Abstract
Background
Tumor necrosis factor (TNF) antagonists [e.g., TNF soluble receptor (TNFsr)] improved survival in preclinical but not clinical sepsis trials. However fluid support—itself beneficial—is standard clinically but rarely employed in pre-clinical sepsis models. We hypothesized that these therapies may not have additive benefit.
Methods and results
Antibiotic-treated rats (n = 156) were randomized to intratracheal or intravenous Escherichia coli challenges (>LD50) and either placebo or TNFsr and 24 h fluid treatments alone or together. The survival effects of these therapies did not differ significantly comparing challenge routes. When averaged across route, while TNFsr or fluid alone decreased the hazard ratio of death significantly [ln ± standard error (SE): −0.65 ± 0.30 and -0.62 ± 0.30, respectively, p ≤ 0.05], together they did not (p = 0.16). Furthermore, the observed effect of TNFsr and fluid together on reducing the hazard ratio was significantly less than estimated (−0.37 ± 0.29 versus −1.27 ± 0.43, respectively, p = 0.027) based on TNFsr and fluid alone. While each treatment increased central venous pressure at 6 and 24 h, the observed effects of the combination were also less than estimated ones (p ≤ 0.0005). Conclusions: The individual survival benefits of TNFsr and fluids were not additive in this rat sepsis model. Investigating new sepsis therapies together with conventional ones during preclinical testing may be informative.
Keywords: Sepsis, Bacterial infection, Pneumonia, TNF soluble receptor, Fluid support, Rat
Introduction
While tumor necrosis factor α (TNF) is central to the innate immune response, its excessive release has been implicated in the pathogenesis of septic shock [1–4]. Supporting this concept, TNF inhibitors improved survival in animal sepsis models [5, 6]. Based in part on such studies, TNF directed antibodies (TNF-Ab) and soluble receptors (TNFsr) were tested clinically in sepsis. However no anti-TNF agent improved survival significantly in any clinical sepsis trial [6]. One explanation for these divergent effects is that variables influencing these agents in patients were not tested in animal studies [6–8]. Notably, fluid support while standard clinically is not routinely employed in sepsis models [9]. However, the beneficial effects of both fluids and anti-TNF agents are believed to be related in part to their effects on hemodynamic function. Furthermore, some studies suggest that fluid therapy may itself have anti-inflammatory effects [10, 11]. Despite such potentially overlapping actions, whether the benefits of fluid and anti-TNF agents are additive has not been tested.
We showed previously in antibiotic-treated rats that both TNFsr and fluid support with 24 h normal saline infusion (NS) when administered individually improved survival with highly lethal intratracheal (IT) or intravenous (IV) E. coli challenges [6, 12]. Our primary objective in the present study was to employ this rat model to test whether similar TNFsr and fluid treatments would have additive beneficial effects on survival during sepsis arising from either extravascular or intravascular routes of infection. A secondary objective was to investigate whether these therapies had effects on other laboratory measures that would provide a basis for any observed survival effects. To test a potential physiologic basis, we performed serial hemodynamic and arterial blood gas measures, and in some animals with IT challenge alone, lung lavage protein and lung wet to dry weight ratios. To investigate whether alterations in host defense or inflammatory responses might also provide such a basis, we measured complete blood counts, and again with IT challenge alone, blood and lung bacteria counts, plasma cytokine and nitric oxide levels, and lung lavage cell numbers.
Methods
Animal care
All studies were approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health.
Study design
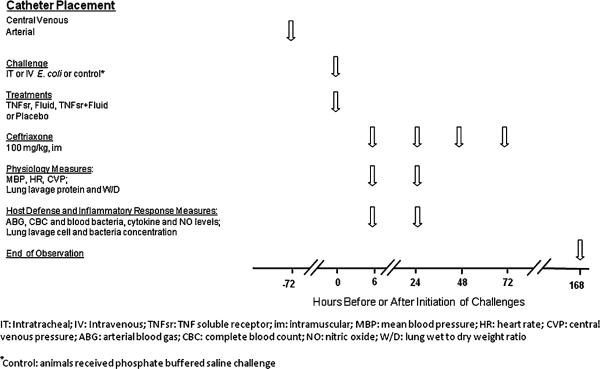
This study was designed to investigate whether TNFsr and fluid treatment would have additive effects on survival and other measures in sepsis arising via either extravascular or intravascular routes of infection. Briefly, anesthetized male Sprague-Dawley rats (n = 156) with indwelling central venous and carotid arterial catheters were randomized to E. coli challenge (0.5 ml) administered either intratracheally (IT, 60 × 109 CFU/kg) or intravenously (IV, 2 × 109 CFU/kg) (Fig. 1). E. coli doses were designed to produce greater than 50% lethality rates. Animals were also randomized to receive either: (1) placebo alone [human serum albumin (HSA), 250 μg/kg, IV] immediately (0 h) after E. coli, (2) p80 TNF receptor:Fc fusion protein (TNFsr, Immunex, Seattle, WA, 250 μg/kg, IV) alone at 0 h, (3) HSA at 0 h followed by normal saline (NS) infusion (20 ml/kg/h for 24 h, termed fluids) started 3 h after E. coli, or (4) TNFsr at 0 h and fluid started at 3 h. These regimens of TNFsr or fluids alone were studied because they had previously been shown to improve survival in this E. coli challenged rat model [6, 12]. Mean arterial blood pressure (MBP), heart rate (HR), central venous pressure (CVP), arterial blood gas with lactate, and complete blood count were obtained at 6 and 24 h after challenge (i.e., before and following the anticipated onset of lethality in the model, respectively) [13]. Hemodynamic measures alone were also obtained at 12 h. Animals alive after 168 h were considered survivors. At 6 h, all animals began treatment with ceftriaxone (100 mg/kg, intramuscular, daily for 4 days). To estimate the effects of E. coli alone, instrumented noninfected animals (n = 12) challenged with phosphate buffered saline (PBS) and otherwise untreated were similarly studied and observed over 168 h.
Fig. 1.
Interventions and measures and their timing in this study. Some physiology and host defense and inflammatory response measures were only performed in animals challenged with intratracheal E. coli (see “Methods”)
In additional experiments, to evaluate the effects of fluids and TNFsr, alone or together, on a broader group of physiologic and host defense and inflammatory response measures, animals (n = 100) were challenged with IT E. coli and randomized to the same treatments as above. Resources only permitted investigation of a single infection route, and it was felt that the IT route was most relevant. At 6 h in randomly selected animals (n = 30) and at 24 h in all remaining animals (n = 32), quantitative blood bacteria counts, and plasma cytokine, total protein, and nitric oxide levels were obtained in addition to hemodynamic, arterial blood gas, and complete blood count measures [13]. Animals were then sacrificed, and isolated lungs were lavaged for cell, protein, and bacteria analysis or were prepared for wet to dry weight ratio determinations [14]. Because sacrifice of animals was required at 6 or 24 h for these measures, survival was not assessed. Finally, noninfected animals were challenged with PBS and studied at 6 or 24 h (n = 10 at each time point) to estimate the effects of IT E. coli alone in these experiments.
Bacterial inoculation and treatments
Escherichia coli 0111:B4 was stored and prepared as previously described [14]. Ketamine anesthesia was employed in experiments assessing survival at 168 h, while isoflurane was employed in other experiments [13–15]. TNFsr and fluid support were administered as previously described [6, 12].
Laboratory measures
Hemodynamic, arterial blood gas, complete blood count, quantitative bacteria, lung lavage cell and protein, and lung wet to dry weight ratio measures were determined as previously described [13]. Cytokines including interleukin-1α (IL-1α), IL-1β, IL-5, IL-6, IL-10, IL-13, IL-17, tumor necrosis factor α (TNFα), migratory inhibitory protein 1α (MIP-1α), interferon gamma-induced protein (IP-10), keratinocyte-derived cytokine (KC), mouse homolog of human chemokine gro-alpha (CXCL1), eotaxin, regulated on activation normal T-cell expressed and secreted (RANTES), granulocyte macrophage colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF), monocyte chemotactic protein-1 (MCP-1), and interferon c (INFc) were measured using Procarta Rat Cytokine Profiling kits (Affymetrix, Santa Clara, CA). To assess nitric oxide levels, plasma nitrite/nitrate was measured with a fluorometric assay kit (Cayman Chemical, Ann Arbor, MI).
Statistics
All data were analyzed using SAS version 9.2. A Cox proportional-hazards model employing a PROC PHREG procedure (taking into account the influence of weekly experiments) was used to compare the effects of the following groups or interventions on survival in E. coli challenged animals: (1) treatment with TNFsr and fluid alone or together versus placebo control, (2) treatment with TNFsr and fluid alone or together with IT versus IV infection, and (3) observed versus estimated effects of treatment with TNFsr and fluid together. This procedure provides an estimate of the effect of treatment on survival, presented here as ln(hazard ratio of death) ± standard error (SE) [12, 16]. Other laboratory data were analyzed using three-way or two-way analysis of variance (ANOVA) accounting for infection route (IT versus IV), challenge (E. coli versus PBS) or treatment (fluid or TNFsr alone or together versus placebo), and time of measurement (6 versus 24 h for all measures). The effects of TNFsr and fluid alone or together on survival and CVP were not different across infection route, and these were combined for analysis. For clarity of presentation, the effects of treatment were calculated by subtracting the mean of the placebo-treated E. coli animals from the mean of the TNFsr- and fluid-treated groups. Data were log transformed where appropriate. For all laboratory measures, the observed effects of fluids and TNFsr together were compared with the sum of the effects of the treatments alone (termed estimated effect). All results were expressed as mean ± standard error of the mean (SEM), and p values ≤0.05 were considered significant. Finally, to further investigate the relationship between CVP and survival in the model, CVP in survivors and nonsurvivors from placebo-treated, IT and IV E. coli challenged animals observed for 168 h was also analyzed at 6 and 12 h together.
Results
Effects of E. coli challenge
All noninfected animals survived. Challenge with IT or IV E. coli in placebo-treated animals produced lethality rates close to 80% (Fig. 2 a, b). Compared with noninfected animals, IT E. coli increased alveolar to arterial oxygen gradient, hemoglobin, blood and lung bacteria counts, lung leukocyte and wet to dry weight ratios, three plasma cytokines (MIP-1α, MCP-1, and IP-10), and nitric oxide levels and decreased MBP, HR, and blood neutrophils and lymphocytes at 6 or 24 h or both (p ≤ 0.05) (comparisons not shown). At 6 and 24 h, IT E. coli first increased and then decreased plasma TNF and IL-6 (p ≤ 0.05 for the time interaction, data not shown). Intravenous E. coli increased HR and decreased blood neutrophils, lymphocytes, and platelets at 6 or 24 h or both (p ≤ 0.05).
Fig. 2.
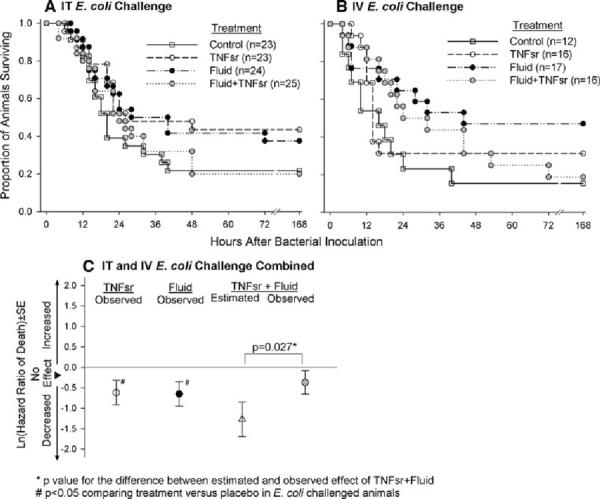
a, b Proportional survival over 168 h in animals challenged with intratracheal (IT) or intravenous (IV) E. coli and then treated with either placebo (human serum albumin), soluble tumor necrosis factor soluble receptor (TNFsr) alone, fluid therapy alone, or TNFsr and fluids together (see “Methods” for the actual regimens). c Effects of TNFsr and fluids alone or together on ln(hazard ratio of death) ± SE in animals averaged over the IT or IV routes of infection (see Table 1 for the effects of therapies with individual routes of infection)
Effect of therapies on survival
In E. coli challenged animals, compared with placebo, treatment with either TNFsr, or fluids, or both together produced effects on survival and the hazard ratio of death with both IT and IV E. coli that were similarly on the side of benefit, but none of which were significant (Fig. 2a, b; Table 1). Since these effects did not differ comparing IT and IV infection, we averaged over the two routes to further explore the individual and combined effects of these therapies (Fig. 2c). Both TNFsr and fluid alone improved survival and decreased the hazard ratio of death significantly (ln ± SE; −0.65 ± 0.30 and −0.62 ± 0.30, respectively, p ≤ 0.05 for each). In contrast, while TNFsr and fluid together had an effect on the side of benefit, this was not significant (−0.37 ± 0.29, p = 0.16). Furthermore, the observed effect of TNFsr and fluid together on reducing the hazard ratio of death was significantly less (p = 0.027) than the estimated one based on the observed effects of TNFsr and fluids alone (−1.27 ± 0.43). Thus, the individual beneficial effects of TNFsr and fluid on survival in this model were not additive.
Table 1.
Observed effects (calculated compared with placebo) of TNFsr and fluid alone or combined (TNFsr + fluid) and the estimated effect of the combination (based on the observed effects of the each treatment alone) on the In(hazard ratio of death)(hazard ratio) over 168 h and on the central venous pressure (CVP) 6 or 24 h after E. coli challenge via intratracheal (IT) or intravenous (IV) route
| Parameter | Route | Time (h) | TNFsr | Fluid | TNFsr + fluid |
p-Value# | |
|---|---|---|---|---|---|---|---|
| Estimated | Observed | ||||||
| Hazard ratio | IT | 168 | −0.71 ± 0.39 | −0.48 ± 0.37 | −1.19 ± 0.54 | −0.40 ± 0.36 | 0.063 |
| IV | 168 | −0.48 ± 0.47 | −0.95 ± 0.50 | −1.43 ± 0.69 | −0.39 ± 0.46 | 0.220 | |
| CVP | IT | 6 | 1.54 ± 0.51** | 1.48 ± 0.48** | 3.02 ± 0.87 | 1.27 ± 0.49* | 0.010 |
| IT | 24 | 3.32 ± 0.97** | 3.14 ± 0.97** | 6.46 ± 1.69 | 2.84 ± 0.98** | 0.009 | |
| IV | 6 | 1.62 ± 0.55* | 1.66 ± 0.54** | 3.27 ± 0.97 | 0.94 ± 0.55 | 0.003 | |
| IV | 24 | 3.17 ± 1.57* | 4.66 ± 1.37** | 7.83 ± 2.66 | 2.93 ± 1.41* | 0.010 | |
p < 0.05,
p < 0.01 comparing treatment versus placebo in E. coli challenged animals
p value for comparison of the estimated versus observed effects of TNFsr + fluid in combination
Effect of therapies on central venous pressure and hemoglobin
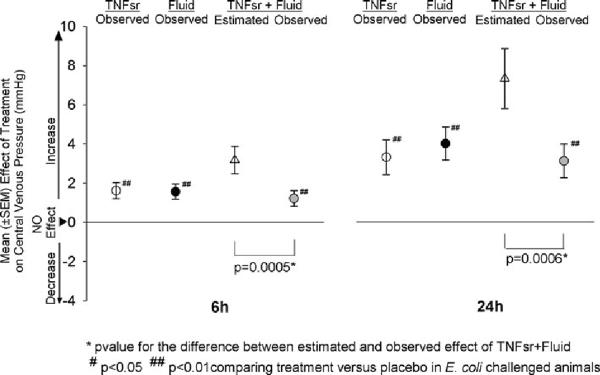
In animals receiving either IT or IV E. coli challenge, compared with placebo treatment, TNFsr and fluid alone or together significantly increased CVP at 6 and 24 h [p ≤ 0.05 for all comparisons except for TNFsr and fluid together with IV E. coli at 6 h (p = 0.14)] (Table 1). However, similar to their effect on survival, the observed effect of TNFsr and fluid together on CVP was less than the one estimated based on the observed effects of each individual treatment for either route of infection or when combined across route (p ≤ 0.014; Table 1; Fig. 3). Thus, just as with survival, increases in CVP with TNFsr and fluid alone in this model were not additive when the therapies were combined. The potential importance of increased CVP for survival in this model is reflected by the finding that, in placebo-treated animals with either IT or IV E. coli and observed for 168 h, CVP at 6 and 12 h was higher in survivors than in nonsurvivors [mean difference (± SEM)] (+0.44 ± 0.25 mmHg) in a pattern that approached significance (p = 0.086 when averaged over route and time).
Fig. 3.
Effect of tumor necrosis factor soluble receptor (TNFsr) alone, fluids alone, or TNFsr and fluids together on central venous pressure (CVP) at 6 or 24 h in animals averaged over intratracheal and intravenous E. coli challenges (see Table 1 for the effects of therapies with individual routes of infection)
Potentially consistent with the effect of therapy on CVP, when averaged over the two routes of infection at 24 h, TNFsr alone, fluids alone, and the combination reduced hemoglobin concentrations (mean effect compared with placebo ± SEM; −1.51 ± 1.07, −2.43 ± 1.02, and −2.58 ± 1.07 g/dL, respectively) in patterns significant for the latter two (p = 0.02 for both) but not for TNFsr alone (p = 0.16). As with CVP, this observed change with the therapies together was not as great as the estimated one (−3.94 ± 1.86 g/dL), although the difference was not significant (p = 0.345).
Effect of therapies on other laboratory measures
With IT E. coli, compared with placebo, TNFsr both alone and with fluid increased MBP significantly (p ≤ 0.05 averaged over 6 and 24 h), while all three treatments increased HR at 6 h (p ≤ 0.05; Table 2). Treatment with TNFsr alone and with fluid increased measured TNF at 6 and 24 h (p ≤ 0.002), but fluid alone had opposing effects, decreasing TNF early and increasing it late (p = 0.001 for the time interaction). Treatment with fluid alone or with TNFsr decreased nitric oxide levels but not significantly (p = 0.078 and 0.066, respectively, averaged over 6 and 24 h). With IV E. coli, both fluid alone and with TNFsr decreased circulating neutrophils significantly (p ≤ 0.05 averaged over 6 and 24 h), while all three treatments were associated with decreases in circulating lymphocytes at 6 h but increases at 24 h (p ≤ 0.05 for the time interactions). Compared with placebo, TNFsr and fluid either alone or together did not significantly alter any other parameter measured for either route of infection throughout.
Table 2.
Mean (± SEM) physiology and host defense and inflammatory response measures 6 or 24 h after intratracheal or intravenous challenge with E. coli and treatment with either placebo, TNFsr, fluid, or TNFsr and fluid together
| Hours after initiation of challenge | ||||||||
|---|---|---|---|---|---|---|---|---|
| Measures | 6 h |
24 h |
||||||
| Placebo | TNFsr | Fluid | TNFsr + fluid | Placebo | TNFsr | Fluid | TNFsr + fluid | |
| Intratracheal E. coli challenge | ||||||||
| n = 24–27 | n = 23–24 | n = 26–28 | n = 24–26 | n = 14–17 | n = 14–19 | n = 17–20 | n = 14–18 | |
| CVP | 0.3 ± 0.4 | 1.8 ± 0.4## | 1.8 ± 0.4## | 1.6 ± 0.4# | −0.5 ± 0.6 | 2.8 ± 0.6## | 2.7 ± 0.6## | 2.4 ± 0.6## |
| Hb | 12.5 ± 0.5 | 12.9 ± 0.5 | 12.5 ± 0.5 | 12.1 ± 0.5 | 15.1 ± 0.6 | 13.7 ± 0.6 | 13.5 ± 0.5 | 13.3 ± 0.6 |
| MBP | 101 ± 2 | 109 ± 2## | 103 ± 2 | 108 ± 2# | 98 ± 3 | 102 ± 3 | 102 ± 3 | 105 ± 3 |
| HR | 258 ± 10 | 297 ± 11## | 286 ± 10# | 286 ± 10## | 282 ± 17 | 295 ± 16 | 283 ± 15 | 235 ± 15 |
| IL-1β | 2.41 ± 0.35 | 2.76 ± 0.13 | 2.45 ± 0.19 | 2.94 ± 0.54 | 2.54 ± 0.13 | 3.19 ± 0.46# | 2.79 ± 0.09 | 2.62 ± 0.12 |
| TNFα | 3.52 ± 0.19 | 3.79 ± 0.24 | 2.77 ± 0.22 | 4.19 ± 0.25 | 0.74 ± 0.33 | 2.75 ± 0.53## | 2.08 ± 0.21# | 2.18 ± 0.52# |
| NO | 3.93 ± 0.30 | 4.00 ± 0.26 | 3.87 ± 0.26 | 3.70 ± 0.30 | 5.02 ± 0.26 | 5.10 ± 0.37 | 4.55 ± 0.28 | 3.77 ± 0.37## |
| Intraveous E. coli challenge | ||||||||
| n = 7–10 | n = 12–15 | n = 11–16 | n = 10–14 | n = 2–4 | n = 5 | n = 8–11 | n = 6–9 | |
| CVP | 1.0 ± 0.6 | 4.5 ± 0.8# | 2.7 ± 0.5## | 1.9 ± 0.5 | 1.3 ± 0.9 | 4.5 ± 0.8# | 6.0 ± 0.6## | 4.3 ± 0.6# |
| Hb | 13.4 ± 0.5 | 13.0 ± 0.4 | 11.4 ± 0.4# | 12.1 ± 0.5 | 11.6 ± 1.0 | 11.3 ± 0.6 | 9.3 ± 0.5 | 9.1 ± 0.6 |
| HR | 432 ± 23 | 456 ± 19 | 418 ± 18 | 419 ± 20 | 466 ± 37 | 519 ± 33 | 380 ± 22 | 358 ± 24# |
| NEU | −0.9 ± 0.2 | −1.2 ± 0.2 | −1.5 ± 0.2# | −1.5 ± 0.2# | 1.5 ± 0.4 | 1.9 ± 0.3 | 1.0 ± 0.2 | 0.6 ± 0.2# |
| LYM | −1.6 ± 0.4 | −2.1 ± 0.3 | −2.1 ± 0.3 | −2.4 ± 0.3 | −0.7 ± 0.7 | 1.4 ± 0.4# | 0.8 ± 0.3 | 1.2 ± 0.4# |
p < 0.05,
p < 0.01 comparing TNFsr, fluid or TNFsr + fluid versus placebo in E. coli challenged animals
CVP central venous pressure (mmHg), Hb hemoglobin (g/dL), MBP mean blood pressure (mmHg), HR heart rate (beats per minute), IL-1β interleukin-1β [ln(pg/ml)], TNFα tumor necrosis factor α [ln(pg/ml)], NO nitric oxide [ln(mM)], NEU neutrophils [ln(103/μL)], LYM lymphocytes [ln(103/μL)]
Discussion
In this rat model, across the two routes of infection, treatment with TNFsr or fluid alone increased survival significantly. However, when these therapies were combined, their beneficial effects were not additive. In a prior systematic review of preclinical (23 publications) and clinical (12 publications) trials testing TNF antagonists for sepsis, only one of the preclinical trials incorporated fluid support in the study design [6]. However, fluid administration has been employed for patients with sepsis for several decades [17]. The present findings support the possibility that TNF antagonists were more beneficial in preclinical compared with clinical sepsis trials because the former did not account for the possibility that the beneficial effects of fluid and anti-TNF agents were not additive. Interestingly, in the one preclinical trial noted in the prior systematic review that employed fluid support in both control and treated groups, TNF-Ab did not show benefit [18].
Improved survival with TNFsr and fluid alone in the present study appears in part related to the effects of each therapy on increasing CVP. Reductions in CVP are reported to be associated with worsened outcome in sepsis [19–21]. Consistent with that, we showed previously in this rat model that increasing mortality rates with increasing IT E. coli doses were associated with dose-dependent decreases in CVP [22]. Furthermore, in another study in rats receiving either low or high doses of IV E. coli, CVP was higher in animals with less severe compared with more severe infection [23]. Finally, in the present study in E. coli animals treated with placebo, CVP measures were higher in survivors compared with nonsurvivors. Importantly, fluid support to increase CVP has been reported to improve survival in both clinical and preclinical sepsis studies [20, 24]. Prior preclinical studies have also suggested that TNF inhibition can increase CVP [25]. Consistent with increased CVP in the present study and the expanded intravascular fluid volume this might reflect, at 24 h each therapy was also associated with reductions in hemoglobin concentration. Increased CVP with TNFsr may have been related to inhibition of inflammatory injury to the systemic endothelium, improved vascular integrity, and inhibition of extravascular fluid losses [1, 4]. Increased CVP with fluids was most likely related to direct expansion of the intravascular space. However, reductions in nitric oxide with fluids at 6 and 24 h may have also contributed to increased CVP levels, since nitric oxide can dilate venous capacitance vessels [26].
While TNFsr and fluids both alone and together increased CVP, their observed effects together were not additive and were significantly less than those estimated based on their individual effects. Thus, to the extent that increased CVP may have added to improved survival with the two agents, their inability to produce additive CVP increases may have been why their effects on survival also were not additive. Absence of an additive effect of the two therapies on CVP may have been because volume expansion related to fluid administration either limited TNFsr and TNF interactions or increased the clearance of the two [27]. Alternatively, inhibition of inflammatory renal injury by TNFsr may have increased clearance of the administered fluid [28, 29].
Several studies in trauma models have suggested that fluid therapy may itself have anti-inflammatory effects [10, 11]. In the present study, fluid therapy both alone and in combination with TNFsr reduced nitric oxide increases associated with IT E. coli challenge. Fluids did not alter total circulating protein levels, suggesting that their effect on NO levels was not due to hemodilution. Fluids alone were also associated with biphasic changes in TNF levels, decreasing them early but increasing them late. However, fluid therapy did not alter other cytokine levels. Absence of such an effect was not due to insensitivity of these tests, since they did detect the effects of both E. coli and time.
There are limitations to the present study. First, as an animal study its results are not directly applicable to patients. Confirmation of the present findings, possibly in a large animal model permitting the kind of invasive hemodynamic monitoring and titration of therapies that occurs clinically, would be informative. It is possible that fluid therapy titrated based on CVP or other measures as is done clinically might result in fewer potentially competing effects between the two therapies and produce additive benefits. Second, while the TNFsr treatment employed was a human Fc fusion protein, the control was human albumin. While earlier experiments showing the effectiveness of this agent in mice employed human IgG as a control, the ideal placebo for this therapy would be another type of human Fc fusion protein [30]. Finally, conventional hemodynamic support in patients sometimes includes not only fluids but other interventions such as vasopressors and inotropes. Whether an agent such as TNFsr would have added to the potential benefits of these other therapies, alone or with fluids, requires study.
In conclusion, anti-TNF therapies appeared beneficial in many published preclinical sepsis trials but not in clinical trials. In contrast to preclinical sepsis models however, fluid support, which is itself beneficial, is standard therapy in patients with sepsis including those in trials. In the present study, the individual survival benefits of TNFsr and fluids were not additive in this rat sepsis model. These findings raise the possibility that failure of fluid and anti-TNF therapies to have additive beneficial effects in sepsis may provide one basis for the differing results comparing published preclinical and clinical sepsis trials. Investigating whether potential new sepsis therapies and conventional ones such as fluid therapy have additive beneficial effects during preclinical testing may be informative.
Acknowledgments
This work was supported by NIH intramural fund.
Footnotes
Conflict of interest None of the authors have a commercial or other association that might pose a conflict of interest.
References
- 1.Vicaut E, Hou X, Payen D, Bousseau A, Tedgui A. Acute effects of tumor necrosis factor on the microcirculation in rat cremaster muscle. J Clin Invest. 1991;87:1537–1540. doi: 10.1172/JCI115165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989;169:823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichacker PQ, Hoffman WD, Farese A, Banks SM, Kuo GC, MacVittie TJ, Natanson C. TNF but not IL-1 in dogs causes lethal lung injury and multiple organ dysfunction similar to human sepsis. J Appl Physiol. 1991;71:1979–1989. doi: 10.1152/jappl.1991.71.5.1979. [DOI] [PubMed] [Google Scholar]
- 4.Naziri W, Joshua IG. Role of tumor necrosis factor-alpha in small intestinal microcirculation. Am Surg. 1998;64:203–209. discussion 209–210. [PubMed] [Google Scholar]
- 5.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 6.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 7.Bagby GJ, Plessala KJ, Wilson LA, Thompson JJ, Nelson S. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991;163:83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Karzai W, Cui X, Mehlhorn B, Straube E, Hartung T, Gerstenberger E, Banks SM, Natanson C, Reinhart K, Eichacker PQ. Protection with antibody to tumor necrosis factor differs with similarly lethal Escherichia coli versus Staphylococcus aureus pneumonia in rats. Anesthesiology. 2003;99:81–89. doi: 10.1097/00000542-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Piper RD, Cook DJ, Bone RC, Sibbald WJ. Introducing critical appraisal to studies of animal models investigating novel therapies in sepsis. Crit Care Med. 1996;24:2059–2070. doi: 10.1097/00003246-199612000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Dorresteijn MJ, van Eijk LT, Netea MG, Smits P, van der Hoeven JG, Pickkers P. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. J Endotoxin Res. 2005;11:287–293. doi: 10.1179/096805105X58715. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MA, Chou MC, Spain DA, Downard PJ, Qian Q, Cheadle WG, Garrison RN. Fluid resuscitation attenuates early cytokine mRNA expression after peritonitis. J Trauma. 1996;41:622–627. doi: 10.1097/00005373-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Sherer K, Li Y, Cui X, Li X, Subramanian M, Laird MW, Moayeri M, Leppla SH, Fitz Y, Su J, Eichacker PQ. Fluid support worsens outcome and negates the benefit of protective antigen-directed monoclonal antibody in a lethal toxin-infused rat Bacillus anthracis shock model. Crit Care Med. 2007;35:1560–1567. doi: 10.1097/01.CCM.0000266535.95770.A2. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Li X, Haley M, Fitz Y, Gerstenberger E, Banks SM, Eichacker PQ, Cui X. DTPA Fe(III) decreases cytokines and hypotension but worsens survival with Escherichia coli sepsis in rats. Intensive Care Med. 2006;32:1263–1270. doi: 10.1007/s00134-006-0234-2. [DOI] [PubMed] [Google Scholar]
- 14.Haley M, Parent C, Cui X, Kalil A, Fitz Y, Correa-Araujo R, Natanson C, Danner RL, Banks SM, Eichacker PQ. Neutrophil inhibition with l-selectin-directed MAb improves or worsens survival dependent on the route but not severity of infection in a rat sepsis model. J Appl Physiol. 2005;98:2155–2162. doi: 10.1152/japplphysiol.01241.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cui X, Zeni F, Vodovitz Y, Correa-de-Araujo R, Quezado M, Roberts A, Wahl S, Danner RL, Banks SM, Gerstenberger E, Fitz Y, Natanson C, Eichacker PQ. TGF-beta1 increases microbial clearance but worsens lung injury during Escherichia coli pneumonia in rats. Cytokine. 2003;24:115–127. doi: 10.1016/j.cyto.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Cui X, Li Y, Li X, Haley M, Moayeri M, Fitz Y, Leppla SH, Eichacker PQ. Sublethal doses of Bacillus anthracis lethal toxin inhibit inflammation with lipopolysaccharide and Escherichia coli challenge but have opposite effects on survival. J Infect Dis. 2006;193:829–840. doi: 10.1086/500468. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Stack AM, Saladino RA, Thompson C, Sattler F, Weiner DL, Parsonnet J, Nariuchi H, Siber GR, Fleisher GR. Failure of prophylactic and therapeutic use of a murine anti-tumor necrosis factor monoclonal antibody in Escherichia coli sepsis in the rabbit. Crit Care Med. 1995;23:1512–1518. doi: 10.1097/00003246-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 21.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 22.Cui X, Solomon S, Natanson C, Fitz Y, Danner RL, Gerstenberger E, Banks S, Eichacker PQ. Oligonuleotide microarrays identify genes, including one for TNFsr, with changes in expression dependent on infection dose of E. coli in rats. Am J Crit Care Med. 2002;165:A521. [Google Scholar]
- 23.Cui X, Parent C, Macarthur H, Ochs SD, Gerstenberg E, Solomon S, Fitz Y, Danner RL, Banks SM, Natanson C, Salvemini D, Eichacker PQ. Severity of sepsis alters the effects of superoxide anion inhibition in a rat sepsis model. J Appl Physiol. 2004;97:1349–1357. doi: 10.1152/japplphysiol.01161.2003. [DOI] [PubMed] [Google Scholar]
- 24.Natanson C, Danner RL, Reilly JM, Doerfler ML, Hoffman WD, Akin GL, Hosseini JM, Banks SM, Elin RJ, MacVittie TJ, et al. Antibiotics versus cardiovascular support in a canine model of human septic shock. Am J Physiol. 1990;259:H1440–H1447. doi: 10.1152/ajpheart.1990.259.5.H1440. [DOI] [PubMed] [Google Scholar]
- 25.Evans DA, Jacobs DO, Revhaug A, Wilmore DW. The effects of tumor necrosis factor and their selective inhibition by ibuprofen. Ann Surg. 1989;209:312–321. doi: 10.1097/00000658-198903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon KS, Pang CC. Venodilator action of an organotransition-metal nitrosyl complex. Eur J Pharmacol. 2002;436:107–110. doi: 10.1016/s0014-2999(01)01612-0. [DOI] [PubMed] [Google Scholar]
- 27.Bemelmans MH, Gouma DJ, Buurman WA. Influence of nephrectomy on tumor necrosis factor clearance in a murine model. J Immunol. 1993;150:2007–2017. [PubMed] [Google Scholar]
- 28.Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW. Endotoxemic renal failure in mice: role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int. 2001;59:2243–2249. doi: 10.1046/j.1523-1755.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Zolty E, Falk S, Basava V, Reznikov L, Schrier R. Pentoxifylline protects against endotoxin-induced acute renal failure in mice. Am J Physiol Renal Physiol. 2006;291:F1090–F1095. doi: 10.1152/ajprenal.00517.2005. [DOI] [PubMed] [Google Scholar]
- 30.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]