Abstract
Objective
W1-mAb is a chimpanzee-derived monoclonal antibody to protective antigen that improved survival when administered before anthrax lethal toxin challenge in rats. To better define W1-mAb’s efficacy for anthrax, we administered it after initiation of 24-hr infusions of edema toxin and lethal toxin either alone or together in rats or following anthrax spore challenge in mice.
Interventions
W1-mAb or placebo treatment.
Methods and Main Results
In toxin-challenged rats treated with placebo, survival rates were lower with edema toxin (500 μg/kg) compared to lethal toxin either alone (175 μg/kg) or with edema toxin (175 μg/kg each) (8%, 33%, and 32%, respectively), but the median time to death was longer (36, 11, and 9 hrs, respectively) (p ≤ .01 for all comparisons). W1-mAb administered up to 12 hrs after edema toxin and 6 hrs after lethal toxin increased survival and reduced hypotension (p ≤ .01). However, only administration of W1-mAb at 0 hrs improved these variables with lethal toxin and edema toxin together (p ≤ .0002). In C57BL/6J mice challenged with anthrax spores subcutaneously, compared to placebo treatment (0 of 15 animals survived), W1-mAb administered beginning 24 hrs after challenge increased survival (13 of 15 survived) (p ≤ .0001).
Conclusion
While rapidity of lethality may influence the effectiveness of delayed W1-mAb treatment, these rat and mouse studies provide a basis for further exploring this agent’s usefulness for anthrax.
Keywords: Bacillus anthracis, lethal and edema toxins, protective-antigen-directed mAb, treatment, rodent
Shock with Bacillus anthracis is difficult to manage with conventional therapies as recently highlighted by the fatal outbreak of anthrax in injection drug abusers in Europe and the case of gastrointestinal anthrax in the United States (1–5). Developing effective adjunctive treatments for life-threatening disease is important.
B. anthracis produces two toxins, lethal toxin (LT), comprised of lethal factor and protective antigen (PA), and edema toxin (ET), comprised of edema factor (EF) and PA (6). PA mediates cellular uptake of the toxic factors (7, 8). Lethal factor is an endopeptidase that inactivates mitogen-activated protein kinase kinases (9–12). ET has strong adenyl cyclase activity that increases intracellular cyclic adenosine monophosphate to high levels (13). Since both toxins likely contribute to anthrax pathogenesis and may have additive effects, therapies inhibiting PA may be useful (14, 15). One such agent is a chimpanzee-derived monoclonal antibody against PA (W1-mAb) (16). Pretreatment with W1-mAb was protective in LT-challenged Fischer rats.
However, there are unanswered questions regarding W1-mAb’s efficacy, such as the following: Will it improve survival with ET alone or in combination with LT? Will it have a benefit if administered after the onset of cardiovascular dysfunction caused by these toxins? Will it be effective administered following anthrax spore challenge? We therefore investigated these questions in rats challenged with 24-hr ET or LT infusions alone or in combination and in mice challenged with spores of a Sterne-like B. anthracis strain.
MATERIALS AND METHODS
Animal Care
The protocol used in this study was approved by the Animal Care and Use Committee of the Clinical Center, National Institutes of Health (Bethesda, MD).
Design of Toxin-Challenged Rat Studies
Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) (n = 453) weighing between 250 and 280 g, with central venous and systemic arterial catheters, were briefly anesthetized with isoflurane for connection to infusion lines and transducers (see the electronic supplement for more detailed methods [Supplement Digital Content 1, http://links.lww.com/CCM/A238]). They were then challenged with 24-hr infusions of: 1) ET (EF, 500 μg/kg, with double the amount of PA), 2) LT (lethal factor, 175 μg/kg, with double the amount of PA), or 3) LT and ET in combination (lethal factor and EF, 175 μg/kg each, with double the amount of PA). LT and ET were administered together in these doses because lethal factor and EF have similar molar weights and affinities for PA. A control group of animals received 24-hr diluent infusions.
Toxin components were prepared and administered as previously described (17). At a time of 0, 6, or 12 hrs following the start of toxin infusion, animals were randomized to treatment with W1-mAb or diluent (placebo) administered intravenously. Doses of W1-mAb were either equal to (1×) or ten times (10×) the molar dose of PA. All animals received similar volumes of toxin and treatment. Animals not challenged with toxin (controls) were treated with placebo at one of the three treatment times. Immediately before toxin challenge and at 2-hr intervals until catheter disconnection (24 hrs), mean arterial blood pressures (MBPs) and heart rates (HRs) were recorded as previously described (18). In animals receiving treatment at 6 or 12 hrs, hemodynamic measurements at these times were performed immediately before treatment. Animals receiving W1-mAb at 0 hrs had blood drawn at 4, 8, and 24 hrs for measurement of arterial blood gases, complete blood counts, and serum lactate, blood urea nitrogen, creatinine, liver enzyme (alanine and aspartate transaminases), and creatine phosphokinase levels (Trilogy Clinical Chemistry, Drew Scientific, Dallas, TX). The alveolar to arterial oxygen gradient was calculated (18). Animals were observed every 2 hrs for the first 24 hrs, then every 4 hrs from 24 to 48 hrs, and finally every 12 hrs for up to 168 hrs. Weekly experiments were performed, each of which included 24 animals randomized to either diluent challenge and placebo treatment or toxin challenge with placebo or W1-mAb treatment. Additional experiments tested the effects of lower doses of W1-mAb (0.5× and 0.1×) administered at the initiation toxin infusions.
Design of Anthrax-Spore-Challenged Mouse Studies
Spore-sensitive C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) weighing 22–25 g were randomized to receive either 25 or 100 μg of W1-mAb prepared in phosphate-buffered saline and administered intraperitoneally 24 and 3 hrs before or 24 and 48 hrs after subcutaneous challenge with 2 × 107 spores of the capsule-deficient anthrax A35 strain prepared as previously described (19, 20). Another group of spore-challenged animals received placebo (phosphate-buffered saline only) treatment. Animals were observed daily for a total of 10 days and had access to water and feed throughout.
W1-mAb Preparation
Anti-PA neutralizin-antigen binding fragment W1 was generated from chimpanzees that were immunized with PA. The antigen-binding fragment was then converted into a full-length immunoglobulin G with the human r1 heavy chain constant region, as previously described (21). The immunoglobulin G was produced from stably transformed Chinese hamster ovary cells and purified through protein A and size exclusion columns.
Statistical Analysis
The effects of W1-mAb on survival and hemodynamic measures were the primary outcomes for the study. To measure survival in the rat studies, power estimates were conducted and sample sizes calculated. These were reviewed and approved by an Animal Care and Use Committee before the experiments. It was planned at the outset of study that hemodynamic and other measures would be obtained with animals estimated for survival studies. This analysis and factors that later influenced the final number of animals studied are detailed in the electronic supplement (Supplement Digital Content 1, http://links.lww.com/CCM/A238). Kaplan-Meier plots of survival are presented for the toxin challenge studies. The analysis of the median time to death comparing toxins in placebo-treated nonsurvivors was performed using the Wilcoxon signed rank test. The effect of toxin on survival rates in placebo-treated rats and the effect of W1-mAb compared to placebo treatment in spore-challenged mice were analyzed using Fisher’s exact test. The effect of W1-mAb treatment on the odds ratio of survival was analyzed with the Mantel-Haenszel chi-square test. Survival in toxin-challenged animals treated with placebo did not differ significantly compared to that of experiments testing either 1× or 10× W1-mAb doses with each toxin; these data were therefore combined as the 0× group and are shown as single plots in Figure 1. However, the effects of W1-mAb on the odds ratio of survival were calculated and analyzed for each dose of W1-mAb using its own concurrent control. Initial pilot studies assessed the dose response characteristics of the toxin preparations to be used.
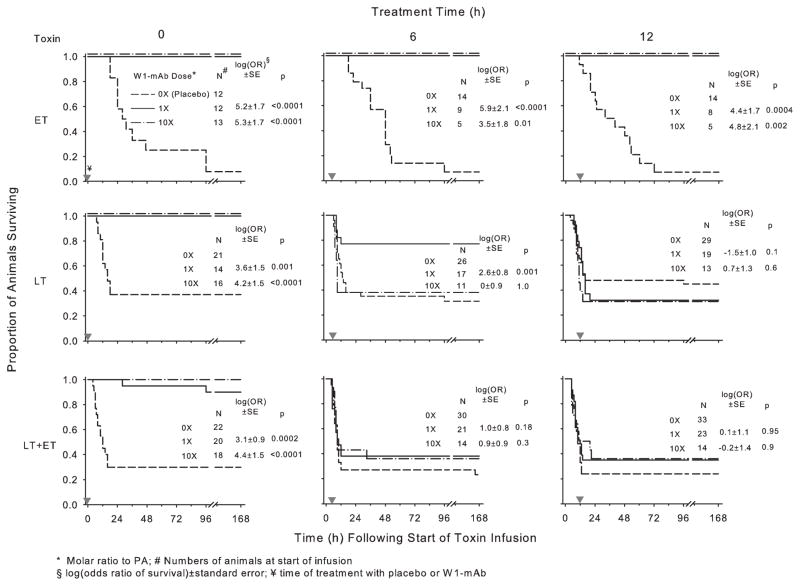
Figure 1.
Survival over time in animals challenged with 24-hr infusions of edema toxin (ET; 500 μg/kg), lethal toxin (LT; 175 μg/kg), or ET and LT in combination (175 μg/kg each) and treated with a chimpanzee-derived monoclonal antibody against protective antigen (W1-mAb; 1× or 10×) or placebo (0×). Treatment was administered at the initiation of toxin infusion (0 hrs) or 6 or 12 hrs after the start of toxin infusion (designated by the gray inverted triangles). The number of animals studied for each combination of toxin and treatment is shown. Since survival in placebo-treated animals studied with either 1× or 10× W1-mAb doses did not differ significantly, these data are combined in the present plots. The corresponding effects of W1-mAb on the log(odds ratio of survival), log(OR), ± SE and the p values for these effects are shown in each panel. The log(OR) values for either the 1× or 10× W1-mAb doses were calculated on the basis of their respective placebo-treated controls (see Materials and Methods). PA, protective antigen.
In studies in rats, other laboratory variables were analyzed using a mixed model with Statistical Analysis System version 9.1 software (SAS Institute, Cary, NC), and least square means (±SEM) were reported. As in prior studies, the effects of treatment on hemodynamic measures (MBP and HR) compared to placebo were analyzed on the basis of changes over 12-hr periods from values measured immediately before treatment (14). Hemodynamic and other laboratory data in toxin-challenged animals treated with placebo did not differ significantly compared to those of experiments testing W1-mAb doses of 1× or 10×, and these placebo data were combined as the 0× group for analysis throughout. Data were log transformed where appropriate. All results are expressed as least square means (±SEM), and two-sided p values of ≤.05 were considered significant. Analysis is unadjusted for multiple comparisons.
RESULTS
Toxin-Challenged Rat Studies
Survival
The number of animals assigned to each group is shown in Figure 1. In animals challenged with toxin and treated with placebo, the overall survival rate with ET alone was lower than with LT alone or LT together with ET (8% vs. 33% or 32%, respectively) (p ≤ .01 for each compared to ET alone) (Fig. 1). However, the median time (range) until death with ET [36 hrs (11–96 hrs)] was significantly longer than that with LT alone [11 hrs (4–132 hrs)] or LT together with ET [9 hrs (4–166 hrs)] (p ≤ .01 for each compared to ET alone).
In animals challenged with ET alone, compared to treatment with placebo, treatment with both doses of W1-mAb (1× and 10×) increased survival whether administered at the initiation of (0 hrs) or 6 or 12 hrs after the start of toxin infusion [log(odds ratio of survival) ±SE ranged from 3.5 ± 1.8 to 5.3 ± 1.7, p ≤ .01 for all] (Fig. 1). In animals challenged with LT alone, treatment with both doses of W1-mAb at 0 hrs significantly increased survival, as did treatment with the 1× dose at 6 hrs (3.6 ± 1.5 and 4.2 ± 1.5, respectively, at 0 hrs and 2.6 ± 0.8 at 6 hrs, p ≤ .001). However, the 10× dose given at 6 hrs and both doses at 12 hrs did not improve survival with LT alone. Finally, in animals challenged with LT and ET together, while both W1-mAb doses increased survival significantly when administered at 0 hrs (3.1 ± 0.9 and 4.4 ± 1.5, respectively, p ≤ .0002), neither did at 6 or 12 hrs.
With each toxin challenge, reducing the dose of W1-mAb with treatment at 0 hrs to either 0.5× or 0.1× was still protective (Table 1). However, these effects were not as pronounced as with the W1-mAb 1× dose.
Table 1.
Summary of the proportion of animals surviving challenge with edema toxin, lethal toxin, or edema and lethal toxins together treated with placebo (0×) or with one of four doses of W1-mAb at the time of infusion start
| Toxin (μg/kg)a | Proportion surviving (%) for each W1-mAb dose
|
||||
|---|---|---|---|---|---|
| 0× | 0.1× | 0.5× | 1× | 10× | |
| Edema toxin (500) | 1 of 12 (8) | 8 of 12 (67)b | Not done | 12 of 12 (100)b | 13 of 13 (100)b |
| Lethal toxin (175) | 7 of 21 (33) | 5 of 15 (33) | 8 of 12 (67)b | 14 of 14 (100)b | 16 of 16 (100)b |
| Edema toxin + lethal toxin (175) | 7 of 22 (32) | 6 of 16 (38) | Not done | 18 of 20 (90)b | 18 of 18 (100)b |
W1-mAb is a chimpanzee-derived monoclonal antibody against protective antigen.
Dose of factor;
p ≤ .01 compared to 0×.
Hemodynamic and Other Laboratory Measurements
For both hemodynamic and other laboratory measurements, the initial number of animals in each group is shown in Figure 1. The number of animals remaining over time for subsequent measures can be determined by the proportional survival curves shown in this figure.
Compared to animals receiving diluent challenge only, ET challenge in animals treated with placebo produced progressive decreases in MBP throughout and increases in HR that returned toward baseline levels later (p < .0001 for the interaction with time for both MBP and HR, Tables 2 and 3). Challenges with LT alone or LT together with ET in animals treated with placebo produced decreases in both MBP and HR that were resolved by 24 hrs (p ≤ .004 for the interaction with time, Tables 2 and 3).
Table 2.
Serial mean (±SEM) arterial blood pressure over 24 hrs of measurement in animals receiving W1-mAb in 1× or 10× molar doses of protective antigen or placebo at the time of (0 hrs) or 6 or 12 hrs after the start of anthrax toxin or diluent infusions
| Challenge | Treatment | Treatment Time (hrs) | Time (hrs) After Initiation of Anthrax Toxin Infusion
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | |||
| Diluent | Placebo | —c | 114 ± 2 | 118 ± 2 | 119 ± 2 | 119 ± 2 | 119 ± 3 | 119 ± 3 | 117 ± 2 | 118 ± 3 | 118 ± 3 | 117 ± 4 | 121 ± 3 | 114 ± 3 | 119 ± 3 |
| ET | Placeboa | 0 | 118 ± 4b | 113 ± 5 | 105 ± 6 | 103 ± 5 | 104 ± 4 | 101 ± 4 | 92 ± 6 | 95 ± 5 | 86 ± 6 | 90 ± 5 | 87 ± 5 | 81 ± 8 | 70 ± 9 |
| 1× | 119 ± 3 | 122 ± 4 | 118 ± 4 | 120 ± 4 | 114 ± 3 | 115 ± 5 | 114 ± 4 | 114 ± 5 | 114 ± 5 | 112 ± 6 | 117 ± 6 | 110 ± 7 | 109 ± 6 | ||
| 10× | 120 ± 4 | 126 ± 3 | 123 ± 3 | 121 ± 4 | 117 ± 4 | 112 ± 3 | 112 ± 4 | 111 ± 3 | 114 ± 3 | 112 ± 3 | 112 ± 4 | 120 ± 6 | 112 ± 5 | ||
| Placeboa | 6 | 115 ± 2 | 115 ± 3 | 107 ± 3 | 104 ± 4 | 105 ± 3 | 103 ± 3 | 105 ± 3 | 93 ± 4 | 91 ± 3 | 86 ± 3 | 86 ± 4 | 79 ± 5 | 76 ± 4 | |
| 1× | 120 ± 4 | 114 ± 4 | 103 ± 4 | 101 ± 3 | 104 ± 3 | 104 ± 4 | 104 ± 4 | 99 ± 4 | 98 ± 3 | 95 ± 2 | 98 ± 3 | 94 ± 3 | 90 ± 3 | ||
| 10× | 112 ± 2 | 124 ± 7 | 109 ± 6 | 98 ± 6 | 97 ± 7 | 94 ± 7 | 94 ± 6 | 96 ± 9 | 98 ± 9 | 95 ± 9 | 95 ± 9 | 94 ± 9 | 93 ± 9 | ||
| Placeboa | 12 | 110 ± 4 | 121 ± 4 | 111 ± 4 | 107 ± 5 | 105 ± 4 | 96 ± 6 | 94 ± 6 | 91 ± 5 | 88 ± 5 | 83 ± 5 | 74 ± 8 | 78 ± 5 | 73 ± 9 | |
| 1× | 113 ± 3 | 109 ± 3 | 101 ± 2 | 105 ± 5 | 100 ± 5 | 108 ± 4 | 105 ± 5 | 100 ± 4 | 96 ± 2 | 95 ± 5 | 98 ± 7 | 94 ± 7 | 94 ± 6 | ||
| 10× | 114 ± 2 | 123 ± 11 | 125 ± 10 | 108 ± 5 | 109 ± 4 | 108 ± 5 | 106 ± 3 | 103 ± 3 | 102 ± 3 | 97 ± 3 | 91 ± 4 | 96 ± 2 | 91 ± 2 | ||
| LT | Placeboa | 0 | 112 ± 3 | 120 ± 5 | 114 ± 4 | 102 ± 5 | 98 ± 7 | 94 ± 8 | 97 ± 10 | 105 ± 10 | 105 ± 7 | 110 ± 6 | 111 ± 7 | 105 ± 3 | 109 ± 5 |
| 1× | 108 ± 4 | 113 ± 5 | 111 ± 4 | 112 ± 4 | 111 ± 5 | 113 ± 5 | 112 ± 4 | 109 ± 5 | 109 ± 4 | 107 ± 4 | 107 ± 5 | 101 ± 4 | 100 ± 5 | ||
| 10× | 112 ± 2 | 118 ± 3 | 114 ± 4 | 113 ± 4 | 108 ± 3 | 108 ± 3 | 105 ± 3 | 106 ± 2 | 109 ± 3 | 115 ± 4 | 111 ± 3 | 110 ± 4 | 111 ± 5 | ||
| Placeboa | 6 | 117 ± 2 | 120 ± 3 | 121 ± 3 | 115 ± 4 | 102 ± 5 | 105 ± 6 | 101 ± 9 | 105 ± 8 | 114 ± 4 | 109 ± 5 | 113 ± 5 | 112 ± 5 | 121 ± 7 | |
| 1× | 114 ± 3 | 121 ± 3 | 117 ± 3 | 112 ± 5 | 104 ± 5 | 108 ± 4 | 110 ± 4 | 109 ± 4 | 111 ± 4 | 113 ± 3 | 112 ± 4 | 111 ± 3 | 106 ± 2 | ||
| 10× | 113 ± 2 | 119 ± 3 | 119 ± 4 | 105 ± 6 | 109 ± 7 | 107 ± 4 | 109 ± 7 | 113 ± 6 | 116 ± 7 | 117 ± 3 | 116 ± 7 | 115 ± 6 | 113 ± 5 | ||
| Placeboa | 12 | 116 ± 2 | 121 ± 3 | 114 ± 3 | 110 ± 2 | 106 ± 3 | 101 ± 5 | 103 ± 5 | 112 ± 4 | 112 ± 4 | 109 ± 4 | 113 ± 4 | 111 ± 4 | 110 ± 5 | |
| 1× | 111 ± 2 | 117 ± 2 | 115 ± 3 | 112 ± 3 | 107 ± 4 | 95 ± 5 | 102 ± 7 | 95 ± 8 | 101 ± 10 | 104 ± 10 | 119 ± 5 | 117 ± 6 | 107 ± 4 | ||
| 10× | 115 ± 4 | 118 ± 3 | 106 ± 4 | 101 ± 6 | 99 ± 6 | 99 ± 6 | 107 ± 5 | 114 ± 6 | 112 ± 4 | 118 ± 6 | 128 ± 4 | 122 ± 14 | 124 ± 6 | ||
| ET + LT | Placeboa | 0 | 114 ± 2 | 117 ± 3 | 113 ± 3 | 100 ± 3 | 93 ± 6 | 90 ± 7 | 95 ± 6 | 102 ± 6 | 110 ± 5 | 107 ± 7 | 109 ± 2 | 107 ± 3 | 106 ± 6 |
| 1× | 122 ± 3 | 116 ± 4 | 118 ± 4 | 119 ± 4 | 116 ± 4 | 115 ± 4 | 115 ± 4 | 118 ± 4 | 115 ± 5 | 111 ± 5 | 119 ± 4 | 120 ± 4 | 115 ± 5 | ||
| 10× | 116 ± 3 | 120 ± 3 | 114 ± 3 | 117 ± 3 | 118 ± 3 | 118 ± 4 | 120 ± 5 | 123 ± 3 | 120 ± 5 | 123 ± 4 | 124 ± 4 | 124 ± 3 | 121 ± 4 | ||
| Placeboa | 6 | 118 ± 2 | 126 ± 2 | 114 ± 3 | 98 ± 3 | 86 ± 4 | 106 ± 4 | 110 ± 3 | 101 ± 6 | 111 ± 3 | 109 ± 2 | 111 ± 4 | 106 ± 4 | 106 ± 3 | |
| 1× | 118 ± 3 | 127 ± 3 | 119 ± 3 | 101 ± 4 | 93 ± 5 | 94 ± 7 | 100 ± 9 | 110 ± 8 | 116 ± 4 | 113 ± 4 | 108 ± 8 | 115 ± 4 | 114 ± 5 | ||
| 10× | 115 ± 5 | 122 ± 3 | 115 ± 3 | 96 ± 7 | 94 ± 9 | 117 ± 3 | 112 ± 5 | 120 ± 5 | 122 ± 6 | 122 ± 7 | 126 ± 6 | 125 ± 10 | 123 ± 9 | ||
| Placeboa | 12 | 115 ± 3 | 124 ± 3 | 117 ± 3 | 96 ± 3 | 89 ± 4 | 91 ± 5 | 99 ± 6 | 114 ± 4 | 114 ± 8 | 107 ± 3 | 103 ± 6 | 109 ± 4 | 101 ± 6 | |
| 1× | 114 ± 3 | 120 ± 2 | 115 ± 2 | 97 ± 5 | 91 ± 6 | 112 ± 7 | 110 ± 7 | 107 ± 8 | 111 ± 5 | 106 ± 6 | 112 ± 6 | 113 ± 7 | 109 ± 7 | ||
| 10× | 116 ± 4 | 125 ± 4 | 114 ± 5 | 100 ± 6 | 101 ± 4 | 105 ± 4 | 102 ± 7 | 96 ± 9 | 101 ± 9 | 98 ± 10 | 105 ± 3 | 108 ± 4 | 101 ± 4 | ||
W1-mAb is a chimpanzee-derived monoclonal antibody against protective antigen.
Compared to diluent challenge, edema toxin with placebo treatment produced progressive decreases in the mean arterial blood pressure throughout (p < .0001 for the interaction with time averaged over placebo groups), while challenges with lethal toxin alone or lethal toxin together with edema toxin produced decreases in mean arterial blood pressure that were resolved by 24 hrs (p ≤ .004 for the interaction with time averaged over respective placebo groups). See Results for a full description of these comparisons;
bold values were employed to compare over similar periods of time (12 hrs) the effects of W1-mAb administered at different time points. The levels of significance for the overall effects of W1-mAb and interaction of this effect and time are shown in Figure 2;
treatment at 0, 6, or 12 hrs (see Material and Methods).
Table 3.
Serial mean (±SEM) heart rate over 24 hrs of measurement in animals receiving W1-mAb in 1× or 10× molar doses of protective antigen or placebo at the time of (0 hrs) or 6 or 12 hrs after the start of anthrax toxin or diluent infusions
| Challenge | Treatment | Treatment Time (hrs) | Time (hrs) After Initiation of Anthrax Toxin Infusion
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | |||
| Diluent | Placebo | —c | 425 ± 8 | 397 ± 6 | 405 ± 8 | 405 ± 7 | 408 ± 8 | 365 ± 10 | 371 ± 9 | 352 ± 9 | 363 ± 7 | 355 ± 9 | 366 ± 10 | 361 ± 73 | 385 ± 73 |
| ET | Placeboa | 0 | 416 ± 13b | 445 ± 16 | 509 ± 18 | 538 ± 18 | 542 ± 17 | 502 ± 16 | 475 ± 29 | 471 ± 12 | 443 ± 19 | 434 ± 24 | 416 ± 27 | 457 ± 18 | 435 ± 25 |
| 1× | 411 ± 14 | 397 ± 12 | 432 ± 14 | 438 ± 20 | 435 ± 17 | 422 ± 17 | 430 ± 21 | 453 ± 16 | 449 ± 21 | 465 ± 17 | 488 ± 15 | 503 ± 12 | 530 ± 15 | ||
| 10× | 438 ± 14 | 408 ± 13 | 423 ± 13 | 415 ± 8 | 395 ± 14 | 376 ± 18 | 368 ± 13 | 360 ± 18 | 366 ± 18 | 378 ± 28 | 367 ± 15 | 393 ± 14 | 398 ± 16 | ||
| Placeboa | 6 | 411 ± 13 | 427 ± 22 | 490 ± 13 | 504 ± 18 | 515 ± 17 | 471 ± 16 | 474 ± 16 | 444 ± 20 | 406 ± 23 | 385 ± 21 | 382 ± 18 | 373 ± 18 | 374 ± 30 | |
| 1× | 440 ± 13 | 458 ± 10 | 509 ± 11 | 543 ± 25 | 546 ± 25 | 494 ± 18 | 511 ± 16 | 500 ± 17 | 509 ± 17 | 500 ± 10 | 509 ± 10 | 511 ± 14 | 503 ± 25 | ||
| 10× | 408 ± 10 | 424 ± 19 | 488 ± 16 | 503 ± 22 | 512 ± 24 | 460 ± 29 | 456 ± 33 | 432 ± 44 | 440 ± 43 | 452 ± 30 | 464 ± 32 | 460 ± 29 | 464 ± 33 | ||
| Placeboa | 12 | 425 ± 10 | 431 ± 13 | 491 ± 13 | 510 ± 13 | 526 ± 19 | 469 ± 24 | 455 ± 26 | 464 ± 20 | 442 ± 28 | 447 ± 30 | 440 ± 31 | 400 ± 37 | 381 ± 34 | |
| 1× | 435 ± 11 | 430 ± 9 | 490 ± 8 | 523 ± 15 | 533 ± 17 | 490 ± 22 | 510 ± 19 | 470 ± 15 | 453 ± 9 | 465 ± 12 | 463 ± 13 | 503 ± 8 | 511 ± 20 | ||
| 10× | 432 ± 17 | 432 ± 17 | 478 ± 20 | 518 ± 14 | 525 ± 8 | 496 ± 16 | 504 ± 22 | 472 ± 8 | 464 ± 17 | 468 ± 14 | 468 ± 12 | 468 ± 12 | 503 ± 17 | ||
| LT | Placeboa | 0 | 408 ± 11 | 367 ± 10 | 415 ± 11 | 351 ± 14 | 305 ± 27 | 278 ± 38 | 287 ± 30 | 320 ± 36 | 367 ± 29 | 330 ± 17 | 340 ± 23 | 380 ± 12 | 383 ± 9 |
| 1× | 425 ± 15 | 348 ± 8 | 392 ± 12 | 396 ± 9 | 399 ± 6 | 340 ± 20 | 335 ± 12 | 338 ± 13 | 334 ± 15 | 338 ± 15 | 350 ± 15 | 357 ± 13 | 386 ± 14 | ||
| 10× | 423 ± 12 | 365 ± 11 | 397 ± 11 | 381 ± 11 | 391 ± 11 | 353 ± 14 | 337 ± 13 | 328 ± 12 | 323 ± 8 | 357 ± 15 | 337 ± 8 | 347 ± 11 | 374 ± 9 | ||
| Placeboa | 6 | 436 ± 8 | 395 ± 9 | 412 ± 11 | 380 ± 11 | 333 ± 17 | 300 ± 24 | 318 ± 24 | 340 ± 18 | 342 ± 16 | 349 ± 17 | 353 ± 13 | 356 ± 22 | 396 ± 19 | |
| 1× | 441 ± 9 | 398 ± 12 | 396 ± 12 | 362 ± 17 | 352 ± 20 | 329 ± 18 | 360 ± 17 | 331 ± 13 | 332 ± 13 | 336 ± 7 | 344 ± 13 | 358 ± 8 | 345 ± 7 | ||
| 10× | 453 ± 11 | 404 ± 12 | 420 ± 15 | 358 ± 24 | 350 ± 31 | 388 ± 10 | 392 ± 19 | 388 ± 30 | 360 ± 13 | 396 ± 12 | 392 ± 10 | 374 ± 16 | 388 ± 5 | ||
| Placeboa | 12 | 415 ± 14 | 400 ± 8 | 403 ± 7 | 367 ± 13 | 358 ± 15 | 313 ± 21 | 348 ± 21 | 351 ± 12 | 339 ± 13 | 338 ± 18 | 352 ± 24 | 335 ± 13 | 362 ± 18 | |
| 1× | 443 ± 11 | 389 ± 8 | 386 ± 8 | 347 ± 11 | 336 ± 21 | 257 ± 28 | 304 ± 26 | 288 ± 21 | 312 ± 5 | 360 ± 28 | 348 ± 17 | 324 ± 13 | 344 ± 18 | ||
| 10× | 460 ± 11 | 402 ± 8 | 413 ± 8 | 360 ± 25 | 338 ± 29 | 290 ± 36 | 440 ± 40 | 355 ± 10 | 345 ± 17 | 370 ± 13 | 370 ± 31 | 410 ± 10 | 400 ± 53 | ||
| ET + LT | Placeboa | 0 | 430 ± 9 | 419 ± 9 | 437 ± 19 | 387 ± 19 | 390 ± 18 | 367 ± 30 | 398 ± 32 | 410 ± 12 | 396 ± 20 | 428 ± 24 | 448 ± 16 | 468 ± 8 | 492 ± 19 |
| 1× | 415 ± 10 | 393 ± 11 | 398 ± 11 | 407 ± 11 | 392 ± 11 | 381 ± 15 | 378 ± 12 | 383 ± 15 | 389 ± 17 | 393 ± 18 | 388 ± 21 | 438 ± 22 | 452 ± 21 | ||
| 10× | 427 ± 11 | 390 ± 9 | 408 ± 7 | 402 ± 7 | 401 ± 7 | 364 ± 8 | 375 ± 11 | 360 ± 10 | 357 ± 12 | 359 ± 7 | 354 ± 10 | 381 ± 11 | 402 ± 12 | ||
| Placeboa | 6 | 420 ± 7 | 411 ± 9 | 443 ± 12 | 392 ± 22 | 396 ± 20 | 426 ± 21 | 480 ± 21 | 438 ± 31 | 473 ± 20 | 480 ± 20 | 473 ± 14 | 498 ± 19 | 493 ± 17 | |
| 1× | 420 ± 11 | 396 ± 10 | 408 ± 9 | 366 ± 23 | 378 ± 26 | 310 ± 33 | 378 ± 23 | 356 ± 12 | 368 ± 13 | 355 ± 17 | 358 ± 15 | 373 ± 21 | 425 ± 27 | ||
| 10× | 432 ± 14 | 431 ± 12 | 468 ± 9 | 393 ± 33 | 400 ± 44 | 407 ± 14 | 400 ± 21 | 407 ± 12 | 428 ± 20 | 440 ± 11 | 396 ± 12 | 452 ± 12 | 410 ± 6 | ||
| Placeboa | 12 | 425 ± 8 | 415 ± 7 | 433 ± 8 | 372 ± 18 | 360 ± 21 | 359 ± 25 | 412 ± 24 | 454 ± 26 | 460 ± 23 | 457 ± 24 | 469 ± 28 | 466 ± 34 | 471 ± 26 | |
| 1× | 408 ± 11 | 397 ± 9 | 423 ± 8 | 387 ± 20 | 359 ± 25 | 364 ± 31 | 373 ± 30 | 420 ± 39 | 417 ± 17 | 414 ± 15 | 432 ± 25 | 477 ± 28 | 440 ± 15 | ||
| 10× | 439 ± 9 | 427 ± 15 | 454 ± 15 | 452 ± 20 | 438 ± 17 | 417 ± 20 | 426 ± 26 | 397 ± 22 | 400 ± 34 | 380 ± 40 | 465 ± 37 | 432 ± 50 | 490 ± 13 | ||
W1-mAb is a chimpanzee-derived monoclonal antibody against protective antigen.
Compared to diluent challenge, edema toxin with placebo produced increases in heart rate that returned toward baseline levels later (p <.0001 for the interaction with time averaged over placebo groups), while challenges with lethal toxin alone or lethal toxin together with edema toxin produced decreases in mean arterial blood pressure that were resolved by 24 hrs (p ≤ .004 for the interaction with time averaged over respective placebo groups). See Results for a full description of these comparisons;
bold values were employed to compare over similar periods of time (12 hrs) the effects of W1-mAb administered at different time points. The levels of significance for the overall effects of W1-mAb and interaction of this effect and time are shown in Figure 3; ctreatment at 0, 6, or 12 hrs (see Material and Methods).
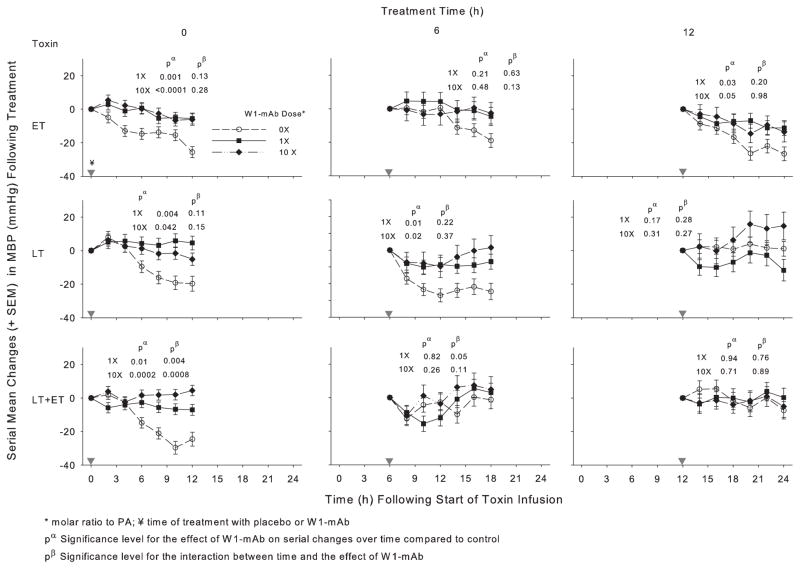
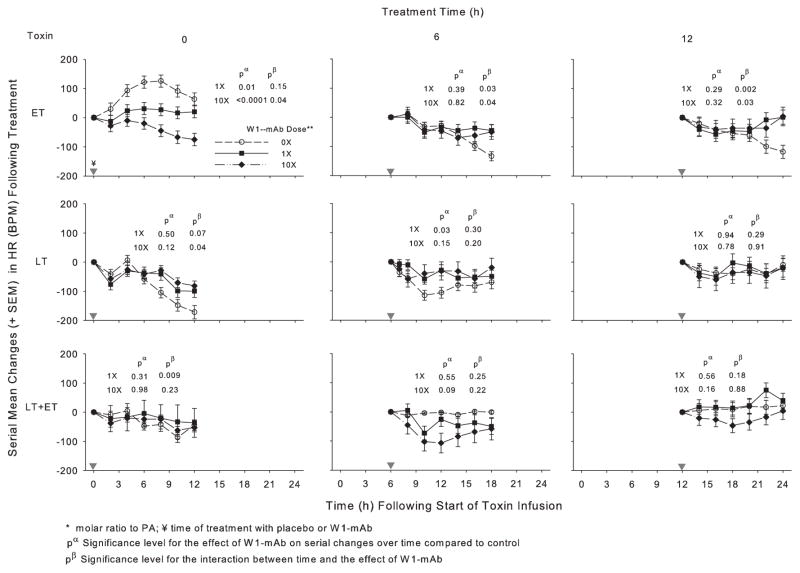
In animals challenged with ET alone, compared to placebo treatment, 1× or 10× doses of W1-mAb treatment at 0, 6, or 12 hrs increased the MBP over subsequent 12-hr periods, and these changes were significant with both the 0- and 12-hr treatments (p ≤ .05) (Fig. 2, Table 2). With ET challenge, 1× or 10× W1-mAb treatment at 0 hrs decreased the HR (p ≤ .01) while treatment at 6 or 12 hrs caused later increases (p ≤ .04 for the interaction with time) (Fig. 3, Table 3). In animals challenged with LT alone, compared to placebo treatment, 1× or 10× W1-mAb treatment at 0 or 6 hrs but not 12 hrs increased the MBP (p ≤ .04). With LT challenge alone, 1× W1-mAb treatment at 6 hrs increased the HR (p = .03). In animals challenged with LT and ET together, compared to placebo, 1× or 10× W1-mAb treatment at 0 hrs but not later times increased the MBP (p ≤ .01); neither dose significantly altered the HR at any time.
Figure 2.
Effect of 1× or 10× doses of a chimpanzee-derived monoclonal antibody against protective antigen (W1-mAb) compared to placebo (0×) on serial changes in mean arterial blood pressure (MBP; mm Hg) over the 12-hr period immediately following treatment at the time of (0 hrs) or 6 or 12 hrs after the start of 24-hr infusions of edema toxin (ET; 500 μg/kg), lethal toxin (LT; 175 μg/kg), or ET and LT together (175 μg/kg each). As in prior studies, these changes were calculated on the basis of MBP measures made immediately before treatment (14). Changes in placebo-treated animals in experiments testing the low or high W1-mAb doses with each toxin did not differ significantly, and these were combined for analysis and presentation for each time point. In each plot, pα and pβ demonstrate the level of significance for the effect of W1-mAb across the time points and for the interaction between these effects and time, respectively. The inverted gray triangles demarcate when treatment was administered. PA, protective antigen.
Figure 3.
Effect of 1× or 10× doses of a chimpanzee-derived monoclonal antibody against protective antigen (W1-mAb) compared to placebo (0×) on serial changes in heart rate [HR; beats per minute (BPM)] over the 12-hr period immediately following treatment at the time of (0 hrs) or 6 or 12 hrs after the start of 24-hr infusions with edema toxin (ET; 500 μg/kg), lethal toxin (LT; 175 μg/kg), or ET and LT together (175 μg/kg each). As in prior studies, these changes were calculated on the basis of HR measures made immediately before treatment (14). Changes in placebo animals in experiments testing the low or high W1-mAb doses with each toxin did not differ significantly, and these were combined for analysis and presentation. In each plot, pα and pβ demonstrate the level of significance for the effect of W1-mAb across the time points and for the interaction between these effects and time, respectively. The inverted gray triangles demarcate when treatment was administered. PA, protective antigen.
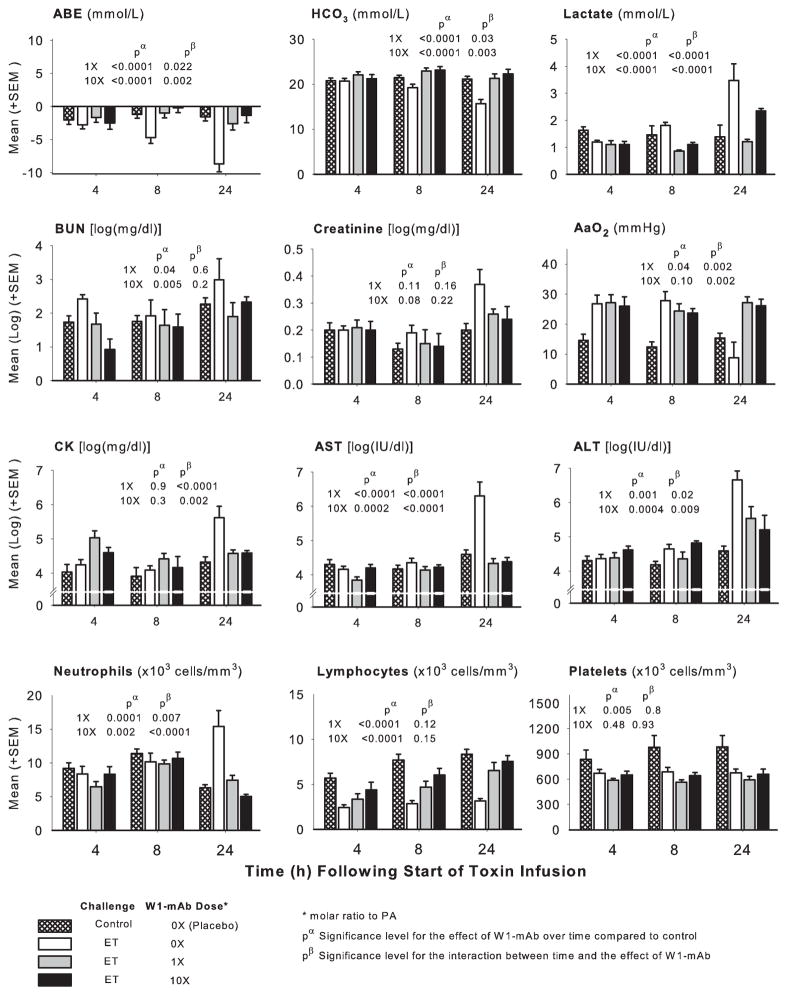
Across measurement times (4, 8, and 24 hrs), compared to diluent challenge, ET challenge alone in animals treated with placebo decreased arterial base excess, bicarbonate, lymphocytes, and platelets and increased lactate, blood urea nitrogen, creatinine, aspartate transaminase, alanine transaminase, creatine phosphokinase, and neutrophils either throughout or in patterns that increased over time (p ≤ .02 for the effect of toxin or for the interaction with time) (Fig. 4). ET had variable effects on the alveolar to arterial oxygen gradient, first increasing and then decreasing it (p = .001 for the interaction with time). In animals challenged with ET alone, compared to treatment with placebo, W1-mAb treatment with either 1× or 10× doses at time 0 hrs increased the pH, arterial base excess, and bicarbonate and decreased lactate, blood urea nitrogen, aspartate transaminase, alanine transaminase, creatine phosphokinase, and neutrophils overall or at later time points (p ≤ .05 for the effect of W1-mAb or for the interaction with time) (Fig. 4). Both W1-mAb doses had variable effects on the alveolar to arterial oxygen gradient, decreasing it at 8 hrs and increasing it at 24 hrs (p= .002 for the interaction with time). Low- but not high-dose W1-mAb decreased platelets (p = .005). Rapid lethality with LT infusion alone or together with ET resulted in small sample sizes and less robust analysis of these laboratory measures. These data are described and presented in Table E1 (see Supplement Digital Content 2, http://links.lww.com/CCM/A237).
Figure 4.
Serial mean (±SEM) arterial base excess (ABE), bicarbonate (HCO3), lactate, blood urea nitrogen (BUN), creatinine, aspartate and alanine transaminases (AST and ALT, respectively), creatine phosphokinase (CK), alveolar to arterial oxygen gradient (AaO2), and circulating neutrophils, lymphocytes, and platelets at 4, 8, and 24 hrs in animals challenged with diluent alone (crosshatched bars) or with 24-hr edema toxin (ET) infusions and treated at the start of ET with placebo (open bars) or a chimpanzee-derived monoclonal antibody against protective antigen (W1-mAb), 1× (gray bars) or 10× (black bars). The pα and pβ values demonstrate the level of significance for the effect of W1-mAb across the time points compared to placebo in ET animals and for the interaction between these effects and time, respectively. PA, protective antigen.
Spore-Challenged Mouse Studies
In animals treated with placebo, subcutaneous challenge with anthrax A35 spores was associated with a 100% mortality rate (0 of 15 animals survived; deaths occurred between 48 and 148 hrs). Treatment with W1-mAb improved survival whether it was administered 24 and 3 hrs before spore infection (10 of 10 animals survived when receiving a 25-μg dose at each time point) or 24 and 48 hrs after spore infection (8 of 10 survived when receiving 25 μg of antibody at each time point, and 5 of 5 animals survived when receiving 100 μg at each time point) (p < .0001 for all compared to placebo).
DISCUSSION
The present investigation provides data supporting the potential effectiveness of W1-mAb as a toxin-directed therapy for anthrax. Compared to high lethality rates in animals challenged with ET and treated with placebo, all animals receiving either the low or high dose of W1-mAb survived even when treatment was delayed for 12 hrs. W1-mAb also improved survival significantly if started 6 hrs after the initiation of LT alone. Finally, W1-mAb improved survival when administered at the start of challenge with LT and ET in combination. Of note, while the dose of the ET employed produced greater overall lethality compared to LT, the time to death with ET alone was significantly longer. In fact, lethality with some LT doses alone or in combination with ET was largely complete by 12 hrs after the initiation of challenge. Therefore, more rapid lethality with LT vs. ET may underlie the greater apparent efficacy that delayed W1-mAb treatment had with ET. It is also possible that LT and ET together had effects that were even more resistant to the protective effects of later W1-mAb treatment than LT alone. Importantly, W1-mAb provided substantial protection in spore-challenged mice, even when treatment was delayed until 24 hrs after the spore challenge.
Improved survival with W1-mAb treatment in the rat model appears related in part to improved hemodynamic function. Increased intracellular cyclic adenosine monophosphate is closely associated with systemic vasodilation and increased cardiac pacemaker cell activity (22, 23). Progressive hypotension and rapid increases in HR after the start of ET challenge in the model are consistent with EF’s strong adenyl cyclase activity (17). However, treatment with W1-mAb as late as 12 hrs increased blood pressure, and early treatment reduced tachycardia. W1-mAb was also associated with reduced acidosis and improved renal and hepatic function with ET.
Different from ET, hypotension with LT is likely related to both peripheral vascular dysfunction and direct myocardial injury (24–28). Inappropriate reductions in HR may also contribute to shock with LT (17, 18). Notably, W1-mAb treatment as late as 6 hrs after the initiation of LT increased blood pressure and the HR. Finally, early treatment with W1-mAb before substantial mortality had occurred increased blood pressure following challenge with both toxins together. As with survival, more rapid mortality may have obscured potential beneficial hemodynamic effects with W1-mAb when administered following LT alone or in combination with ET. It is possible that, during active infection, either with slower increases in LT levels or with concentrations possibly not as great as those present here, the W1-mAb doses employed in rats or higher ones may be effective. Consistent with this, W1-mAb improved survival with spore challenge even when treatment was delayed for 24 hrs.
Effective dosing requiring the least amount of agent will facilitate inclusion of toxin-directed therapy in biodefense stockpiles. In these rat experiments, a dose of W1-mAb equal to a molar equivalent of PA (i.e., 1×) appeared as effective as the 10× dose. In fact, reducing the dose further to 0.1× with ET and 0.5× with LT still provided some protection. Further defining the dose of W1-mAb effective during live bacterial infection is important. It will also be important to determine the optimal storage conditions and shelf-life of such preparations as well as the resources necessary for their administration to large numbers of patients. Ultimately, whether antibody preparations can be effectively administered in mass casualty situations will require careful analysis.
Both PA-directed monoclonal antibody preparations (i.e., Raxi-mAb) and pooled human antiserum (anthrax immunoglobulin) are available for application clinically (29, 30). Although further study is needed, W1-mAb may represent another potential PA-directed agent. At present, only anthrax immunoglobulin has been applied clinically either in several isolated cases or in patients from the recent United Kingdom anthrax outbreak. This combined experience has yet to be reported (5, 30). How monoclonal antibody preparations compare with pooled antiserum has not been tested.
There are limitations to the present investigations. Neither of the models employed included the full range of virulence factors present in clinical anthrax infection (e.g., the polyglutamate capsule that protects vegetative bacterial forms from host clearance). Also, neither model employed the conventional therapies employed in patients. The importance of including such interventions preclinically is emphasized by experience with another PA-directed antibody that was beneficial following spore challenge in the absence but not the presence of antibiotics (29). However, high survival with antibiotics in that study may have masked the benefit of treatment. Thus, to determine whether a toxin-directed treatment improves outcome together with antibiotics, preclinical models should incorporate bacterial challenges producing measurable lethality despite antibiotic doses similar to those employed in seriously ill patients. It is also possible that, during anthrax infection, early hemodynamic support and antibiotic treatment would slow clinical deterioration and increase the effectiveness of delayed toxin-directed treatment. Another limitation in the present study is that central nervous system penetration of W1-mAb was not tested. This would be important to determine on the basis of the poor outcome associated with anthrax infection complicated by meningitis. Finally, with regard to statistical methods, bias may have been introduced into this study since some research assistants were not blinded to the experimental conditions. Also, no adjustments were made for multiple comparisons.
In conclusion, in rats challenged with ET or LT infusions and in mice challenged with live anthrax spores, W1-mAb improved survival whether administered before or after challenge. Chimpanzee immunoglobulins such as W1-mAb are very similar to human immunoglobulins and have been shown to have potentially useful clinical applications (31). This observation and the present findings in rat and mouse models provide a basis for further exploring the usefulness of W1-mAb for the treatment of anthrax.
Acknowledgments
Dr. Altaweel, Dr. Johnson, and Dr. Su received funding from the National Institutes of Health (Bethesda, MD). Dr. Moayeri and Dr. Leppla have a pending patent from the U.S. Government.
Supported, in part, by the Critical Care Department, Clinical Center, National Institutes of Health (Bethesda, MD).
We thank Ling Huang, Steve Burke, and Valentina Ciccarone from MacroGenics (Rockville, MD) for production of the W1 immunoglobulin employed in these studies.
Footnotes
Presented, in part, at the 2007 American Thoracic Society meeting, San Francisco, CA, May 18–23, 2007.
The remaining authors have not disclosed any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ccmjournal.com).
For information regarding this article, peichacker@mail.cc.nih.gov
References
- 1.Barakat LA, Quentzel HL, Jernigan JA, et al. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA. 2002;287:863– 868. doi: 10.1001/jama.287.7.863. [DOI] [PubMed] [Google Scholar]
- 2.Holty JE, Bravata DM, Liu H, et al. Systematic review: A century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 3.Jernigan JA, Stephens DS, Ashford DA, et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klempner MS, Talbot EA, Lee SI, et al. Case records of the Massachusetts General Hospital. Case 25-2010 A 24-year-old woman with abdominal pain and shock. N Engl J Med. 2010;363:766–777. doi: 10.1056/NEJMcpc1003887. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay CN, Stirling A, Smith J, et al. An outbreak of infection with Bacillus anthracis in injecting drug users in Scotland. Euro Surveill. 2010;15:19465. doi: 10.2807/ese.15.02.19465-en. pii. [DOI] [PubMed] [Google Scholar]
- 6.Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott JL, Mogridge J, Collier RJ. A quantitative study of the interactions of Bacillus anthracis edema factor and lethal factor with activated protective antigen. Biochemistry. 2000;39:6706– 6713. doi: 10.1021/bi000310u. [DOI] [PubMed] [Google Scholar]
- 8.Mogridge J, Cunningham K, Collier RJ. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002;41:1079–1082. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
- 9.Duesbery NS, Vande Woude GF. Anthrax lethal factor causes proteolytic inactivation of mitogen-activated protein kinase kinase. J Appl Microbiol. 1999;87:289–293. doi: 10.1046/j.1365-2672.1999.00892.x. [DOI] [PubMed] [Google Scholar]
- 10.Ho DT, Bardwell AJ, Abdollahi M, et al. A docking site in MKK4 mediates high affinity binding to JNK MAPKs and competes with similar docking sites in JNK substrates. J Biol Chem. 2003;278:32662–32672. doi: 10.1074/jbc.M304229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rainey GJ, Young JA. Antitoxins: Novel strategies to target agents of bioterrorism. Nat Rev Microbiol. 2004;2:721–726. doi: 10.1038/nrmicro977. [DOI] [PubMed] [Google Scholar]
- 12.Vitale G, Pellizzari R, Recchi C, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 13.Leppla SH. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Li Y, Moayeri M, et al. Late treatment with a protective antigen-directed monoclonal antibody improves hemodynamic function and survival in a lethal toxin-infused rat model of anthrax sepsis. J Infect Dis. 2005;191:422–434. doi: 10.1086/427189. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney DA, Cui X, Solomon SB, et al. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J Infect Dis. 2010;202:1885–1896. doi: 10.1086/657408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Moayeri M, Crown D, et al. Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody. Infect Immun. 2009;77:3902–3908. doi: 10.1128/IAI.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X, Li Y, Li X, et al. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis. 2007;195:572–580. doi: 10.1086/510856. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, Moayeri M, Li Y, et al. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R699–R709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Sa Q, Koehler TM, et al. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell Microbiol. 2006;8:1634–1642. doi: 10.1111/j.1462-5822.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 20.Pomerantsev AP, Sitaraman R, Galloway CR, et al. Genome engineering in Bacillus anthracis using Cre recombinase. Infect Immun. 2006;74:682– 693. doi: 10.1128/IAI.74.1.682-693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Moayeri M, Zhou YH, et al. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J Infect Dis. 2006;193:625– 633. doi: 10.1086/500148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 23.Murray KJ. Cyclic AMP and mechanisms of vasodilation. Pharmacol Ther. 1990;47:329–345. doi: 10.1016/0163-7258(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 24.Lips DJ, Bueno OF, Wilkins BJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 25.Moayeri M, Crown D, Dorward DW, et al. The heart is an early target of anthrax lethal toxin in mice: A protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;5:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell NH, Wilkins BJ, York A, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warfel JM, Steele AD, D’Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol. 2005;166:1871–1881. doi: 10.1016/S0002-9440(10)62496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi O, Watanabe T, Nishida K, et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migone TS, Subramanian GM, Zhong J, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JJ, Pesik N, Quinn CP, et al. A case of naturally acquired inhalation anthrax: Clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect Dis. 2007;44:968–971. doi: 10.1086/512372. [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich PH, Moustafa ZA, Harfeldt KE, et al. Potential of primate monoclonal antibodies to substitute for human antibodies: Nucleotide sequence of chimpanzee Fab fragments. Hum Antibodies Hybridomas. 1990;1:23–26. [PubMed] [Google Scholar]