right middle occipital gyrus (BA 19) | 44 | −82 | 2 | 4.23 |
| Temporal | ||||
| Left middle temporal gyrus (BA 37) | −58 | −52 | 6 | 3.57 |
| Subcortical | ||||
| Left thalamus | −16 | −22 | −4 | 3.48 |
| Right thalamus | 8 | −12 | 0 | 3.75 |
| 4 | −20 | 2 | 3.38 | |
| Main effects of non-switch trials greater than switch trials | ||||
| Frontal | ||||
| Left medial frontal gyrus (BA 10) | −14 | 54 | 14 | 5.12 |
| Occipital | ||||
| Left cuneus (BA 18) | −10 | −100 | 20 | 7.83 |
3.2.2 Parametric effects of stimulus complexity
Increased complexity (Figure 4A, Table 2A) was characterized by significantly greater activity in bilateral middle/inferior frontal gyri (BA 6/9/46/47), right medial frontal cortex (BA 8, pre-SMA), bilateral superior parietal lobes (BA 7), bilateral inferior parietal lobes (BA 40), bilateral caudate, bilateral thalamus, bilateral anterior insula, bilateral middle/inferior occipital gyri, and bilateral fusiform/lingual gyri. Increased complexity also was characterized by lower activity in bilateral anterior frontal gyri (BA 8/9), anterior cingulate, inferior parietal gyri (BA 40), posterior cingulate (BA 31), posterior insula (BA 13), and middle temporal gyri (BA 22).
Figure 4. A: Parametric effects of stimulus complexity.
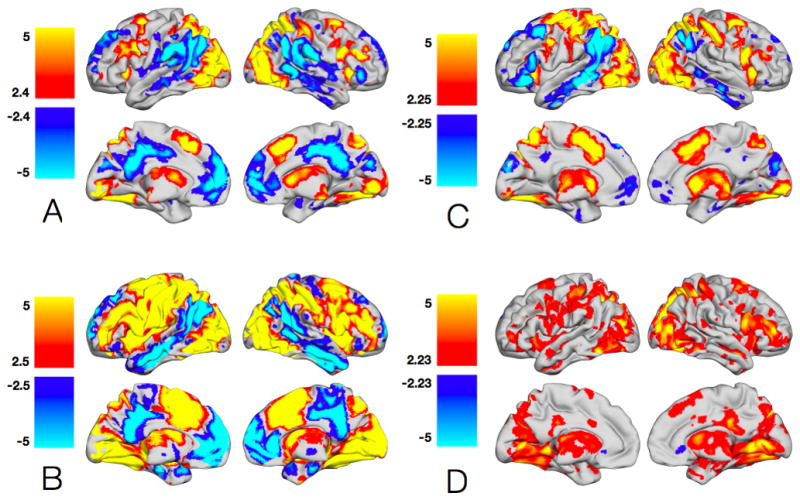
Regions with greater activity for trials with high stimulus complexity are shown in YELLOW/ORANGE, while regions with greater activity for trials with low stimulus complexity are shown in GREEN/BLUE. B: Effects of stimulus complexity on switching. Stimulus complexity related regions with greater activity during switch trials are shown in YELLOW/ORANGE. Stimulus complexity related regions with greater activity during non-switch trials are shown in GREEN/BLUE. C: Parametric effects of response complexity. Regions with greater activity for trials with greater number of potential response options are shown in YELLOW/ORANGE, while regions with greater activity for trials with fewer number of response options are shown in GREEN/BLUE. D: Effects of response complexity on switching. Response complexity related regions with greater activity during switch trials are shown in YELLOW/ORANGE. Response complexity related regions with greater activity during non-switch trials are shown in GREEN/BLUE. All t-maps have been thresholded at p < 0.05, corrected for multiple comparisons (FDR). Color bars are expressed in terms of t-scores, and images are displayed in normal convention.
Table 2. A: Peak stereotactic coordinates (MNI) for the parametric effects stimulus complexity.
Regions with greater activity for trials with high stimulus complexity are listed first, followed by those with greater activity for trials with low stimulus complexity. B: Peak stereotactic coordinates (MNI) for modulatory effects of stimulus complexity on switching. Regions with greater activity during switch trials are listed first, followed by regions with greater activity during non-switch trials. Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via False Discovery Rate (FDR) at p < 0.05.
| Region | X | Y | Z | T | |
|---|---|---|---|---|---|
| TABLE 2A | |||||
| Main effects of increasing stimulus complexity | |||||
| Frontal | |||||
| Right medial frontal gyrus (BA 8) | 2 | 24 | 48 | 6.88 | |
| Left middle frontal gyrus (BA 6) | −24 | 4 | 52 | 6.80 | |
| Right middle frontal gyrus (BA 6) | 26 | 4 | 52 | 7.39 | |
| Right middle frontal gyrus (BA 46) | 46 | 34 | 24 | 6.71 | |
| Left middle/inferior frontal gyrus (BA 9) | −42 | 6 | 28 | 8.21 | |
| Right inferior frontal gyrus (BA 9) | 46 | 10 | 28 | 7.07 | |
| Left insula | −32 | 22 | 0 | 5.53 | |
| Right inferior frontal gyrus/insula (BA 47) | 32 | 24 | −4 | 6.75 | |
| Parietal | |||||
| Left superior parietal lobe/precuneus (BA 7) | −22 | −62 | 54 | 12.08 | |
| Right superior parietal lobe/precuneus (BA 7) | 24 | −62 | 54 | 13.92 | |
| Left inferior parietal lobe (BA 40) | −40 | −38 | 42 | 6.43 | |
| Right inferior parietal lobe/postcentral gyrus (BA 40) | 46 | −32 | 46 | 8.56 | |
| Occipital | |||||
| Left cuneus (BA 19) | −26 | −86 | 22 | 12.88 | |
| Right middle occipital gyrus (BA 19) | 30 | −82 | 22 | 12.12 | |
| Left middle/inferior occipital gyrus (BA 19) | −36 | −76 | −8 | 8.99 | |
| Right middle/inferior occipital gyrus (BA 19) | 42 | −80 | −4 | 7.88 | |
| Left lingual gyrus (BA 17) | −6 | −94 | −6 | 7.46 | |
| Right lingual gyrus (BA 18) | 8 | −84 | −6 | 7.91 | |
| Subcortical | |||||
| Left caudate | −6 | 4 | 12 | 4.39 | |
| Right caudate | 8 | 10 | 10 | 5.05 | |
| Left thalamus | −12 | −22 | 14 | 4.17 | |
| Right thalamus | 20 | −24 | 16 | 3.95 | |
| Right caudate/thalamus | 12 | −6 | 18 | 4.09 | |
| Main effects of decreasing stimulus complexity | |||||
| Frontal | |||||
| Left superior frontal gyrus (BA 8/9) | −14 | 50 | 44 | 5.41 | |
| Right superior frontal gyrus (BA 8/9) | 12 | 52 | 44 | 4.97 | |
| Left medial frontal gyrus/anterior cingulate (BA 10) | −4 | 52 | 0 | 6.19 | |
| −10 | 60 | 10 | 7.06 | ||
| Left insula (BA 13) | −40 | −4 | 14 | 4.80 | |
| Right insula (BA 13) | 42 | −8 | 14 | 4.27 | |
| Parietal | |||||
| Left cingulate gyrus (BA 31) | −12 | −28 | 44 | 6.36 | |
| Right cingulate gyrus (BA 31) | 6 | −26 | 44 | 6.65 | |
| Right posterior cingulate (BA 31) | −2 | −46 | 30 | 6.20 | |
| Left angular gyrus | −54 | −66 | 32 | 6.87 | |
| Left inferior parietal lobe (BA 40) | −64 | −46 | 34 | 7.68 | |
| Left inferior parietal lobe/postcentral gyrus (BA 40) | −62 | −28 | 22 | 6.57 | |
| Right inferior parietal lobe/postcentral gyrus (BA 40) | 62 | −26 | 22 | 8.45 | |
| Right supramarginal gyrus (BA 40) | 62 | −52 | 32 | 7.00 | |
| Occipital | |||||
| Right cuneus (BA 19) | 4 | −84 | 28 | 4.44 | |
| Left parahippocampus (BA 34) | −20 | −10 | −20 | 3.05 | |
| Right parahippocampus (BA 34) | 22 | −10 | −20 | 2.99 | |
| Temporal | |||||
| Left middle temporal gyrus (BA 22) | −64 | −54 | 10 | 5.43 | |
| Right middle temporal gyrus (BA 21/22) | 66 | −50 | 4 | 7.11 | |
| Right superior temporal gyrus | 54 | 0 | 2 | 5.25 | |
| TABLE 2B | |||||
| Modulatory effects of stimulus complexity on switch trials | |||||
| Frontal | |||||
| Left middle frontal gyrus (BA 6) | −28 | −2 | 66 | 11.55 | |
| Right middle frontal gyrus (BA 6) | 32 | 4 | 62 | 11.88 | |
| Right middle frontal gyrus/cingulate (BA 6/8/32) | 6 | 20 | 50 | 13.18 | |
| Right inferior frontal gyrus (BA 9/44) | 58 | 12 | 16 | 12.59 | |
| Right inferior frontal gyrus (BA 44) | 58 | 12 | 16 | 12.59 | |
| Left insula (BA 13) | −34 | 16 | 4 | 10.78 | |
| Left insula/putamen (BA 13) | −40 | −4 | 10 | 15.05 | |
| Right insula (BA 13) | 38 | 18 | 0 | 11.46 | |
| Left precentral gyrus (BA 4/6) | −42 | −18 | 62 | 13.60 | |
| Left precentral gyrus | −38 | −8 | 64 | 11.76 | |
| Right precentral/postcentral gyrus (BA 6) | 44 | −8 | 34 | 2.46 | |
| Parietal | |||||
| Left precentral gyrus (BA 3) | −32 | −14 | 70 | 14.36 | |
| Left postcentral gyrus/inferior parietal lobe (BA 4/40) | −46 | −36 | 54 | 17.67 | |
| Left inferior parietal lobe (BA 2/40) | −42 | −38 | 48 | 18.44 | |
| Right inferior parietal lobe | 46 | −34 | 48 | 16.95 | |
| Left superior parietal lobe/precuneus (BA 7) | −24 | −64 | 60 | 16.54 | |
| Right superior parietal lobe/precuneus (BA 7) | 24 | −64 | 60 | 15.62 | |
| Occipital | |||||
| Left middle occipital gyrus | −46 | −74 | −10 | 13.60 | |
| Right inferior occipital gyrus | 44 | −82 | −6 | 15.85 | |
| Right cuneus (BA 17) | 2 | −94 | −2 | 17.81 | |
| Subcortical | |||||
| Left thalamus | −20 | −30 | 4 | 10.87 | |
| −12 | −22 | 8 | 10.59 | ||
| Right thalamus | 22 | −30 | 0 | 10.83 | |
| 14 | −12 | 10 | 6.82 | ||
| Right putamen | 22 | 0 | 12 | 9.28 | |
| Left cerebellum | −30 | −84 | −22 | 17.60 | |
| Right cerebellum | 24 | −72 | −18 | 16.02 | |
| Modulatory effects of stimulus complexity on non-switch trials | |||||
| Frontal | |||||
| Left medial frontal gyrus/anterior cingulate (BA 9/10/24/32) | −4 | 60 | 12 | 12.14 | |
| Left anterior cingulate (BA 24) | −2 | 34 | 0 | 8.62 | |
| Parietal | |||||
| Left cingulate/precuneus (BA 31) | −2 | −44 | 40 | 9.31 | |
| Left cingulate | 4 | −22 | 42 | 7.34 | |
| Left supramarginal gyrus/inferior parietal lobe/superior temporal gyrus (BA 39) | −48 | −72 | 42 | 12.66 | |
| Right inferior parietal lobe/superior temporal gyrus (BA 39) | 50 | −68 | 42 | 8.28 | |
| Temporal | |||||
| Left middle/inferior temporal gyrus (BA 21) | −60 | −18 | −16 | 8.99 | |
| Right middle/inferior temporal gyrus (BA 21) | 52 | −14 | −24 | 8.89 | |
| Left parahippocampus | −24 | −18 | −22 | 6.85 | |
| Right paradhippocampus | 26 | −16 | −26 | 6.15 | |
3.2.3 Modulatory effects of stimulus complexity on set switching
A number of brain regions identified above as being related to stimulus complexity (Figure 4B, Table 2B) modulated brain activity during switch trials compared with non-switch trials. These regions encompassed frontal, motor, and parietal control areas, including the right anterior cingulate (BA 32), bilateral MFG (BA 8), right postcentral gyrus (BA 2), bilateral cerebellum, left anterior insula (BA 13), bilateral posterior cuneus, and thalamus, as well as more extensive activity in bilateral middle and medial frontal gyri (BA 6). There were also effects of stimulus complexity on non-switch trials relative to switch trials in bilateral medial frontal cortex/anterior cingulate (BA 9/10/24/32), left posterior cingulate (BA 31), bilateral inferior parietal lobes (BA 39), bilateral middle temporal gyri (BA 21), and bilateral parahippocampi.
3.2.4 Parametric effects of number of response options
An increased number of response options (Figure 4C, Table 3A) was characterized by greater activity in numerous regions throughout the brain. These included bilateral anterior cingulate (BA 32), middle/inferior frontal and precentral gyri (BA 6), SMA (BA 6), postcentral gyri (BA 2), superior parietal lobes (BA 7), inferior parietal lobes (BA 40), caudate, thalamus, anterior insulae (BA 13), and middle occipital gyri (BA 19), as well as left putamen, and right lingual gyrus. A decreased number of response options was characterized by relatively lower activity in bilateral superior frontal gryi (BA 8/9), several regions in bilateral inferior parietal lobes (BA 40), bilateral middle temporal gyri (BA 22), and left cuneus (BA 19).
Table 3. A: Peak stereotactic coordinates (MNI) for the parametric effects response complexity.
Regions with greater activity for trials with a larger number of potential response options are listed first, followed by those with greater activity for trials with a smaller number of potential response options. B: Peak stereotactic coordinates (MNI) for modulatory effects of response complexity on switching. Regions with greater activity during switch trials are listed first, followed by regions with greater activity during non-switch trials. Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via FDR at p < 0.05.
| Region | X | Y | Z | T |
|---|---|---|---|---|
| TABLE 3A | ||||
| Main effects of increasing number of response options | ||||
| Frontal | ||||
| Left medial frontal gyrus (BA 32) | −6 | 6 | 52 | 7.72 |
| Right cingulate (BA 32) | 8 | 18 | 46 | 6.35 |
| Left middle frontal gyrus (BA 6) | −24 | −2 | 50 | 7.78 |
| Right middle frontal gyrus (BA 6) | 26 | 2 | 58 | 7.81 |
| Left inferior frontal/precentral gyrus (BA 6/9) | −50 | 4 | 34 | 5.47 |
| Right inferior frontal/precentral gyrus | 48 | 8 | 30 | 7.29 |
| Left inferior frontal gyrus/insula | −32 | 22 | −2 | 5.63 |
| Right inferior frontal gyrus/insula (BA 13) | 34 | 22 | 2 | 6.37 |
| Parietal | ||||
| Left superior parietal lobe (BA 7) | −24 | −58 | 56 | 10.49 |
| Right superior parietal lobe/precuneus (BA 7) | 26 | −58 | 50 | 12.31 |
| Left inferior parietal lobe/postcentral gyrus (BA 2/40) | −46 | −32 | 40 | 7.04 |
| Right inferior parietal lobe/postcentral gyrus (BA 40) | 40 | −36 | 46 | 8.51 |
| Occipital | ||||
| Left middle occipital gyrus (BA 19) | −38 | −88 | 10 | 9.55 |
| Right middle occipital gyrus (BA 19) | 34 | −84 | 20 | 10.08 |
| Left middle occipital gyrus | −44 | −74 | −8 | 7.59 |
| Right middle occipital gyrus | 52 | −62 | −10 | 7.13 |
| Right lingual gyrus | 14 | −92 | −6 | 7.76 |
| Subcortial | ||||
| Left caudate | −10 | 8 | 2 | 5.06 |
| Right caudate | 10 | 10 | 0 | 6.17 |
| Left putamen | −10 | 8 | 2 | 5.06 |
| Left thalamus | −12 | −20 | 10 | 5.60 |
| Right thalamus | 8 | −14 | 10 | 5.63 |
| 8 | −26- | 6 | 6.01 | |
| Main effects of decreasing number of response options | ||||
| Frontal | ||||
| Left superior frontal gyrus (BA 8) | −10 | 46 | 50 | 4.82 |
| Left superior frontal gyrus (BA 9) | −16 | 56 | 34 | 5.21 |
| Right superior frontal gyrus (BA 8/9) | 20 | 48 | 42 | 3.78 |
| Left middle frontal gyrus (BA 8) | −40 | 16 | 48 | 6.03 |
| Left inferior frontal gyrus (BA 45/47) | −50 | 30 | −6 | 6.55 |
| Parietal | ||||
| Left inferior parietal lobe (BA 40) | −52 | −60 | 48 | 6.84 |
| Left inferior parietal lobe (BA 40) | −52 | −58 | 36 | 7.73 |
| Right inferior parietal lobe (BA 40) | 56 | −60 | 42 | 6.53 |
| Occipital | ||||
| Left cuneus (BA 19) | 0 | −90 | 24 | 4.39 |
| Temporal | ||||
| Left middle temporal gyrus (BA 21/22) | −58 | −44 | −4 | 7.27 |
| Right middle temporal gyrus (BA 21/22) | 68 | −40 | −2 | 4.20 |
| TABLE 3B | ||||
| Modulatory effects of number of response options on switching | ||||
| Frontal | ||||
| Left superior/middle frontal gyrus (BA 6) | −22 | −2 | 62 | 3.36 |
| Right superior frontal gyrus (BA 6) | 12 | 12 | 70 | 4.36 |
| Right middle frontal gyrus (BA 6) | 32 | 8 | 60 | 4.48 |
| Left middle/inferior frontal gyrus (BA 6/9) | −48 | 4 | 44 | 3.98 |
| Right middle/inferior frontal gyrus (BA 9/44) | 54 | 12 | 20 | 4.41 |
| Right middle/inferior frontal gyrus (BA 46) | 50 | 38 | 14 | 4.40 |
| Right inferior frontal gyrus (BA 45) | 40 | 14 | 16 | 4.19 |
| Left precentral gyrus (BA 6) | −54 | 2 | 16 | 3.38 |
| Left insula (BA 13) | −28 | 20 | −2 | 3.98 |
| Parietal | ||||
| Left postcentral gyrus (BA 2) | −54 | −24 | 52 | 3.71 |
| Left superior parietal lobe/precuneus (BA 7) | −22 | −62 | 56 | 5.35 |
| Right superior parietal lobe/precuneus (BA 7) | 22 | −66 | 54 | 5.93 |
| Right superior parietal lobe | 28 | −74 | 46 | 6.87 |
| Left inferior parietal lobe (BA 40) | −44 | −40 | 46 | 4.83 |
| Right inferior parietal lobe (BA 40) | 46 | −44 | 56 | 5.24 |
| Right cingulate (BA 23) | 8 | −28 | 28 | 4.80 |
| Temporal | ||||
| Left superior temporal gyrus (BA 22) | −48 | −14 | −4 | 4.55 |
| Subcortical | ||||
| Left putamen/globus pallidus | −28 | −18 | −6 | 4.57 |
| Right putamen | 18 | 4 | −8 | 3.94 |
| Right caudate | 10 | 8 | 10 | 3.88 |
| Left thalamus | −10 | −24 | 12 | 4.39 |
| Right thalamus | 12 | −20 | 12 | 3.87 |
3.2.5 Modulatory effects of the number of response options on set switching
Several brain regions, identified above as being engaged by number of response options, modulated activity during switch trials compared with non-switch trials (Figure 4D, Table 3B). These regions included bilateral superior/middle/inferior frontal gyri (BA 6/9/44/46), superior parietal lobes (BA 7), inferior parietal lobes (BA 40), bilateral putamen/globus pallidus, thalamus, left insula, left postcentral gyrus (BA 2), and right caudate. In contrast, no such significant effect of the number of response options was observed for non-switch trials compared with switch trials.
3.2.6 Parametric effects of number of preceding non-switch trials
A greater number of non-switch trials preceding a given trial (Figure 5, Table 4) was characterized by increased activity in bilateral superior frontal gyri (BA 8/9), medial frontal gyri (BA 10), inferior frontal gyri (BA 47), anterior cingulate (BA 24), pre-/postcentral gyri (BA 3/4), inferior parietal lobes (BA 39/40), cuneus (BA 18), middle temporal gyri (BA 21/22), right posterior cingulate (BA 30), right caudate, and right thalamus. An increased number of preceding non-switch trials did not significantly reduce BOLD response in any brain regions.
Figure 5. Parametric effects of number of preceding non-switch trials.
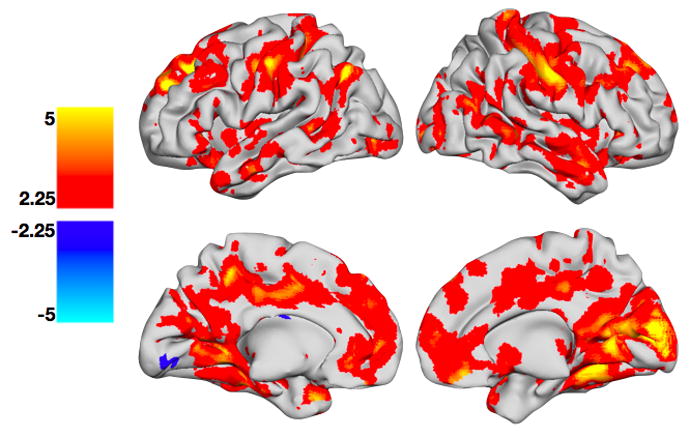
Regions with greater activity for trials preceded by a greater number of non-switch trials are shown in YELLOW/ORANGE, while regions with greater activity for trials preceded by a lesser number of non-switch trials are shown in GREEN/BLUE. All t-maps have been thresholded at p < 0.05, corrected for multiple comparisons (FDR). Color bars are expressed in terms of t-scores, and images are displayed in normal convention.
Table 4. Peak stereotactic coordinates (MNI) for the parametric effects of the number of non-switch trials preceding a given trial.
Regions with greater activity for trials preceded by a greater number of non-switch trials are listed first, followed by regions with greater activity for trials preceded by a lesser number of non-switch trials. Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via FDR at p < 0.05.
| Region | X | Y | Z | T |
|---|---|---|---|---|
| TABLE 4 | ||||
| Main effects of increasing number of preceding non-switch trials | ||||
| Frontal | ||||
| Left middle/superior frontal gyrus (BA 8/9) | −20 | 34 | 40 | 5.07 |
| Left superior frontal gyrus | −18 | 50 | 32 | 5.13 |
| Right superior frontal gyrus (BA 8) | 14 | 48 | 46 | 4.62 |
| Left medial frontal gyrus (BA 10) | −10 | 54 | 4 | 3.93 |
| Right medial frontal gyrus (BA 10) | 8 | 56 | 10 | 3.44 |
| Right medial frontal gyrus (BA 11) | 4 | 42 | −18 | 3.63 |
| Left inferior frontal gyrus (BA 45/47) | −46 | 18 | 4 | 3.73 |
| −44 | 26 | 2 | 3.79 | |
| Right middle/inferior frontal gyrus (BA 11/47) | 44 | 32 | −2 | 3.80 |
| Left inferior frontal gyrus/insula (BA 47) | −30 | 14 | −20 | 4.00 |
| Right insula (BA 13) | 38 | 0 | −2 | 3.87 |
| Left anterior cingulate | −10 | 34 | 2 | 4.36 |
| Right anterior cingulate (BA 24) | 8 | 38 | 2 | 3.17 |
| Left precentral/postcentral gyrus (BA 3/4) | −34 | −20 | 48 | 4.13 |
| −50 | −16 | 46 | 4.35 | |
| Parietal | ||||
| Left postcentral gyrus (BA 40) | −34 | −38 | 56 | 3.72 |
| Right postcentral gyrus (BA 3/4) | 32 | −30 | 52 | 4.38 |
| Right postcentral gyrus (BA 2) | 44 | −28 | 48 | 4.46 |
| Right precentral/postcentral gyrus (BA 4) | 58 | −16 | 36 | 4.82 |
| Left inferior parietal lobe (BA 39) | −40 | −70 | 40 | 4.99 |
| Left precuneus (BA 7) | −18 | −46 | 46 | 4.41 |
| Right posterior cingulate (BA 30) | 12 | −64 | 8 | 5.09 |
| Occipital | ||||
| Left cuneus | −10 | −68 | 6 | 3.85 |
| Right cuneus (BA 18) | 12 | −94 | 6 | 5.23 |
| Left parahippocampus/lingual gyrus | −18 | −52 | 0 | 4.38 |
| Temporal | ||||
| Left superior temporal gyrus (BA 38) | −26 | 10 | −28 | 3.32 |
| Left middle temporal gyrus | −56 | −28 | −8 | 3.41 |
| Left middle/superior temporal gyrus (BA 21/22) | −58 | −44 | 4 | 3.73 |
| Right middle temporal gyrus (BA 21) | 54 | 8 | −28 | 3.32 |
| Right middle temporal gyrus (BA 21/22) | 52 | −36 | 2 | 3.81 |
| Right middle/superior temporal gyrus (BA 39) | 54 | .62 | 16 | 4.09 |
| Left parahippocampus | −20 | −42 | −8 | 3.54 |
| Right parahippocampus/fusiform gyrus | 30 | −52 | −10 | 5.14 |
| Subcortical | ||||
| Right caudate | 10 | 8 | −6 | 3.32 |
| Right thalamus | 12 | −26 | 0 | 3.72 |
3.2.7 Effects of number of preceding non-switch trials on set switching
There were no significant modulatory effects of the number of preceding non-switch trials when comparing switch or non-switch trials.
DISCUSSION
The purposes of this study were to identify the neural correlates of set-shifting, unconfounded by common paradigm design aspects, and to determine whether these stimulus design aspects (largely uncontrolled in previous functional neuroimaging studies of set shifting), such as stimulus complexity, motor response complexity, and stimulus ordering, could account for neural activity in some of the brain regions frequently but inconsistently attributed to mental set switching. The study had several important, novel findings. First, we have demonstrated that when several stimulus and paradigm design aspects were effectively balanced within the fMRI paradigm, set switches among different stimulus attributes appeared to be executed by a predominantly left-lateralized fronto-parietal cortical control network. This is a subset of brain regions identified in previous meta-analyses as engaged for set switching (Buchsbaum et al., 2005; Wager et al., 2004). Second, our analyses also helped clarify the somewhat ambiguous role of brain regions such as the anterior cingulate cortex and striatum in set switching. Specifically, we found evidence that neither of these regions was involved in the direct execution of a set switch. Instead, their engagement appeared to be more related to mediating interference induced by complex stimuli. Finally, we observed that there was a demonstrable effect of stimulus ordering on set switching brain function. This particular paradigm design aspect may represent an additional, largely unexplored, cognitive component of mental set switching, as the behavioral and neural correlates suggested a form of attentional disengagement not previously described.
4.1 Main effects of switching
By controlling for the paradigm and stimulus design aspects of stimulus complexity, motor response complexity, and stimulus ordering, we were able to identify a number of brain regions that appeared to be, in the context of this study, engaged to execute a mental set switch. In addition, activity in these regions could not easily be attributed to the three experimental factors. This suggests that, in comparison to meta-analyses of previous functional neuroimaging set-shifting studies that find a somewhat larger handful of regions, these regions might be more central to attribute set-shifting. This predominantly left lateralized fronto-parietal network included the dorsolateral prefrontal cortex, inferior frontal gyrus, and inferior parietal lobe and was in line with previous summaries of published results (e.g., Buchsbaum et al., 2005; Wager et al., 2004). In particular, the current results confirmed a role for the prefrontal cortex, especially the dorsolateral prefrontal cortex in switching when stimulus selection was the only issue (Rushworth et al., 2002b; Rushworth et al., 2004; van Veen et al., 2001). Likewise, fMRI studies in healthy participants have associated hemodynamic activity in the left inferior frontal gyrus to conflict resolution in switch trials (Badre and Wagner, 2006, 2007). Similar studies in patients with damage to this region have also noted impairment in task-switching performance, specifically in the ability to select and maintain task-set (Aron et al., 2004; Mecklinger et al., 1999; Rogers et al., 1998). The inferior parietal lobes have associated with the selection of stimulus-response sets (Corbetta and Shulman, 2002).
4.2 Parametric effects of stimulus complexity
As hypothesized, increased stimulus complexity resulted in engagement of additional frontal and parietal regions, including the medial frontal gyrus, dorsal caudate, and superior parietal lobules. The presence of activity in the medial frontal gyrus and dorsal caudate (discussed below in terms of their shared roles in overcoming both stimulus and response complexity) confirms our second aim that activity in these regions may be related more to other paradigm design considerations than to executing the mental set-shift.
4.3 Parametric effects of response complexity
Stimuli with an increased number of motor response options engaged additional motor planning and programming regions, including the medial frontal gyrus, premotor cortices, sensorimotor cortices, superior parietal lobules, and ventral striatum. Activity in several of the same regions, including medial frontal cortex, superior parietal lobules, and striatum, again suggests that these regions that they play a role in overcoming general stimulus-response set interference more than executing mental set switches, as discussed below. Additionally, the differences between the neural correlates of increased stimulus complexity and motor response complexity, including that increased response complexity engaged motor performance regions and ventral striatum, confirm our hypothesis, as well as behavioral work done by Kiesel et al., (2010), that these two phenomena are capturing different neural effects.
4.4 Role of anterior cingulate cortex in switching and stimulus and response complexity
In contrast to several previous mental set switching studies (Braver et al., 2003; Derrfuss et al., 2004; Rubia et al., 2006; Smith et al., 2004), we did not observe activity in anterior cingulate cortex that was related to executing attribute-based mental set switches. There also was no evidence for striatal engagement during switching once major paradigmatic factors were controlled for, adding to both previous and current evidence that striatal involvement in switching is perhaps only required to inhibit competing cognitive or motor responses (Cools, 1980; Mink, 1996). The anterior cingulate cortex and striatum, in addition to other regions commonly associated with motor planning, programming, and performance, appeared to be engaged more to overcome intra-stimulus interference either due to increased stimulus complexity or increased motor response complexity. In several previous studies, the anterior cingulate cortex has been demonstrated to be active only during switches with changes in response set or conflicts between possible response sets (Botvinick et al., 2004; Rushworth et al., 2002a; Rushworth et al., 2004; van Veen et al., 2001). A recent study examining mechanisms of cognitive flexibility found that activity in the anterior cingulate was related more to switching between response sets than switching between stimulus attributes (Kim et al., 2011). Several authors also proposed that the neighboring pre-SMA region is involved in such interference resolution (Ridderinkhof et al., 2004; Rushworth et al., 2008; Ullsperger and von Cramon, 2001), as it is thought to be engaged in change-of-plan and stop tasks (Aron and Poldrack, 2006; Curtis, 2006; Li et al., 2006; Nachev et al., 2005). Neural activity in these medial frontal cortical regions has been related to inhibiting responses, switching between tasks, and switching between stimulus-response sets (Brass and von Cramon, 2002; Crone et al., 2006; Garavan et al., 2003; Rushworth et al., 2002a; Wager et al., 2004). Neither the pre-SMA nor the anterior cingulate cortex has been shown to be engaged in switching when the switching entails only how stimuli are processed without any stimulus-response set changes (Rushworth et al., 2002b, 2005). In general, the role of the anterior cingulate cortex and pre-SMA in task control involves anticipatory preparation and selection of task set, while their role in response competition can be explained by need to select or re-select partially ambiguous stimulus-response sets (Rushworth et al., 2004). This fits well with our results showing medial frontal engagement associated with stimulus and motor response complexity and not switching per se.
4.5 Role of striatum in switching and mediating stimulus and response complexity
Striatal activity was also associated to stimulus and motor response complexity but not switching itself. Although dorsal striatal activity was noted both for trials with increased stimulus complexity and trials with increased motor response complexity, only trials with increased motor response complexity engaged regions of the ventral striatum, including the putamen. At least one previous study has shown that the caudate is active during the cognitive decision to shift, but not during the simple execution of a shift (Monchi et al., 2006). Activity in the caudate has additionally been seen when evaluation and decision to change set is required but not during blocks of continuous shifting (Grahn et al., 2008). Its engagement in association with increased stimulus complexity and increased number of response options also suggests a potential role for the caudate in mediating conflict and interference. In contrast, activity in bilateral putamen has been predominantly associated with motor performance (Lehericy et al., 1998; Maillard et al., 2000; Witt et al., 2008), having strong connections to the sensorimotor strip, SMA, and anterior cingulate cortex (Di Martino et al., 2008). The ventral striatum has been associated with cortical regions engaged in executive functions, decision making, and motor planning (Di Martino et al., 2008), however, since activity in the ventral striatum was only observed during trials of increased number of motor response options, its observed activity here was most likely related to motor planning.
4.6 Additional regions engaged in mediating stimulus and response complexity
Several other brain regions exhibited activity apparently related more to stimulus and motor response complexity than switching itself, including middle and medial frontal gyri and superior parietal lobe. The bilateral premotor and supplementary motor cortices have been previously associated with motor programming and planning (Grafton et al., 1992; Passingham, 1985; Roland, 1984; Roland et al., 1980; Wise et al., 1997), in addition to the execution of simple, voluntary movements in general (Witt et al., 2008). Both left and right superior parietal lobes, extending into the inferior parietal lobes and precuneus, have been connected to both mediating stimulus-response set conflict or interference and selecting the appropriate stimulus-response set (Hazeltine et al., 2000; Jiang and Kanwisher, 2003). The superior parietal lobes, in conjunction with the supplementary motor area, left cingulate, and left middle and bilateral inferior frontal gyri, have also been linked to processing increasingly complex stimuli (Kroger et al., 2002), lending further evidence that increased stimulus complexity and increased motor response complexity require additional resources to overcome the conferred interference.
4.7 Summary of parametric effects of stimulus and response complexity
Our careful task design and use of parametric analyses to control for various stimulus and paradigm design aspects suggests that anterior cingulate, striatum, middle and medial frontal gyri, and superior parietal lobule play more of a role in facilitating switches by mediating stimulus-induced interference rather than in executing the switch itself. Therefore, while previous studies linking activity in these brain regions to set switching were not incorrect, they did not uncover the specificity of arguably distinct cognitive demands that engaged those regions. We observed that activity in these regions was also modulated for switch trials. Qualitative comparison of the results for parametric effects of stimulus and response complexity to their respective modulatory effects on switching strongly suggests that activity in these regions increased. However, the limitation of our parametric analysis framework made it impossible to precisely confirm this. Future experiments that specifically seek to describe how these complexities influence switch-related activation could confirm this observation that inter-trial interference (i.e., switching) necessitates greater engagement of the brain these regions. It is noteworthy that brain regions exhibiting increased activity during non-switch trials mirrored those active during trials with overall reduced stimulus and motor response complexity and included many regions though to comprise the default mode network (i.e., superior/medial frontal gyri, posterior cingulate gyri, and inferior parietal lobes; (Damoiseaux et al., 2006; Greicius et al., 2003; Gusnard and Raichle, 2001; Raichle et al., 2001; van den Heuvel et al., 2009). This suggests that the additional cortical and motor planning control, afforded by the anterior cingulate cortex, striatum, and superior parietal lobe, was only needed to overcome the interference posed by highly complex stimuli when a switch was being performed. During non-switch trials, the observed lesser degree of “deactivation” within regions of the default mode network could be interpreted in a similar manner as Eichele et al. (2008) and Weissman et al. (2006), wherein reduced deactivation was taken as an indication of reduced attention to the task at hand. If correct, the current results suggest that the less complex the stimulus, the more likely it was for participants to lose focus on the task at hand if the behavioral set remained unchanged across subsequent trials.
Behaviorally, both increased stimulus and response complexity resulted in both overall slowing of performance regardless of trial type and increased switch costs, paralleling the neuroimaging results. This indicated that increased cognitive resources are recruited to process the most complex stimuli. Both stimulus complexity and the number of potential response options exhibited similar behavioral and neuroimaging correlates, which was not wholly unexpected given that the two were linked in the paradigm employed in this study, i.e., the number of potential response options on a given stimulus, for the most part, paralleled the number of dimensions on which the stimulus differed. However, these two design aspects were not significantly correlated and did not measure the same cognitive phenomenon. Thus, they should not be conflated. Notable differences in how these two factors modulated brain activity included engagement of the ventral striatum and more posterior aspects of middle and medial frontal regions for trials with increased motor response complexity compared with trials of increased stimulus complexity (Sections 3.2.2–3.2.5; Figure 3; Tables 2–3). Additionally, it should also be noted that for trials with minimal response complexity (i.e., only one possible motor response option), switch costs were effectively eliminated. As described previously (Figure 1, Section 2.7), the most complex stimulus could have only one response option while, a relatively simple stimulus could have three potential response options. Additionally, as reviewed in the Introduction (Section 1.4), it is possible to design a mental set-shifting paradigm that uses very complex stimuli with very simple motor response patterns and vice versa.
4.8 Effects of stimulus ordering
With a greater number of preceding non-switch trials, the data suggested there was disengagement of the cognitive control brain regions normally observed active during switching in favor of regions related to attention reorientation, task reversal, and motor programming and performance. In contrast to our initial prediction that increasing the number of preceding non-switch trials to a switch trial would engage similar brain regions to that previously observed for task automaticity and habituation, no significant switch-elicited activity was observed in dorsolateral prefrontal cortex. Also, only a small area of the left inferior parietal lobe was observed to exhibit significant activity. Additionally, unlike with the two previously considered paradigmatic design aspects, there was virtually no overlap between the parametric effects of increasing number of preceding non-switch trials and either the main effects of switching or the main effects of stimulus complexity or number of motor response options. The uniqueness of the effect the number of preceding non-switch trials had on switches suggested that it, like task set reconfiguration (Allport et al., 1994; Meiran, 1996; Rogers and Monsell, 1995) and task set inertia (Rogers and Monsell, 1995), may represent another component of cognitive theories of mental set switching. The neural correlates of this phenomenon indicate that the fronto-parietal cognitive control network proposed to underlie mental set switching disengaged quickly in the face of increasingly longer trains of non-switch trials and was not fully re-engaged during switch trials preceded by long trains of non-switch trials. Behaviorally, this was reflected in overall improved performance with increased number of preceding non-switch trials up to three preceding non-switch trials. For switch trials preceded by four or more non-switch trials, there appeared to be a resetting of switch costs and overall performance back to the level of that associated with switch trials preceded by only one non-switch trial.
Although the overall pattern of neural activity for the main effects of the number of preceding non-switch trials was widespread, the areas of activity with the largest signal change and extent included the anterior frontal cortex, the sensorimotor strip, and secondary visual areas. A few mental set switching fMRI studies have noted increased hemodynamic activity in the anterior PFC (Braver et al., 2003; Rubia et al., 2006; Smith et al., 2004). Braver et al., (2003), using a block design, noted that activity in the anterior PFC was sustained during blocks in which participants had to randomly switch between two tasks. They related activity in the anterior PFC to the increased working memory and attentional demands of the switching task. Rubia et al., (2006) noted increased anterior PFC activity in adults during switch trials compared with non-switch trials. Of note, this particular study employed an event-related design in which switch trials were purposefully preceded by no fewer than four non-switch trials, such that they might have captured activity in the anterior PFC of a similar origin to what was observed in this present study. However, Derfuss et al., (2004), in employing an event-related design in which switch trials could be preceded by up to five non-switch trials, failed to note any significant neural activity in anterior PFC. Activity in this region has been linked to numerous higher-order cognitive functions. As reviewed by (Ramnani and Owen, 2004), the anterior PFC (BA 10) has been linked to processing internal states (Christoff and Gabrieli, 2000), prospective memory (Burgess et al., 2001), episodic memory retrieval (Konishi et al., 2000; McDermott et al., 2003; Ranganath and Paller, 2000; Rugg et al., 1998), branching and reallocating attention (Baker et al., 1996; Braver and Bongiolatti, 2002; Koechlin et al., 1999; Koechlin et al., 2000; Sakai and Passingham, 2003; Sylvester et al., 2003), and relational integration (Braver et al., 2003; Kroger et al., 2002). Neural activity in BA 10 has also been proposed in relation to motor response programming (Peterson et al., 1999). As no significant trial type modulation was observed for this effect, it was difficult to determine whether the increased activity in anterior PFC observed here was related to maintaining attention during repetitive trains of non-switch trials or to reorienting attention during a switch trial preceded by a long train of non-switch trials. The activity observed in the anterior PFC also extended inferiorly into the orbitofrontal cortices, which have been linked to both response inhibition and task reversal (Elliott and Deakin, 2005). This again indicated that switch trials performed after a long succession of non-switch trials required additional cognitive control from regions not typically observed during the main effects of switching (Buchsbaum et al., 2005; Wager et al., 2004). In contrast to the increased cognitive control afforded by the anterior prefrontal cortex, the presence of significant activity in the sensorimotor strip, in the relative absence of activity in the motor planning/programming regions noted above for increased stimulus and response complexity, suggested that only those regions associated with motor performance were needed when the target stimulus remained the same across several trials.
CONCLUSIONS
Our findings emphasize that merely describing mental set switching in terms of simple comparisons between switch and non-switch trials is inadequate in terms of accurately delimitating the neural correlates of switching. This study more thoroughly unravels the growing number of theoretically distinct cognitive phenomena at play during the act of executing a mental set switch. This study has several strengths including its large sample size, well-balanced paradigm, and its use of the relatively novel analytic technique that controlled for paradigmatic design aspects by including them as parametric modulation terms within the statistical parametric model. It is hoped that other researchers are encouraged to explore how stimulus-response set complexity affects the brain function elicited during mental set switching, both to replicate our results as well as to consider types of mental set switching other than attribute switching. One limitation of our study which presents an avenue for future work lies in further characterizing the modulatory effects of stimulus-response complexity on switching, in particular, determining whether, as suggested by the results presented here, brain regions engaged in overcoming increased stimulus-response interference are also engaged more by switch trials than non-switch trials or vice versa. Additional work on the effects of stimulus ordering (i.e., the number of non-switch trials preceding a switch trial) on set switching would be useful to determine if this phenomenon does, as we have suggested, represent an additional cognitive component of mental set switching. Finally, while controlling for paradigmatic design considerations like stimulus-response set complexity, stimulus ordering, and experimental timing is not a trivial design consideration, future mental set switching research should pay attention to these factors to ensure that the effects described in this study do not become inextricably entangled in what are perceived as the main effects of switching.
Highlights.
Set-switching is subserved by a mostly left-lateralized fronto-parietal network.
Several brain regions were recruited solely to overcome intra-stimulus interference.
Stimulus ordering may represent another cognitive component of mental set-switching.
Acknowledgments
This research was supported by the National Institutes of Health under grant R01-MH081969 (PI: MCS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Suzanne T. Witt, Email: stwitt@harthosp.org.
Michael C. Stevens, Email: msteven@harthosp.org.
References
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, editor. Attention and Performance XV. The MIT Press; Cambridge, MA: 1994. pp. 421–452. [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asari T, Konishi S, Jimura K, Miyashita Y. Multiple components of lateral posterior parietal activation associated with cognitive set shifting. Neuroimage. 2005;26:694–702. doi: 10.1016/j.neuroimage.2004.12.063. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci U S A. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cereb Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Davidson MC, Hara Y, Thomas KM, Martinez A, Galvan A, Halperin JM, Rodriguez-Aranda CE, Tottenham N. Early development of subcortical regions involved in non-cued attention switching. Dev Sci. 2004;7:534–542. doi: 10.1111/j.1467-7687.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci. 2002;22:8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. PSYCHOBIOLOGY-AUSTIN. 2000;28:168–186. [Google Scholar]
- Cools AR. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: facilitation of processes involved in selecting the best strategy in a stressful situation. Behav Brain Res. 1980;1:361–378. doi: 10.1016/0166-4328(80)90035-2. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, Biswal B, Walters J, Castellanos F, Milham M. Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex. 2008;18:2735. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: evidence from functional magnetic resonance imaging studies in healthy human subjects. Int Rev Neurobiol. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders. 1996 doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME. Human functional anatomy of visually guided finger movements. Brain. 1992;115 ( Pt 2):565–587. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci. 2000;12(Suppl 2):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher N. Common neural substrates for response selection across modalities and mapping paradigms. J Cogn Neurosci. 2003;15:1080–1094. doi: 10.1162/089892903322598067. [DOI] [PubMed] [Google Scholar]
- Kalkut EL, Han SD, Lansing AE, Holdnack JA, Delis DC. Development of set-shifting ability from late childhood through early adulthood. Arch Clin Neuropsychol. 2009;24:565–574. doi: 10.1093/arclin/acp048. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I. Control and interference in task switching--a review. Psychol Bull. 2010;136:849–874. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Cilles SE, Gold BT. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J Neurosci. 2011;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci U S A. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Kubler A, Dixon V, Garavan H. Automaticity and reestablishment of executive control-an fMRI study. J Cogn Neurosci. 2006;18:1331–1342. doi: 10.1162/jocn.2006.18.8.1331. [DOI] [PubMed] [Google Scholar]
- Lehericy S, van de Moortele PF, Lobel E, Paradis AL, Vidailhet M, Frouin V, Neveu P, Agid Y, Marsault C, Le Bihan D. Somatotopical organization of striatal activation during finger and toe movement: a 3-T functional magnetic resonance imaging study. Ann Neurol. 1998;44:398–404. doi: 10.1002/ana.410440319. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Loose R, Kaufmann C, Tucha O, Auer DP, Lange KW. Neural networks of response shifting: influence of task speed and stimulus material. Brain Res. 2006;1090:146–155. doi: 10.1016/j.brainres.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Maillard L, Ishii K, Bushara K, Waldvogel D, Schulman AE, Hallett M. Mapping the basal ganglia: fMRI evidence for somatotopic representation of face, hand, and foot. Neurology. 2000;55:377–383. doi: 10.1212/wnl.55.3.377. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Mecklinger AD, von Cramon DY, Springer A, Matthes-von Cramon G. Executive control functions in task switching: evidence from brain injured patients. J Clin Exp Neuropsychol. 1999;21:606–619. doi: 10.1076/jcen.21.5.606.873. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology-Learning Memory and Cognition. 1996;22:1423–1442. [Google Scholar]
- Meiran N. Modeling cognitive control in task-switching. Psychol Res. 2000;63:234–249. doi: 10.1007/s004269900004. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J, Fukuyama H, Shibasaki H. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–199. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Cortical mechanisms and cues for action. Philos Trans R Soc Lond B Biol Sci. 1985;308:101–111. doi: 10.1098/rstb.1985.0013. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Paller KA. Neural correlates of memory retrieval and evaluation. Brain Res Cogn Brain Res. 2000;9:209–222. doi: 10.1016/s0926-6410(99)00048-8. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology-General. 1995;124:207–230. [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121 ( Pt 5):815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Roland PE. Organization of motor control by the normal human brain. Hum Neurobiol. 1984;2:205–216. [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Allan K, Frith CD, Frackowiak RS, Dolan RJ. Neural correlates of memory retrieval during recognition memory and cued recall. Neuroimage. 1998;8:262–273. doi: 10.1006/nimg.1998.0363. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002a;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. J Cogn Neurosci. 2002b;14:1139–1150. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Passingham RE, Nobre AC. Components of attentional set-switching. Exp Psychol. 2005;52:83–98. doi: 10.1027/1618-3169.52.2.83. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Croxson PL, Buckley MJ, Walton ME. Ventrolateral and medial frontal contributions to decision-making and action selection. In: Bunge SA, Wallis JD, editors. Neuroscience of rule-guided behavior. Oxford University Press; New York: 2008. pp. 129–157. [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp. 2004;21:247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Buchel C, Dolan RJ. Switching between the forest and the trees: brain systems involved in local/global changed-level judgments. Neuroimage. 2001;13:56–67. doi: 10.1006/nimg.2000.0678. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]