Abstract
Karenia brevis, the harmful alga associated with red tide, produces brevetoxins (PbTxs). Exposure to these toxins can have a negative impact on marine wildlife and serious human health consequences. The elimination of PbTxs is critical to protect the marine environment and human health. TiO2 photocatalysis under 350 nm and solar irradiation leads to significant degradation of PbTxs via first order kinetics. ELISA results demonstrate TiO2 photocatalysis leads to a significant decrease in the bioactivity of PbTxs as a function of treatment time. Experiments conducted in the presence of synthetic seawater, humic material and a hydroxyl scavenger showed decreased degradation. PbTxs are highly hydrophobic and partition to organic microlayer on the ocean surface. Acetonitrile was employed to probe the influence of an organic media on the TiO2 photocatalysis of PbTxs. Our results indicate TiO2 photocatalysis may be applicable for the degradation of PbTxs.
Keywords: TiO2 photocatalysis, brevetoxin, hydroxyl radical, advanced oxidation, photosensitization, singlet oxygen
1. Introduction
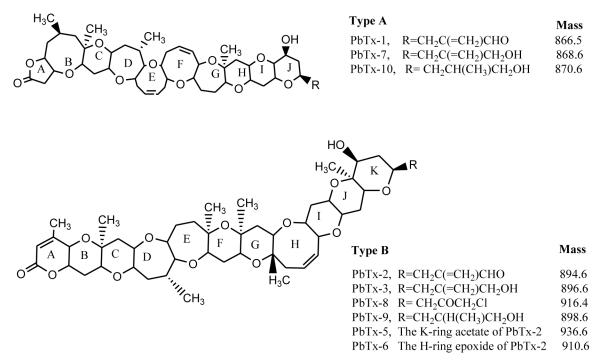
Algae are unicellular plants found throughout aquatic environments. Although algae are vitally important for aquatic ecosystems, unfortunately some species synthesize potent toxins, which can have a devastating impact on marine wildlife and coastal species of fish, birds, mammals, etc [Hallegraeff et al., 1995]. Karenia Brevis is a marine alga prevalent in the Gulf of Mexico. Under specific conditions of temperature and salinity, the K. brevis cells bloom rapidly reaching cell counts in excess of 106 cells/L [Backer et al., 2005; Raloff, 2005]. The phenomenon is referred to as harmful algal bloom (HAB). The fragile cells are ruptured by waves and release photosynthetic colored pigments in water leading to water discoloration, commonly referred to as red tide. K. brevis synthesize brevetoxins (PbTxs), a suite of complex polyethers with molecular weights around 900 Da (Fig. 1) [Fleming et al., 2005].
Figure 1.
General structures of type-A and B brevetoxins
PbTxs are grouped as type A and B based on their backbone structures. PbTx-1 (A-type) and PbTx-2 (B-type) are considered the parent algal toxins from which others are derived [Abraham et al., 2006]. Although found as a major constituent of bloom waters, PbTx-2 is often converted to PbTx-3 and other brevetoxin metabolites [Radwan et al., 2006]. Shellfish (clams, mussels, crabs, shrimp) feed on phytoplankton containing elevated numbers of K. brevis cells during HABs. Under such conditions, bioaccumulation of PbtTxs occurs without apparent ill effects on the shellfish and thus the PbTxs are passed further up the food chain. In higher organisms, PbTxs act as neurotoxins by interfering with voltage-sensitive Na+ channels in cellular membranes, leading to excessive influx of Na+ ions and cell death [Gawley et. al, 1992; Baden et. al, 2003; LePage et. al, 2003]. K. brevis blooms have been responsible for massive mortalities of waterfowl, fish (~ 100 tons per day), and other marine life [Baden et al., 1979; Tester et al., 1992]. In humans, ingestion of PbTx contaminated shellfish causes a temporary illness called “neurotoxic shellfish poisoning” (NSP), characterized by numbness in tongue, lips, throat, disorientation, etc [LePage et al., 2003]. Exposure to brevetoxin containing aerosol causes eye and respiratory irritation [Hua et. al, 1999, LePage et. al, 2003]. PbTxs induce toxicity at nanomolar concentrations.
Red tides regularly threaten living marine animals, result in closures of commercial harvesting of fish and shellfish, and have a negative impact on recreational activities and tourism. The state of Florida imposes a regulatory action limit at ≤ 5000 K. brevis cells/L for harvesting and shellfish collection [Abraham et al., 2006; Tester et al., 1992; Raloff, 2005]. Red tides are often large and occur in open marine waters; hence the options for water treatment are limited and represent a unique challenge. The marine waters during a red tide event are heavily loaded with biomaterials, pigments, humics and dissolved organic materials, such that the associated toxins represent only a small fraction of the organic material present. Under such conditions, traditional adsorption and oxidation water treatments are not effective or economically feasible.
Innovative photochemical and advanced oxidation processes (AOPs) have received tremendous attention for the treatment of a variety of toxins including those from HABs [Song et al., 2006; Antoniou et al., 2008; Osugi et al., 2008]. AOPs involve the generation of reactive oxygen species, primarily hydroxyl radicals that can oxidize a wide variety of organic compounds [Glaze et al., 1987; Fox et al., 1993; Kamat, 1993]. TiO2 photocatalysis, an AOP, is particularly attractive for such an application because TiO2 is environmentally safe, inexpensive, and readily available. TiO2 is a semi-conductor with the band-gap of 3.2 eV. Upon irradiation of TiO2 with photons (≤ 385 nm), excitation of a valence band electron to the conduction band can be achieved, generating an electron/hole (e−/h+) pair (eq. 1). The e−/h+ pair leads to the formation of hydroxyl radicals and superoxide anion radicals, (eqs. 2 and 3). Hydroxyl radicals can lead to the mineralization of a variety of organic pollutants (eq. 4). The oxidation of organic compounds can also occur via h+ directly as an alternative degradation pathway [Fox et al., 1993].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
TiO2 photocatalysis has been used for the destruction of biological toxins, organic impurities, oxidizable inorganic species, and inactivation of pathogenic microorganism from drinking and waste water [Pichat et al., 2000; Ryu et al., 2004; Ferguson et al., 2005; Zhou et al., 2007]. We conducted TiO2 photocatalytic studies on PbTxs under a variety of conditions, including under solar irradiation, UV (350 nm) irradiation, in deionized water, acetonitrile, synthetic seawater, and in the presence of humic substances. The mechanism of TiO2 photocatalytic degradation of PbTxs was investigated using t-butyl alcohol as a hydroxyl radical scavenger. Brevetoxins have limited availability and thus PbTx-2 and PbTx-3 (both type B brevetoxins) were used interchangeably and will be referred to as PbTx for the purposes of this paper. We report herein PbTx is degraded by TiO2 photocatalysis under a variety of conditions, including under solar irradiation, in acetonitrile, and in synthetic seawater. Hydroxyl radical appears to play a major role in oxidative degradation. Our results suggest solar initiated TiO2 photocatalysis may have limited applicability for the degradation of PbTxs in marine estuaries.
2. Materials and Methods
2.1 Materials
PbTx-2 and PbTx-3 were provided by Center for Marine Science, University of North Carolina and by ARCH culture facility (director: Professor Kathleen Rein) at Florida International University. Titanium dioxide powder (Degussa P-25), a mixture of 80 % anatase and 20 % rutile, with an average surface area of 50 m2/g was used as a photocatayst. HPLC grade methanol, acetonitrile, tert-butanol, and formic acid were purchased from Fisher Scientific and were used as received. The humic acid was purchased from Fluka. Aqueous based experiments were conducted in Milli-Q water. The seawater based experiments were conducted in artificial seawater.
2.2. Sample preparation
Aqueous or organic solutions of desired brevetoxins containing 50 mg TiO2 /L solvent were prepared in polypropylene tubes and solution agitated using an ultrasonic cleaning bath for 15 minutes to achieve homogeneous dispersion of catalyst. An aliquot was collected before irradiation, and the solution was transferred to a 30 mL borosilicate glass test tube (15×1.5 cm; wavelength cut-off ~ 280 nm) for irradiation. The solution was gently purged with air before and during irradiation unless indicated otherwise.
2.3. Irradiation
A Rayonet photochemical reactor (Model RPR 100, manufactured by Southern New England Ultraviolet Company, Brandford, CT) which can hold up to 16 lamps was used for our experiments. The reactor was equipped with phosphor coated-low pressuremercury lamps (RPR 3500 Å) that emit in the UVA region between 310-390 nm with the maximal output at 350 nm. The spectral distribution graphs of irradiance for these UV lamps are available from the company website www.rayonet.org/spectral-graphs.htm. In these studies, two and sixteen lamps were employed for experiments in DI water and acetonitrile solvents, respectively. The solar experiments were conducted under direct sunlight from 12:00-14:00 in clear days of March, 2006 (Miami, FL). The intensity of artificial as well as sunlight was measured using Ocean Optics Spectrophotometer (USB 2000). The intensity was 230 μ Watt/cm2 for two lamps, 990 μ Watt/cm2 for 16 lamps, and 190 μ Watt/cm2 for sunlight (measured between 300-400 nm).
2.4. Sample workup
After irradiation at given time intervals, aliquots (0.5 mL) were collected and transferred to 2 mL polypropylene centrifuge tubes. To extract adsorbed PbTxs from the catalyst surface, the 50:50 v/v methanol was added (recovery > 85%). The mixture was subjected to centrifugation (3500 rpm) for 15 min to separate TiO2 from the solution. The supernatant was carefully withdrawn for HPLC analyses, and appropriately diluted for determination of biological activity.
2.5. Analysis
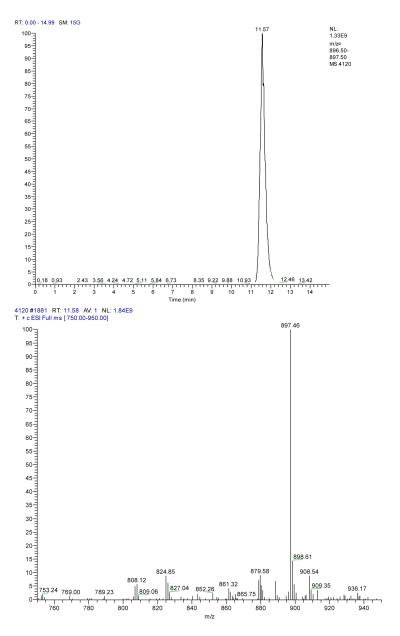
The analyses of PbTx samples were conducted using the high-performance liquid chromatograph (HPLC), Beckman Coutler System Gold with Binary Pump 126, Autosampler 507, and PDA Detector 168. The separation was carried out using an ODS2 C-18 reverse-phase column (250 mm × 4.6 mm) and PbTx monitored at 215 nm. The eluent was a mixture of methanol and water (85 % MeOH: 15 % H2O) at a flow rate of 1 mL/min. The LCQ Deca Finnigan’s Liquid Chromatograph, equipped with mass spectrometer was also employed for further analyses. The mass spectrometer was tuned with diluted standard solution (10 μg/mL) using flow injection at 15 μL/min. The ionspray voltage was set at 4.5 kV and capillary temperature at 275 °C. The solvents used were acetonitrile (solution A) and 0.1% formic acid in water (solution B) run isocratically in proportion of 85% A and 15% B at the flow of 0.5 mL/min. The mass spectrometer LC-MS was controlled by Xcalibur software (version 2), also used for data acquisition and processing. The separation was carried out using an ODS2 C-18 reverse-phase column (250 mm × 4.6 mm). The electropherogram and product ion scan spectrum of PbTx-3 are shown in Fig. 2. The mass to charge ratio of PbTx-3 molecular ion peak is 897. ELISA measurements were performed using a method described by Naar et al. (2002).
Figure 2.
LC-MS-ESI+ spectra of PbTx-3. Mobile phase: 85 % acetonitrile and 15% aqueous solution containing 0.1% TFA at 0.5 mL/min using C-18 column.
3. Results and discussion
3.1. TiO2 photocatalytic degradation of PbTxs
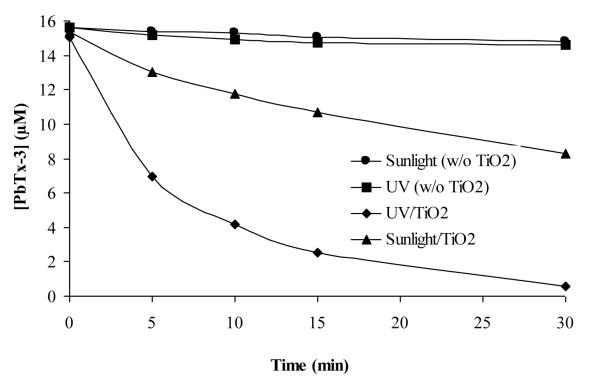
TiO2 photocatalysis experiments were conducted on aqueous solutions of PbTx-3 (16 μM) under UV (350 nm) irradiation. The concentration of PbTx-3 was monitored by HPLC-PDA as a function of irradiation time. The initial rate of degradation is rapid and ~ 95% degradation is observed within 30 min [Fig. 3]. Under UV photocatalysis the rate of degradation decreases significantly after the first 25-50 % of the reaction. The observed decrease is likely due to the reaction products competing for the reactive oxygen species and/or adsorption sites at the surface of the catalyst. To explore the applicability of solar irradiation, experiments were also carried out using sunlight as the irradiation source, illustrated in Fig. 3. Although the rate of degradation is slower under solar irradiation, 50 % degradation was still observed within 30 mins. Control experiments conducted in the absence of TiO2 and with TiO2 in the dark confirm degradation by direct photolysis and losses due to adsorption of PbTx onto the surface of the catalyst are insignificant.
Figure 3.
TiO2 photocatalysis of PbTx3 with UV lamp (350 nm) and sunlight, [PbTx-3]0= 16 μM, [TiO2] = 50 mg/L. The intensities of the UV and sunlight were 230 and 190 μ Watt/cm2, respectively.
3.2. Kinetic Study
Detailed evaluation of the reaction kinetics can provide valuable mechanistic information and the parameters critical for the assessment of TiO2 photocatalysis as a potential treatment process. While TiO2 photocatalysis is a heterogeneous process, the degradation of organic substrates has been reported to follow first order type kinetics under dilute conditions [Lu et al., 1993; Beightol et al., 1997]. The first order kinetic rate equation is represented by eq 6.
| (6) |
To evaluate the effect of initial PbTx concentration on the reaction kinetics of TiO2 photocatalysis, the concentration of PbTx-3 solution was varied in aqueous media while keeping the other reaction conditions constant. The concentration was varied within a range 2 - 40 μM based on solubility and our limits of detection. Experiments were conducted at 2, 16, and 36 μM to have a good distribution of concentrations over the entire range of concentrations amenable to our experimental procedures. The kinetics were evaluated under UV (350 nm) and solar irradiation for the first 20-30 % of the reaction in an attempt to minimize interferences from the reaction by-products competing for the reactive species or active sites at the surface of the catalyst. The PbTx-3 concentrations at specific treatment times were determined and results plotted as ln([PbTx-3]t/[PbTx-3]0) vs. irradiation time. The plots have a linear relationship for UV (350 nm) and solar experiments at different initial concentrations of PbTx-3 [table 1]. Although the rate constants for a true first order process do not change with the concentration of the substrate, we observe a decrease in the rate constant with increasing concentration of PbTx-3 under our experimental conditions. Such kinetic behavior has been reported previously for the photocatalysis of a number of substrates and has been rationalized based on a number of possible explanations, including multilayer adsorption, limitations in the number of reactive species or active sites available during irradiation and/or strong adsorption of the reaction products [Sabin et al., 1992; O’Shea et al., 1997]. Under our experimental conditions using dilute concentrations, we suspect the observed kinetic behavior is the result of competitive adsorption by the reaction byproducts.
Table 1.
First order kinetic parameters for TiO2 photocatalysis of PbTx-3 under UV (350 nm) and solar irradiation.
| UV (350 nm) | Sunlight | ||||
|---|---|---|---|---|---|
| [PbTx-3] (μM) |
k (min−1) | R2 | [PbTx-3] (μM) |
k (min−1) | R2 |
| 2.0 | 0.48 ± 0.02 | 0.99 | 4.0 | 0.023± 0.03 | 0.98 |
| 14.0 | 0.05 ± 0.01 | 0.98 | 15.0 | 0.010± 0.01 | 0.98 |
| 36.0 | 0.04 ± 0.01 | 0.99 | 20.0 | 0.001± 0.01 | 0.98 |
The experiments were conducted in triplicate with the standard deviation listed as the uncertainty.
3.3. Matrix Effects
3.3.1. Effect of environmental factors
Humic acids and substances (HS), ubiquitous in oceanic waters, typically possess high molecular weight, strong light absorbing properties and sensitize photochemical and photo-oxidative processes [Cooper et al., 1989; Minghou et al., 1983; Zeng et al., 2002]. Because of their light absorbing nature, HS may play an important role in the photochemical fate of PbTxs. Absorption of sunlight by HS leads to an excited state (eq 7). The excited state can transfer the energy to molecular oxygen to yield singlet oxygen, or undergo electron transfer to form radicals such as superoxide anion radical (eq 8). These reactive species can subsequently lead to the degradation of PbTx (eq 9). The excited state HS can sensitize the excitation of TiO2 (eq 10), and could enhance the efficiency of potential TiO2 photocatalytic treatment applications (eq 11).
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
Humic substances have been shown to enhance the photolysis of organic contaminants in water [Welker et al., 2000; Zeng et al., 2002]. One report has appeared on the photochemical fate of PbTx but it was limited to the direct photolysis at 254 nm [Hardman et al., 2004]. In the present study, we chose to explore the role of humic acid (HA) in the photochemical fate of PbTxs, and investigate their effect on TiO2 photocatalysis. Irradiations of PbTx-3 by UV (350 nm) were conducted in the presence of HA. A control experiment, irradiating PbTx-3 with UV (350 nm) light in the absence of HA, results in no significant degradation of PbTxs. However, significant degradation of PbTx-3 in the presence of HA was observed, indicating that HA initiated photochemical processes may be critical in the environmental fate of PbTxs.
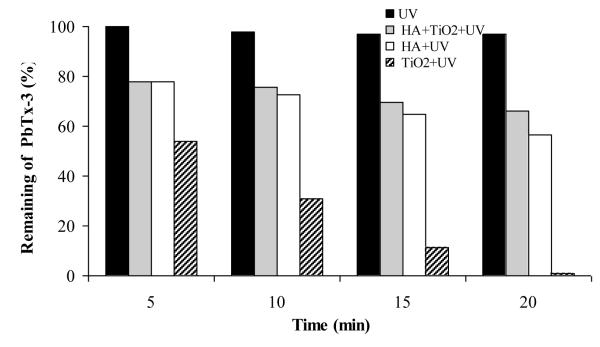
The effect of humic substances on TiO2 photocatalysis of PbTx was studied by conducting experiments in the presence of TiO2 and HA. The results are represented in Fig 4. While HA* has the potential to act as photosensitizer and promote TiO2 mediated oxidative processes, our observations indicate the presence of HA strongly inhibits the TiO2 photocatalysis of PbTx, relative to TiO2 photocatalysis conducted in the absence of HA, but modest degradation is still observed. The inhibition may be due to HA scavenging of the reactive oxygen species responsible for the degradation of PbTx, and attenuation of the light by the strong light-absorbing HA substrates. Our results demonstrate HA inhibits the effectiveness of TiO2 photocatalysis of PbTxs, but HA initiated photochemical processes alone may be critical in the environmental fate of PbTxs.
Figure 4.
Effect of humic substances on the degradation of PbTx-3 under UV (350 nm, intensity = 230 μWatt/cm2), [PbTx-3]0 =2.5 μM, [humic acid]0 = 400 mg/L and [TiO2]0 = mg/L
3.3.2. Effect of Seawater
The rates of TiO2 photocatalytic degradation can be slowed by the presence of ions present in seawater [Kamble et al., 2007; Puraja et al., 2007; Lu et al., 2009]. To assess the potential application of TiO2 photocatalysis in the presence of appropriate ions, experiments were conducted on PbTx-3 in synthetic seawater using UV (350 nm) and solar irradiation. The degradation followed first order kinetics and rate constants are included in table 2. The observed degradation rates are slower in the seawater matrix compared to de-ionized water. The slower degradation in seawater is likely due to ions, such as chloride, etc which can compete for adsorption sites on the surface of the catalyst, may scavenge valence band holes preventing direct hole oxidation [Minero et al., 1997] [Kamble et al., 2007; Puraja et al., 2007; Lu et al., 2009], or react with hydroxyl radicals to produce less reactive alternative radicals such as Cl−., and CO3.−. While a number of these ions may reside in or partition to the SML, their concentrations in the ocean surface microlayer (where Pbtxs are concentrated) should be low relative to the number of ions present in bulk seawater.
Table 2.
Summary of observed kinetic parameters for TiO2 photocatalysis of PbTx(B) under different experimental conditions.
| Conditions min |
Conc (μM) |
Intensity (μWatt/cm2) |
k (10−3/min) | t 1/2, | |
|---|---|---|---|---|---|
| UV/DI water | 11.0 | 230 | 79.0 | 9 | |
| Sunlight/DI water | 17.0 | 190 | 17.3 | 41 | |
| UV/Seawater | 8.5 | 230 | 4.20 | 165 | |
| Sunlight/Seawater | 11.6 | 190 | 3.30 | 231 | |
| UV/Acetonitrile | 16.0 | 990 | 52.0 | 16 | |
First order rate constant based on the disappearance of [PbTx] as a function of irradiation time
Under our experimental conditions and at the natural pH of seawater (i.e, the gulf coast waters of Florida have a solution pH ~7.7) [Smith et al., 2007] the surface charge of TiO2 is minimal. At pH=7.7, the TiO2 surface possesses an overall slight negatively charge. Under such conditions adsorption of common anions, chloride, phosphate, and nitrate should be reduced because of the electrostatic repulsion between negatively charged TiO2 surface and the anion. On the other hand cations, Ca+, Na+ may exhibit modest adsorption because of the electrostatic attraction between negatively charged surface and the cationic species, however such species are not susceptible to oxidation under our experimental conditions. The functional groups present in PbTx are not amenable to ionization under the neutral conditions or slightly basic conditions of seawater. We expect the strong hydrophilic character of PbTx will promote adsorption onto the surface of TiO2 under neutral or near neutral pH conditions even in the presence of ions. While the presence of these ions in seawater has a pronounced effect on the rates, degradation of PbTx-3 can still be achieved by solar TiO2 photocatalysis.
3.3.3. Effect of Organic media
PbTxs have polycyclic ether functionality, possess hydrophobic character and have limited solubility in aqueous media. The hydrophobic properties of PbTxs may lead to their partitioning to the thin organic matrix on the top of marine water bodies commonly referred to as the sea-surface microlayer (SML) which is composed of fatty acids, lipids, total carbohydrates, dissolved organic carbon, particulate organic carbon (POM), mono and polysaccharides, hydrocarbons, hydrophobic humic acids, dissolved inorganic nutrients such as NH4+, NO3−, NO2−, PO43−, etc [Duursma, pg 305]. A study by Rumbold et al. (1999) on the SML in Florida Keys revealed highest levels of PbTxs in the surface organic layer. With this in mind TiO2 photocatalysis of PbTx was carried-out in an organic matrix. While it is extremely difficult to mimic the complex composition of the organic microlayer, we chose to employ acetonitrile as our solvent, because like SML it is polar and has the properties to solvate and/or suspend the organic substrates often associated with SML. The acetonitrile solutions used in these experiments also contain a significant amount of water (~1 mM) relative high compared to the concentration of PbTx. A suspension of TiO2 containing 16 μM PbTxs in acetonitrile was purged with air and irradiated at 350 nm. The results are summarized in table 2 and demonstrate the 1st order kinetic plot of PbTxs concentration as a function of irradiation time. The control experiments indicate no appreciable degradation of PbTx in the absence of UV, TiO2, or oxygen. In general, the rates of degradation of PbTx in acetonitrile were slower than in aqueous media. Because of the slower rate of degradation, higher light intensity was employed. The difference between the rate constants in aqueous versus organic media can be attributed to changes in the adsorption of PbTxs and/or a change in the degradation process. The hydrophobic substrate, PbTx, is expected to partition to the surface of the catalyst to a greater extent in water (hydrophilic solvent) compared to an organic solvent.
3.4. Mechanistic and biological activity studies
A number of reactive oxygen species, including superoxide anion radical and hydroxyl radical are produced during TiO2 photocatalysis. Hydroxyl radical and superoxide anion radical are produced at the surface where they can react with adsorbed substrates (at the liquid-solid interface) or diffuse away from the surface and react with substrates in the solution. The partitioning of the reaction processes occurring at or near the surface is strongly influenced by strength of adsorption and reactivity parameters.
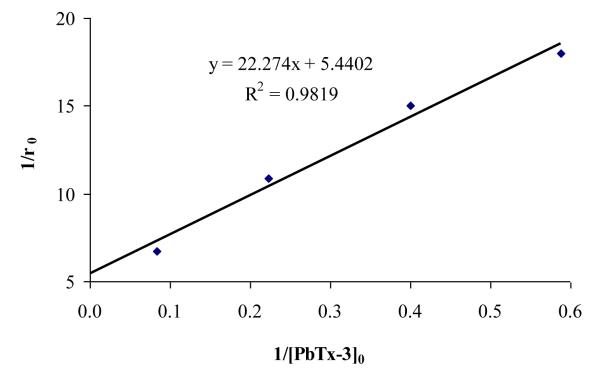
To evaluate the role of adsorption and reactivity, we chose to employ the Langmuir-Hinshelwood (L-H) kinetic model that has been extensively used for heterogeneous TiO2 photocataylsis to evaluate the reactions occurring at or near the surface of the photocatalyst [Wang et al. 2008; Uyguner et al. 2004]. The L-H model assumes non-competitive monolayer adsorption between products and starting material, unique adsorption sites, and no interaction between the adsorption sites [Atkins, pg 999; Evgenidou et.al., 2007]. The L-H equation is represented by eq 12 where C0 is initial PbTx-3 concentration, r0 is an initial degradation rate, kr is apparent reaction coefficient, and K is apparent equilibrium constant.
| (12) |
Application of the L-H kinetic model requires evaluation of the kinetics over a significant range of concentration. We chose to conduct the L-H experiments in an organic solvent, acetonitrile, as a mimic of the surface microlayer (SML) of the ocean, since PbTxs tend to partition into the SML. The enhanced solubility of PbTxs in organic solvents relative to aqueous media enabled L-H experiments to be conducted over a wider range of initial concentration (5.5-65 μM). In cases where the plot for the L-H equation is linear, the primary mechanism of degradation is assumed to occur at the surface of the catalyst as opposed to in the bulk solution [Schwarz et al., 1997]. Our results (Fig. 5) indicate linear relationship for the plot of initial degradation rates vs. different initial concentrations. The kinetic parameters kr and K, determined from the slope and intercept of the linear plot, are 0.19 M−1s−1 and 0.25 M−1, respectively. Application of the L-H kinetic model for TiO2 photocatalysis represents saturated kinetic parameters and has been the subject of recent criticism [Emeline et al., 2005]. While previous studies have focused on the application of the L-H model in aqueous media, our studies demonstrate the L-H type kinetics are also exhibited during TiO2 photocatalysis in acetonitrile. The apparent kinetic parameters obtained from our studies can be used for predicting treatment times under a given set of conditions over a range of concentrations.
Figure 5.
B>: L-H Kinetic Plot: Reciprocal plot of the initial reaction rate (1/r0) vs 1[PbTx- 3]0. [TiO2]= 50 mg/L, λ=350 nm, intensity = 990 μ Watt/cm2.
3.4.1. Role of hydroxyl radical
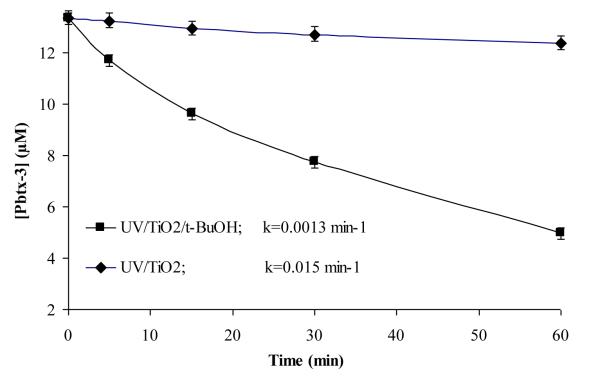
Hydroxyl radical is often considered the dominant oxidant in TiO2 photocatalytic oxidation reactions [Fox et al., 1993; Kamat, 1993; Dong et al., 1998; Xu et al., 2007]. Due to high oxidation potential of +2.8 V vs. NHE, hydroxyl radical is capable of oxidizing a wide spectrum of organic substrates. Under ambient conditions, the surface of TiO2 is saturated with hydroxyl groups as a precursor to the formation of hydroxyl radicals. The acetonitrile employed in our studies contained significant concentrations of water (~ 1 mM) relative to the concentrations of the target compound (~16 μM). Thus, the number of hydroxyl radicals potentially generated under our conditions exceeds the number of target molecules by several orders of magnitude. To probe the role of HO•, the scavenger t-BuOH was added to the reaction solution. The absolute HO• rate constant with t-BuOH is 6.0 × 108 M−1s−1 [Buxton et al., 1988] in aqueous media. Although the scavenging ability in acetonitrile may be different than in aqueous media, we expect t-BuOH will be adsorbed at the surface of TiO2 through hydrogen bonding interactions with the surface hydroxyl groups. Since hydroxyl radicals are also generated at the surface where t-BuOH is expected to be localized, t-BuOH should be an effective scavenger of HO• in acetonitrile. With this in mind, 5% v/v t-BuOH and PbTx-3 was added to the TiO2 suspension. The solution was purged with air and subjected to UV (350 nm) irradiation. A control experiment, without t-BuOH was conducted under the same conditions and the results are summarized in Fig. 6. The presence of the hydroxyl radical scavenger dramatically reduces the PbTxs degradation (by ten fold), providing evidence of hydroxyl radical participation in degradation of PbTxs in acetonitrile. It is also possible that t-BuOH may scavenge the hole directly, inhibiting the formation of hydroxyl radical and/or shutting down the possibility of direct hole oxidation of PbTx. In our experimental conditions, direct hole oxidation of PbTx seems less probable than hydroxyl radical mediated oxidation given the concentration of water competing for the valence band hole is orders of magnitude higher than PbTxs.
Figure 6.
The effect of hydroxyl radical scavenger tert-butanol on the rate of degradation of PbTx-3. The experiments were conducted under UV (intensity=990 μ Watt/cm2) in acetonitrile. [PbTx-3]= 16 μM, [t-BuOH] = 5%v/v, [TiO2]= 50 mg/L, air saturated.
While product studies were not feasible, the ether linkages are reactive towards hydroxyl radicals and the most predominant functional group present in PbTxs. We therefore expected PbTx to exhibit hydroxyl radical mediated degradation similar to other alkyl ethers. Based on our extensive studies of hydroxyl radical degradation of alkyl ethers [Cooper et al., 2009; O’Shea et al., 1997, 2001, 2002; Mezyk et al., 2004], we expect fragmentation of the PbTx fused ring systems with the formation of new carbonyl functionality. Fragmentation of the ring system will break the structural integrity (rigidity) of PbTxs critical to their biological activity. Such products should be more susceptible to biodegradation and exhibit dramatically reduced toxicity compared to the parent compound.
3.4.2. Biological Activity analysis using ELISA
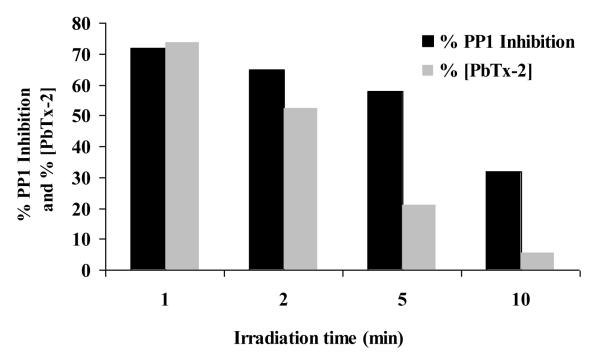
The biological activities of PbTx solutions following TiO2 photocatalytic treatment were evaluated using ELISA measurements. This immunological assay utilizes anti-brevetoxin antibodies to quantify the total PbTx-like molecules (parent toxin and related products) in a sample at ng/mL levels. A calibration curve was established to measure the percentage inhibition of binding as a function of PbTx concentration used as an antibody (standard). The observed inhibition of a binding antigen of PbTx-2 using immunoassay ELISA for the solution prior to treatment was 75 %. The results are plotted in Fig 7. The inhibition following treatment for 10 mins is approximately half of the bioactivity of the solution prior to irradiation. TiO2 photocatalysis dramatically reduces the concentration of PbTx measured by HPLC with appropriate dilution for corresponding ELISA measurements. The biological activity of the sample decreases steadily as a function of treatment time, but the decrease does not parallel the concentration of PbTx, indicating the byproducts formed during the early stages of photocatalysis are also biologically active. The ELISA employed is sensitive towards all PbTx like molecules, including variants with slight structural differences. Our observations suggest that PbTx-2 is converted to initial oxidation products, which maintain modest biological activity. Continued treatment is required to further reduce the biological activity.
Figure 7.
The percent inhibition of antigen detected using ELISA, and the % concentration of treated PbTx-2 samples detected using HPLC-UV as a function of irradiation time. UV (350 nm, intensity=230 μWatt/cm2), [PbTx-2]0= 42 nM, [TiO2]= 50 mg/L, air saturated.
4. Conclusion
TiO2 photocatalysis leads to the rapid degradation of the problematic PbTxs in aqueous and organic media. The degradation was fastest under UV irradiation, but solar irradiation also leads to the destruction of PbTxs. The degradation follows a first-order kinetic type process and hydroxyl radical seems to play a major role in degradation of PbTxs. Although the biological activity of the treated solution did not directly parallel the PbTx concentration, the toxicity of PbTx solution measured by ELISA was dramatically reduced during TiO2 photocatalysis. Humic substances and the components (ions) of seawater reduce the efficiency of the TiO2 photocatalytic degradation, but degradation is still observed. Although not practical to treat large bodies of marine water with TiO2 photocatalysis, it may be feasible to treat a heavily populated stretch of beach, a shellfish collection area, or a popular recreation site in an attempt to mitigate the human heath effects of PbTxs. We envision a treatment process would require the attachment of the photocatalyst to a floating substrate (glass beads). The floating substrates could be applied and maintained at the surface where the concentrations of the PbTxs are highest in the SML, the solar flux is greatest, and inhibitory effects of ions and humic substances below the SML will have a reduced effect. TiO2 photocatalysis can effectively destroy and detoxify PbTxs in DI water, but the treatment of seawater will require further development of catalyst and catalytic systems, which are less susceptible to inhibition by ions and humic materials present in seawater. Despite these challenges, the development of solar activated catalyst on floating substrates may offer a potential method for the degradation of PbTxs in marine estuaries.
Acknowledgements
We gratefully acknowledge the financial support of Council for International Exchange of Scholars (Fulbright program). This research was funded in part by the U.S. Environmental Protection Agency (RD-83322301) and NIH/NIEHS (ARCH S11ESO11181).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Abraham WM, Baden DG. Aerosolized Florida Red Tide Toxins and Human Health Effects. Oceanogr. 2006;19(2):107–109. doi: 10.5670/oceanog.2006.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeline AV, Ryabchuk VK, Serpone N. Dogmas and Misconceptions in Heterogeneous Photocatalysis. Some Enlightened Reflections. J. Phys. Chem. B. 2005;109:18515–18521. doi: 10.1021/jp0523367. [DOI] [PubMed] [Google Scholar]
- Antoniou MG, Shoemaker JA, de la Cruz AA, Dionysiou DD. LC/MS/MS structure elucidation of reaction intermediates formed during the TiO2 photocatalysis of microcystin –LR. Toxicon. 2008;51(6):1103–1118. doi: 10.1016/j.toxicon.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Atkins P, Paula J. Physical chemistry. 7th ed W. H. Freeman and Company; New York: 2001. [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational Exposure to Aerosolized Brevetoxins during Florida Red Tide events. Harmful Alg. 2003;2(1):19–28. [Google Scholar]
- Baden DG, Mende TJ, Block RE. In: Toxic Dinoflagellate Blooms. Taylor DL, Seliger HH, editors. Elsevier; New York: 1979. pp. 327–331. [Google Scholar]
- Buxton GV, Greenstock CL, Helman WP, Ross AP. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (.OH/.O−) in Aqueous Solution. J. Phys. Chem. Ref. Data. 1988;17:513–886. [Google Scholar]
- Cooper WJ, Cramer CJ, Martin NH, Mezyk SP, O’Shea KE, von Sonntag C. Free Radical Mechanisms for the Treatment of Methyl tert-Butyl Ether (MTBE) via Advanced Oxidation/Reductive Processes in Aqueous Solutions. Chem. Rev. 2009;109:1302–1345. doi: 10.1021/cr078024c. [DOI] [PubMed] [Google Scholar]
- Cooper WJ, Zika RG, Petasne RG, Fisher AM. Aquatic humic substances: Influence on fate and treatment of pollutants. In: Suffet IH, MacCarthy P, editors. Am. Chem. Soc. Washington, DC: 1989. pp. 333–362. [Google Scholar]
- Dong C, Chen CW, Huang CP. Removal of 3-chlorophenol from Water by Photocatalytic Oxidation. Hazard. Waste Hazard. Mater. 1998;30:479–489. [Google Scholar]
- Duursma EK, Dawson R. Elsevier Oceanography Series. Scientific Publishing Company; Amsterdam: 1981. Marine Organic Chemistry: Evolution, Composition, Interactions and Chemistry of Organic Matter in Seawater. lookup in google for composition of sea surface microlayer. [Google Scholar]
- Evgenidou E, Konstantinou I, Fytianos K, Poulios I, Albanis T. Photocatalytic Oxidation of Methyl Parathion over TiO2 and ZnO Suspensions. Catal. Today. 2007;124(3-4):156–162. [Google Scholar]
- Ferguson MA, Hoffman MR, Hering JG. TiO2-photocatalyzed As(III) Oxidation in Aqueous Suspensions: Reaction Kinetics and Effects of Adsorption. Environ. Sci. Technol. 2005;39(6):1880–1886. doi: 10.1021/es048795n. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of Aerosolized Florida Red Tide Toxins: Exposures and Effects. Environ. Health Perspect. 2005;113(5):618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Dulay MT. Heterogeneous Photocatalysis. Chem. Rev. 1993;93(1):341–357. [Google Scholar]
- Gawley RE, Rein KS, Kinoshita M, Baden DG. Binding of Brevetoxins and Ciguatoxin to the Voltage-sensitive Sodium Channel and Conformational Analysis of Brevetoxin B. Toxicon. 1992;30:780–785. doi: 10.1016/0041-0101(92)90014-v. [DOI] [PubMed] [Google Scholar]
- Glaze WH, Kang JW, Chapin DH. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Oz. Sci. Eng. 1987;9(4):335–52. [Google Scholar]
- Habibi MH, Vosooghian H. Photocatalytic degradation of some organic sulfides as environmental pollutants using titanium dioxide suspension. Journal of Photochemistry and Photobiology A: Chemistry. 2005;174:45–52. [Google Scholar]
- Hallegraeff GM. Manual on Harmful Marine Algae. In: Hallegraeff GM, Anderson DM, Cembella AD, editors. IOC Manuals and Guides. Intergovernmental Oceanographic Commission; Paris: 1995. pp. 1–22. [Google Scholar]
- Hardman RC, Cooper WJ, Bourdelais AJ, Gardinali PR, Baden DG. Brevetoxin Degradation and By-product Formation via Natural Sunlight. In: Steidinger KA, Landsberg JH, Yomas CR, Vargo GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and Intergovernmental Oceanographic Commission of UNESCO; 2004. p. 153. [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Cole RB. Solution Reactivity of Brevetoxins As Monitored by Electrospray Ionization Mass Spectrometry and Implications for Detoxification. Chem. Res. Toxicol. 1999;12(12):1268–1277. doi: 10.1021/tx9900876. [DOI] [PubMed] [Google Scholar]
- Kamat PV. Photochemistry on Nonreactive and Reactive (Semiconductor) Surfaces. Chem. Rev. 1993;93(1):267–300. [Google Scholar]
- Kamble SP, Sawant SB, Pangarkar VG. Heterogeneous photocatalytic degradation of p-toluenesulfonic acid using concentrated solar radiation in slurry photoreactor. J. Hazard. Mater. 2007;140:149–154. doi: 10.1016/j.jhazmat.2006.06.064. [DOI] [PubMed] [Google Scholar]
- LePage KT, Baden DG, Murray TF. Brevetoxin derivatives act as partial agonists at neurotoxin site 5 on the voltage-gated Na+ channel. Brain Research. 2003;959(1):120–127. doi: 10.1016/s0006-8993(02)03737-x. [DOI] [PubMed] [Google Scholar]
- Lu MC, Roam GD, Chen JN, Huang CP. Factors affecting the photocatalytic degradation of dichlorvos over titanium dioxide supported on glass. J. Photochem. Photobiol., A: Chem. 1993;76(1-2):103–110. [Google Scholar]
- Lu Chung-Shin, Chen Chiing-Chang, Mai Fu-Der, Li Hua-Kuang. Identification of the degradation pathways of alkanolamines with TiO2 photocatalysis. J. Hazard. Mater. 2009;165(1-3):306–316. doi: 10.1016/j.jhazmat.2008.09.127. [DOI] [PubMed] [Google Scholar]
- Mezyk SP, Jones J, Cooper WJ, Tobien T, Nickelsen MG, Adams JW, O’Shea KE, Bartels DM, Wishart J, Tornatore PM, Newman KS, Gregoire KD, Weidman J. Radiation Chemistry of Methyl tert-Butyl Ether in Aqueous Solution. Environ. Sci Technol. 2004;38(14):3994–4001. doi: 10.1021/es034558t. [DOI] [PubMed] [Google Scholar]
- Minero C, Maurino V, Pelizzetti E. Photocatalytic Transformations of Hydrocarbons at the Sea Water/Air Interface under Solar Radiation. Mar. Chem. 1997;58(3-4):361–372. [Google Scholar]
- Mohammed OS, Ahmed SA, Mostafa FM, Abdel-Wahab AA. TiO2 Nanoparticles-Photocatalytic Oxidation of Selected Cycloalkanols. International Journal of Photoenergy. 2007;2008:1–11. [Google Scholar]
- Minghou J, Wenda C, Lejun H. Studies on Marine Humic Substances I. Isolation of Humic Substances from Seawater With an Effective Adsorbent, GDx-102 Adsorbtion Resin. Chin. J. Ocean. Limn. 1(2):370–379. [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ. Health Perspect. 2002;110(2):179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis D, Pelizzetti E, Serpone N. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 1991;25(9):1522–1529. [Google Scholar]
- O’Shea KE, Beightol S, Garcia I, Kalen DV, Cooper WJ. Photocatalytic decomposition of organophosphonates in irradiated TiO2 suspensions. J. Photochem. Photobiol. A: Chem. 1997;107(1-3):221–226. [Google Scholar]
- O’Shea KE, Garcia I, Aguilar M. TiO2 photocatalytic degradation of dimethyl- and diethyl- methylphosphonate, effects of catalyst and environmental factors. Res. Chem. Intermed. 1997;23(4):325–339. [Google Scholar]
- O’Shea KE, Kim DK, Wu T, Cooper WJ, Mezyk SP. The degradation of MTBE-BTEX mixtures by gamma radiolysis: A kinetic modeling study. Rad. Phys. Chem. 2002;65(4-5):343–347. [Google Scholar]
- O’Shea KE, Wu T, Cooper WJ. TiO2 Photocatalysis of Gasoline Oxygenates. Kinetic Parameters and Effects of Catalyst Types and Loading on Degradation of MTBE. In: Diaz AF, Drogos DL, editors. Oxygenates in Gasoline. Washington DC: 2001. p. 165. (ACS Symposium Series No. 799). [Google Scholar]
- Osugi ME, Zanoni MVB, Chenthamarakshan CR, de Tacconi NR, Woldemariam GA, Mandal SS, Rajeshwar K. Toxicity assessment and degradation of disperse azo dyes by photoelectrocatalytic oxidation on Ti/TiO2 nanotubular array electrodes. J. Adv. Oxidation Technol. 2008;11(3):425–434. [Google Scholar]
- Pichat P, Disdier J, Hoang-Van C, Mas D, Goutailler G, Gaysse C. Purification/Deodorization of Indoor Air and Gaseous Effluents by TiO2 Photocatalysis. Catal. Today. 2002;63(2-4):363–369. [Google Scholar]
- Pujara K, Kamble SP, Pangarkar VG. Photocatalytic degradation of phenol-4-sulfonic acid using an artificial UV/TiO2 system in a slurry bubble column reactor. Ind. Eng. Chem. Res. 2007;46:4257–4264. [Google Scholar]
- Radwan FFY, Ramsdell JS. Characterization of In Vitro Oxidative and Conjugative Metabolic Pathways for Brevetoxin (PbTx-2) Toxicol. Sci. 2006;89(1):57–65. doi: 10.1093/toxsci/kfj013. [DOI] [PubMed] [Google Scholar]
- Raloff J. Toxic Surfs: Homing in on an Alga’s Threat—and Therapeutic Promise Science News online. 2005;168(4):56. http://www.phschool.com/science/science_news/articles/toxic_surfs.html. [PMC free article] [PubMed] [Google Scholar]
- Rumbold D, Snedaker S. Sea-Surface Microlayer Toxicity of the Florida Keys. Mar. Environ. Res. 1999;47(5):457–472. [Google Scholar]
- Ryu J, Choi W. Effects of TiO2 Surface Modifications on Photocatalytic Oxidation of Arsenite: The Role of Superoxides. Environ. Sci. technol. 2004;38(10):2928–2933. doi: 10.1021/es034725p. [DOI] [PubMed] [Google Scholar]
- Sabin F, Turk T, Vogler A. Photooxidation of organic compounds in the presence of titanium dioxide: determination of the efficiency. J. Photochem. Photobiol. A: Chem. 1992;63(1):99–106. [Google Scholar]
- Schwarz PF, Turro NJ, Bossmann SH, Braun AM, Abdel Wahab AA, Durr H. A New Method To Determine the Generation of Hydroxyl Radicals in Illuminated TiO2 Suspensions. J. Phys. Chem. B. 1997;101:7127–7134. [Google Scholar]
- Song W, De la Cruz AA, Rein KS, O’Shea KE. Ultrasonically Induced Degradation of Microcystin-LR and -RR: Identification of Products, Effect of pH, Formation and Destruction of Peroxides. Environ. Sci. Technol. 2006;40(12):3941–3946. doi: 10.1021/es0521730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester PA, Steindinger KA. Gymnodinium Breve Red Tide Blooms: Initiation, Transport, and Consequences of Surface Circulation. Limnol Oceanogr. 1997;42(2):1039–1051. [Google Scholar]
- Uyguner CS, Bekbolet M. Photocatalytic Degradation of Natural Organic Matter: Kinetic Considerations and Light Intensity Dependence. Int. J. Photoenergy. 2004;6(2):73–80. [Google Scholar]
- Uyguner CS, Bekbolet M. A comparative study on the photocatalytic degradation of humic substances of various origins. Desalination. 2005;176:167–176. [Google Scholar]
- Wang CC, Lee CK, Lyu MD, Juang LC. Photocatalytic Degradationof C.I. Basic Violet 10 using TiO2 Catalysts Supported by Y Zeolite: An Investigation of the Effects of Operational Parameters. Dyes Pigm. 2007;76(3):817–824. [Google Scholar]
- Welker M, Steinberg C. Rates of Humic Substance Photosensitized Degradation of Microcystin - LR in Natural Waters. Environ. Sci. Technol. 2000;34(16):3415–3419. [Google Scholar]
- Xu T, Cai Y, O’Shea KE. Adsorption and Photocatalyzed Oxidation of Methylated Arsenic Species in TiO2 Suspensions. Environ. Sci. Technol. 2007;41(15):5471–5477. doi: 10.1021/es0628349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K, Hwang HM, Yu H. Effect of Dissolved Humic Substances on the Photochemical Degradation Rate of 1 - Aminopyrene and Atrazine. Int. J. Mol. Sci. 2002;3(10):1048–1057. [Google Scholar]