Abstract
Background
To evaluate whether cumulative lead dose from environmental exposures is associated with cognitive function and decline, and whether persistent, reversible, or progressive effects are indicated.
Methods
We used longitudinal linear modeling to evaluate associations of tibia lead concentration with cognitive function and decline in socio-demographically diverse, community-dwelling adults, aged 50-70 years, randomly selected from neighborhoods in Baltimore. Six summary measures of cognitive function were created from standard tests in these domains: language, processing speed, eye-hand coordination, executive functioning, verbal memory and learning, and visual memory.
Results
The mean (SD) tibia lead level was 18.8 (11.6) μg/g. In models adjusting for demographic characteristics, socioeconomic status (SES), and race/ethnicity, higher tibia lead was associated with a progressive decline in eye-hand coordination in all subjects; stratified analysis substantiated this association only in African-Americans. In all subjects, tibia lead was associated with persistent effects with worse cognitive function in all six domains, but these associations weakened after increasing covariate control. In fully adjusted stratified analysis, persistent effects were present in whites in eye-hand coordination, executive functioning, and verbal memory and learning.
Conclusions
The study presents the strongest adult evidence to date in a diverse population of the impacts of cumulative lead dose on cognitive function independent of SES. As the study population was relatively young and the average total duration of follow-up short (< 30 months), the findings may represent the lower bound of what the impact of cumulative lead dose might be on the cognitive function of older Americans.
Introduction
Evidence is increasing that lead exposure is associated with adverse cognitive outcomes in adults.1 This observation has public health importance because during the middle of the 20th century, the U.S. population was exposed to unprecedented levels of lead in both occupational settings and the general environment, the latter because, before 1980, lead was extensively used in commercial products including gasoline, paint, food cans, and water pipes.2-4 As a consequence, the current population of older Americans has accumulated significant lifetime lead doses.5,6 Because this population is also expected to have longer survival than previous cohorts, the potential exists that high lead exposure and longer survival combine to produce a significant epidemic of cognitive impairment. This report evaluates whether cumulative lead dose is adversely associated with trajectories of cognitive function in 50-70 year old adults with primarily non-occupational lead exposures in the Baltimore Memory Study (BMS).4,7
Several cross-sectional and longitudinal studies of occupationally-exposed workers have demonstrated associations between cognitive test scores and recent (blood lead) and/or cumulative (tibia lead) dose.1,5,8-17 There are, however, only two such epidemiologic studies of community residents with environmental exposure: the BMS and the Normative Aging Study (NAS).18,19 Both have reported significant cross-sectional associations between cumulative lead dose and results of neurobehavioral tests spanning a variety of cognitive domains as well as a joint cognitive measure (Mini-Mental State Examination score [MMSE]14). However in the NAS, associations were inconsistent across domains and, in some cases, outlier-dependent;14,18 whereas in the BMS, associations were consistent across multiple domains, but were substantially attenuated after adjusting for race/ethnicity.4 The NAS has also reported longitudinal associations between cumulative lead dose and adverse changes in MMSE14 and seven tests measuring attention/working memory, short-term memory, and visuospatial ability18 but few besides the MMSE association achieved statistical significance. In summary, while the current evidence from general population studies is suggestive, it is sparse, inconsistent and mainly from cross-sectional analysis. Therefore, new evidence from longitudinal studies is important.
This report presents longitudinal analyses that augment existing evidence in two ways. First, the NAS mainly included only white men.11,15 The BMS includes both sexes and is racially/ethnically diverse. In light of the historical and persistent disparities in lead exposure by race/ethnicity and socioeconomic status,20,21 it is imperative to include disadvantaged subpopulations in research on lead-associated risk. Second, our analyses specifically aimed to distinguish four competing hypotheses regarding the associations (absence, reversible, persistent, or progressive) of cumulative lead dose and cognitive decline.22
Methods
Study population and Design
The BMS is a longitudinal cohort study of urban-dwelling persons designed to evaluate potential determinants of cognitive decline in mid- and later-life.4,7,23,24 In brief, households with telephone numbers in 65 contiguous neighborhoods of Baltimore were randomly selected; 18,826 households were telephoned to assess eligibility and interest in the study. Neighborhoods were diverse in race/ethnicity and the socioeconomic profiles of residents. Persons aged 50-70 years and residing in Baltimore for at least five years were eligible. Among the 2,351 randomly chosen residents meeting these criteria, 1,140 (48.5%) were subsequently enrolled in the study and completed a first assessment. As previously discussed,7 we examined the representativeness of our study sample by comparing our study subjects to the distribution of residents in our target neighborhoods by sex and race/ethnicity using 2000 Census data.25 These data suggested that African-American men were underrepresented and white women, overrepresented, in our sample. We believe that two main factors could have influenced the representativeness of our study sample regarding race/ethnicity: differential phone ownership and differential participation rates. We could not easily evaluate the first, but for the second, we examined enrollment success in our study neighborhoods by race/ethnicity using 2000 Census data. We found no apparent trend in enrollment success by the proportion of African-Americans in neighborhoods, supporting that the study subjects represented the source population with telephones.
Subjects were scheduled for two follow-up visits at approximately 14-month intervals. Of those enrolled, 1,033 (90.6%) completed the second study visit and 943 (82.7%) completed the third study visit. Of the 197 who did not return for visit three, 101 (9% of those enrolled) refused, 23 (2%) were too ill, 21 (2%) were deceased, 38 (3%) were lost to follow-up, and 14 (1%) had moved out of state. All participants provided written informed consent and were paid $50 for their time at each visit. The study was approved by the Committee for Human Research at the Johns Hopkins Bloomberg School of Public Health.
Analytic Outcome: Cognitive Function
At each study visit, participants completed an approximately 90-minute battery of 20 standardized tests designed to assess a broad range of cognitive domains, provide multiple measures of each domain, and minimize differential item bias by race/ethnicity or socioeconomic status.7,23,24 In examination of longitudinal univariate summary statistics, we identified a problem with one test (the Rey complex figure copy task) that arose due to an unanticipated inconsistency in the scoring procedure after the first study visit. We thus excluded it from the longitudinal analysis and analyzed six cognitive domains: language (Boston naming test, letter fluency, category fluency), processing speed (inverse of simple reaction time), eye-hand coordination (Purdue pegboard dominant hand, non-dominant hand, and both hands, trail-making test A), executive functioning (Purdue pegboard assembly minus both hands, Stroop C form minus A form, trail-making test B minus A), verbal memory and learning (Rey auditory verbal learning test immediate recall, delayed recall, recognition), and visual memory (Rey complex figure delayed recall, symbol digit). We derived a summary score for each domain by first negating where necessary to ensure that higher values indicated better performance, then standardizing each test in the domain to a z-score, and finally averaging z-scores across the tests.4,26
Primary Risk Factor: Lifetime Cumulative Lead Dose
The specific aim of this analysis was to characterize associations of cumulative lead dose with cognitive function and decline. Lead accumulates in bone and its residence time in cortical bone is measured in decades.27,28 We measured cortical bone (tibia) lead concentration (in units of μg lead per gram bone mineral) to estimate cumulative lead dose at visit two, using 109Cd K-shell X-ray fluorescence.29,30 This is a well-validated and reliable method for measurement of lead in bone 5,31,32; however, measurement imprecision can result in a negative estimate of the tibia lead concentration for low concentrations. Because it has been suggested that these negative estimates accurately rank individuals with respect to their underlying tibia lead concentrations,33 a common practice has been to retain the values for analysis.4,5 Early in our analysis, we evaluated the influence of the 45 negative concentrations; these exhibited strong associations with cognitive scores in virtually all domains, but in the opposite direction to those observed, or where none existed, with the non-negative range of concentrations. This raised concern about the validity of these negative values and analyses including them, so they were subsequently primarily excluded, leaving 965 individuals. Analyses including all data are provided as an online supplement.
Other Potential Determinants of Cognitive Function or Lead Dose
We sought to control for potential confounders of the lead-cognition relation, which were organized into two tiers for adjustment: “base” characteristics (age, sex, neurobehavioral testing technician) and cultural/socioeconomic characteristics (race/ethnicity, educational attainment, household wealth).7,34 Age, sex, and race/ethnicity were self-reported, the latter using the categories for the 2000 US Census. Socioeconomic characteristics were assessed using an instrument developed for the study.35 Educational attainment was assessed as a nine-level ordinal index and household wealth was measured in dollars of income plus assets.7,34
Conceptual Framework
Tibia lead concentrations vary considerably between individuals and are thought to validly and reliably estimate lifetime cumulative dose. For our study subjects, daily lead dosing from environmental sources was high for many decades, peaked around 1970, and then declined. We consider the large-scale reductions in environmental lead levels as an exposure “remission.” We evaluated four competing hypotheses on the consequences for cognition of such cumulative dose against our data:
H0 (none): lead dose is not associated with cognitive status or trajectory.
H1 (reversible): lead dose is associated with cognitive dysfunction but recovery occurs after exposure remission.
H2 (persistent): higher lead doses are associated with cognitive dysfunction and decrements persist over time.
H3 (progressive): higher lead doses are associated with cognitive dysfunction and the magnitude of the decrement increases over time.
H1 and H3 involve a changing magnitude of decrement over time; we henceforth refer to these as “changing effect” hypotheses.
Statistical Analyses
In initial analyses distributional skewing was identified, crude longitudinal trends and bivariable relationships between cognitive outcomes, time and other predictor variables described, and variable distributions compared across participant dropout patterns. Outlier values were validated. Household wealth was log-transformed. To limit the analytic influence of tibia lead outliers, we truncated the three highest outliers (148, 90, and 85 μg/g) to the next highest value (63.3 μg/g) a priori.
We employed marginal longitudinal linear regression to analyze relationships between lead and cognition, i.e. “generalized estimating equations” (GEE),36,37 allowing “unstructured” serial correlation among the three study visits. With approximately normally distributed data, this is essentially equivalent to maximum likelihood fitting, which assures robustness of findings to incomplete participation and dropout up to “missing at random.”38 Q-normal plots suggested approximate normality in the visual memory domain and a slight departure from this in the other domains. To achieve valid inferences in this scenario, we report tests and confidence intervals employing “robust” standard errors, using the “sandwich” estimator.39
Each domain summary score was separately regressed on tibia lead and other potential determinants identified a priori, as well as time since baseline visit (in years) and interactions between lead and time. Age, lead, and household wealth were modeled as continuous variables, and educational attainment ordinally. Following on findings of attenuation of lead associations in previous studies and as motivated by our study's focus on health disparities, race/ethnicity-stratified analyses were conducted for white and African-American participants (all others comprised only 5% of our subjects). Bivariable plots suggested threshold and plateau relationships of lead with cognitive outcomes, so these were modeled as a linear spline40 with a knot at 15 μg/g (the approximate nonlinear inflection point). For analyses including negative tibia lead measurements, reported as an online supplement, an additional knot was placed at 0. Relations with log-household-wealth were modeled as a linear spline (knot at 95th percentile, approximately $106) to limit the influence of very wealthy households. Models controlling only for “base” covariates, and then also for “cultural / SES” covariates (full model), were compared.
To build our longitudinal models, we first tested the presence of interactions between time and all lead spline terms, using nested model comparison. This distinguishes a progressive or reversible hypothesis (“changing” lead effect: alternative) against a persistent or “none” hypothesis (“non-changing” effect: null). We compared findings across base and full models; conclusions were comparable. Next, we reported findings or, for a null finding at the first step, proceeded to test for persistent versus “none” lead effect using models with no lead-by-time interactions. Results of the latter analysis were also compared across “base” and “full” models. The two primary hypotheses were evaluated with joint null hypothesis tests for both lead coefficients (main term and spline), or their interactions with time since baseline, using the Wald testing method for GEE.41 Then, where the lead spline term was not statistically significantly different from 0, we eliminated it to report strengths of association in a simpler model. This procedure yields primary hypothesis tests as free of subjectivity as possible, while allowing simplified interpretation and added precision for description of association strengths where simplification appeared appropriate. Because there is no norm for what constitutes harmful tibia lead exposure, we report associations per inter-quartile range (IQR) of lead doses in our sample. In our previous cross sectional work a nearly identical IQR increase in tibia lead concentrations was associated with a lowering of cognitive test scores equivalent to 22% to 60% of the IQR range of age (54.0 to 62.4 years) across the different domains of cognitive function,4 suggesting our selected unit was clinically meaningful.
We undertook extensive model-checking and sensitivity analyses to ensure that our fitted models faithfully represented the data. Residual and partial residual plots were examined.42 In isolated cases where poor fit was suggested, we enriched models to account for discrepancies. We also conducted analyses assessing test-retest effects (by including both “visit” indicators and time-since-baseline), including random effects for resident neighborhood, and constricting household wealth to the range shared by whites and African-Americans. In no case was there a substantive change in findings in the lead-cognition relations.
Results
Description of Study Subjects
Approximately two-thirds of participants were female, 55% were of white race/ethnicity, and 40% were African-American (Table 1). Participants varied considerably in educational attainment. Mean age and geometric mean household wealth were approximately 60 years and $200,000 respectively. The proportion of subjects with complete visit data, those who dropped out of the study after the baseline visit, and those who dropped out after the second visit did not differ by sex (p > 0.05), but those who dropped out were younger, had a higher proportion of African-Americans, were less well educated, less wealthy, and had higher tibia lead levels (all p < 0.05). Mean tibia lead concentration (only non-negative values) was 19 μg/g with an IQR of 12.7 μg/g. Persons with negative tibia lead concentrations, excluded in primary analyses, were approximately 20% more likely to be white and more than three times more likely to be of “other” race/ethnicity (p < 0.05); as additional non-significant trends, they were 1.3 years younger on average and 77.8 % female as opposed to 65.2 % in the non-negative range. Magnitudes of association with wealth and educational attainment were negligible.
Table 1.
Demographic and exposure characteristics by study visit, Baltimore Memory Study, 2001-2005. (n=1140)
| Variable | Baseline (n=1140) | 1st Follow-up (n=1033) | 2nd Follow-up (n=943) | Test of difference1 | |||
|---|---|---|---|---|---|---|---|
| Mean / n | SD / % | Mean / n | SD / % | Mean / n | SD / % | p-value | |
| Age (years) | 59.3 | 6.0 | 59.4 | 6.0 | 59.4 | 6.0 | 0.335 |
| 50-54 years | 355 | 31.1 | 315 | 30.5 | 286 | 30.3 | 0.003 |
| 55-59 years | 294 | 25.8 | 268 | 25.9 | 243 | 25.8 | |
| 60-64 years | 239 | 21.0 | 219 | 21.2 | 201 | 21.3 | |
| 65+ years | 252 | 22.1 | 231 | 22.4 | 213 | 22.6 | |
| Duration from V1 (years) | NA | NA | 1.3 | 0.2 | 2.5 | 0.2 | NA |
| Female, N, % | 749 | 65.7 | 684 | 66.2 | 623 | 66.1 | 0.498 |
| Race/ethnicity, N, % | |||||||
| White | 611 | 53.6 | 565 | 54.7 | 530 | 56.2 | 0.012 |
| African-American | 474 | 41.6 | 420 | 40.7 | 371 | 39.3 | |
| Mixed race/ethnicity | 31 | 2.7 | 28 | 2.7 | 25 | 2.7 | |
| Other | 24 | 2.1 | 20 | 1.9 | 17 | 1.8 | |
| Education index, N, % | |||||||
| < High school | 154 | 13.5 | 136 | 13.2 | 115 | 12.2 | 0.001 |
| High school / GED2 | 438 | 38.4 | 392 | 38.0 | 350 | 37.1 | |
| Some college / technical school | 66 | 5.8 | 61 | 5.9 | 59 | 6.3 | |
| College graduate | 136 | 11.9 | 119 | 11.5 | 111 | 11.8 | |
| Postgraduate | 345 | 30.4 | 324 | 31.4 | 307 | 32.7 | |
| Household wealth ($)3 | 190074 | 233500 | 196110 | 245500 | 203358 | 248800 | <0.001 |
| Tibia lead (μg/g)4 | NA | NA | 18.8 | 11.6 | 18.5 | 11.6 | 0.017 |
Chi-squared (% comparison) or ANOVA (mean comparison) across mutually exclusive groups: baseline visit only, baseline and first follow-up visits (only), present at second follow up
GED indicates General Educational Development test completion.
Geometric mean and inter-quartile range
Statistics among those with range-valid measurements (>=0): n=965, 1st follow up; n=892, 2nd follow up
Primary Findings
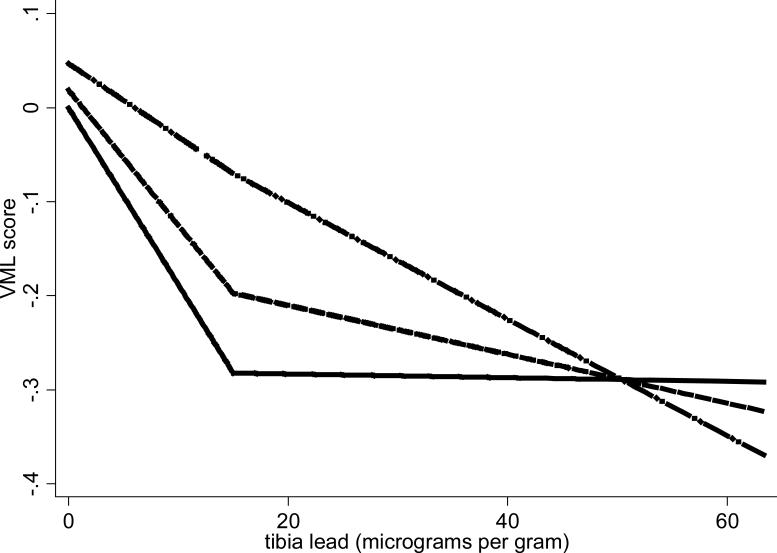
We first distinguished between progressive / reversible (“changing”) effects and no / persistent effects by testing for interaction between time and both lead concentration spline terms in models fully adjusted for base and cultural / SES characteristics (Table 2). Findings from models only adjusted for “base” characteristics were qualitatively identical. The tests generally failed to support effect change, except in the eye-hand coordination (EHC) domain, where compelling support was observed for a progressive effect. Because there was no strong indication for a spline term in this domain, we present linearly modeled effects in Table 3: baseline associations between domain scores and increasing lead concentrations, and estimates of effect change (linear versions of the spline interactions provided in Table 2). We found that the rate of decline in EHC domain z-score per 12.7 μg/g (IQR) worsened by 0.019 units per year (95% CI [0.007, 0.031]) (Table 3) in the whole cohort; in the African-American cohort, the estimated worsened by decline rate of 0.032 units per year per 12.7 μg/g (95% CI [0.012, 0.052]). We found one additional, but considerably less compelling, indication of changing effect: Among whites, there was a suggestion of an interaction between time and lead dose in the verbal memory and learning domain (departure from parallelism over time illustrated in Figure 1; inference for magnitude and significance of the departure, in Table 2). For this domain spline terms were indicated, thus are retained in Table 3. Rather than clear evidence of progression, the estimated temporal changes suggested lessening practice effect, or convergence in repeated performances, with increasing lead dose, and finally, a crossover into worsening performance over time at the highest lead doses.
Table 2.
Tests of changing effect hypotheses from fully-adjusted models, Baltimore Memory Study, 2001-2005. The p-value evaluates whether there is evidence of a progressive or reversible effect.
| Domain | Low-lead linear1 Beta (95% CI) | High-lead linear spline1 Beta (95% CI) | Test statistic2 (2 df) | P-value |
|---|---|---|---|---|
| All study subjects, N = 9643 | ||||
| Language | -0.015 (-0.050, 0.020) | 0.014 (-0.028, 0.056) | 1.0 | 0.60 |
| Processing speed | -0.011 (-0.084, 0.061) | 0.008 (-0.084, 0.100) | 0.26 | 0.88 |
| Eye-hand coordination | -0.036 (-0.075, 0.004) | 0.020 (-0.027, 0.068) | 11 | 0.0035 |
| Executive functioning | -0.001 (-0.041, 0.039) | -0.008 (-0.062, 0.047) | 0.59 | 0.74 |
| Verbal memory & learning | 0.033 (-0.017, 0.083) | -0.053 (-0.116, 0.009) | 3.2 | 0.20 |
| Visual memory | 0.017 (-0.038, 0.071) | -0.022 (-0.091, 0.048) | 0.38 | 0.83 |
| African-Americans subjects only, N = 394 | ||||
| Language | -0.010 (-0.082, 0.062) | 0.006 (-0.078, 0.091) | 0.46 | 0.79 |
| Processing speed | -0.020 (-0.175, 0.134) | 0.023 (-0.161, 0.207) | 0.07 | 0.97 |
| Eye-hand coordination | -0.055 (-0.142, 0.033) | 0.028 (-0.069, 0.125) | 10 | 0.0062 |
| Executive functioning | 0.065 (-0.025, 0.154) | -0.091 (-0.203, 0.021) | 2.7 | 0.27 |
| Verbal memory & learning | -0.010 (-0.097, 0.077) | 0.003 (-0.109, 0.115) | 0.41 | 0.82 |
| Visual memory | 0.013 (-0.094, 0.120) | -0.011 (-0.138, 0.116) | 0.11 | 0.94 |
| White subjects only, N = 517 | ||||
| Language | -0.025 (-0.068, 0.017) | 0.022 (-0.033, 0.076) | 2.2 | 0.34 |
| Processing speed | -0.015 (-0.097, 0.067) | 0.011 (-0.101, 0.123) | 0.33 | 0.85 |
| Eye-hand coordination | -0.030 (-0.075, 0.014) | 0.029 (-0.028, 0.086) | 2.3 | 0.32 |
| Executive functioning | -0.041 (-0.083, 0.002) | 0.052 (-0.003, 0.107) | 3.5 | 0.17 |
| Verbal memory & learning | 0.056 (-0.004, 0.116) | -0.086 (-0.166, -0.007) | 5.9 | 0.051 |
| Visual memory | 0.034 (-0.038, 0.106) | -0.053 (-0.145, 0.039) | 1.4 | 0.49 |
Low-lead linear term estimates the yearly change in the adjusted lead relation over lead values between 0 and 15 μg/g; high-lead linear spline term estimates the difference between the yearly change in the adjusted lead relation over lead values greater, and less than, 15 μg/g. Lead relation is given as units of domain score per 12.7 μg/g (IQR) of tibia lead.
Bivariate Wald test for the null hypothesis that both lead coefficients equal 0, with chi-square reference distribution
The stratified total does not add up to this grand total because 53 subjects of mixed race/ethnicity or other race/ethnicity were not included in the two strata.
Table 3.
Estimates of magnitudes of the “progressive” tibia lead associations with outcomes, Baltimore Memory Study, 2001-2005. Results are presented only for results with associated p-values < 0.10 in Table 2. Where not noted relationships are linear.
| Domain | Effect | Base Model1 Mean (95% CI) domain score difference per 12.7 μg/g tibia lead2 | Race- and SES-adjusted3 Mean (95% CI) domain score difference per 12.7 μg/g tibia lead2 |
|---|---|---|---|
| All study subjects, N = 964 | |||
| Eye-hand coordination | Baseline lead comparison Yearly difference amplification |
-0.089 (-0.139, -0.039) -0.019 (-0.031, -0.007) |
-0.005 (-0.052, 0.042) -0.019 (-0.031, -0.007) |
| African-Americans subjects only, N = 394 | |||
| Eye-hand coordination | Baseline lead comparison Yearly difference amplification |
0.010 (-0.072, 0.092) -0.032 (-0.052, -0.012) |
0.028 (-0.052, 0.108) -0.032 (-0.052, -0.012) |
| White subjects only, N = 517 | |||
| Eye-hand coordination | Baseline lead comparison Yearly difference amplification |
-0.076 (-0.136, -0.016) -0.009 (-0.024, 0.006) |
-0.052 (-0.109, 0.005) -0.009 (-0.024, 0.006) |
| Verbal memory & learning4 | Baseline lead comparison – low to moderate Baseline lead comparison – high vs. low/mod Yearly difference amplification – low to moderate Yearly difference amplification – high vs. low/mod |
-0.257 (-0.446, -0.067) 0.217 (-0.057, 0.491) 0.056 (-0.004, 0.116) -0.085 (-0.160, -0.010) |
-0.239 (-0.423, -0.055) 0.236 (-0.035, 0.508) 0.056 (-0.004, 0.116) -0.086 (-0.161, -0.012) |
The Base Model adjusted for age, sex and interviewer.
12.7 μg/g is the IQR of tibia lead among individuals with non-negative concentrations (n=965).
The race/ethnicity and SES-adjusted models include all base model adjustments. They do not include race/ethnicity after stratification.
Test for adequacy of linear model was rejected, with 95% CI for spline term equal to (-0.166, -0.007); “low to moderate” term estimates the lead relation over lead values between 0 and 15 μg/g; “high vs. low/mod” term estimates the difference between the lead relation over lead values greater, and less than, 15 μg/g.
Figure 1.
Graphical display of the estimated decline in verbal memory and learning with increasing tibia lead levels by study visit in subjects of white race/ethnicity only, Baltimore Memory Study, 2001-2005. The solid line represents the fully adjusted association of tibia lead with the verbal memory and learning cognitive domain score at baseline, the dashed line the same association at the first follow-up visit, and the dash-dot line at the second follow-up visit. First and second follow-up curves are estimated for respective timings of 1 year and 2.5 years from baseline.
In the absence of clear progressive (except for EHC) or reversible effects, we simplified to a model without time-by-lead interactions and proceeded to distinguish a persistent lead effect from no effect (Table 4) using “joint” tests for absence of both lead terms (main term and spline for time-invariant association between cognitive outcomes and lead concentration). Because no spline term differed statistically significantly from 0, we report magnitudes of association (confidence intervals in Table 4) from simplified, linear models. In analysis of all subjects, findings were similar to those reported previously in cross-sectional analyses,4 with persistent associations between increasing lead dose and worse cognitive performance that were large and statistically significant in all domains, but gradually diminished as adjustments for SES and race/ethnicity were made. Effect sizes were reduced by 75% or more, and no effect retained statistical significance, upon full adjustment for both SES and race/ethnicity. However, stratified analyses provided novel insights, in whites: After full adjustment, joint tests were suggestive of a persistent association between increasing lead dose and worsening cognition in the three domains of EHC (p = 0.07), executive functioning (p = 0.05), and verbal memory and learning (p = 0.03). The upper spline term was not significant for any of these domains; upon its removal, mean cognitive function was found to worsen, for both EHC and executive function, by 0.064 z-score points (with respective 95% CIs of [0.009, 0.118] and [0.011, 0.116]), and for verbal memory and learning, by 0.076 z-score points (95% CI [-0.001, 0.153]) per IQR additional lead dose. Findings in analyses including negative tibia lead measurements (see online supplement) did not materially differ from those we have just reported.
Table 4.
Models evaluating the persistence of the lead-associated cognitive effects, Baltimore Memory Study, 2001-2005.
| Mean (95% CI) domain score difference with 12.7 μg/g tibia lead difference2 | Joint test1 | ||||
|---|---|---|---|---|---|
| Domain | Base Model3 | SES-adjusted3 | Race-adjusted3 | Race- and SES-adjusted3 | p-value |
| All subjects, N = 964 | |||||
| Language | -0.1207 (-0.1829, -0.0584) | -0.0254 (-0.0752, 0.0244) | -0.0127 (-0.065, 0.0396) | 0.0216 (-0.0232, 0.0664) | 0.5 |
| Processing speed | -0.0851 (-0.1523, -0.0179) | -0.0254 (-0.0901, 0.0393) | -0.0127 (-0.0774, 0.052) | 0.0064 (-0.0584, 0.0711) | 0.88 |
| Executive functioning | -0.1219 (-0.1667, -0.0771) | -0.0622 (-0.1045, -0.0199) | -0.0483 (-0.0881, -0.0084) | -0.0305 (-0.0678, 0.0069) | 0.26 |
| Verbal memory & learning | -0.1359 (-0.1981, -0.0737) | -0.0737 (-0.1334, -0.0139) | -0.061 (-0.1232, 0.0013) | -0.0406 (-0.1029, 0.0216) | 0.16 |
| Visual memory | -0.1207 (-0.1754, -0.0659) | -0.0622 (-0.117, -0.0075) | -0.0495 (-0.1043, 0.0052) | -0.0318 (-0.0865, 0.023) | 0.21 |
| African-American subjects only, N = 394 | |||||
| Language | 0.0495 (-0.0376, 0.1367) | 0.0648 (-0.0099, 0.1394) | NA | NA | 0.23 |
| Processing speed | 0.0076 (-0.082, 0.0972) | 0.0216 (-0.0680, 0.1112) | NA | NA | 0.83 |
| Executive functioning | 0.0025 (-0.0572, 0.0623) | 0.0089 (-0.0484, 0.0661) | NA | NA | 0.86 |
| Verbal memory & learning | -0.0038 (-0.1009, 0.0933) | 0.0064 (-0.0907, 0.1034) | NA | NA | 0.96 |
| Visual memory | 0.0165 (-0.0532, 0.0862) | 0.0241 (-0.0431, 0.0913) | NA | NA | 0.23 |
| White subjects only, N = 517 | |||||
| Language | -0.0724 (-0.1371, -0.0077) | -0.0241 (-0.0764, 0.0281) | NA | NA | 0.33 |
| Processing speed | -0.0394 (-0.129, 0.0502) | -0.0241 (-0.1162, 0.068) | NA | NA | 0.83 |
| Eye-hand coordination | -0.0876 (-0.1449, -0.0304) | -0.0635 (-0.1183, -0.0087) | NA | NA | 0.071 |
| Executive functioning | -0.0927 (-0.1475, -0.0379) | -0.0635 (-0.1158, -0.0112) | NA | NA | 0.053 |
| Verbal memory & learning | -0.1054 (-0.1851, -0.0258) | -0.0762 (-0.1534, 0.001) | NA | NA | 0.034 |
| Visual memory | -0.094 (-0.1811, -0.0069) | -0.0648 (-0.1544, 0.0248) | NA | NA | 0.31 |
Bivariate Wald tests for the null hypothesis that both linear and spline lead coefficients equal 0, with chi-square reference distribution.
12.7 μg/g is the IQR of tibia lead among individuals with non-negative concentrations (n=965)
The Base Model adjusted for age, sex and interviewer. All subsequent models included these as adjustments.
Discussion
In this study designed to distinguish among absent, reversible, persistent, and progressive associations of cumulative lead dose on six cognitive domains over time, we found that higher tibia lead levels were associated with a progressive decline in eye-hand coordination (EHC), that was statistically significant in the full cohort and in African-Americans. Similar to the previous results from the cross-sectional analysis of first visit data,4 persistent associations of higher tibia lead concentrations with worse cognitive function were consistently found in all domains. These weakened after increasing control for SES and race/ethnicity. However, the increased power of the three visits of data allowed us to complete a stratified analysis. In whites, even after full adjustment, persistent effects were substantiated in EHC, executive functioning, and verbal memory and learning. These robust and consistent findings are the strongest evidence to date concerning cumulative lead dose and cognitive function in adults. They support and augment evidence from the only prior longitudinal study of cumulative lead dose and cognitive function in adults,11,14,15,18 but in a sample that is considerably more diverse in its sex and racial/ethnic composition.
Although the strongest findings on persistence were observed in whites, we believe it mistaken to conclude that there are not more general implications of the findings. As discussed in our prior cross-sectional publications in this population,4,43 there are many possible reasons why associations might attenuate after adjustment for race/ethnicity and SES even if cumulative lead dose contributes to cognitive decline in all subjects (e.g., unmeasured effect modification, reciprocal effects43). Detailed additional discussion is beyond the present scope, but other explanations for our failure to substantiate an association of lead among African-Americans, if an underlying association exists, include the “weathering” hypothesis,44 social stratification,45 survivor bias and out selection,46,47 cumulative disadvantage,48 and difficulty in “unchaining” the effect of a single health insult in the presence of multiple health insults.49-51 We believe these considerations leave well open that cumulative lead dose may contribute to decrements in cognitive function in adults regardless of race/ethnicity.
Data from multiple visits provides longitudinal evidence. Longitudinal data more strongly support causal inferences than cross-sectional data by establishing temporality and allowing comparisons treating individuals as their own controls. The strongest inferences possible in our study were tied to progressive effects, which involve within-person changes in cognitive outcomes over time. These were observed only in isolated instances, perhaps due to the relatively short duration of follow-up. The primary benefit of longitudinal data for analyses of persistent effect is to increase precision in characterizing cognitive functioning and, where persistence is observed, demonstrate stability over time in the relations of lead dose and cognitive test scores.
There were several strengths of this study. It is the largest study of bone lead and cognitive decline to date; it randomly selected study subjects to be representative of its sampling frame; it is diverse by sex, race/ethnicity, and SES; it rigorously measured SES; it rigorously assessed cognition in multiple domains; it measured a large number of covariates; it is longitudinal with reasonably successful follow-up; and it employed theoretically driven, state-ofthe-art longitudinal analyses. The main limitation was loss of subjects from the studied cohort due to initial refusal to participate, uncompleted or negative tibia lead measurements and dropout during the study. Loss of data by all of these mechanisms impacted power and precision for analyses and has the potential to bias findings. Because recruitment achieved designed targets, and loss to follow-up was at the rate anticipated in initial study design, we do not consider power loss related to sample size a primary concern.
Biases are not as easily dismissed, as data loss was systematically related to both demographic factors and tibia lead. It is possible that refusal or dropout could have related to cognitive status independently of measured characteristics. We consider the most likely scenario of informative data loss to be a higher likelihood of dropout among of individuals with impaired cognition, both theoretically and because mean cognitive performance improved over rounds in most domains. We believe such a mechanism would have tended to mask progressive or persistent effects due to loss of individuals with impaired cognition, rather than create spurious ones. By this standard, the persistence findings are particularly compelling. Then, it must be noted that the primary analytic plan was to not stratify, and that we made multiple comparisons by domains and by strata. Among 18 fully-adjusted joint tests of persistence (6 domains by total, white and African-American samples), 1.8 positive findings are anticipated (at alpha level = 0.10); by comparison, our three positive findings is suggestive but not overwhelmingly conclusive of a signal. We consider weight to be added in that all three findings were in a single cohort (whites) and were strengthened upon eliminating spline terms not supported by the data.
We believe it critical that public health researchers continue to characterize the cognitive effects of cumulative lead dose and, if present, evaluate possible interventions to prevent or mitigate them. Our study subjects were born between 1930 and 1950. Lead use in gasoline increased dramatically during the subsequent decades of 1950 to 1970 in the U.S., peaking in 1969.2 We estimate that this use distributed, on average, approximately 800,000 μg of lead per person into the environment each year during these decades. Although general population surveillance studies were not performed until the late 1970s, data within single cities suggest that mean blood lead levels in 1965 were as high as 25 μg/dL in the general population.2 By the time NHANES II measured blood lead levels in the general population in the late 1970s, use of lead in gasoline had declined considerably, but mean blood lead levels still exceeded 15 μg/dL across virtually all age and sex groups in the U.S. With growing numbers of older Americans achieving increasing life expectancies, population aging will soon collide with the peak lead dosing experienced by this cohort. It is therefore critical to characterize the late life consequences of early-, mid-, and late-life lead exposures. Our study contributes to this developing understanding.
In conclusion, this study provides the strongest evidence to date that cumulative lead dose is associated with adverse effects on cognitive function that are at least persistent in nature, decades after the majority of lifetime dose was achieved. While there may be a signal of progressive effect in one cognitive domain, the relatively short follow-up duration and the relatively low average age of study subjects makes conclusions about the existence of a progressive effect uncertain at this time. The data suggest that a proportion of what has been termed “normal cognitive aging” may in fact be due to lifetime exposure to neurotoxicants such as lead. As persons with the highest cumulative lead doses age, the role of lead dose on cognitive aging could not only become more apparent but have important public health consequences which we are only beginning to understand.
Supplementary Material
Acknowledgments
None.
Sources of financial support: This work was supported by R01 AG19604 from the National Institute on Aging.
References
- 1.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115(3):483–92. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren C. Brush with death: a social history of lead poisoning. Johns Hopkins University Press; Baltimore: 2000. [PubMed] [Google Scholar]
- 3.Annest JL, Pirkle JL, Makuc D, Neese JW, Bayse DD, Kovar MG. Chronological trend in blood lead levels between 1976 and 1980. N Engl J Med. 1983;308(23):1373–7. doi: 10.1056/NEJM198306093082301. [DOI] [PubMed] [Google Scholar]
- 4.Shih RA, Glass TA, Bandeen-Roche K, Carlson MC, Bolla KI, Todd AC, Schwartz BS. Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology. 2006;67(9):1556–62. doi: 10.1212/01.wnl.0000239836.26142.c5. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115(3):455–62. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz BS, Hu H. Adult lead exposure: time for change. Environ Health Perspect. 2007;115(3):451–4. doi: 10.1289/ehp.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, Bressler J, Shi W, Bandeen-Roche K. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect. 2004;112(3):314–20. doi: 10.1289/ehp.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz BS, Lee BK, Bandeen-Roche K, Stewart W, Bolla K, Links J, Weaver V, Todd A. Occupational lead exposure and longitudinal decline in neurobehavioral test scores. Epidemiology. 2005;16(1):106–13. doi: 10.1097/01.ede.0000147109.62324.51. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz BS, Lee BK, Lee GS, Stewart WF, Lee SS, Hwang KY, Ahn KD, Kim YB, Bolla KI, Simon D, Parsons PJ, Todd AC. Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with neurobehavioral test scores in South Korean lead workers. Am J Epidemiol. 2001;153(5):453–64. doi: 10.1093/aje/153.5.453. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, Gordon PB, Links JM, Todd AC. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology. 2000;55(8):1144–50. doi: 10.1212/wnl.55.8.1144. [DOI] [PubMed] [Google Scholar]
- 11.Payton M, Riggs KM, Spiro A, 3rd, Weiss ST, Hu H. Relations of bone and blood lead to cognitive function: the VA Normative Aging Study. Neurotoxicol Teratol. 1998;20(1):19–27. doi: 10.1016/s0892-0362(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WF, Schwartz BS, Simon D, Bolla KI, Todd AC, Links J. Neurobehavioral function and tibial and chelatable lead levels in 543 former organolead workers. Neurology. 1999;52(8):1610–7. doi: 10.1212/wnl.52.8.1610. [DOI] [PubMed] [Google Scholar]
- 13.Stokes L, Letz R, Gerr F, Kolczak M, McNeill FE, Chettle DR, Kaye WE. Neurotoxicity in young adults 20 years after childhood exposure to lead: the Bunker Hill experience. Occup Environ Med. 1998;55(8):507–16. doi: 10.1136/oem.55.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisskopf MG, Wright RO, Schwartz J, Spiro A, 3rd, Sparrow D, Aro A, Hu H. Cumulative lead exposure and prospective change in cognition among elderly men: the VA Normative Aging Study. Am J Epidemiol. 2004;160(12):1184–93. doi: 10.1093/aje/kwh333. [DOI] [PubMed] [Google Scholar]
- 15.Wright RO, Tsaih SW, Schwartz J, Spiro A, 3rd, McDonald K, Weiss ST, Hu H. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology. 2003;14(6):713–8. doi: 10.1097/01.EDE.0000081988.85964.db. [DOI] [PubMed] [Google Scholar]
- 16.Lindgren KN, Masten VL, Ford DP, Bleecker ML. Relation of cumulative exposure to inorganic lead and neuropsychological test performance. Occup Environ Med. 1996;53(7):472–7. doi: 10.1136/oem.53.7.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleecker ML, Lindgren KN, Ford DP. Differential contribution of current and cumulative indices of lead dose to neuropsychological performance by age. Neurology. 1997;48(3):639–45. doi: 10.1212/wnl.48.3.639. [DOI] [PubMed] [Google Scholar]
- 18.Weisskopf MG, Proctor SP, Wright RO, Schwartz J, Spiro A, 3rd, Sparrow D, Nie H, Hu H. Cumulative lead exposure and cognitive performance among elderly men. Epidemiology. 2007;18(1):59–66. doi: 10.1097/01.ede.0000248237.35363.29. [DOI] [PubMed] [Google Scholar]
- 19.Weisskopf MG, Proctor SP, Wright RO, Schwartz J, Spiro A, 3rd, Sparrow D, Nie H, Hu H. Cumulative Lead Exposure and Cognitive Performance Among Elderly Men. Epidemiology. 2006 doi: 10.1097/01.ede.0000248237.35363.29. [DOI] [PubMed] [Google Scholar]
- 20.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). Jama. 1994;272(4):284–91. [PubMed] [Google Scholar]
- 21.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991-1994. Environ Health Perspect. 1998;106(11):745–50. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart WF, Schwartz BS. Effects of lead on the adult brain: A 15-year exploration. Am J Ind Med. 2007;50(10):729–39. doi: 10.1002/ajim.20434. [DOI] [PubMed] [Google Scholar]
- 23.Schafer JH, Glass TA, Bolla KI, Mintz M, Jedlicka AE, Schwartz BS. Homocysteine and cognitive function in a population-based study of older adults. J Am Geriatr Soc. 2005;53(3):381–8. doi: 10.1111/j.1532-5415.2005.53153.x. [DOI] [PubMed] [Google Scholar]
- 24.Weil M, Bressler J, Parsons P, Bolla K, Glass T, Schwartz B. Blood mercury levels and neurobehavioral function. Jama. 2005;293(15):1875–82. doi: 10.1001/jama.293.15.1875. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Census Bureau . Census 2000 Summary File 3 - Maryland. U.S. Census Bureau; Washington, DC: 2002. [Google Scholar]
- 26.Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64(7):810–8. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- 27.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106(1):1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd AC, Landrigan PJ. X-ray fluorescence analysis of lead in bone. Environ Health Perspect. 1993;101(6):494–5. doi: 10.1289/ehp.93101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd AC, McNeill FE. In vivo measurements of lead in bone using a 109Cd “spot” source. In: Ellis KJ, Eastman JD, editors. Human Body Composition Studies. Plenum Press; New York: 1993. pp. 299–302. [DOI] [PubMed] [Google Scholar]
- 30.Todd AC, Parsons PJ, Carroll S, Geraghty C, Khan FA, Tang S, Moshier EL. Measurements of lead in human tibiae. A comparison between K-shell x-ray fluorescence and electrothermal atomic absorption spectrometry. Phys Med Biol. 2002;47(4):673–87. doi: 10.1088/0031-9155/47/4/309. [DOI] [PubMed] [Google Scholar]
- 31.Chettle DR, Scott MC, Somervaille LJ. Lead in bone: sampling and quantitation using K X-rays excited by 109Cd. Environ Health Perspect. 1991;91:49–55. doi: 10.1289/ehp.919149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd AC, McNeill FE, Fowler BA. In vivo X-ray fluorescence of lead in bone. Environ Res. 1992;59(2):326–35. doi: 10.1016/s0013-9351(05)80039-8. [DOI] [PubMed] [Google Scholar]
- 33.Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol. 1995;40(9):1475–85. doi: 10.1088/0031-9155/40/9/007. [DOI] [PubMed] [Google Scholar]
- 34.Glass TA, Rasmussen MD, Schwartz BS. Neighborhoods and obesity in older adults: the Baltimore Memory Study. Am J Prev Med. 2006;31(6):455–63. doi: 10.1016/j.amepre.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz BS, Glass TA, Bolla KI, Stewart WF, Glass G, Rasmussen M, Bressler J, Shi W, Bandeen-Roche K. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ Health Perspect. 2004;112(4):314–320. doi: 10.1289/ehp.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. Oxford University Press; Oxford, England: 1994. [Google Scholar]
- 37.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 38.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7:305–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- 39.Royall RM. Model robust confidence internals using maximum likelihood estimators. Int Stat Rev. 1986;54:221–226. [Google Scholar]
- 40.Wegman EJ, Wright IW. Splines in statistics. J Am Stat Assoc. 1983;78:351–365. [Google Scholar]
- 41.Rotnitsky A, Jewell NP. Hypothesis testing of regression parameters in semiparametric generalized linear models for cluster correlated data. Biometrika. 1990;77:485–497. [Google Scholar]
- 42.Bandeen-Roche K, Hall CB, Stewart WF, Zeger SL. Modelling disease progression in terms of exposure history. Stat Med. 1999;18(21):2899–916. doi: 10.1002/(sici)1097-0258(19991115)18:21<2899::aid-sim203>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Martin D, Glass TA, Bandeen-Roche K, Todd AC, Shi W, Schwartz BS. Association of blood lead and tibia lead with blood pressure and hypertension in a community sample of older adults. Am J Epidemiol. 2006;163(5):467–78. doi: 10.1093/aje/kwj060. [DOI] [PubMed] [Google Scholar]
- 44.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–33. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J Health Soc Behav. 1994;35(3):213–34. [PubMed] [Google Scholar]
- 46.Berkman L, Singer B, Manton K. Black/white differences in health status and mortality among the elderly. Demography. 1989;26(4):661–78. [PubMed] [Google Scholar]
- 47.Glymour MM. Invited commentary: when bad genes look good - APOE*E4, cognitive decline, and diagnostic thresholds. Am J Epidemiol. 2007;165(11):1239–46. doi: 10.1093/aje/kwm092. author reply 1247. [DOI] [PubMed] [Google Scholar]
- 48.Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, Salonen JT. Socioeconomic position across the lifecourse and cognitive function in late middle age. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):S43–51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- 49.Lupien SJ, Nair NP, Briere S, Maheu F, Tu MT, Lemay M, McEwen BS, Meaney MJ. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10(2):117–39. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- 50.Koppe JG, Bartonova A, Bolte G, Bistrup ML, Busby C, Butter M, Dorfman P, Fucic A, Gee D, van den Hazel P, Howard V, Kohlhuber M, Leijs M, Lundqvist C, Moshammer H, Naginiene R, Nicolopoulou-Stamati P, Ronchetti R, Salines G, Schoeters G, ten Tusscher G, Wallis MK, Zuurbier M. Exposure to multiple environmental agents and their effect. Acta Paediatr Suppl. 2006;95(453):106–13. doi: 10.1080/08035320600886646. [DOI] [PubMed] [Google Scholar]
- 51.Weiss B, Bellinger DC. Social ecology of children's vulnerability to environmental pollutants. Environ Health Perspect. 2006;114(10):1479–85. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.