Abstract
Background
Prior studies indicate that a subset of patients diagnosed with ST-segment elevation myocardial infarction (STEMI) will have an initial non-diagnostic ECG during evaluation. However, the timing of diagnostic ECG changes in this group is unknown. Our primary aim was to describe the timing of ECG diagnosis of STEMI in patients whose initial ECG was non-diagnostic. Secondarily, we sought to compare the delivery of ACC/AHA guidelines-based care and in-hospital outcomes in this group compared to patients diagnosed with STEMI on initial ECG.
Methods
We analyzed data from 41,560 patients diagnosed with STEMI included in the NCDR® ACTION Registry®-GWTG™ from 01/2007 to 12/2010. We divided this study population into two groups: those diagnosed on initial ECG (N= 36,994) and those with an initial non-diagnostic ECG that were diagnosed on a follow-up ECG (N= 4,566).
Results
In general, baseline characteristics and clinical presentations were similar between the two groups. For patients with an initial non-diagnostic ECG, 72.4% (N= 3,305)had an ECG diagnostic for STEMI within 90 minutes of their initial ECG. There did not appear to be significant differences in the administration of guidelines-recommended treatments for STEMI, in-hospital major bleeding (p 0.926), or death (p 0.475) between these groups.
Conclusions
In a national sample of patients diagnosed with STEMI, 11.0% had an initial non-diagnostic ECG. Of those patients, 72.4% had a follow-up diagnostic ECG within 90 minutes of their initial ECG. There did not appear to be clinically meaningful differences in guidelines-based treatment or major in-hospital outcomes between patients diagnosed with STEMI on an initial versus follow-up ECG.
The 12-lead electrocardiogram(ECG) is one of the corner stones of the initial evaluation for acute myocardial infarction (AMI). However, ECG findings during AMI can vary substantially depending on the type, stage, and extent of infarction and timing of ECG acquisition.1–3 Several studies cited in the current American College of Emergency Physicians (ACEP) and American College of Cardiology (ACC)/American Heart Association (AHA) recommendations regarding serial ECG monitoring in patients being evaluated for acute coronary syndromes (ACS) indicate that a subset of patients ultimately diagnosed with ST-segment elevation myocardial infarction (STEMI) will have an initial non-diagnostic ECG.4,5 However, little has been reported about the timing of diagnostic ST-segment elevation in those with initial non-diagnostic ECGs. The primary aim of this study was to describe the timing of ECG diagnosis of STEMI in patients with an initial non-diagnostic ECG. The secondary objectives were to determine whether the delay in diagnosis of STEMI for patients with an initial non-diagnostic ECG resulted in differing administration of guidelines-recommended treatments and in-hospital outcomes compared to patients whose initial ECG was diagnostic. Given the importance of timely recognition of STEMI, further characterization of the diagnostic time course in patients with delayed ECG diagnosis, along with its association with treatment and outcomes, is warranted.
Methods
Study Population
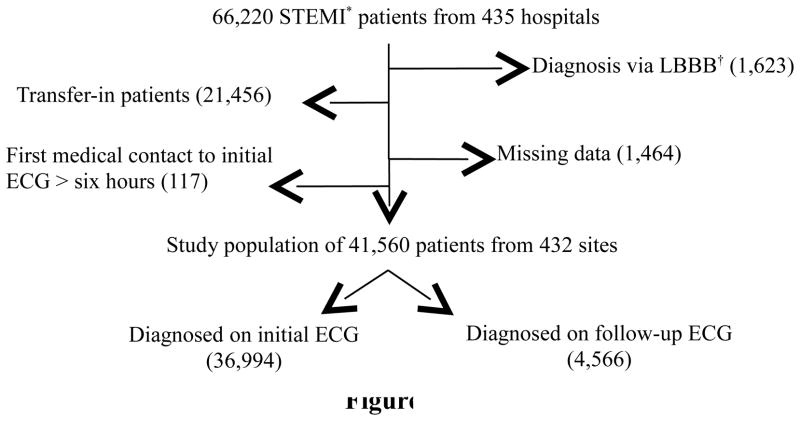
We performed an observational analysis using data from the NCDR ACTION Registry-GWTG, a nationally representative, quality improvement AMI registry. It is a voluntary registry that currently receives data from over 600 participating hospitals throughout the United States; details on the data collection process have been previously reported.6 All patients with the diagnosis of STEMI using the data collection form 2.0 (long form) were identified in the database from January 1st, 2007, through December 31st, 2010, producing a starting population of 66,220 patients from 435 hospitals. Diagnostic criteria for STEMI were based on registry protocol: (1) ischemic symptoms at rest, lasting ≥10 minutes, occurring within 72 hours prior to admission and (2) persistent ST segment elevation ≥1 mm in 2 or more contiguous ECG leads, including posterior leads V7–V9. For study purposes, ECGs meeting criteria (2)were considered diagnostic for STEMI; ECGs without these changes were considered non-diagnostic, regardless of other findings (T-wave inversions, ST-segment depression (aside from true posterior MIs), Q-waves). To be included in the study, diagnostic changes for STEMI had to be present on at least one ECG obtained during evaluation. Those noted to have left bundle branch blocks (LBBB) were excluded (N=1,623) due to the high likelihood that the LBBB would persist on follow-up ECGs and potentially affect ST-segment interpretation. Patients transferred-in to facilities participating in the registry were excluded as well to avoid heterogeneity in the reported timing of events (N= 21,456). Patients with missing data regarding the timing of ECG acquisition were also excluded (N=1,464), along with those whose time from initial medical contact to initial ECG was over six hours (N=117). This left a study population of 41,560 STEMI patients from 432 sites (Figure 1).
Figure 1.
Study Population. *STEMI, ST-segment elevation myocardial infarction; †LBBB, left bundle branch block
We divided the study population into two groups: those with diagnostic criteria for STEMI present on the initial ECG(N= 36,994) obtained during evaluation and those who developed diagnostic criteria on a follow-up ECG obtained after an initial non-diagnostic ECG (N= 4,566). The initial ECG was defined as the first ECG obtained during patient evaluation, which could have been obtained either during pre-hospital evaluation or after arrival at the treating facility’s emergency department (ED). Baseline characteristics, timing of ECG aquisition, initial presentation variables, treatment, and in-hospital outcomes were determined for both groups. Treatments investigated included class I recommendations from the ACC/AHA 2009 update on care of patients with STEMI and the 2008 ACC/AHA performance measures for patients with AMI, along with use of glycoprotein IIb-IIIa inhibitors, beta blockers, and statins.5, 7 Patients were considered to have received a pharmacologic intervention if administered within 24 hours of presentation and a non-pharmacologic intervention if performed at any point during the index hospital stay, unless otherwise indicated. Patients with documented contra-indications to a medication or procedure were excluded from calculations for that intervention. Initial and peak troponin ratio values were defined as the initial or peak value divided by the local laboratory upper limit of normal.8 Door-to-balloon and door-to-needle times were calculated in the traditional manner for patients with findings diagnostic for STEMI on initial ECG and from the time of time of diagnostic ECG-to-needle and diagnostic ECG-to-balloon in the group with an initial non-diagnostic ECG.
Statistical Methods
Categorical variables were presented as frequencies and percentages while continuous variables were presented as medians (interquartile range). Comparisons between groups with and without an initial diagnostic ECG were made using the Pearson chi-square test for categorical variables and the chi-square rank based group means score statistic, which is equivalent to a Wilcoxon test, for continuous variables. Unadjusted and adjusted analyses were performed to assess for potential differences in risk of in-hospital mortality and major bleeding episodes between groups using the logistic generalized estimating equations (GEE) modeling method with an exchangeable working correlation matrix to account for within-hospital clustering.9 Adjusted analyses included covariates from the validated ACTION-GWTG mortality and major bleeding models.10, 11 Current rates of missing data from this database are low, averaging <5% across all variables; missing continuous variables were set to the variable median, while missing categorical variables were set to the most frequent group unless otherwise noted.
The ACTION Registry–GWTG served as the hospital data collection evaluation mechanism and registry coordinating center for the American Heart Association’s Mission: Lifeline program. The Duke Clinical Research Institute (DCRI) served as the data analysis center and had an agreement to analyze the aggregate de-identified data for research purposes. All participating institutions were required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. The Institutional Review Board at Wake Forest University Health Sciences approved this study for waiver of consent and waiver of HIPPA authorization.
Vovici (Dulles, Virginia) and ROK (Charleston, South Carolina) served as the Mission: Lifeline data collection tools. All analyses were performed using SAS software (version 9.2, SAS Institue, Cary, North Carolina, USA).
Results
Baseline Characteristics and Presentation
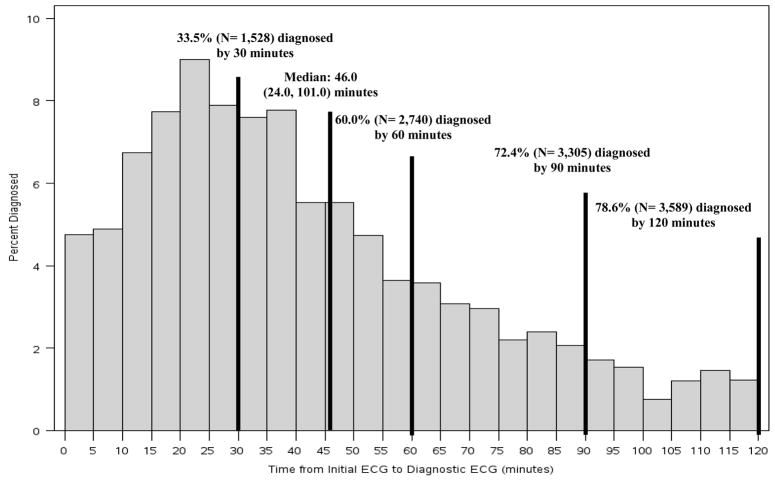
With the exception of smoking and male gender, patients with a non-diagnostic initial ECG more often had traditional coronary artery disease (CAD) risk factors and risk equivalents compared with those with an initial diagnostic ECG, though these differences were small (Table I). Patients presenting with an initial non-diagnostic ECG also had longer median times from symptom onset to initial medical contact (72.0 versus 67.8 minutes, p 0.043) and arrival at the treating facility(97.2 versus 93.0 minutes, p 0.002)compared with those diagnosed on initial ECG, though they were more likely to have their first ECG obtained prior to hospital arrival (40.0% versus 36.4%, p <0.001)(Table II). For patients with an initial non-diagnostic ECG, median time from initial ECG to follow-up diagnostic ECG was 46.0 (24.0, 101.0) minutes, with a range of 0 to 2,910 minutes. Of these patients, 32.0% (1,463)developed diagnostic ECG criteria for STEMI within 30 minutes, 60.0% (2,740)within 60 minutes, 72.4% (3,305) within 90 minutes, and 78.6% (4,566) within 120 minutes of the initial non-diagnostic ECG (Figure 2).
Table I.
Baseline characteristics for STEMI* patients according to timing of diagnostic ECG
| Variable† | Diagnosed on Initial ECG (N=36,994) | Diagnosed on Follow-up ECG (N=4,566) | p-value |
|---|---|---|---|
| Median Age (years) | 60.0 (52.0, 71.0) | 61.0 (52.0, 72.0) | 0.016 |
| Male gender | 25,996 (70.3) | 3,053 (66.9) | <0.001 |
| Caucasian race | 30,618 (82.8) | 3,729 (81.7) | 0.016 |
| Median body mass index (kg/m2) | 28.1 (25.0, 32.0) | 28.2 (25.0, 32.2) | 0.077 |
| Current/recent smoker (<1 year) | 15,982 (43.2) | 1,787 (39.1) | <0.001 |
| Hypertension | 23,413 (63.3) | 3,055 (66.9) | <0.001 |
| Dyslipidemia | 19,611 (53.0) | 2,623 (57.5) | <0.001 |
| Chronic lung disease | 3,468 (9.4) | 492 (10.8) | 0.002 |
| Diabetes mellitus | 8,372 (22.6) | 1,170 (25.6) | <0.001 |
| Prior myocardial infarction | 7,351 (19.9) | 994 (21.8) | 0.004 |
| Congestive heart failure | 1,872 (5.1) | 346 (7.6) | <0.001 |
| Prior revascularization | 9,044 (24.5) | 1,209 (26.5) | 0.004 |
| Atrial fibrillation or flutter | 1,487 (4.0) | 245 (5.4) | <0.001 |
| Cerebrovascular disease | 2,707 (7.3) | 372 (8.2) | 0.045 |
| Peripheral artery disease | 2,051 (5.5) | 312 (6.8) | <0.001 |
STEMI, ST-segment elevation myocardial infarction;
Reported as number (%) unless noted to be median values (IQR)
Table II.
Clinical presentation variables according to initial versus follow-up ECG diagnosis of STEMI*
| Variable† | Diagnosed on Initial ECG (N= 36,994) | Diagnosed on Follow-up ECG (N= 4,566) | p-value |
|---|---|---|---|
| Mode of transport to hospital | <0.001 | ||
| Self or family | 14,519 (39.3) | 1,910 (41.8) | |
| Emergency medical services | 21,309 (59.0) | 2,598 (57.7) | |
| First ECG obtained | <0.001 | ||
| Pre-hospital | 13,456 (36.4) | 1,827 (40.0) | |
| After arrival to hospital | 23,493 (63.5) | 2,737 (59.9) | |
| Median time from symptom onset to first medical contact (minutes) | 68.8 (30.0, 171.0) | 72.0 (31.2, 181.8) | 0.043 |
| Median time from initial medical contact to first ECG (minutes) | 10.0 (4.0, 27.0) | 9.0 (4.0, 22.0) | <0.001 |
| Median time from symptom onset to arrival at enrolling facility (minutes) | 93.0 (55.8, 189.0) | 97.2 (54.6, 210.0) | 0.002 |
| Median time from arrival in ED‡ to first ECG (minutes) | 6.0 (2.0,11.0) | 7.0 (3.0, 13.0) | <0.001 |
| Median time from arrival in ED to diagnostic ECG (minutes) | 6.0 (2.0, 11.0) | 47.0 (16.0, 116.0) | <0.001 |
| ECG within 10 minutes of arrival | 17,560 (73.7) | 1,891 (68.1) | <0.001 |
| Signs of congestive heart failure | 2,986 (8.1) | 463 (10.1) | <0.001 |
| Hypotensive (systolic blood pressure ≤ 90 mm Hg) | 2,616 (7.1) | 273 (6.0) | 0.006 |
| Cardiogenic shock | 3,057 (8.3) | 396 (8.7) | 0.357 |
| Heart rate >100 beats per minute | 5,931 (16.0) | 702 (15.4) | 0.244 |
| Median initial hemoglobin (g/dL) | 14.5 (13.2, 15.6) | 14.3 (13.0, 15.4) | <0.001 |
| Initial troponin ratio within normal limits | 19,799 (53.5) | 2,679 (58.7) | <0.001 |
| Median initial troponin ratio | 1.0 (0.2, 7.6) | 0.8 (0.2, 3.9) | <0.001 |
| Median peak troponin ratio | 132.7 (30.9, 519.9) | 79.5 (16.9, 362.0) | <0.001 |
| Teaching hospital | 8,356 (22.6) | 1,194 (26.2) | <0.001 |
| Hospital services available | <0.001 | ||
| Neither PCI§ nor surgery | 896 (2.4) | 146 (3.2) | |
| PCI and surgery | 31,825 (86.0) | 3,810 (83.4) |
STEMI, ST-segment elevation myocardial infarction;
Reported as number (%) unless noted to be median values (IQR);
ED, Emergency Department;
PCI, percutaneous coronary intervention
Figure 2.
Time from initial ECG to follow-up diagnostic ECG in STEMI patients with an initial non-diagnostic ECG.
Treatment and Outcomes
Patients diagnosed on a follow-up ECG after an initial non-diagnostic ECG were statistically less likely to receive many of the ACC/AHA class I recommended interventions or meet several of the guidelines-based performance measures for STEMI compared with those diagnosed on initial ECG (Table III). They also less often under went diagnostic coronary catheterization, less frequently had diagnostic ECG-to-balloon times ≤ 90 minutes for those undergoing primary PCI, and were less likely to receive reperfusion overall (thrombolytic therapy or primary PCI). However, the overall differences in treatment administration and performance measure attainment between the two groups were small.
Table III.
Treatment according to initial versus follow-up ECG diagnosis of STEMI*
| Variable† | Diagnosed on Initial ECG (N= 36,994) | Diagnosed on Follow-up ECG (N=4,566) | p-value |
|---|---|---|---|
| Aspirin | 35,640 (98.4) | 4,366 (98.1) | 0.234 |
| Clopidogrel (patients undergoing primary PCI‡) | 3,459 (81.8) | 513 (80.6) | 0.049 |
| Prasugrel (patients undergoing primary PCI) | 2,933 (8.1) | 293 (6.5) | 0.001 |
| Glycoprotein IIb-IIIa Inhibitor | 24,437 (67.6) | 2,811 (63.5) | <0.001 |
| Heparin (unfractionated or low molecular weight) | 30,658 (85.6) | 3,861 (85.4) | 0.001 |
| Bivalirudin | 8,713 (23.7) | 1,042 (23.0) | 0.299 |
| Beta Blocker | 28,746 (90.9) | 3,455 (89.7) | 0.010 |
| ACE-I/ARB§ (for patients with measured LVEF|| ≤ 40%) | 18,074 (52.5) | 2,121 (50.7) | 0.022 |
| Statin | 25,072 (69.9) | 2,946 (67.1) | <0.001 |
| Non-invasive stress testing | 869 (2.4) | 129 (2.8) | 0.048 |
| Primary thrombolytic therapy | 622 (1.9) | 86 (2.3) | 0.145 |
| Median door-to-needle time (minutes) | 22.0 (13.0, 36.0) | 23.0 (14.0, 54.0) | 0.274 |
| Door-to-needle time ≤ 30 minutes | 378 (68.2) | 24 (52.2) | 0.030 |
| Contraindications to reperfusion therapy | 5,093 (13.8) | 824 (18.1) | <0.001 |
| Diagnostic coronary catheterization | 34,435 (93.1) | 4,172 (91.4) | <0.001 |
| Median time from arrival to diagnostic catheterization (minutes) | 48.0 (34.2, 64.8) | 96.0 (60.0, 172.2) | <0.001 |
| Vessel(s) with ≥ 70% stenosis on diagnostic catheterization | |||
| Left main | 1,096 (3.6) | 179 (4.8) | <0.001 |
| Proximal left anterior descending | 11,046 (36.2) | 1,325 (36.2) | 0.883 |
| Mid-distal left anterior descending | 13,558 (45.2) | 1,767 (48.3) | <0.001 |
| Circumflex and branches | 12,554 (39.2) | 1,676 (43.0) | <0.001 |
| Right coronary artery and branches | 20,919 (63.7) | 2,361 (59.4) | <0.001 |
| PCI (primary, facilitated, or rescue) | 30,702 (90.6) | 3,633 (88.5) | <0.001 |
| Primary PCI | 29,995 (94.0) | 3,453 (92.1) | <0.001 |
| Median door-to-balloon time (minutes) | 61.0 (47.0, 76.0) | 64.0 (50.0, 78.0) | <0.001 |
| Door-to-balloon time ≤ 90 minutes | 23,390 (90.0) | 2,350 (86.3) | <0.001 |
| Primary PCI with stenting | 27,282 (91.0) | 3,120 (90.4) | 0.255 |
| Bare-metal stent | 12,044 (44.2) | 1,340 (43.0) | 0.202 |
| Drug-eluting stent | 15,388 (56.4) | 1,805 (57.9) | 0.122 |
| Coronary artery bypass graft surgery | 1,920 (5.2) | 247 (5.4) | 0.543 |
| Revascularization | 31,641 (93.3) | 3,766 (91.7) | <0.001 |
| Reperfusion | 30,282 (94.9) | 3,498 (93.3) | <0.001 |
STEMI, ST-segment elevation myocardial infarction;
Reported as number (%) unless noted to be median values (IQR);
PCI, percutaneous coronary intervention;
ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensinogen receptor blocker;
LVEF, left ventricular ejection fraction
There also did not appear to be any statistically significant differences in the odds of major in-hospital clinical events between the groups (Table IV). The incidence of in-hospital major bleeding in those diagnosed on a follow-up ECG was 10.8% (N=491/4,566) compared to 10.1% (N= 3,753/36,994) in those diagnosed on initial ECG with an adjusted odds ratio of 1.00(0.91, 1.11). The incidence of in-hospital death for those diagnosed on a follow-up ECG was 6.5% (N=278/4,566) compared to 5.8% (N= 2,012/36,994) for those diagnosed on initial ECG, with an adjusted odds ratio of 1.06 (0.90, 1.24).
Table IV.
In-hospital outcomes according to diagnosis of STEMI* on initial versus follow-up ECG
| Variable | Unadjusted OR† | p-value | Adjusted OR† | p-value |
|---|---|---|---|---|
| Death‡ | 1.14 (1.00, 1.30) | 0.046 | 1.06 (0.90, 1.24) | 0.475 |
| Major bleeding event§ | 1.07 (0.96, 1.18) | 0.211 | 1.00 (0.91, 1.11) | 0.926 |
STEMI, ST-segment elevation myocardial infarction;
Patient group diagnosed on initial ECG was used as comparison group
Variables in the ACTION-GWTG in-hospital mortality model included: age, prior peripheral artery disease, systolic blood pressure at presentation, heart failure on presentation (heart failure only, shock only, or heart failure with shock versus none), heart rate on presentation, initial troponin ratio, and initial serum creatinine.
Variables in the ACTION-GWTG in-hospital major bleeding model included: age, female gender, weight, diabetes, prior peripheral artery disease, home Warfarin use, heart rate on presentation, systolic blood pressure on presentation (SBP ≤ 130 mmHg, SBP 130–160 mmHg, and SBP ≥ 160 mmHg), heart failure on presentation (heart failure only, shock only, or heart failure with shock versus none), baseline hemoglobin <12 g/dL (versus ≥ 12 g/dL), and initial serum creatinine.
Discussion
In a national sample, we found that 11.0% of STEMI patients had an initial non-diagnostic ECG and that 72.4% of this patient group had a diagnostic ECG obtained within 90 minutes of their initial non-diagnostic ECG. There did not appear to be clinically relevant differences in baseline comorbidities, clinical presentation, guidelines-based treatment administration, or in-hospital outcomes between those diagnosed with STEMI on initial versus follow-up ECG.
Serial ECGs in the Evaluation of Patients with Suspected ACS
Several studies have evaluated the benefit of obtaining serial ECGs to assess for dynamic changes in patients with suspected ACS. In one study, serial ECG changes (including ST-segment elevation or depression, T-wave inversion, or new Q-waves) had a 39% sensitivity and 88% specificity for the diagnosis of AMI.12 In a similar study, 18/67 patients ultimately diagnosed with an AMI developed ST-segment elevation on a follow-up ECG obtained 3 hours after the initial ECG.13 Fesmire and colleagues reported that serial ECG monitoring for one hour after an initial non-diagnostic ECG identified an additional 33 patients with ST-segment elevation out of 204 ultimately diagnosed with AMI, though the timing distribution of ST-segment elevation was not reported.14 Our study adds to the body of literature supporting ECG surveillance of patients being evaluated for ACS. Also, while our study found some dissimilarity in clinical characteristics and presentation variables between those with and without initial diagnostic ST-segment elevation, the differences for individual comorbidities and presentation variables were small and unlikely to be clinically significant. This argues that the decision for futher ECG monitoring in patients being evaluated for ACS who have an initial non-diagnostic ECG cannot be based on past medical history and presentation variables alone and should be generally employed for patients in this group.
However, several important limitations of our study restrict our ability to infer optimal follow-up ECG timing in this population. First, it is not known under what context the repeat ECGs were obtained (change in clinical status, protocol, etc). Second, these were site-reported ECG results without central confirmation by an ECG core laboratory. Finally, it is unknown how many ECGs were obtained prior to the diagnostic ECG in the delayed diagnosis group, nor if any treatment was administered prior to or during follow-up ECG acquisition. Thus, an important caveat to these observations is that obtaining serial ECGs should not supersede clinical judgement. However, one possible inference from the data presented in Figure 2 is that obtaining follow-up or serial ECGs at 15–30 minute intervals from the initial non-diagnostic ECG could identify a significant number of STEMI patients earlier in their clinical presentation, which would support current ACC/AHA and ACEP recommendations for serial ECG monitoring in the evaluation of ACS patients.4, 15
Physiology of Delayed Diagnostic ST-segment Elevation
Some potential physiologic explanations for delayed ST-segment elevation may include the presence or absence of collateral circulation to the infarct artery territory, the contribution of vasoconstriction in the setting of an acutely occluding vessel, or the progressive nature of plaque rupture. There is also the possibility that patients with delayed ST-segment elevation experience stuttering occlusion of the culprit vessel(s), leading to a delay in electrographic evidence of transmural infarction. This hypothesis could be supported by the fact that patients in our study with a delayed ECG diagnosis had longer times from symptom onset to first medical contact and arrival at a treating facility (Table II).
Effects of an Initial Non-Diagnostic ECG on Treatment and Outcomes
While there were statistically significant differences in the administration of many of the ACC/AHA guidelines-recommended treatments for management of STEMI between patients diagnosedon an initial versus follow-up ECGs, these differences were small and not likely clinically relevant. While there may be worry that STEMI patients with a non-diagnostic initial ECG might not have timely follow-up ECGs and go unrecognized for a prolonged period without treatment, our data did not support this concern. It is possible that routine ECG acquisition in the Emergency Department per ACC/AHA and ACEP guidelines resulted in early recognition of dynamic ST-segment elevation indicative of STEMI in patients with initial non-diagnostic ECGs. It is also possible that patients with delayed ECG criteria have a more stuttering presentation, with frequent symptoms or changes in hemodynamic stability that prompted repeat ECG evaluation. It was also noted that, after adjustment for differences in baseline characteristics, there were no statistically significant differences in in-hospital death or major bleeding episodes between those with and without an initial diagnostic ECG, indicating that patients with delayed ECG diagnosis of STEMI do not appear to be at an increased risk for poor in-hospital outcomes compared to those with diagnostic changes noted on their initial ECG.
Limitations and Strengths
Using data from this type of registry introduces selection bias as hospitals that self-select for this type of registry tend to be larger tertiary referral centers and are more likely to follow guidelines-based care recommendations, especially since data on their performance is being reported. Another constraint is that outcomes in the registry are limited to in-hospital events and causes of death are not differentiated. Despite these limitations, the registry reflects a broad experience in actual clinical practice and provides insight into the need for serial assessment of patients with suspected ACS. Future directions include investigating whether routinely obtaining serial 12-lead ECGs, continuous ST-segment monitoring, or 12-lead ECGs with clinical status changes alone would be the most additive in risk prediction models for patients being evaluated for ACS.
Conclusion
ECG surveillance during the evaluation of ACS is warranted, with 11.0% of STEMI patients in a national database presenting with an initial non-diagnostic ECG. However, determining which patients with an initial non-diagnostic ECG need additional ECG surveillance cannot be ascertained from patient comorbidities or presentation characteristics alone. While this study does not provide evidence for the optimal timing of follow-up ECGs in this patient group, it does show that 72.4% of STEMI patients with an initial non-diagnostic ECG had a diagnostic ECG within 90 minutes of their initial ECG. It was also encouraging to note that the use of guidelines-based therapies and outcomes in STEMI patients with an initial non-diagnostic ECG was similar to patients diagnosed on initial ECG.
Acknowledgments
Funding Sources: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32 HL076132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. The ACTION Registry-GWTG is an initiative of the American College of Cardiology Foundation and the American Heart Association with partnering support from the Society of Chest Pain Centers, the American College of Emergency Physicians, and the Society of Hospital Medicine. The registry is sponsored in part by the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership.
Footnotes
Disclosures: None of the authors have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karlson BW, Herlitz J, Wiklund O, et al. Early prediction of acute myocardial infarction from clinical history, examination and electrocardiogram in the emergency room. Am J Cardiol. 1991;68:171–175. doi: 10.1016/0002-9149(91)90739-8. [DOI] [PubMed] [Google Scholar]
- 2.McQueen MJ, Holder D, El-Maraghi NR. Assessment of the accuracy of serial electrocardiograms in the diagnosis of myocardial infarction. Am Heart J. 1983;105:258–261. doi: 10.1016/0002-8703(83)90524-0. [DOI] [PubMed] [Google Scholar]
- 3.Nowakowski JF. Use of cardiac enzymes in the evaluation of acute chest pain. Ann Emerg Med. 1986;15:354–360. doi: 10.1016/s0196-0644(86)80584-4. [DOI] [PubMed] [Google Scholar]
- 4.American college of emergency physicians. Clinical policy: Critical issues in the evaluation and management of adult patients presenting with suspected acute myocardial infarction or unstable angina. Ann Emerg Med. 2000;35:521–525. [PubMed] [Google Scholar]
- 5.Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with st-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Peterson ED, Roe MT, Rumsfeld JS, et al. A call to ACTION (acute coronary treatment and intervention outcomes network): A national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–499. doi: 10.1161/CIRCOUTCOMES.108.847145. [DOI] [PubMed] [Google Scholar]
- 7.Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with st-elevation and non-st-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association task force on performance measures (writing committee to develop performance measures for st-elevation and non-st-elevation myocardial infarction) Circulation. 2008;118:2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 8.Roe MT, Peterson ED, Li Y, et al. Relationship between risk stratification by cardiac troponin level and adherence to guidelines for non-st-segment elevation acute coronary syndromes. Arch Intern Med. 2005;165:1870–1876. doi: 10.1001/archinte.165.16.1870. [DOI] [PubMed] [Google Scholar]
- 9.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 10.Chin CT, Chen AY, Wang TY, et al. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: The acute coronary treatment and intervention outcomes network (ACTION) registry-get with the guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113–122. doi: 10.1016/j.ahj.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Mathews R, Peterson ED, Chen AY, et al. In-hospital major bleeding during st-elevation and non-st-elevation myocardial infarction care: Derivation and validation of a model from the ACTION registry®-GWTG™. Am J Cardiol. 2011;107:1136–1143. doi: 10.1016/j.amjcard.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Hedges JR, Young GP, Henkel GF, et al. Serial ecgs are less accurate than serial ck-mb results for emergency department diagnosis of myocardial infarction. Ann Emerg Med. 1992;21:1445–1450. doi: 10.1016/s0196-0644(05)80057-5. [DOI] [PubMed] [Google Scholar]
- 13.Young GP, Gibler WB, Hedges JR, et al. Serial creatine kinase-mb results are a sensitive indicator of acute myocardial infarction in chest pain patients with nondiagnostic electrocardiograms: The second emergency medicine cardiac research group study. Acad Emerg Med. 1997;4:869–877. doi: 10.1111/j.1553-2712.1997.tb03812.x. [DOI] [PubMed] [Google Scholar]
- 14.Fesmire FM, Percy RF, Bardoner JB, et al. Usefulness of automated serial 12-lead ecg monitoring during the initial emergency department evaluation of patients with chest pain. Ann Emerg Med. 1998;31:3–11. doi: 10.1016/s0196-0644(98)70274-4. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-st-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;123:e426–579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]