Abstract
Postrhinal cortex, the rodent homolog of the primate parahippocampal cortex, processes spatial and contextual information. Our hypothesis of postrhinal function is that it serves to encode context, in part, by forming representations that link objects to places. We recorded postrhinal neuronal activity and local field potentials (LFPs) in rats trained on a two-choice, visual discrimination task. As predicted, a large proportion of postrhinal neurons signaled object-location conjunctions. In addition, postrhinal LFPs exhibited strong oscillatory rhythms in the theta band, and many postrhinal neurons were phase locked to theta. Although correlated with running speed, theta power was lower than predicted by speed alone immediately before and after choice. However, theta power was significantly increased following incorrect decisions, suggesting a role in signaling error. These findings provide evidence that postrhinal cortex encodes representations that link objects to places and suggest that postrhinal theta modulation extends to cognitive as well as spatial functions.
The predominant view of medial temporal lobe function emphasizes that spatial and non-spatial information reach the hippocampus through segregated parahippocampal pathways (e.g. Eichenbaum et al., 2007; Knierim et al., 2006). Spatial and contextual information is conveyed to the hippocampus by the postrhinal (POR) cortex (parahippocampal cortex (PHC) in the primate brain) and the medial entorhinal cortex (MEC); whereas non-spatial information is conveyed by the perirhinal cortex (PER) and the lateral entorhinal cortex (LEC). These two pathways, however, are not completely segregated. For example, intrinsic entorhinal connections span the LEC and MEC in both rats and monkeys (Chrobak and Amaral, 2007; Dolorfo and Amaral, 1998). In addition, in both species, the PER located in the nonspatial pathway is reciprocally connected with the POR/PHC in the spatial pathway (Burwell and Amaral, 1998b; Suzuki and Amaral, 1994b).
Given the anatomical evidence for nonspatial input to the spatial pathway, it is not surprising that the PHC is implicated in a variety of higher order cognitive functions, some of which are not strictly limited to the spatial domain. These functions include visual scene processing (Epstein et al., 1999), processing of objects in large spaces (Maguire et al., 1998), binding of objects and contexts (Hayes et al., 2007), retrieval of spatial context (Burgess et al., 2001), object location processing (Bohbot et al., 1998) and episodic memory (e.g. Gabrieli et al., 1997; Ranganath et al., 2004). Evidence from neuroimaging studies suggests that activity in the PHC increases when individuals are presented with objects that have strong contextual associations (Aminoff et al., 2007; Bar and Aminoff, 2003; Bar et al., 2008). In addition, Mullally and Maguire (2011) provided evidence that the PHC is more active in response to objects that are larger and stationary (spatially defining) as opposed to objects that are smaller and more portable (spatially ambiguous). Results of experimental lesion studies in rats also suggest that the POR processes information about objects, especially with respect to place or context (Gaffan et al., 2004; Norman and Eacott, 2005).
Based on the above review, a reasonable hypothesis is that the POR and PHC represent contexts and scenes, in part, by encoding the spatial layout of objects in the local environment. To test this hypothesis, we recorded from POR neurons during performance on a visual discrimination task in which rats learned object discriminations in multiple places (Figure 1). Stimuli were pairs of two-dimensional (2D) objects back-projected onto the floor of a bow-tie shaped testing area in a novel apparatus, the floor projection maze (Furtak et al., 2009). The location of stimulus presentation alternated by trial between the east and west sides of the maze. We predicted that POR neurons would signal the presence of conjunctions of objects and places as well as particular locations.
Figure 1.
Behavioral task. A. Schematic of Floor Projection Maze with images back-projected by a mirror. B. Top down view of an animal in the maze. A food port was located behind each of the four stimulus presentation areas. C. Pathways for a rat during shaping (left) and training (right). D. The two discrimination problems. E. Sequence of trials and analysis epochs. Trials alternated from east to west. An east trial was initiated when the rat was in the west food port area. The rat then moved to the ready position, facing east to wait for stimulus presentation. A choice was made by approaching a stimulus. Food was delivered at the port behind the correct stimulus. Presence in the east food area initiated a west trial, and so forth. Analysis epochs (500 msec) are indicated by grey blocks. See Table S1 for details of shaping procedures.
Consistent with our prediction, POR cells indeed signaled the conjunction of objects and locations. This finding argues against a strict functional segregation of spatial and nonspatial input to the hippocampus and provides evidence that context may be encoded upstream of the hippocampus.
Results
Animals were trained on two discrimination problems, each consisting of a pair of 2D visual stimuli (Figure 1D) back-projected onto the floor of the maze (Figure 1A). Object pairs were presented in two locations (east and west) to allow assessment of conjunctions of object-location selectivity. After a series of shaping steps (Table S1 and Supplementary Text), rats were trained on the final task in which presentation of object pairs alternated from east to west by trial (Figure 1B and 1E). Each new trial was signaled by the onset of white noise when the rat was in the reward area on the side of the maze opposite the side on which stimuli would next be presented (Figure 1E). Stimuli were presented when the rat had remained still in the ready position for a variable time (500–700 ms). The rat made a choice by approaching one of the two stimuli. A correct choice was followed by chocolate milk reward delivered in the reward area at a location behind the correct stimulus. If the rat first approached the incorrect stimulus, the trial terminated and no reward was provided. Initially, the two problems were presented in blocks of 10 trials. Following surgery, implanted rats were retrained on the blocked-trial version of the task until performing at >70% accuracy. They were then placed on a random-trial version of the task. Single unit and local field potential (LFP) recordings were obtained during daily sessions of 100 trials. All sessions in which the animal performed at or above 65% correct were analyzed. If performance dropped below 65%, rats were returned to blocked trials until accuracy improved. Preliminary analyses indicated no differences in selectivity between blocked and random sessions, so data from both session types were combined for all analyses. Stereotrodes were lowered at the end of daily recording sessions, and no attempt was made to hold single units across sessions.
For recorded sessions, percent correct ranged from 65–79% (69%±1%). Mean latencies to choice were 3.67±0.10 s for correct trials and 2.95±0.17 s for incorrect trials. Median latencies were 2.45 s for correct trials and 0.89 s for incorrect trials. Once well-trained, rats exhibited highly stereotyped pathways when approaching the chosen object, and nearly always checked the other food port before returning to the ready position (Figure 1C, right).
Postrhinal correlates of object, location, and egocentric response
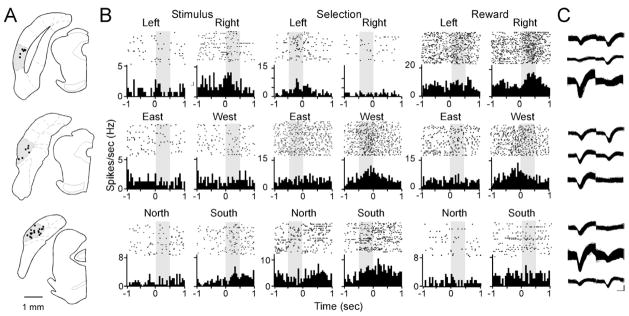
We recorded 97 well-isolated cells from 31 stereotrodes implanted in the POR of five animals during 32 sessions (electrode tip locations, Figure 2A). The mean firing rate per session for all cells was 3.66±0.29 Hz (range, 0.55–15.64 Hz). Firing rates were analyzed separately for three behaviorally relevant epochs of time (Figure 1E): the “stimulus” epoch, the 500 ms following stimulus presentation; the “selection” epoch, the 500 ms before stimulus choice; and the “reward” epoch, the 500 ms following stimulus choice during which reward was delivered.
Figure 2.
Histology and examples of neuronal correlates. A. Location of stereotrode tips in coronal sections of POR (shown in grey) between −7.70 to −8.80 mm relative to bregma. B. Example behavioral correlates of postrhinal cells during the stimulus (left), selection (center), and reward (right) epochs. Raster plots and peri-event histograms are shown for representative examples of cells with task-related firing patterns. For the stimulus epoch, time 0 is stimulus onset. For the selection and reward epochs, time 0 is choice. The upper row shows cells that fired more during left or right responses for correct trials. Examples of cells with spatial selectivity are shown in the middle and bottom rows. C. Waveforms for the isolated cells shown in B. For each row, the upper, middle, and lower waveforms correspond to the left, center, and right histograms and rastergrams. Time bins = 0.05 s, scale bar = 250 us, 100 μV.
Behavioral correlates were determined by factorial analysis of variance (ANOVA) of correct trials (side × object × response). Analyses were restricted to correct trials because low numbers of incorrect trials resulted in low sampling of some trial types. Of the 97 cells isolated, 71 met an analysis criterion of at least three correct trials for each of the eight trial types and a minimum of 20 spikes in the epoch analyzed (stimulus, selection, or reward). Of those 71 cells, 14 cells were recorded on stereotrodes in which one wire was compromised. All cells including those 14 cells were determined by autocorrelation analysis and cluster separation to be well isolated (Figure 2C).
Of the 71 criterion cells, 55 (77%) displayed selectivity as demonstrated by main effects or interactions of object, side, and response in at least one epoch. For example, some cells showed selectivity for a side of the maze (west, Figure 3A, left), a particular object (object 1, Figure 3B, left), a particular object in a particular location (object 2 in the southeast, Figure 3B, right), or an egocentric response (right response, Figure 3C, right).
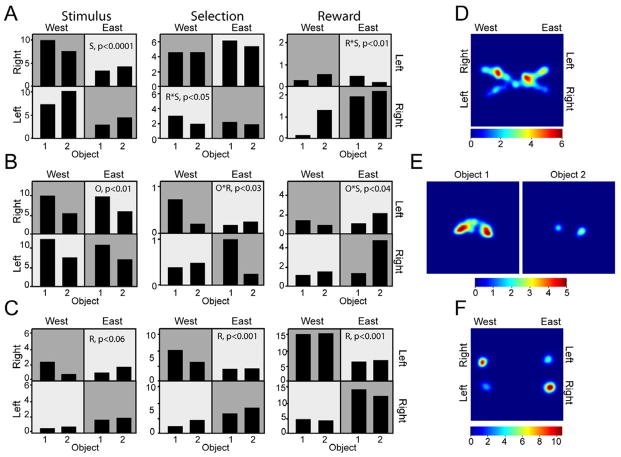
Figure 3.
Histograms and spatial plots for cells selective for location, object, and response. A–C. The left, center, and right histograms are from the stimulus, selection, and reward epochs, respectively, for correct trials. Firing rate is plotted as a function of location (east or west), response (left shown in light grey, right shown in dark grey), and object (1 vs. 2). P-values are inset for each example. A. Location correlates. The left cell was selective for side (S) and fired more during west trials. The center cell showed response x side (R*S) selectivity such that it fired more in the north, i.e. for right responses in the west and for left responses in the east (see also D). The right cell also exhibited R*S selectivity such that it fired more to right responses in the east. B. Object (O) and object-location conjunctions. The left cell fired more to object 1 than 2 overall (see also E). The center cell showed an object x response (O*R) selectivity such that it fired more to object 1 for right responses. The cell on the right exhibited object x side (O*S) selectivity such that it fired significantly more to object 2 in the east. C. Response (R) selectivity emerging across epochs (see also F). This cell most likely correlates with an egocentric right response. D–F. Spatial firing rate maps for representative cells. D. Location selective cell (same as A, center) that fires more in the north during the selection epoch when animals have left the ready position and are approaching the object on the west right or east left. E. Object selective cell (same as B, left) that fires more for object 1 than object 2 during the stimulus epoch when animals are just leaving the ready position near the center of the maze. F. Response selective cell (same as C, right) fires more to right responses during the reward epoch when the animal is at the reward location.
We predicted that POR cells would show patterns of activity consistent with representing conjunctions of 2D objects and places. As expected, a number of POR cells (25/71, 35%) showed selectivity for both object and location in at least one behavioral epoch. Numbers of such cells were roughly equal across epochs (Table 1). Object-location conjunction cells were of three types. The first type, cells with object × side interactions, fired more to an object depending on the side of the maze on which it was presented. For example, a cell might fire more to object 1 in the east and object 2 in the west. A second type of object-location conjunction cell, identified by an object × response interaction, was similar except that the location of firing for a particular pattern was located on the diagonal. For example, a cell would fire more to object 1 when it appeared on the right, regardless of the side of the maze (Figure 3B, center). We considered these cells to be object-location cells because they also fired more to one object than the other in specific locations (e.g., in the northwest and southeast). Finally, we observed cells that fired preferentially to a particular object only when it was in a single quadrant. These cells were identified by a significant object × side × response interaction. The example cell shown in Figure 3B (right) fired preferentially to object 2 only when it was located in one of the four quadrants.
Table 1.
Response patterns of POR cells by Factorial ANOVA
| Significant Main Effects and Interactions | Cells by Epoch (n=71)
|
||
|---|---|---|---|
| Stimulus | Selection | Reward | |
| Object-Location Conjunctions | 7 | 11 | 8 |
| Object*Side | 1 | 3 | 1 |
| Object*Response | 1 | 3 | 1 |
| Object*Side*Response | 1 | 1 | |
| Multiple Object/Response | 1 | 3 | |
| Multiple Object/Side/Response | 4 | 3 | 3 |
| Object Correlates | † | † | 2 |
| Location Correlates | 5 | 13 | 16 |
| Side | 2 | 4 | 6 |
| Side*Response | 1 | 6 | 5 |
| Multiple Side/Response | 2 | 3 | 5 |
| Response Correlates | 3 | 10 | 15 |
| Total Selective Cells (of 71) | 15 | 34 | 41 |
Numbers of cells that exhibited significant main effects or interactions during each of the three behavioral epochs (Stimulus, Selection, and Reward). The 71 cells were analyzed separately for each epoch. Within each epoch, most cells exhibited a single main effect or interaction, but some cells exhibited multiple main effects and/or interactions (shown in italics). For example, two cells in the Multiple Side/Response category showed a main effect of response and a response*side interaction.
Under Object Correlates, one cell in the Stimulus and one in the Selection epochs showed a main effect of Object, but each of those cells had multiple main effects and/or interactions and are thus counted in one of the multiple categories. See Table S2 for laminar differences and S3 for stability across epochs.
Because the POR is implicated in the processing of spatial and contextual information, we also predicted neural correlates of specific locations. There were two types of location correlates identified by factorial ANOVA. Selectivity for side was indicated by increased firing on the east or the west side of the maze (Figure 2B, middle panels; Figure 3A, left). A conjunction of side and response could indicate selectivity for the north or south of the maze (Figure 2B, lower; Figure 3A, center) or for a single quadrant (Figure 3A, right). Overall, 41% of cells meeting criterion (29/71) exhibited a main effect of side or a side × response interaction in at least one epoch. During the stimulus epoch, when the rat was positioned near the center of the maze, five cells demonstrated such location correlates (Table 1). This is interesting because, at stimulus onset, the animal was in the center of the maze viewing the location in which the object had appeared, but was not physically in the location. During the selection and reward epochs, when the animals were approaching or were in the location of a stimulus, more cells showed selectivity for location — 13 and 16 during selection and reward, respectively (Table 1). These results suggest that attending to a particular location from a distance does control activity of POR cells, but not as robustly as the animal’s physical location, at least in this task.
Four cells (6%) exhibited a main effect of object, in that firing rate was significantly higher to one of the two correct objects (Figure 3B, left). Two of those cells, however, also showed conjunctive selectivity in that they also exhibited a significant effect or interaction for some other aspect of the task (Table 1).
Unexpectedly, a large proportion of POR cells showed selectivity for a left vs. right response regardless of the identity of the correct object or the side of the maze on which it was presented (Figure 2B, upper; Figure 3C; Table 1). Of the 71 cells meeting criterion, 49% (35) exhibited a main effect of response in at least one epoch. The cell shown in Figure 3C fired more for right than left responses. The effect was marginally significant during the stimulus epoch and significant during the selection and reward epochs. It might be argued that the response cells were simply signaling two different spatial locations rather than the egocentric response. If that were the case, we would expect to see roughly equal numbers of cells that signal the two locations in the north (left east and right west) or the two locations in the south (east right and west left). We quantified the number of cells with a significant side × response interaction that fired more in the north or the south and observed 0, 4, and 2 such cells during stimulus, selection, and reward epochs, respectively. In contrast, we observed 3, 10, and 15 response cells during the same epochs (Table 1), suggesting that these cells encode something other than location, most likely egocentric responses.
We were interested in whether differences in patterns of selectivity depended upon the laminar location of cells. Of our 71 criterion cells, 32 were in superficial layers, 18 were in deep layers and the layer of the remaining 21 cells could not be precisely determined. Whether or not a cell showed selectivity for egocentric responses, particular objects, or object-location conjunctions was not influenced by laminar location (Table S2). As might be predicted by connectivity, however, cells in deep layers were more likely to exhibit spatial selectivity (χ2(1)=3.125, p < 0.039). Deep layers are targeted by subicular input (Kloosterman et al., 2003). In addition, although the posterior parietal cortex projects to superficial and deep layers, the deep layers are preferentially targeted (Burwell and Amaral, 1998a).
In general, the proportion of cells showing some type of selectivity differed significantly across epochs (χ2(2)=12.07, p < 0.002), such that the numbers increased as the trial progressed, from 15 cells during presentation of the stimulus to 34 and 41 during selection and reward, respectively (Table 1). Numbers of cells exhibiting object and object-location conjunctions were not significantly different across epochs (p = 0.60). Cells showing egocentric response correlates, however, increased significantly across epochs (χ2(2)=7.79, p < 0.02). Numbers of cells showing location correlates were marginally significantly different across epochs (χ2(2)=5.71, p < 0.06). Thus, location and response correlates were more evident in the selection and reward epochs.
To understand the stability of behavioral correlates, we examined patterns of selectivity across the three epochs. Of the 55 cells that were responsive in at least one epoch, 26 (47%) exhibited selectivity only in a single epoch and 29 (53%) exhibited selectivity in 2 or 3 epochs (Table S3). Of the 29 cells selective in more than one epoch, 11 exhibited similar patterns of selectivity across epochs. Of those, 6 were stable for location, 4 for response, and one for object-location selectivity. The remaining 18 of 29 cells changed patterns of selectivity across epochs. In all cases the change was between stimulus/selection epochs and the reward epoch when the stimulus was no longer visible. One third of these cells (6) changed from object-location to location selectivity and one third (6) changed from object-location to response selectivity. Three cells changed from some type of location selectivity to object-location selectivity in that the cell acquired selectivity for a particular object in the same location. Finally, three cells changed from selectivity for one location or response to selectivity for another location or response during the reward epoch. Thus, location and response cells tended to be stable across epochs, and cells that exhibited object location-selectivity changed between the stimulus/selection epochs and the reward epoch, when the stimulus was no longer visible.
Theta modulation in POR
Because brain oscillations, particularly in the theta and gamma ranges, are thought to represent or encode various important aspects of memory and cognition, we conducted multitaper spectral analyses of the POR LFP signals, focusing on the theta and gamma bands. The power spectrum of 42 LFPs (21 sessions from five rats) was calculated over the entire session. Theta rhythms were defined as 6–12 Hz oscillations, low gamma as 30–50 Hz, and high gamma as 70–110 Hz.
For POR LFPs, power in the theta range was higher than that expected from a 1/f power spectrum for 37 of 42 LFPs (88%, Figure 4A). To examine whether there were any task-dependent variations in the theta-band LFP signal, we calculated the power spectrum for each of the four task epochs, averaging across all trials of a session. Theta power differed across epochs in 90% of the LFPs (38/42). Specifically, theta power was greater for the task-related epochs (ready position, stimulus, and selection) when the animal was waiting for or processing the visual stimulus, as compared to the non-task-related reward epoch, when the stimulus was no longer relevant (Figure 4B).
Figure 4.
Increased theta power in POR. Examples are from three representative LFPs from three different animals (left, center, and right panels, respectively). A. Power spectra showed increased power in the theta band in 88% of the LFPs. B. Event related analysis showing higher power in the theta band for the three task relevant epochs prior to choice as compared to the reward epoch in 90% of LFPs.
Theta oscillations in the hippocampus are strongly modulated by the speed at which an animal moves (reviewed in Buzsaki, 2005), so we next asked if there were systematic changes in running speed across epochs, and if POR theta oscillations were also modulated by speed. Figure 5A shows examples of event triggered average running speed for three representative animals. Rats were required to be in the ready location for 500–700 ms prior to stimulus onset, so speed was low during that time (Figure 5A, upper panels). Immediately after presentation of the stimulus, animals began to move toward the choice point. Speed tended to be highest during the selection epoch, prior to choice, and lowest during the reward epoch when animals were checking the reward port for food (Figure 5A, lower panels). We found a strong correlation between running speed and the amplitude of theta oscillations (Figures 5B and 5C). Running speed was an excellent predictor of theta power in the non-task epoch, as well as the ready and stimulus periods of the task. However, theta power during both the selection and reward epochs was significantly lower than expected based on running speed alone (Figure 5C). Thus, theta power was additionally modulated (in this case, decreased) by the selection and reward phases of the task.
Figure 5.
Theta power and running speed. A. Event-triggered running speed for all trials for the ready (red), stimulus (orange), selection (green), and reward (blue) epochs for the same three animals and LFP sessions shown in Figure 4. B. Theta (upper) and running speed (lower) during periods when an animal was moving at high speed (left) and low speed (right). Theta power increases at faster running speeds. C. Running speed vs. average normalized theta power for all LFPs. The overall speed-theta relationship is shown in grey. To calculate this overall relationship, each session was binned into 250 ms time bins and the mean speed and mean theta power was computed for each bin. Data were then grouped by speed and the mean theta power was computed at each speed. Although running speed was correlated with theta power, overall, power in the theta band was lower than would be expected based on running speed alone for the selection (green) and reward (blue) epochs. See also Supplemental Text.
An analysis of correct vs. incorrect trials revealed that running speed was significantly slower for incorrect trials as compared to correct trials during the selection epoch (t=17.40, n=21 sessions, p < 0.0001), but not for any other epoch (Figure 6A). Interestingly, for the reward epoch, in which running speed was exactly the same for correct and incorrect trials (p=0.58, Figure S1), theta power was significantly greater following incorrect choices (t=−3.88, n=42 LFPs, p < 0.0004; Figure 6B).
Figure 6.
Theta power and running speed for correct versus incorrect trials. A. Running speed during the selection epoch. Animals ran significantly more slowly during the selection phase of incorrect trials compared to correct trials. Note that the selection epoch occurred before any cues indicated whether the animals had made a correct or incorrect decision. B. Normalized theta power for correct vs. incorrect trials during the reward epoch for all LFPs. Theta power was greater during incorrect trials for 80% of LFPs, even though there were no significant differences in running speed during the reward epoch. See also Figure S1 and Supplemental Text.
Finally, using circular statistics, we examined whether the activity of individual cells was modulated by POR theta. Of 69 cells recorded during LFP sessions, 26 (38%) showed significant phase-locking (Figure 7). This proportion was similar whether phase locking was determined using a local (same stereotrode) or non-local field potential (different stereotrode, but also in POR). Figure 7A shows three examples of cells phase locked to theta. Although some cells were phase locked to the peak of theta (5/26; 19%), most were phase locked to the trough (21/26; 81%). We examined the possibility that the cells phase locked to the peak of theta are interneurons by assessing a spike width parameter, the duration from trough to peak. Four of the 26 phase locked cells (15%) exhibited narrow spike widths, with a mean trough to peak duration of 135.2 μs, and were thus classified as putative fast-spiking inhibitory interneurons. The remaining phase locked cells (22 of 26) had broad spike widths, with a mean trough to peak duration of 425 μs, and were classified as putative excitatory cells. Three of the 4 putative interneurons were phase locked to the peak of theta oscillations. Twenty of 22 excitatory cells were phase locked to the trough of theta. These results are similar to observations of the distinct theta phases of identified pyramidal cells and fast-spiking parvalbumin-positive basket cells in the hippocampus (Klausberger and Somogyi, 2008).
Figure 7.
Phase-locking to theta in POR. A. Spike phase-locking to extracellular theta oscillations was observed in 38% of the 69 cells recorded simultaneously with the 42 LFPs. Examples shown here are from the same three representative LFPs shown in Figure 4 and Figure 5A. Left panel: mean phase = 152°, kappa = 1.25, ln(Rayleigh’s Z) = 6.57; Middle panel: Mean phase = 211°, kappa = 0.43, ln(Rayleigh’s Z) = 5.88; Right panel: Mean phase = 23°, kappa = 0.39, ln(Rayleigh’s Z) = 4.36. B. Distribution of Rayleigh’s Z for all cells recorded with LFPs. The Z-value distributions are shown in log form because some cells were strongly significant. The orange line is at ln(3) = 1.098 on the graph to indicate threshold for significance. Of the 69 cells, 26 showed significant phase locking to theta. C. Mean kappa score (orange line) and distribution for the 26 significantly phase-locked cells. All values of kappa > 1 are included in the right-most bin. D. Mean phase (orange line) and distribution of the 26 significantly phase-locked cells. E. Two POR cells recorded on the same stereotrode are phase locked to different phases of theta oscillations showing quite different phase preferences. For results of analyses of gamma see Figure S2 and Supplemental Text.
The circular mean preferred phase of all cells was 197.05±16.06° (17° or ~5 ms after the trough, Figure 7D). Interestingly, in some cases, POR cells recorded on the same stereotrode sometimes exhibited very different phase-locking preferences (Figure 7E). For 7 of 11 LFPs (64%) with simultaneously recorded cells, the preferred phase of significantly phase locked cells differed by 40° or less. For the remaining 4 (36%) LFPs, phase preferences differed by greater than 100°. In three of those cases, preferred phases were split between the trough and peak of theta. The broad distribution of phase preferences suggests that POR cells comprise different cell types that may play different roles in theta-based information processing.
Gamma modulation in POR
Gamma power was not visually increased compared to the 1/f power log decay either for low gamma or high gamma. Because of a substantial level of 60 cycle noise, we were not able to conduct event related analyses of gamma power. We did, however, examine whether the activity of individual cells was modulated by low gamma or high gamma. Of 69 cells for which LFPs were available, 64% showed significant phase-locking to low gamma (Figure S2A) and 93% showed significant phase-locking to high gamma (Figure S2B). In contrast to phase locking to theta, spiking of individual cells was phase locked only to the same-electrode LFP and not the non-local field potential (recorded on a different POR electrode than the cell). There were no task differences in phase locking to low or high gamma. Numbers of cells phase locked to gamma were similar across epochs, correct vs incorrect trials, and task vs. non-task phases.
Discussion
Anatomical, functional imaging, and experimental lesion evidence supports the hypothesis that the POR in the rodent brain and the PHC in the primate brain are involved in processing information about space, places, scenes, and contexts. There is little agreement, however, about the relevance of individual objects to representations of places and contexts. We used single unit recording in rats performing a novel visual discrimination task to test the hypothesis that the POR encodes contextual information, in part, by combining spatial information with object information to form representations that link objects to places. We found that a substantial proportion of POR cells exhibited object-location conjunctive encoding. We also report, for the first time, that POR LFPs show increased power in the theta band, that the activity of individual cells is modulated by theta, and that POR theta modulation is associated with both spatial and non-spatial behavior.
POR neuronal activity links objects to places
Object-location conjunctions were identified in more than a third of all recorded POR cells, nearly half of responsive cells, and in roughly equal numbers across the stimulus, selection, and reward epochs. The occurrence of such correlates during the stimulus epoch is especially interesting, because during that epoch the animal is not physically located at the position of the object. Rather, the animal is in the center of the maze, viewing the object from a distance, as if viewing a scene.
Our identification of object-location cells in POR is consistent with experimental lesion studies in which POR damage in rats and posterior PHC damage in monkeys impaired performance on object-place tasks (Gaffan et al., 2004; Malkova and Mishkin, 2003). Our findings extend a report that PHC neurons in monkeys respond to both object and spatial stimuli (Sato and Nakamura, 2003); selectivity for particular stimulus in different locations, as shown in the present study, was not tested.
The observation of object-location correlates in the POR argues against a strict functional segregation of spatial and nonspatial input to the hippocampus. Our findings also provide evidence that the spatial layout of objects in local contexts may be encoded upstream of the hippocampus in the POR rather than configured in the hippocampus.
POR and visuospatial attention
In addition to linking objects to places, available evidence suggests that the POR contributes to processing information about context by modulating attention to changes in the environment. In rodents, POR lesions alter performance in attentional orienting (Bucci and Burwell, 2004). The human PHC is also implicated in attention; activity in the parahippocampal place area attenuates for repeated scenes, but only when the scenes were attended during initial and repeated presentations (Yi and Chun, 2005). In monkeys, neuronal activity in PHC is altered by changes in the context (Vidyasagar et al., 1991) and by changes to stimuli in the periphery (Sato and Nakamura, 2003), suggesting a role in bottom-up, stimulus driven attention.
The rodent POR and primate PHC have anatomical connections with structures implicated in visuospatial attention, including the pulvinar and the posterior parietal cortex (Broussard et al., 2006; Oleksiak et al., 2011; Posner and Petersen, 1990). The POR has robust reciprocal connections with the lateral posterior nucleus of the thalamus (Burwell et al., 1995). This structure is considered to be homologous to the primate pulvinar (Kamishina et al., 2009; Mason and Groos, 1981), which is strongly connected with the monkey PHC(Baleydier and Mauguiere, 1985). Both the POR in the rat and the PHC in the monkey are strongly interconnected with the posterior parietal cortex (Agster and Burwell, 2009; Burwell and Amaral, 1998a; Munoz and Insausti, 2005; Suzuki and Amaral, 1994a). Interestingly, in monkeys performing a delayed match to sample task, activity in the posterior parietal cortex increased before activity in the medial temporal lobe increased (Saalmann et al., 2007).
In the present study, the location selective cells in the POR exhibited selectivity for the locations in which objects appeared, regardless of the identity of the object. Some cells even signaled location when the animal was viewing the location from a distance. These findings are consistent with an interpretation that POR signals attention directed to particular locations. Taken together, the evidence suggests that the POR, based on posterior parietal input, monitors the environmental context for changes and deploys attention to locations in which changes are likely to occur.
Egocentric response correlates in the POR
A number of cells were selective for egocentric response to the left or to the right, regardless of the identity of the object or the side of the maze on which it was presented. The number of cells exhibiting this phenomenon was greater during the selection and reward epochs. During these epochs the animal was either in motion performing the egocentric response or at the final location targeted by the egocentric response. Consistent with our finding, POR damage in rats has been shown to cause deficits in egocentric responses (Gaffan et al., 2004), and PHC neurons in monkeys respond to egocentric views (Rolls and O’Mara, 1995). Functional neuroimaging and neuropsychological studies in humans during performance on a navigation task also provide evidence that PHC has a role in egocentric spatial learning (Weniger and Irle, 2006; Weniger et al., 2010). Correlates of egocentric responses and views in POR and PHC may reflect input from the posterior parietal cortex, which is implicated in the attentional encoding of salient locations and objects in order to guide perception and action (e.g. Gottlieb et al., 2009). Indeed, posterior parietal neurons in rats do show correlates of egocentric responses (McNaughton et al., 1994). Thus, it may be that the posterior parietal input to POR supports both attention to particular locations and egocentric responses in the local context.
Theta modulation in POR
Theta oscillations are implicated in a number of cognitive and sensorimotor functions, but the most prevalent theories suggest theta is important for learning and memory (but see Kelemen et al., 2005; Ward, 2003). In our study, theta oscillations were prominent in the large majority of postrhinal LFPs, manifiesting as clear ~8 Hz rhythms in the time domain and as prominent increases in 6–12 Hz power in the frequency domain. Similar to hippocampal and entorhinal theta, POR theta power was strongly correlated with running speed, providing evidence for POR’s role in spatial information processing. Importantly, theta oscillations during the selection and reward phases had lower power than expected based on the rat’s running speed during those epochs, suggesting a possible role of theta modulation in choice behavior (Womelsdorf et al., 2010b).
An analysis of correct vs. incorrect trials indicated that theta power during the reward epoch was significantly increased following an incorrect choice. This difference was not due to differences in spatial behavior, as spatial behavior was well controlled in our study (Figure 1C, right, and Supplemental Text). In the absence of another explanation, our finding is consistent with a role for theta in cognition, e.g. in signaling prior error (Jacobs et al., 2006; Womelsdorf et al., 2010a), and suggests that theta oscillations in the POR are important for decision making and error processing, at least with respect to objects and locations.
A substantial proportion of POR neurons were phase-locked to theta, primarily at the trough of theta oscillations, which is when these cells are expected to be most depolarized by local theta-frequency synaptic inputs. A small subset of neurons fired close to the peak of theta oscillations. It is possible that the theta sinks in these cases are in layers distant from the location of the cell resulting in theta oscillation phase reversal as a function of cortical depth, as has been observed in the hippocampus (Buzsaki, 2002). Alternatively, this subset of cells could represent fast-spiking interneurons. Consistent with the latter possibility, we found that 3 out of 4 putative fast-spiking interneurons with narrow waveforms were phase locked to the peak of theta. Such opposite theta phase relationships for pydamidal cells and subsets of interneurons have been observed in the hippocampus (Klausberger and Somogyi, 2008). Indeed, we observed neurons recorded on the same electrode that had very different phase relationships (Figure 7E), an observation that cannot be explained by the phase reversal of theta as a function of cortical depth.
The robust theta modulation in the POR is interesting given that theta is proposed to coordinate activity across distant brain structures (Jutras and Buffalo, 2010; Klimesch et al., 2010). As an example, hippocampal theta rhythms are thought to coordinate activity between the hippocampus and associated regions in the service of episodic memory (Buzsaki, 2002, 2005; Jacobs et al., 2006). A recent relevant paper provided evidence that face-location associative learning was mediated by theta power in the parahippocampal gyrus (Atienza et al., 2011). As in the hippocampus, POR theta oscillations are probably dependent on theta-frequency inputs from multiple generators. Indeed, the POR is strongly interconnected with regions that show robust theta modulation, including the PER, entorhinal cortex, and hippocampus (Bilkey and Heinemann, 1999; Kerr et al., 2007; Lee et al., 1994; Naber et al., 1997). The POR, but not the PER, receives a strong input from the septum arising almost entirely from the medial septal nucleus (Deacon et al., 1983; Furtak et al., 2007). Taken together, the evidence suggests that POR theta, possibly generated by septal input, is in a position to modulate transmission of incoming non-spatial information from PER and spatial information from the posterior parietal cortex.
POR and context
Visual information is certainly critical for representations of environmental context, and places in the real world comprise a variety of features. Real-world contexts contain large and small objects that may or may not remain in the same location, are often characterized by multimodal features, and demonstrate a variety of sizes and shapes. In addition, many places and objects are imbued with meaning based on personal experience and semantic knowledge. Notably, the POR is the target of heavy input from the PER in both rats and monkeys (Burwell and Amaral, 1998a; Suzuki and Amaral, 1994a). It should not be surprising that damage to either PER or POR causes deficits in contextual learning (e.g. Bucci et al., 2000; Bucci et al., 2002; for PER see also Corodimas and LeDoux, 1995). The robust reciprocal connectivity with PER provides POR with access to information about individual objects, and connections with other medial temporal structures also provide links to mnemonic input. Moreover, the PER is anatomically and functionally integrated with the amygdala, which is involved in emotion processing and reward learning (LeDoux, 2000; Pitkanen et al., 2000). The POR also receives very strong input from retrosplenial cortex and appears to rely on this information for contextual learning (Keene and Bucci, 2008; Robinson et al., 2012). Thus, the POR is optimally situated to combine object and pattern information from the PER with incoming contextual and spatial information from retrosplenial and posterior parietal cortices to form complex representations of specific environmental contexts.
The hippocampus is also implicated in contextual learning, so one question of interest is how the processing of contextual information differs between POR and the hippocampus proper. Results of experimental lesion studies of contextual fear conditioning suggest context is processed differently by hippocampus and POR. For example, posttraining lesions of the hippocampus are ineffective 50–100 days after training (Anagnostaras et al., 1999; Maren et al., 1997). In contrast, posttraining PER or POR lesions are effective even 100 days after training (Burwell et al., 2004). Object-location correlates similar to those described here in POR have been observed in the hippocampus. Komorowski (2009) reported that hippocampal cells signaled item-context conjunctions in a biconditional discrimination task in which the place determined which of two odor stimuli would be rewarded. In that study, item-location conjunctions developed over time as animals learned to associate items with reward. We have not examined the emergence of object-location conjunctions in the POR, but other work suggests that changes in the spatial layout of local stimuli result in immediate remapping in the POR (Burwell and Hafeman, 2003). The evidence suggests that POR supports online processing of context and provides representations of the current context to the hippocampus for the purposes of associative learning and episodic memory. This is consistent with the idea that the hippocampus is located above the PER and POR in a hierarchy of associativity (Lavenex and Amaral, 2000).
We suggest that object information in the POR arrives by the well-documented direct PER to POR pathway. Alternatively, it could be that object information arrives at the POR by an indirect pathway that involves both the PER and the hippocampus. Indeed, the PER and POR each have reciprocal connections with the entorhinal cortex and CA1 of the hippocampus, and both project to the subiculum. This alternative view, however, does not account for the function of the direct PER-POR projections. Thus, a related idea is that the PER provides object information directly to the POR and the hippocampus, but for different purposes. Object information provided to the POR would be for the purpose of representing and updating the current context. Given that most of the object information in the POR is also associated with a place, the POR seems optimized for encoding the spatial layout of objects rather than detailed features of objects. At the same time, the PER provides detailed object information to the hippocampus for the purpose of associative learning and episodic memory.
Conclusions
We propose a view of postrhinal and parahippocampal function that provides a reasonable account of the available data across species and approaches. By this view the POR combines object and feature information from the PER with spatial information from retrosplenial and posterior parietal cortices to form complex representations of the spatial layout of specific environmental contexts. Such representations would include the objects and physical features of the environment, as well as the locations of objects and features within the environment.
We further propose that the POR not only maintains a representation of the current context, but also monitors the context for changes, updating the representation of the current context when changes occur. This is consistent with hints from monkey electrophysiology and human imaging studies (Nakamura et al., 1994; Vidyasagar et al., 1991; Yi and Chun, 2005), the anatomy (Burwell et al., 1995), and evidence for a postrhinal role in attentional orienting (Bucci and Burwell, 2004). It may be that the POR signals the PER when changes in features and objects have occurred and require further processing by the PER. In addition, the increased theta in POR may reflect states in which information can be transmitted between PER and POR, as suggested by Nerad and Bilkey (2005). The representation of context maintained in the POR could be referenced for a number of purposes, for example, the facilitation of recognition of an object in a scene or place (Gronau et al., 2007), the use of contextual associations to guide behavior (Badre and Wagner, 2007), or the formation of episodic memories (Eichenbaum et al., 2007).
Our findings, together with studies in rats, monkeys, and humans, suggest a model that could account for the neural basis of context representation. By this model, the parahippocampal cortex is necessary for encoding representations of specific contexts and for updating such representations when changes occur. More specifically, the postrhinal/parahippocampal cortex 1) combines spatial information from posterior parietal and retrosplenial cortices with object information from perirhinal cortex to form representations that link objects to places, 2) collects those object-place associations into representations of a specific context including the spatial layout, 3) monitors the current context for changes, and 4) updates the representation of the current context with identified changes. The representation is made available to other regions for the binding of events with context to form episodes that are located in time, for guiding context-relevant behavior, and for recognizing objects in scenes and contexts.
Experimental Procedures
Subjects
Subjects were five male Long-Evans rats (Charles Rivers Laboratories, Wilmington, MA). Rats were singly housed in a 12:12 hr, light:dark cycle with ad libitum access to water. After arriving in the colony, animals were handled several days per week until the beginning of behavioral training. Prior to training, rats were placed on a feeding schedule to maintain body weight at 85–90% of free feeding weight. All procedures were in accordance with the appropriate institutional animal care and use committee and NIH guidelines for the care and use of animals in research.
Apparatus
The apparatus, the Floor Projection Maze (Figure 1A), consisted of an open field (81.3 × 81.3 cm) in which images were back-projected to the floor and the position of the animal was tracked from above (Furtak et al., 2009). Computer controlled pumps provided food reward (2% fat chocolate milk) to four reward ports. The maze was interfaced with integrated systems for location tracking, neuronal data acquisition, and behavioral control.
Behavioral Training
Rats were trained on a discrimination task in which pairs of 2D visual stimuli were presented on the floor of the exploratory maze. Stimuli were well within the limits of visual acuity for Long-Evans rats (Douglas et al., 2005; Furtak et al., 2009). In all phases of shaping and training in which two objects were presented, the left vs. right location of the correct stimulus was counterbalanced.
Animals were shaped in a number of steps indicated in Table S1. The final stage of shaping was exactly the same as the final task and differed only in the stimuli and the number of problems. Once an animal reached criterion (8 out of 10 trials correct for two consecutive days), the animal was advanced to the object discrimination task.
Animals were trained on two discrimination problems, each consisting of a pair of stimuli. Each stimulus was a high contrast, circular pattern. For each problem, the two stimuli were matched for area of light and dark. The two problem pairs differed in contrast and the ratio of light area to dark area (Figure 1D). Animals began with 10-trial blocks alternating between the two problems for 100 trials. Once an animal reached criterion, 10 consecutive correct trials over 2 blocks, the electrode array was implanted. Following surgery, animals were retrained on blocked trials for 100 trials per day. Recording was initiated as soon as rats were reliably performing the task. When animals were performing at > 75% correct, they were given 100 trials per day of randomly-interleaved presentations of the two problems. If performance dropped, rats were returned to blocked trials until accuracy improved. Recordings were obtained on both blocked and random presentation of stimuli.
Surgery and Histology
Under anesthesia electrodes were stereotaxically implanted in the POR as defined by Burwell (2001). The implanted microdrive assembly was produced in-house and consisted of 8 individually drivable stereotrodes (25 μm nichrome wires, A-M Systems, Inc., Carlsborg, WA). A 2.0 mm craniotomy was prepared at −0.1 mm anterior to and 5.0 mm lateral to lambda, allowing for visualization of the transverse sinus. The electrodes were inserted 300–500 μm anterior to the transverse sinus at a 22° angle along the mediolateral axis with tip pointed in the lateral direction. The electrodes were lowered 300 μm from the cortical surface and secured with dental cement, dental acrylic, and anchor screws. Rats were allowed 7 days to recover prior to behavioral training. At the end of the experiment, animals were given an overdose of Beuthanasia-D (100 mg/kg, i.p.), electrode tip placements were marked with a small lesion, the animals were perfused, and the brains were extracted and prepared for histology and subsequent localization of electrodes. The locations of electrode tips were reconstructed with a light microscope and localized in POR as defined by Burwell (2001). During recording, microdrivers were generally advanced about 1/6 turn or 55.5 μm. Total distance advanced ranged from 333 to 610.5 μm. Given this short distance and the trajectory of electrodes, we assumed that all cells recorded from a particular stereotrode were in the layer in which the tip was histologically located. See Supplementary Experimental Procedures for details.
Electrophysiology
Neuronal activity recorded from stereotrodes (McNaughton et al., 1983) was multiplied by 20 with an operational amplifier at the head stage (HST/8o50-G20-GR, Plexon, Inc., Dallas, TX). Signals were then passed through a differential preamplifier with a gain of 50 (PBX2/16sp-r-G50, Plexon, Inc.). Also at this stage, single-unit activity was filtered between 154–8,800 Hz and LFPs were filtered between 0.7–170 Hz (PBX2/16sp-r-G50, Plexon, Inc.). The signal was then digitized at 40 kHz for single-unit activity and 1 kHz for LFP activity and further amplified for a total gain of 10,000 (MAP system, Plexon, Inc.). Waveforms with signal-to-noise ratios greater than ~3:1 were extracted by real-time thresholding (Sort Client, Plexon, Inc.) and stored along with time stamps of behaviorally relevant events for offline analysis.
Analysis
Spikes associated with putative individual cells were isolated offline for each session using a variety of manual and partially automated techniques for classification based on waveform characteristics (Offline Sorter, Plexon, Inc). Separation of sorted spikes by at least 1 ms was verified by autocorrelograms. Sorted files were corrected for an identified issue of time alignment between spike data and field potential data using FPAlignV1 (Plexon, Inc.) (Nelson et al., 2008). Timestamps for spikes and behaviorally relevant event markers were extracted from sorted files using Neuroexplorer (NEX, Plexon, Inc.).
For each isolated cell, neuronal activity (spikes/s) was analyzed during four, 500 ms epochs: the ready, stimulus, selection, and the reward epochs. We used factorial ANOVA to examine selectivity of cells for behavioral events during the stimulus, selection, and reward epochs of correct trials. Between-trial variables were object (correct stimulus in problem 1 vs. problem 2), side (trials occurring in the west vs. the east), and egocentric response (left vs. right) resulting in eight possible event types for correct trials. Because of occasional side biases, the experimental design was altered such that event types were not counterbalanced for some sessions. For the factorial ANOVA only sessions in which there were at least 3 trials per event were included. In addition, only cells that met a criterion within an epoch of firing at least 20 spikes on at least half the trials were included in the analysis. A Pearson’s chi-square test was used to compare the overall distribution of postrhinal cell selectivity across epochs.
All analyses were conducted using NEX, SPSS (IBM Corporation, Somers, NY), SAS (Version 9.1.3, SAS Institute Inc., Cary, NC) or Matlab (Mathworks, Natick, MA). Level of significance was p < 0.05. Results are reported as mean ± 1 standard error.
LFP Analysis
The Chronux toolbox for Matlab was used for the multitaper spectral analysis of the LFP. The spectrum of each of the 42 LFPs (21 sessions from 5 rats) recorded from the POR was calculated over the entire session. The spectrum was also calculated independently for the task-related epochs defined above. Normalized power in a given frequency band during a particular epoch was calculated by dividing the power during that epoch by the overall power in that frequency band during the entire session. This permitted comparisons of normalized power across sessions and across rats. Spike-LFP phase relationships were analyzed by first filtering each LFP between 6–12 Hz (for theta oscillations), 30–50 Hz (for low gamma) or 70–110 Hz (for high gamma) using a symmetric 4-pole butter worth filter. The filtered LFP was then Z-scored in its entirety, setting the mean of the entire signal at 0 and the standard deviation at 1. The peak and trough of each theta or gamma cycle were identified using an extrema-detection algorithm whose sensitivity was set to detect peak-to-trough or trough-to-peak amplitudes as small as 0.2 Z.
For spike assignments to theta or gamma cycles, the start time of the cycle was defined as the time of the peak. The next peak represented the end time of that cycle. Spikes occurring within the start and end peak were assigned to the cycle. Spike phase was expressed in degrees and calculated as follows: (tspike − tstart)/(tend − tstart) * 360, such that tstart and tend are the start and end times of each theta or gamma cycle, and tspike is the time of the spike.
Circular statistics were used to analyze the spike phase distributions during the various epochs of the task (Siapas et al., 2005; Zar, 1999). In particular, the description of circular distributions formalized by von Mises was used (Zar, 1999). Von Mises distributions are characterized by a circular mean and circular concentration (κ) parameter. The higher the value of κ, the tighter the distribution is around the mean. κ is analogous (but not equivalent) to the inverse of the standard deviation of a normal distribution. The value of κ was thus the most appropriate estimate of the width of the spike phase distribution, and hence an appropriate estimate of the precision of spike times around the mean.
To determine the significance of phase locking to a particular frequency we used Rayleigh’s Z test. The null hypothesis for this Z test is that the circular distribution is uniform at all phases. The p-value for this test is approximated using the term e-Z such that a Z value of 3 and above indicates significant phase locking (Siapas et al., 2005). For cells that showed significant phase-locking, we also calculated mean phase and κ. We did not calculate κ for cells that were not significantly phase-locked because cells with a low number of spikes can exhibit an artificially large κ.
Supplementary Material
Acknowledgments
This work was funded by NSF IOB-0522220 and DARPA N66001-10-C-2010 to RDB and NIH T32-MH019118 and F32-MH084443 to SCF. SCF is now located at the Department of Psychology, California State University-Sacramento and OJA is now located at the Department of Neurology, Massachusetts General Hospital.
Footnotes
Conflicts of Interest: No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza M, Crespo-Garcia M, Cantero JL. Semantic congruence enhances memory of episodic associations: role of theta oscillations. J Cogn Neurosci. 2011;23:75–90. doi: 10.1162/jocn.2009.21358. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J Comp Neurol. 1985;232:219–228. doi: 10.1002/cne.902320207. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. Famous faces activate contextual associations in the parahippocampal cortex. Cereb Cortex. 2008;18:1233–1238. doi: 10.1093/cercor/bhm170. [DOI] [PubMed] [Google Scholar]
- Bilkey DK, Heinemann U. Intrinsic theta-frequency membrane potential oscillations in layer III/V perirhinal cortex neurons of the rat. Hippocampus. 1999;9:510–518. doi: 10.1002/(SICI)1098-1063(1999)9:5<510::AID-HIPO4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Broussard J, Sarter M, Givens B. Neuronal correlates of signal detection in the posterior parietal cortex of rats performing a sustained attention task. Neuroscience. 2006;143:407–417. doi: 10.1016/j.neuroscience.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Burwell RD. Deficits in attentional orienting following damage to the perirhinal or postrhinal cortices. Behav Neurosci. 2004;118:1117–1122. doi: 10.1037/0735-7044.118.5.1117. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114:882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav Neurosci. 2002;116:479–488. [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14:439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998a;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci. 2004;24:11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–588. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Amaral DG. Entorhinal cortex of the monkey: VII. intrinsic connections. J Comp Neurol. 2007;500:612–633. doi: 10.1002/cne.21200. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behav Neurosci. 1995;109:613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol. 1983;220:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Cho CE, Kerr KM, Barredo JL, Alleyne JE, Patterson YR, Burwell RD. The Floor Projection Maze: A novel behavioral apparatus for presenting visual stimuli to rats. J Neurosci Methods. 2009;181:82–88. doi: 10.1016/j.jneumeth.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17:709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Healey AN, Eacott MJ. Objects and positions in visual scenes: effects of perirhinal and postrhinal cortex lesions in the rat. Behav Neurosci. 2004;118:992–1010. doi: 10.1037/0735-7044.118.5.992. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Balan P, Oristaglio J, Suzuki M. Parietal control of attentional guidance: the significance of sensory, motivational and motor factors. Neurobiology of learning and memory. 2009;91:121–128. doi: 10.1016/j.nlm.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronau N, Neta M, Bar M. Integrated contextual representation for objects’ identities and their locations. J Cogn Neurosci. 2007 doi: 10.1162/jocn.2008.20027. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Curr Opin Neurobiol. 2010;20:150–155. doi: 10.1016/j.conb.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamishina H, Conte WL, Patel SS, Tai RJ, Corwin JV, Reep RL. Cortical connections of the rat lateral posterior thalamic nucleus. Brain Res. 2009;1264:39–56. doi: 10.1016/j.brainres.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral neuroscience. 2008;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Moron I, Fenton AA. Is the hippocampal theta rhythm related to cognition in a non-locomotor place recognition task? Hippocampus. 2005;15:472–479. doi: 10.1002/hipo.20071. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P. Oscillatory mechanisms of process binding in memory. Neurosci Biobehav Rev. 2010;34:1002–1014. doi: 10.1016/j.neubiorev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Witter MP, Van Haeften T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J Comp Neurol. 2003;455:156–171. doi: 10.1002/cne.10472. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Burgess N, Donnett JG, O’Keefe J. Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large-scale space. J Cogn Neurosci. 1998;10:61–76. doi: 10.1162/089892998563789. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Mason R, Groos GA. Cortico-recipient and tecto-recipient visual zones in the rat’s lateral posterior (pulvinar) nucleus: an anatomical study. Neurosci Lett. 1981;25:107–112. doi: 10.1016/0304-3940(81)90316-5. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Mizumori SJ, Barnes CA, Leonard BJ, Marquis M, Green EJ. Cortical representation of motion during unrestrained spatial navigation in the rat. Cereb Cortex. 1994;4:27–39. doi: 10.1093/cercor/4.1.27. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, O’Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983;8:391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31:7441–7449. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Naber PA, Caballero-Bleda M, Jorritsma-Byham B, Witter MP. Parallel input to the hippocampal memory system through peri- and postrhinal cortices. Neuroreport. 1997;8:2617–2621. doi: 10.1097/00001756-199707280-00039. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto K, Mikami A, Kubota K. Visual response properties of single neurons in the temporal pole of behaving monkeys. J Neurophysiol. 1994;71:1206–1221. doi: 10.1152/jn.1994.71.3.1206. [DOI] [PubMed] [Google Scholar]
- Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J Neurosci Methods. 2008;169:141–157. doi: 10.1016/j.jneumeth.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerad L, Bilkey DK. Ten- to 12-Hz EEG oscillation in the rat hippocampus and rhinal cortex that is modulated by environmental familiarity. J Neurophysiol. 2005;93:1246–1254. doi: 10.1152/jn.00199.2004. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Oleksiak A, Klink PC, Postma A, van der Ham IJ, Lankheet MJ, van Wezel RJ. Spatial summation in macaque parietal area 7a follows a winner-take-all rule. J Neurophysiol. 2011;105:1150–1158. doi: 10.1152/jn.00907.2010. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, Bucci DJ. Identification of Functional Circuitry between Retrosplenial and Postrhinal Cortices during Fear Conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12076–12086. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, O’Mara SM. View-responsive neurons in the primate hippocampal complex. Hippocampus. 1995;5:409–424. doi: 10.1002/hipo.450050504. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Sato N, Nakamura K. Visual response properties of neurons in the parahippocampal cortex of monkeys. J Neurophysiol. 2003;90:876–886. doi: 10.1152/jn.01089.2002. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994a;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994b;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar TR, Salzmann E, Creutzfeldt OD. Unit activity in the hippocampus and the parahippocampal temporobasal association cortex related to memory and complex behaviour in the awake monkey. Brain Res. 1991;544:269–278. doi: 10.1016/0006-8993(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Weniger G, Irle E. Posterior parahippocampal gyrus lesions in the human impair egocentric learning in a virtual environment. Eur J Neurosci. 2006;24:2406–2414. doi: 10.1111/j.1460-9568.2006.05108.x. [DOI] [PubMed] [Google Scholar]
- Weniger G, Siemerkus J, Schmidt-Samoa C, Mehlitz M, Baudewig J, Dechent P, Irle E. The human parahippocampal cortex subserves egocentric spatial learning during navigation in a virtual maze. Neurobiol Learn Mem. 2010;93:46–55. doi: 10.1016/j.nlm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010a;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front Hum Neurosci. 2010b;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi DJ, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 4. New Jersey: Prentice Hall; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.