Abstract
BACKGROUND
PERP is a p53/p63 regulated gene encoding a desmosomal protein that plays a critical role in cell-cell adhesion and tumor suppression.
STUDY DESIGN
We evaluated PERP expression in different grades of oral dysplasia (34 cases) and at different stages of invasive squamous cell carcinoma (SCC), and correlated the latter with clinical outcome. A tissue microarray (TMA) consisting of non-dysplastic mucosa, carcinoma in situ, SCC and nodal metastases from 33 patients with HPV-negative SCC was stained for PERP and E-cadherin.
RESULTS
Complete loss of PERP expression was associated with worse local control in patients with SCC. The 5-year local control rate was 91% for patients with partial PERP loss versus 31% for those with complete loss (p = 0.01).
CONCLUSIONS
This is the first study to show that loss of PERP expression correlates with the transition to SCC and with increased local relapse in patients with oral cavity SCC.
Keywords: PERP, oral dysplasia, oral cavity, squamous cell carcinoma, local control, p63, p53, desmosomes
INTRODUCTION
While several studies have shown that dysregulation of adherens junctions is associated with tumor development and progression, the role of desmosomal proteins and their loss in carcinogenesis is not well-delineated. 1-3 Investigators from our group have identified PERP (p53 apoptosis effector related to PMP-22) as a p53 and p63 regulated gene. PERP plays an important role in the p53 apoptotic pathway and is also associated with p63-mediated cell-cell adhesion. Initially identified in a screen for p53 target genes induced during p53-mediated apoptosis but not during p53-induced cell cycle arrest, PERP protein has been shown to localize subsequently to the desmosome and to play a critical role in both cell-cell adhesion and tumor suppression.4-6 Loss of PERP leads to dysregulated cell-cell adhesion by causing decreased numbers of desmosomes and destabilization of desmosome complexes, resulting in an oral cavity blistering phenotype.7 In studies of PERP-deficient mice, loss of PERP leads to resistance to development of cutaneous papillomas but promotes the initiation and progression of UVB-induced cutaneous squamous cell carcinoma (SCC). 5, 8 PERP is also involved in mammary gland development and PERP deficiency promotes the development of breast cancers. 6 These findings suggest that the role of cell-cell adhesion and desmosome complexes may vary depending on the stage or context of cancer development. In addition, in both mouse and human cutaneous SCCs, PERP loss has been identified in a step that precedes the loss of E-cadherin. 5
These findings and the frequent mutation of p53 in the development of oral SCC suggest that PERP may play an important role in oral SCC development. In this study, we evaluated the expression of PERP protein as a potential prognostic marker in a subset of head and neck (HN) SCC. We focused our study on resected human papillomavirus negative [(HPV(-)] oral cavity (OC) SCC since they tend to exhibit an orderly progression from epithelial dysplasia to carcinoma in-situ (CIS) to invasive carcinomas.9 Moreover, large resection specimens from these tumors provided access to nearby non-dysplastic mucosa, CIS, invasive SCC, and in some cases nodal metastasis, all within the same patient specimen, thus permitting the study of PERP protein expression within these different components representing tumor progression. We compared the expression of PERP to that of E-cadherin, a marker of adherens junctions that has been shown to be downregulated during epithelial-mesenchymal transition. We hypothesized that PERP loss starting at the basal layer may be associated with oral epithelial dysplasia and that PERP loss could be a predictor of worse outcome in HPV(-) invasive SCC of the oral cavity.
MATERIALS AND METHODS
Case Set Selection
We used formalin-fixed, paraffin-embedded tissues from 32 patients with OC (25 oral tongue, 3 floor of mouth, 2 retromolar trigone, 1 buccal) and HPV(-) OP SCC (1 base of tongue) to construct the first tissue microarray (TMA1). All patients had clinical follow-up (median 57.2 months, range 4.3-107.4 months) and were identified from the Stanford Department of Radiation Oncology Clinical Database. Table 1 shows the patient distribution and treatment types. Most of the patients had OC primary tumors and only 1 had an OP tumor. All cases were negative for p16 (clone E6H4, predilute, Tris, MTM Laboratories), a surrogate marker for high-risk HPV. TMA1 was constructed with cores of non-dysplastic mucosa, CIS, invasive tumor and nodal metastasis from the same cases whenever available. For 6 patients, cores of tumor and non-dysplastic mucosa were also taken from a local recurrence or new primary OC cancer and included in the TMA. The invasive carcinomas were graded as high- or low-grade by a pathologist (CMN) blinded to outcome and to the PERP and E-cadherin results. Low-grade tumors encompassed well- and moderately-differentiated SCC while high-grade encompassed poorly-differentiated SCC. Grading was based on World Health Organization (WHO) criteria for head and neck SCC.10
Table 1.
Patient distribution of 32 patients with invasive SCC (TMA 1)
| Characteristic | Descriptor | N (%) |
|---|---|---|
| Age (median: 57) | < 60 | 18 (56.2) |
| >=60 | 14 (43.8) | |
| Gender | Male | 12 (37.5) |
| Female | 20 (62.5) | |
| Tumor site | Oral cavity | 31 (96.9) |
| Oropharynx | 1 (3.1) | |
| T-Classification | 1-2 | 24 (75) |
| 3-4 | 8 (25) | |
| N-Classification | 0 | 22 (68.8) |
| 1-2a | 7 (21.9) | |
| 2b-c | 3 (9.3) | |
| Treatment | Surgery alone | 15 (46.9) |
| Surgery & RT +/- chemo | 15* (46.9) | |
| Chemoradiotherapy | 2 (6.2) | |
Postoperative radiotherapy (RT) alone in 12, postoperative chemoradiotherapy in 3
The oral dysplasia TMA (TMA2) was constructed from formalin-fixed, paraffin-embedded specimens from 34 patients ranging in age from 34-85 years (mean 58 years) with oral epithelial dysplasia (17 mild, 9 moderate and 8 severe), who were identified from the University of California San Francisco (UCSF) Oral Pathology archives. All cases were reviewed by two pathologists (CSK & RCJ), who were blinded to the PERP results, to confirm the histological diagnoses based on established histological criteria 11. Mild dysplasia was characterized by a proliferation of basal and parabasal layer cells that did not extend beyond the lower third of the epithelium and exhibited minimal cytologic atypia. Moderate dysplasia was defined as cells with more marked cytologic atypia, nuclear pleomorphism, rare suprabasal mitoses and aberrant keratinization extending into the middle third of the epithelium. Severe dysplasia exhibited full thickness cytological atypia with prominent architectural abnormalities and aberrant patterns of keratinization. Carcinoma in situ was characterized by full thickness cytological and architectural changes, including loss of polarity, that were more marked than that seen with severe dysplasia and suggested carcinomatous transformation without evidence of invasion 12. Insufficient clinical follow-up was available to permit clinical outcomes analyses for this set of patients.
The study protocol was approved by the institutional review boards at Stanford University and UCSF.
TMA Construction
Two separate tissue microarrays (TMA) were constructed by using a tissue arrayer (Beecher Instruments, Silver Spring, MD) to create new paraffin blocks from representative 1.0 mm cores taken in duplicate.13 TMA1 was created from the Stanford samples and included two or more cores of non-dysplastic squamous mucosa (33 samples), CIS (21 samples), invasive SCC (35 samples), and metastatic carcinoma within regional lymph nodes (14 samples). Table 2 shows number of evaluable cores. TMA2 contained duplicate cores of the oral epithelial dysplasia samples from UCSF. All cores for TMA2 were evaluable.
Table 2.
Number (%) of evaluable cores for TMA 1
| Non-dysplastic Mucosa (%) | CIS (%) | Invasive Carcinoma (%) | Nodal metastasis (%) | |
|---|---|---|---|---|
| PERP Extent | 30/33 (90.9) | 18/21 (85.7) | 33/35 (94.3) | 13/14 (92.9) |
| PERP Intensity | 31/33 (93.9) | 18/21 (85.7) | 34/35 (97.1) | 14/14 (100) |
| E-cadherin | 31/33 (93.9) | 17/21 (81.0) | 31/35 (88.6) | 14/14 (100) |
Immunohistochemistry
Using immunohistochemistry, TMA1 was stained with antibodies to PERP (1:100, Tris, Abcam, Cambridge, MA) and E-cadherin (1:200, Trilogy, Invitrogen, Camarillo, CA). TMA2 was stained with PERP only.
PERP was graded based on assessment of staining intensity and extent of membrane expression. Cytoplasmic-only staining was scored as negative. In general, cytoplasmic staining was diffuse and could easily be discerned from membrane staining that was present as a dark linear stain along the cell membranes resulting in a reticulated staining pattern. Intensity was scored as strong (3), weak (2), equivocal (1) or negative (0). For the cores of CIS, oral dysplasia, and non-dysplastic squamous mucosa, extent of staining was based on the distribution of mucosal reactivity and scored as full-thickness (membrane staining involving the entire mucosal thickness with occasional sparing of the basal layer), partial-thickness (membrane staining involving only the upper half of the mucosa), or negative (complete loss of PERP). Since parakeratosis represent non-living cells on the surface of the mucosa, areas of parakeratosis were not included in the scoring. Areas of parakeratosis were negative for PERP, reflecting the lack of desmosomes. (Figures 1B, 3D & 3F) For invasive or metastatic SCC where the malignant epithelial cells have lost the orderly arrangement of non-dysplastic or dysplastic mucosa, extent of staining was scored as extensive (> 50%), patchy (<50%), or negative. E-cadherin was scored based on intensity of membrane staining as strong (3), weak (2), equivocal (1) or negative (0).
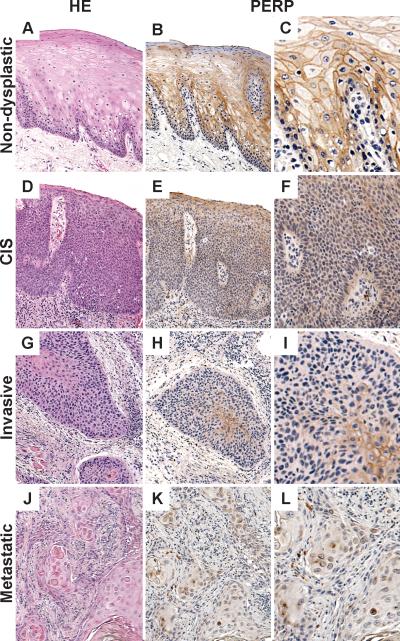
Figure 1.
Representative cores from TMA1: Non-dysplastic mucosa (A) with full-thickness membrane staining with PERP (excluding area of parakeratosis on the surface) (B-C) contrasts with the loss of membrane staining (E-F, H-I, K-L) seen with carcinoma in situ (CIS) (D), invasive cancer (G) and cervical nodal metastasis (J). Cytoplasmic only staining is scored as negative.
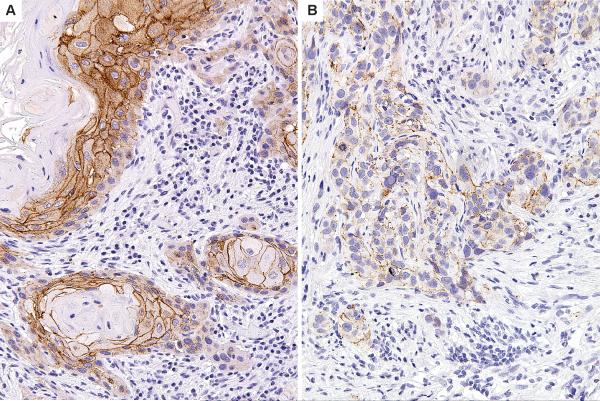
Figure 3.
E-cadherin with strong membranous staining (A) and loss of staining (B) in metastatic squamous cell carcinoma.
Statistical Analysis
The Statview statistical package was used for statistical analysis. (Computing Resource Center, San Monica, CA) Chi square tests were used to correlate marker's staining thickness and/or staining intensity to different pathologic subtypes (non-dysplastic, CIS, invasive, metastasis). Kaplan Meier survival curves were generated for freedom from local relapse (FFLR).14 Log-rank statistics were used to compare survival curves 15.
RESULTS
Extent and intensity of PERP membrane staining (TMA1)
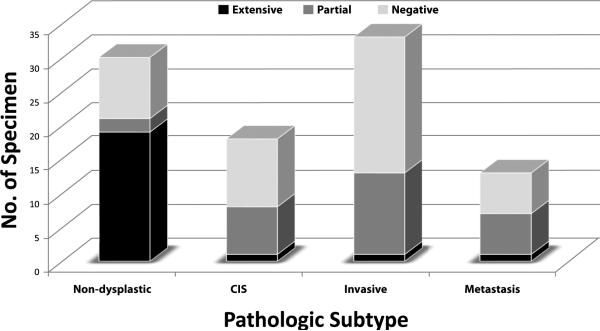
Sixty-three per cent of non-dysplastic squamous mucosa cases exhibit full-thickness membrane staining, extending from the basal layer to the mucosal surface, excluding areas of parakeratosis. (Figures 1-2; Table 3) In contrast, 94% CIS cases exhibit partial to complete loss of PERP. 97% of invasive carcinomas and 92% of lymph node metastases also exhibit loss of PERP, with patchy or no staining in the majority of cases. Only rare cases of CIS, invasive carcinoma, and nodal metastasis show full-thickness or extensive staining with PERP. The difference in the extent of PERP staining between non-dysplastic and malignant tissue is highly statistically significant (p < 0.0001, X2 test).
Figure 2.
Distribution of extent of PERP membrane staining for non-dysplastic, CIS, invasive carcinoma, and metastasis (TMA1)
Table 3.
Distribution of staining pattern and intensity for different markers
| Staining Pattern | Non-dysplastic | CIS | Invasive component | Nodal Metastasis | p-value | |
|---|---|---|---|---|---|---|
| PERP extent | Full/extensive Partial/patchy Negative |
19/30 (63%) 2/30 (7%) 9/30 (30%) |
1/18 (6%) 7/18 (38%) 10/18 (56%) |
1/33 (3%) 12/33 (36%) 20/33 (61%) |
1/13 (8%) 6/13 (46%) 6/13 (46%) |
<0.0001 |
| PERP intensity | Strong Weak Negative |
16/30(53%) 5/30 (13%) 9/30 (30%) |
3/18 (17%) 5/18 (28%) 10/18 (56%) |
7/33 (24%) 6/33 (15%) 20/33 (61%) |
5/13 (38%) 2/13 (15%) 6/13 (46%) |
<0.0001 |
| E-cadherin intensity | Strong Weak Negative |
27/31 (87%) 4/31 (13%) 0/31 (0%) |
14/17 (82%) 3/17 (18%) 0/17 (0%) |
23/31 (74%) 8/31 (26%) 0/31 (0%) |
5/14 (36%) 8/14 (57%) 1/14 (7%) |
0.008 |
Intensity of PERP staining also varies significantly between non-dysplastic and malignant tissue, with 97% of adjacent non-dysplastic mucosa showing strong staining vs. 50% of CIS, 23% of invasive SCC and 29% of metastatic tumors (p < 0.0001, X2 test). (Table 3)
E-cadherin (TMA1)
E-cadherin exhibits extensive membrane staining in non-dysplastic and malignant squamous epithelium but there is decreased intensity of staining in the metastatic lesions, suggesting that decreased E-cadherin staining is a late event. (Table 3) The majority of the non-dysplastic mucosa (87%), CIS (82%) and invasive SCC specimens (74%) retain strong E-cadherin staining while only 36% of the metastatic lesions displayed strong staining (p = 0.008, X2 test). (Figure 3)
Prognostic significance of PERP in invasive SCC (TMA1)
At the latest follow-up, 18 patients from the Stanford group (TMA1) have died, 4 are alive with disease and 10 are alive without cancer. Except for two patients who were lost to follow-up when they moved outside of the country, all living patients have greater than 2 years of follow-up. Twenty patients have failed: 4 local alone, 6 local and nodal, 1 local and distant, 5 nodal alone, 1 nodal and distant, 2 distant alone and 1 all 3 sites of failure. We recorded the date of local, nodal and distant failure independently since they often occurred sequentially, with local relapse generally occurring first followed by either nodal or distant relapse.
We hypothesized that decreased PERP expression in the invasive tumor may correlate with a more clinically aggressive phenotype and thus increased risk of local relapse. For invasive SCC, the extent of staining was scored as extensive (> 50%), patchy (<50%), or negative. Because there was only one invasive tumor with extensive staining, we grouped it together with those with patchy staining. Figure 4 shows the freedom from local relapse by the extent of PERP staining in the invasive tumor component. As shown, 91% of the 14 patients with partial PERP loss retained local control at 5 years compared with only 31% of those with complete PERP loss (n = 18, p = 0.01). Loss of PERP was not associated with aggressive clinical parameters such as larger tumor size or higher histologic grade (data not shown).
Figure 4.
Freedom from local relapse by extent of PERP loss within the invasive component.
The difference between weak and strong PERP staining was not significant. The 5-year local control rate was 71% for patients with strong PERP staining (n = 9), 64% for patients with weak (n = 15) and 17% for those with negative staining (n = 8). The comparison p-value between these three groups was not statistically significant (p = 0.13) but reaches statistical significance (p = 0.047) by combining strong and weak into a single positive group and comparing it with the negative group.
PERP staining extent or intensity did not significantly correlate with either nodal or distant relapse though tumors with negative PERP expression generally had a higher rate of nodal and distant recurrence. E-cadherin also did not correlate with local, nodal or distant relapse. Multivariate analysis was not carried out due to the small number of patients.
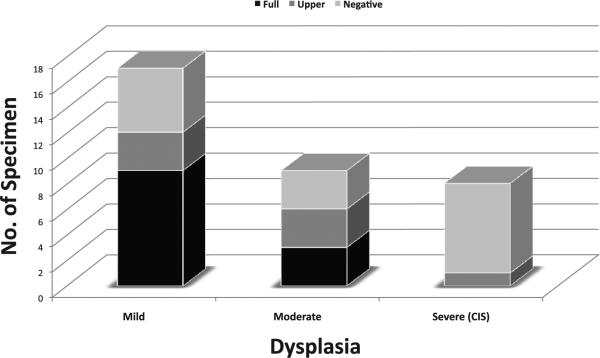
Evaluation of PERP on oral dysplasia TMA (TMA2)
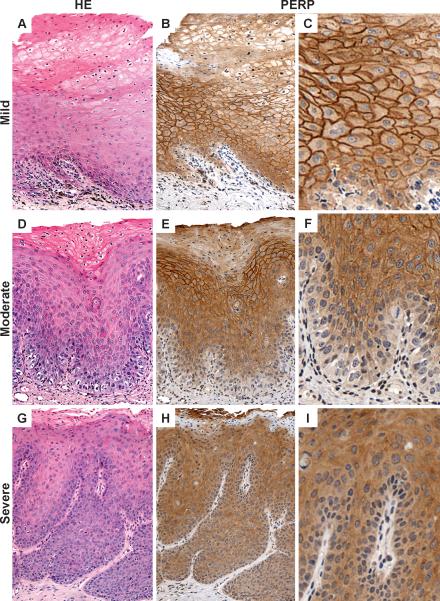
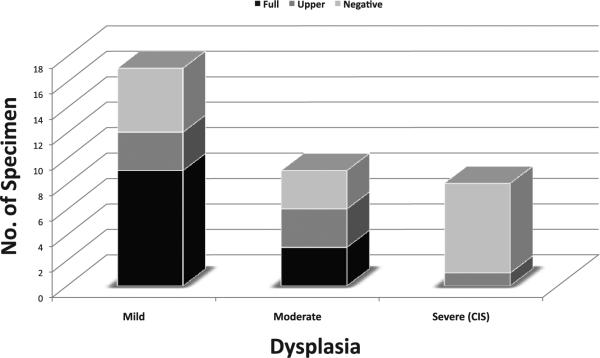
The above observations suggest that PERP loss may be associated with epithelial dysplasia and early invasion. Therefore, we evaluated PERP on an independent TMA that contained mild, moderate and severe oral epithelial dysplasia (TMA2). Figures 5 and 6 show extent of PERP staining for different grades of dysplasia. There is a gradual decrease in the extent of PERP staining with loss starting from the basal cell layer and extending up to the mucosal surface with increasing loss from mild to moderate to severe dysplasia/CIS. The difference in the extent of PERP staining between the 3 groups is statistically significant (p = 0.04, X2 test). By contrast, there is no significant difference in the intensity of PERP staining when CIS is separated from severe dysplasia and compared with mild, moderate and severe dysplasia (p = 0.16, data not shown).
Figure 5.
Representative cores from TMA2: Mild dysplasia (A) with full-thickness membrane staining with PERP (B-C) contrasts with moderate dysplasia (D) showing partial loss of PERP (E-F) and severe dysplasia (G) showing complete loss of PERP (H-I). Cytoplasmic only staining is scored as negative. Areas of parakeratosis on the mucosal surface are not included in the PERP scoring (B, E, H).
Figure 6.
Distribution of extent of PERP membrane staining for mild, moderate, and severe dysplasia (TMA2)
DISCUSSION
This is the first study to evaluate PERP expression in relation to tumor progression and treatment outcomes in head and neck SCC. Invasive SCC of the oral cavity exhibits loss of PERP, which correlates with increased local relapse. There is a higher risk of local relapse with complete loss of PERP than with partial loss. Known clinical and histologic prognostic factors for local relapse in OC tumors include tumor stage, surgical margin status, histologic pattern of invasion, perineural invasion (PNI), lymphovascular invasion (LVI), and the use of postoperative radiation +/- chemotherapy.{Lubek, 2012} In our small series of 36 patients, none of these parameters was associated with risk of local relapse (data not shown). However, most of the patients in our series had T1-2 tumors while only 5 patients had T3-4 tumors. Patients with large tumors (T3-4) or any high risk clinical or pathologic features received adjuvant radiation +/- chemotherapy, which may have obscured the adverse impact of these parameters. Determination of the prognostic impact of PERP, independent of known prognostic factors, requires the evaluation of a much larger cohort of OC patients treated uniformly, a project we hope to complete in the near future.
We also found that in oral cavity cancer, PERP protein loss, specifically a reduction in the extent of mucosal expression, occurs early at the transition to severe dysplasia/CIS. This observation was confirmed on a separate tissue microarray (TMA2) comprising samples from a different set of patients with oral dysplasia. The gradual loss of PERP membrane staining with increasing grade of dysplasia suggests that PERP loss is an important step in tumorigenesis. In contrast, loss of E-cadherin appears to occur at a later point in SCC progression – at the time of nodal metastasis – and did not correlate with local, nodal or distant relapse.
Several DNA microarray gene expression profiling studies evaluating HNSCC have resulted in genetic signatures that at first glance appear to consist of completely different gene sets, but it has been shown that the different genes have related functions, such as encoding proteins involved in cell adhesion, extracellular matrix function, and regulating cell motility.16, 17 Roepman et al identified a genetic signature including genes encoding for proteins involved in cell adhesion, cell growth and maintenance, and extracellular matrix remodeling which is predictive of lymph node metastasis in patients with oral cavity and oropharyngeal SCC. 18 Two-thirds of the genes in the genetic signature were downregulated, suggesting that loss of function may play a greater role in the development of metastasis than upregulation. In a gene expression profiling study of localized esophageal carcinomas, decreased expression of PERP, along with S100A2 and SPRR3, correlated with decreased likelihood of complete remission.19 In a previous study by our group, loss of PERP in a mouse model of UVB-induced skin SCC resulted in the promotion of both tumor initiation and progression. In addition, gene expression profiling of cells derived from the mouse epidermis after inactivation of PERP showed an induction of genes involved in promoting inflammation. 5 Since inflammation is known to play a role in tumorigenesis, the tumor suppressor function of PERP may involve not only cell adhesion but also interactions with the microenvironment. Similarly, PERP deficiency in a mouse breast cancer model correlated with decreased tumor-free survival and decreased latency of tumor initiation. 6 We also found that PERP expression is decreased in human cutaneous SCC and in human breast cancer cell lines.5, 6 Our current study evaluating oral cavity SCC further highlights the importance of PERP loss in promoting tumorigenesis and progression to invasive carcinoma.
Studies evaluating E-cadherin expression have yielded conflicting results. We noted decreased E-cadherin expression in metastatic lesions, which corresponds with other studies that have reported the correlation of E-cadherin loss with the presence of regional lymph node metastases. 20-23 However, Liu et al did not find a correlation between decreased E-cadherin expression and nodal metastasis in patients with oral SCC but did find a correlation with local recurrence and shorter overall and disease-free survival. 24 In contrast, loss of E-cadherin in our study did not correlate with local, nodal or distant relapse. The variability between studies may be due to factors such as differences in the patient populations (i.e. age, alcohol or tobacco use), differences in the tumor (i.e. location, stage, grade), or differences in how E-cadherin is scored.
Oral cavity SCC are typically associated with tobacco smoking, alcohol use, and poor dentition, and only infrequently associated with high-risk HPV. The reported rate of HPV(+) cases among oral cavity SCC ranges from 4% in an IARC (International Agency for Research on Cancer) multicenter oral cancer study25 to 12% in a U.S. study 26. While p16 is well-established as an independent prognostic marker that is predictive of improved prognosis in oropharyngeal SCC, a similar easy-to-use prognostic marker does not exist for oral cavity SCC.27 Whether immunohistochemical stain for PERP will be able to fulfill this role will require further studies.
This is the first study that links PERP loss with early progression and prognosis in oral cavity SCC. Larger prospective validation studies in patients with oral dysplasia as well as those with invasive OC SCC treated uniformly in a clinical trial will be required to validate the role of PERP as a tumor suppressor gene in HNSCCs and to determine its utility as a biomarker for risk assessment of patients with oral epithelial dysplasia.
Acknowledgments
Grant support: PO1-CA67166 (QTL, CSK, HC, SK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
No disclosures (CMN, RCJ, VGB, LDA)
REFERENCES
- 1.Chen YJ, Chang JT, Lee L, Wang HM, Liao CT, Chiu CC, et al. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene. 2007;26:467–476. doi: 10.1038/sj.onc.1209802. [DOI] [PubMed] [Google Scholar]
- 2.Papagerakis S, Shabana AH, Pollock BH, Papagerakis P, Depondt J, Berdal A. Altered desmoplakin expression at transcriptional and protein levels provides prognostic information in human oropharyngeal cancer. Hum Pathol. 2009;40:1320–1329. doi: 10.1016/j.humpath.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Depondt J, Shabana AH, Florescu-Zorila S, Gehanno P, Forest N. Down-regulation of desmosomal molecules in oral and pharyngeal squamous cell carcinomas as a marker for tumour growth and distant metastasis. Eur J Oral Sci. 1999;107:183–193. doi: 10.1046/j.0909-8836.1999.eos1070305.x. [DOI] [PubMed] [Google Scholar]
- 4.Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000;14:704–718. [PMC free article] [PubMed] [Google Scholar]
- 5.Beaudry VG, Jiang D, Dusek RL, Park EJ, Knezevich S, Ridd K, et al. Loss of the p53/p63 regulated desmosomal protein Perp promotes tumorigenesis. PLoS Genet. 2010;6:e1001168. doi: 10.1371/journal.pgen.1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dusek RL, Bascom JL, Vogel H, Baron S, Borowsky AD, Bissell MJ, et al. Deficiency of the p53/p63 target Perp alters mammary gland homeostasis and promotes cancer. Breast Cancer Res. 2012;14:R65. doi: 10.1186/bcr3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, et al. Perp is a p63-regulated gene essential for epithelial integrity. Cell. 2005;120:843–856. doi: 10.1016/j.cell.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Marques MR, Horner JS, Ihrie RA, Bronson RT, Attardi LD. Mice lacking the p53/p63 target gene Perp are resistant to papilloma development. Cancer Res. 2005;65:6551–6556. doi: 10.1158/0008-5472.CAN-05-0366. [DOI] [PubMed] [Google Scholar]
- 9.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 10.Cardesa A, Gale N, Nadal A, Zidar N. In: Squamous Cell Carcinoma. Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. IARC Press; Lyon: 2005. p. 119. [Google Scholar]
- 11.MacDonald DGS, S.M. In: Structural indicators of the high risk lesion. Johnson NW, editor. Cambridge University Press; Cambridge: 1991. pp. 165–187. [Google Scholar]
- 12.Brothwell DJ, Lewis DW, Bradley G, Leong I, Jordan RC, Mock D, et al. Observer agreement in the grading of oral epithelial dysplasia. Community Dent Oral Epidemiol. 2003;31:300–305. doi: 10.1034/j.1600-0528.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu CL, Prapong W, Natkunam Y, Alizadeh A, Montgomery K, Gilks CB, et al. Software tools for high-throughput analysis and archiving of immunohistochemistry staining data obtained with tissue microarrays. Am J Pathol. 2002;161:1557–1565. doi: 10.1016/S0002-9440(10)64434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glanz SAS, B.K. Primer of applied regression analysis of variance. McGraw-Hill, Inc.; New York: 1990. [Google Scholar]
- 15.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–229. [Google Scholar]
- 16.Roepman P, Holstege FC. Tumor profiling turmoil. Cell Cycle. 2005;4:659–660. doi: 10.4161/cc.4.5.1679. [DOI] [PubMed] [Google Scholar]
- 17.Colella S, Richards KL, Bachinski LL, Baggerly KA, Tsavachidis S, Lang JC, et al. Molecular signatures of metastasis in head and neck cancer. Head Neck. 2008;30:1273–1283. doi: 10.1002/hed.20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roepman P, Wessels LF, Kettelarij N, Kemmeren P, Miles AJ, Lijnzaad P, et al. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nat Genet. 2005;37:182–186. doi: 10.1038/ng1502. [DOI] [PubMed] [Google Scholar]
- 19.Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24:259–267. doi: 10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka N, Odajima T, Ogi K, Ikeda T, Satoh M. Expression of E-cadherin, alpha-catenin, and beta-catenin in the process of lymph node metastasis in oral squamous cell carcinoma. Br J Cancer. 2003;89:557–563. doi: 10.1038/sj.bjc.6601124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber GF, Zullig L, Soltermann A, Roessle M, Graf N, Haerle SK, et al. Down regulation of E-Cadherin (ECAD) - a predictor for occult metastatic disease in sentinel node biopsy of early squamous cell carcinomas of the oral cavity and oropharynx. BMC Cancer. 2011;11217:211–218. doi: 10.1186/1471-2407-11-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow V, Yuen AP, Lam KY, Tsao GS, Ho WK, Wei WI. A comparative study of the clinicopathological significance of E-cadherin and catenins (alpha, beta, gamma) expression in the surgical management of oral tongue carcinoma. J Cancer Res Clin Oncol. 2001;127:59–63. doi: 10.1007/s004320000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diniz-Freitas M, Garcia-Caballero T, Antunez-Lopez J, Gandara-Rey JM, Garcia-Garcia A. Reduced E-cadherin expression is an indicator of unfavourable prognosis in oral squamous cell carcinoma. Oral Oncol. 2006;42:190–200. doi: 10.1016/j.oraloncology.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010;23:213–224. doi: 10.1038/modpathol.2009.160. [DOI] [PubMed] [Google Scholar]
- 25.Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 26.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 27.Cao H, Banh A, Kwok S, Shi X, Wu S, Krakow T, et al. Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2012;82:e351–358. doi: 10.1016/j.ijrobp.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]