Abstract
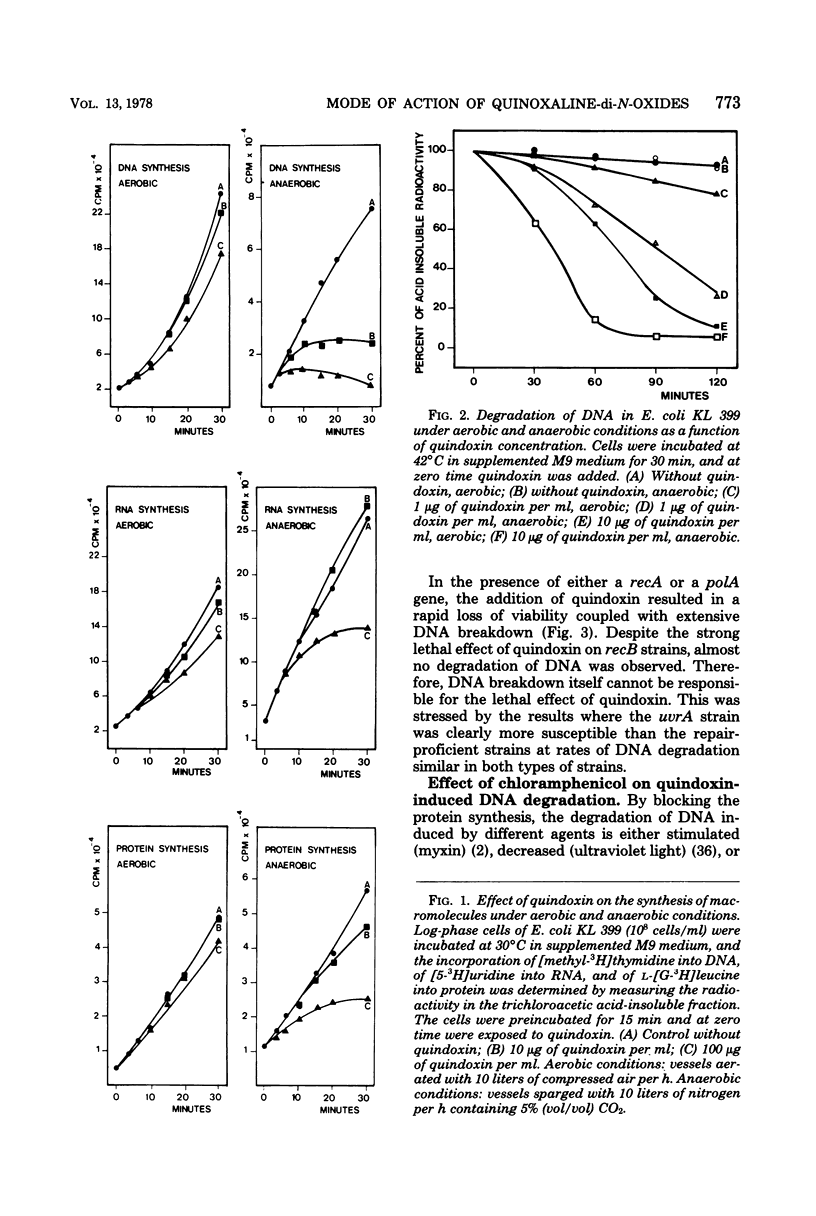
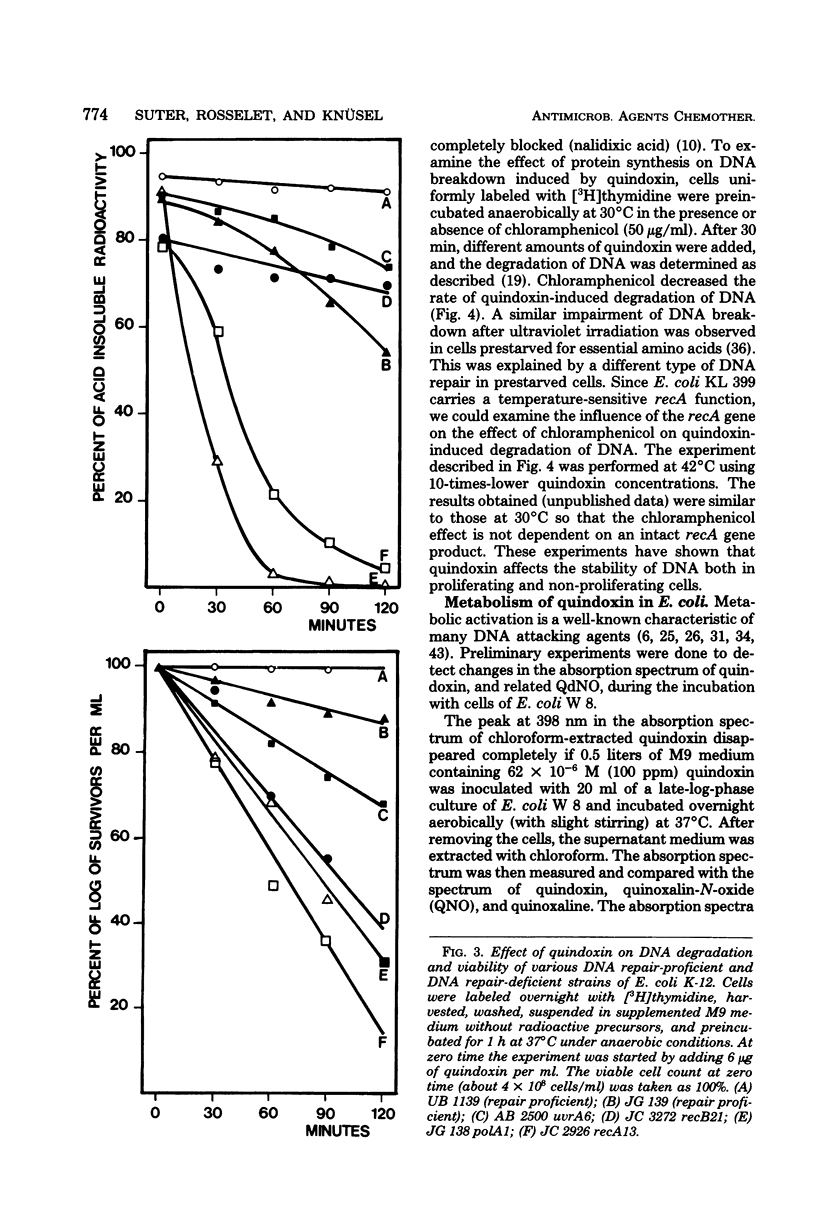
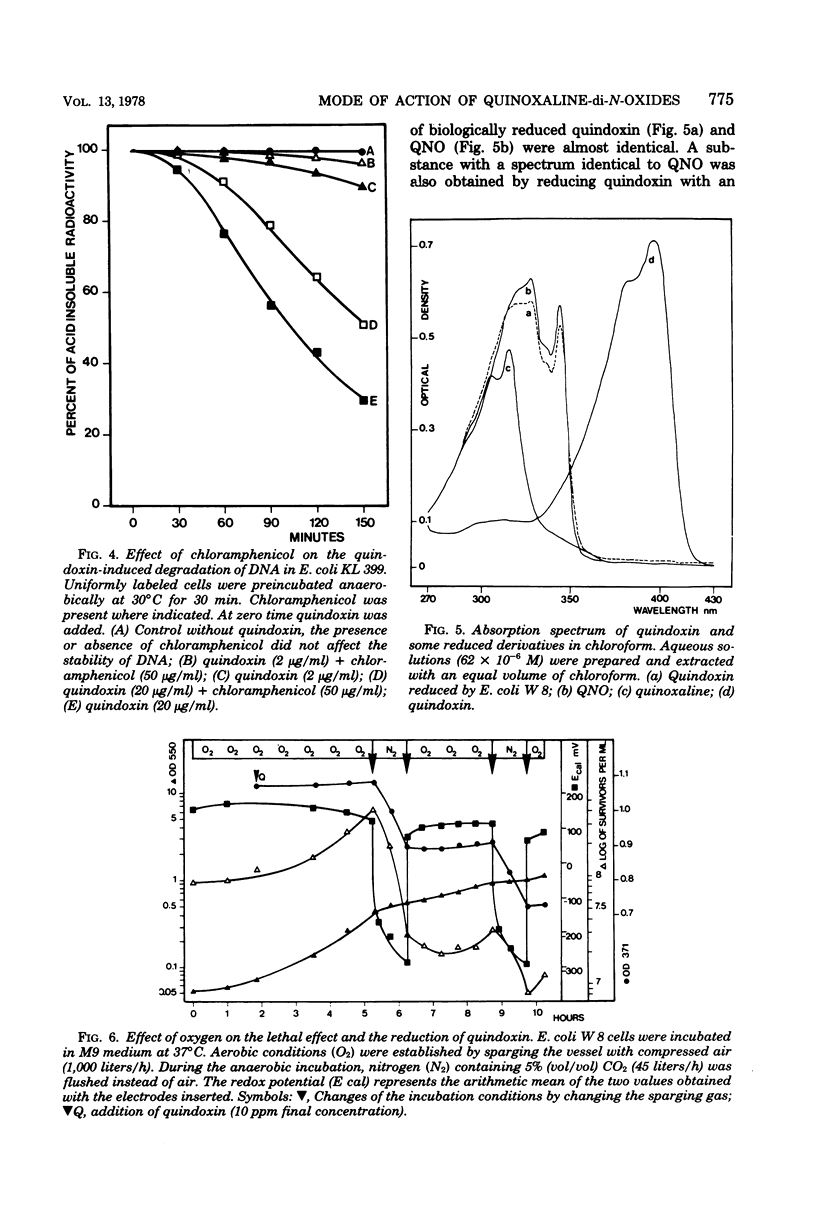
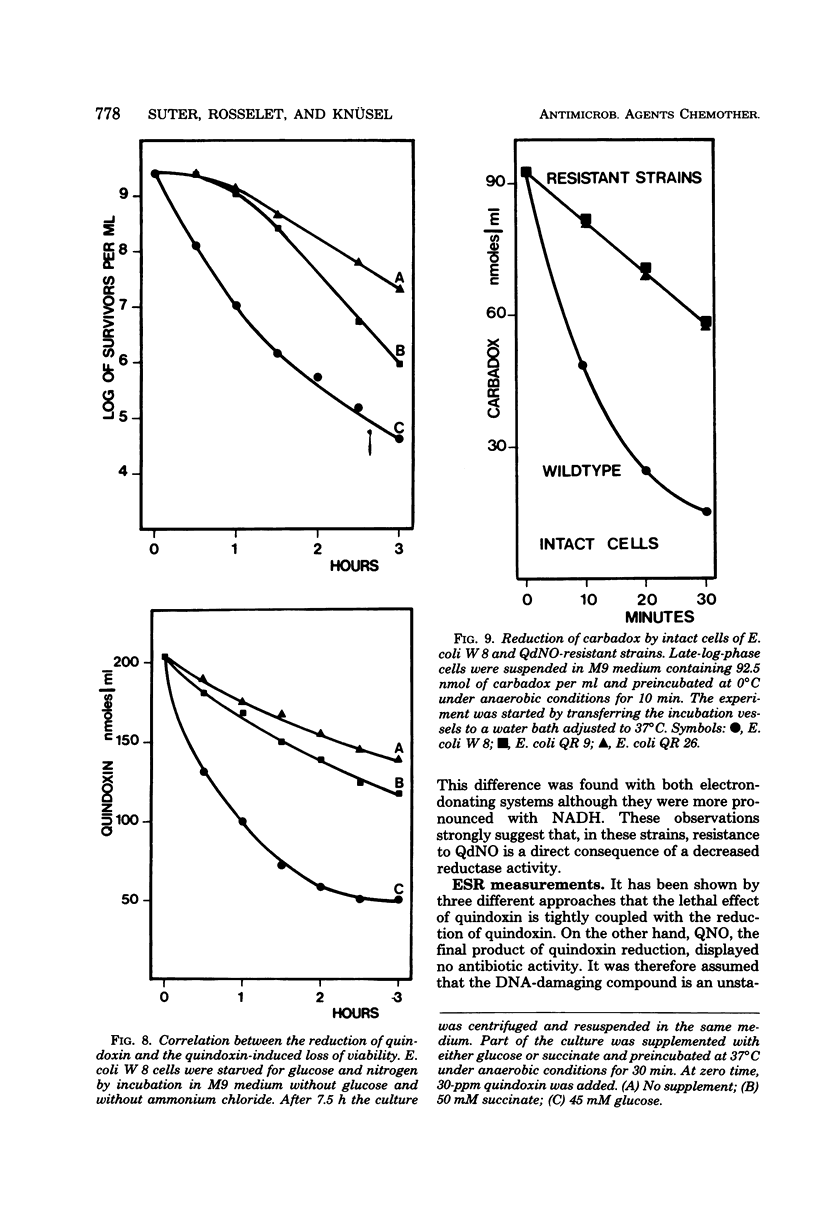
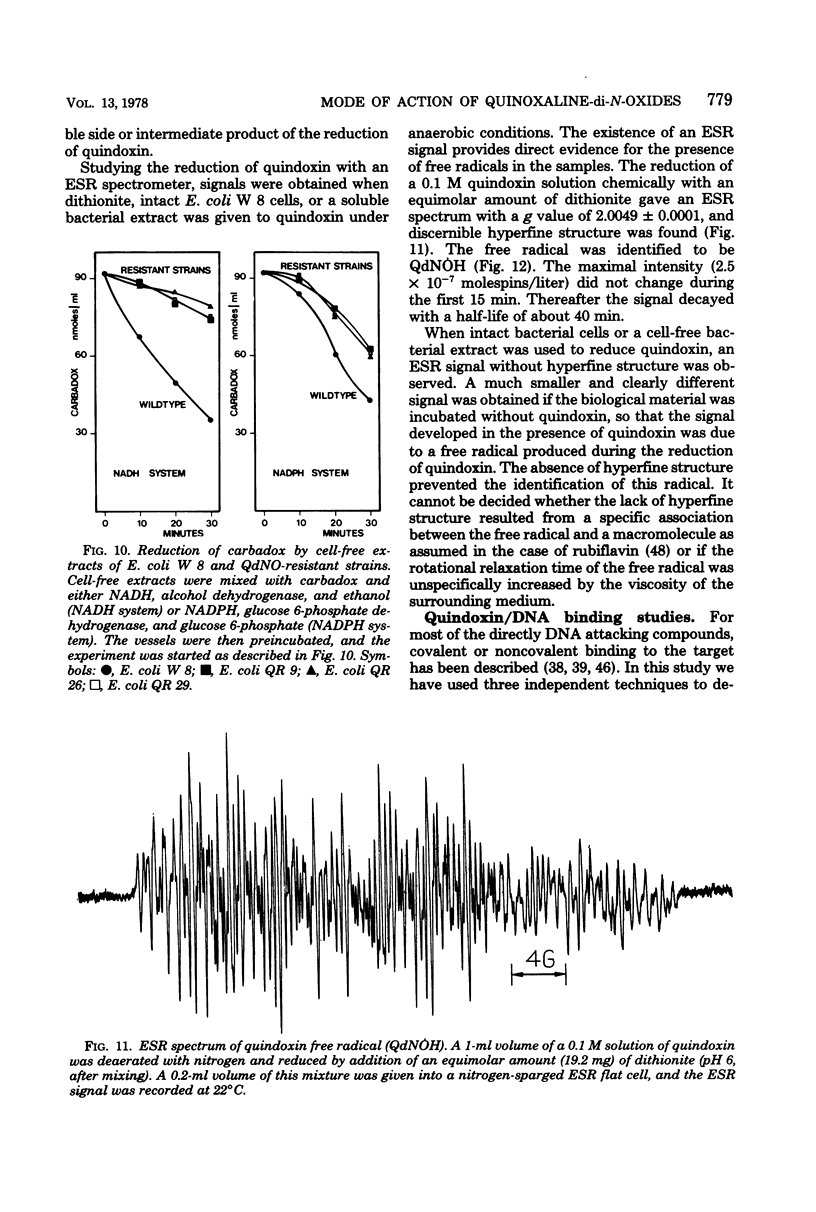
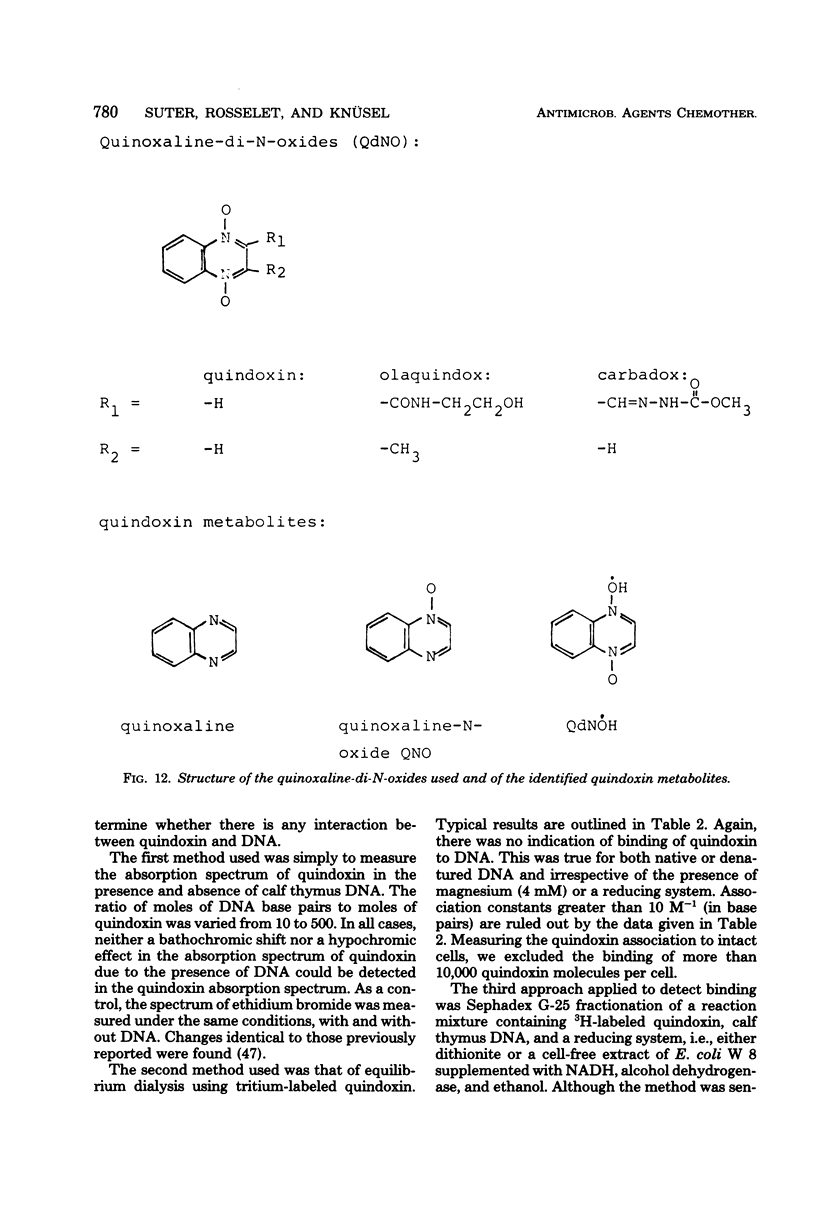
The effect of quindoxin on the synthesis of deoxyribonucleic acid (DNA), ribonucleic acid, and protein in Escherichia coli KL 399 was examined under aerobic and anaerobic conditions. In the absence of oxygen the synthesis of DNA was completely inhibited by 10 ppm of quindoxin, whereas the syntheses of ribonucleic acid and protein were not affected. Quinoxalin-di-N-oxides (QdNO) induce degradation of DNA in both proliferating and non-proliferating cells. polA, recA, recB, recC, exrA, and uvrA mutants were more susceptible than the corresponding repair-proficient strains. All strains were more resistant in the presence of oxygen. Quindoxin was reduced to quinoxalin-N-oxide by intact E. coli cells or by a cell-free E. coli extract. Electron spin resonance measurements demonstrated the generation of free radicals during the reduction of quindoxin. Oxygen or deficiency of energy sources impaired the antibiotic activity and the reduction of QdNO. The QdNO reductase activity was demonstrated to be lower in QdNO-resistant mutants than in the susceptible parent strain. Based on these results it is concluded that an intermediate of reduction, probably a free radical, is responsible for the lethal effect of quindoxin. With three independent techniques no evidence has been found for binding of quindoxin to DNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behki R. M., Lesley S. M. Deoxyribonucleic acid degradation and the lethal effect by myxin in Escherichia coli. J Bacteriol. 1972 Jan;109(1):250–261. doi: 10.1128/jb.109.1.250-261.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome A. W., Bowie R. A. The growth promoting activity of quinoxaline-I, 4-dioxide (quindoxin) in young chicken (Gallus domesticus). Res Vet Sci. 1972 Jul;13(4):330–333. [PubMed] [Google Scholar]
- CONSTANTOPOULOS G., TCHEN T. T. ENHANCEMENT OF MITOMYCIN C-INDUCED BREAKDOWN OF DNA BY INHIBITORS OF PROTEIN SYNTHESIS. Biochim Biophys Acta. 1964 Mar 23;80:456–462. doi: 10.1016/0926-6550(64)90148-3. [DOI] [PubMed] [Google Scholar]
- COULTHARD C. E., HALE L. J. The treatment of experimental bacillary infections of mice with quinoxaline 1:4 di-N-oxide. Br J Pharmacol Chemother. 1955 Sep;10(3):394–396. doi: 10.1111/j.1476-5381.1955.tb00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R., Hasan S. K., Lown J. W., Morgan A. R. The mechanism of the degradation of DNA by streptonigrin. Can J Biochem. 1976 Mar;54(3):219–223. doi: 10.1139/o76-034. [DOI] [PubMed] [Google Scholar]
- Cook T. M., Deitz W. H., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. IV. Effects on the stability of cellular constituents. J Bacteriol. 1966 Feb;91(2):774–779. doi: 10.1128/jb.91.2.774-779.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMERSON P. T., HOWARD-FLANDERS P. POST-IRRADIATION DEGRADATION OF DNA FOLLOWING EXPOSURE OF UV-SENSITIVE AND RESISTANT BACTERIA TO X-RAYS. Biochem Biophys Res Commun. 1965 Jan 4;18:24–29. doi: 10.1016/0006-291x(65)90876-4. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T., McAthey P., Strike P. Some properties of mutants of Escherichia coli K12 defective in DNA polymerase I and exonuclease V. Mol Gen Genet. 1974 Apr 9;130(1):29–38. doi: 10.1007/BF00270516. [DOI] [PubMed] [Google Scholar]
- English A. R., Dunegan C. M. Quinoxaline-1, 4-di-N oxides. I. Inhibition of deoxyribonucleic acid synthesis in Escherichia coli by 2,3-dihydroxymethyl-quinoxaline-1, 4-di-N-oxide. Proc Soc Exp Biol Med. 1970 Feb;133(2):398–400. doi: 10.3181/00379727-133-34481. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Donch J., Greenberg J. Effect of inhibitors of DNA synthesis of UV-sensitive derivatives of Escherichia coli strain K-12. Mutat Res. 1970 Feb;9(2):149–154. doi: 10.1016/0027-5107(70)90053-9. [DOI] [PubMed] [Google Scholar]
- HURST E. W., LANDQUIST J. K., MELVIN P., PETERS J. M., SENIOR N., SILK J. A., STACY G. J. The therapy of experimental psittacosis and lymphogranuloma venereum (inguinale) II. The activity of quinoxaline-1:4-dioxide and substituted and related compounds, with a note on the morphological changes induced in lymphogranuloma virus by these compounds and by antibiotics. Br J Pharmacol Chemother. 1953 Sep;8(3):297–305. doi: 10.1111/j.1476-5381.1953.tb00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. D., Howard-Flanders P. Temperature-sensitive recA mutant of Escherichia coli K-12: deoxyribonucleic acid metabolism after ultraviolet irradiation. J Bacteriol. 1975 Mar;121(3):892–900. doi: 10.1128/jb.121.3.892-900.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C. Repair of genetic material in living cells. Endeavour. 1972 May;31(113):83–87. [PubMed] [Google Scholar]
- Hennessey T. D., Edwards J. R. Antibacterial properties of quindoxin: a new growth-promoting agent. Vet Rec. 1972 Feb 12;90(7):187–191. doi: 10.1136/vr.90.7.187. [DOI] [PubMed] [Google Scholar]
- Hollstein U., Butler P. L. Inhibition of ribonucleic acid synthesis by myxin. Biochemistry. 1972 Apr 11;11(8):1345–1350. doi: 10.1021/bi00758a003. [DOI] [PubMed] [Google Scholar]
- Ishida R., Takahashi T. Increased DNA chain breakage by combined action of bleomycin and superoxide radical. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1432–1438. doi: 10.1016/0006-291x(75)90519-7. [DOI] [PubMed] [Google Scholar]
- JONES W. R., LANDQUIST J. K., STEWART G. T. Synthetic amoebicides. II. The anti-amoebic action of quinoxaline-1:4-dioxide and some derivatives. Br J Pharmacol Chemother. 1953 Sep;8(3):286–289. doi: 10.1111/j.1476-5381.1953.tb00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. E., Horn G. Kontinuierliche Registrierung physikochemischer Messgrössen in Bakterienkulturen. II. Der Einfluss von Gelöstsauerstoff auf Redoxpotentialmessungen in Bakterienkulturen. Z Allg Mikrobiol. 1965;5(1):33–41. doi: 10.1002/jobm.3630050105. [DOI] [PubMed] [Google Scholar]
- Jenkins S. T., Bennett P. M. Effect of mutations in deoxyribonucleic acid repair pathways on the sensitivity of Escherichia coli K-12 strains to nitrofurantoin. J Bacteriol. 1976 Mar;125(3):1214–1216. doi: 10.1128/jb.125.3.1214-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lesley S. M., Behki R. M. Induction of phage formation in lysogenic Escherichia coli by myxin. J Gen Microbiol. 1972 Jun;71(1):195–197. doi: 10.1099/00221287-71-1-195. [DOI] [PubMed] [Google Scholar]
- Lesley S. M., Behki R. M. Mode of action of myxin on Escherichia coli. J Bacteriol. 1967 Dec;94(6):1837–1845. doi: 10.1128/jb.94.6.1837-1845.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Low B., Godson G. N., Birge E. A. Isolation and characterization of an Escherichia coli K-12 mutant with a temperature-sensitive recA- phenotype. J Bacteriol. 1974 Oct;120(1):407–415. doi: 10.1128/jb.120.1.407-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCalla D. R., Reuvers A., Kaiser C. Mode of action of nitrofurazone. J Bacteriol. 1970 Dec;104(3):1126–1134. doi: 10.1128/jb.104.3.1126-1134.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N. S., Gilboe D. P. Binding of streptonigrin to DNA. Biochim Biophys Acta. 1970 Dec 14;224(2):319–327. doi: 10.1016/0005-2787(70)90565-4. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Yamazaki Z., Breter H. J., Zahn R. K. Action of bleomycin on DNA and RNA. Eur J Biochem. 1972 Dec 18;31(3):518–525. doi: 10.1111/j.1432-1033.1972.tb02560.x. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- PARKER C. A. Anaerobiosis with iron wool. Aust J Exp Biol Med Sci. 1955 Feb;33(1):33–37. doi: 10.1038/icb.1955.4. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. The mechanism of DNA breakage by phleomycin in vitro. Nucleic Acids Res. 1976 Apr;3(4):891–901. doi: 10.1093/nar/3.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava B. S. Letter: The yield of single-strand breaks in the DNA of bacteriophage lambda irradiated extra- and intracellularly with gamma-rays in oxic and anoxic conditions. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Oct;26(4):391–394. doi: 10.1080/09553007414551371. [DOI] [PubMed] [Google Scholar]
- Terawaki A., Greenberg J. Post-treatment breakage of mitomycin C induced cross-links in deoxyribonucleic acid of Escherichia coli. Biochim Biophys Acta. 1966 Jun 22;119(3):540–546. doi: 10.1016/0005-2787(66)90130-4. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Mercado C. M., Olson J., Chatterjie N. The mode of interaction of mitomycin C with deoxyribonucleic acid and other polynucleotides in vitro. Biochemistry. 1974 Nov 19;13(24):4878–4887. doi: 10.1021/bi00721a002. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- White H. L., White J. R. Lethal action and metabolic effects of streptonigrin on Escherichia coli. Mol Pharmacol. 1968 Nov;4(6):549–565. [PubMed] [Google Scholar]
- White J. R., Dearman H. H. Generation of free radicals from phenazine methosulfate, streptonigrin, and riboflavin in bacterial suspensions. Proc Natl Acad Sci U S A. 1965 Sep;54(3):887–891. doi: 10.1073/pnas.54.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle D. Today's Job Market, and Tomorrow's. Science. 1964 Aug 14;145(3633):665–665. doi: 10.1126/science.145.3633.665. [DOI] [PubMed] [Google Scholar]