Abstract
Degeneration of the neural retina is the leading cause of untreatable blindness in the developed world. Stem cell replacement therapy offers a novel strategy for retinal repair. Postmitotic photoreceptor precursors derived from the early postnatal (P) retina are able to migrate and integrate into the adult mouse retina following transplantation into the subretinal space, but it is likely that a large number of these cells would be required to restore vision. The adult recipient retina presents a very different environment to that from which photoreceptor precursor donor cells isolated from the developing postnatal retina are derived. Here we considered the possibility that modulation of the recipient environment by ectopic expression of developmentally regulated growth factors, normally present during photoreceptor development, might enhance the migration and integration of transplanted cells into the adult neural retina. Adeno-associated viral (AAV) vectors were used to introduce three growth factors previously reported to play a role in photoreceptor development, IGF1, FGF2, and CNTF, into the adult retina, prior to transplantation of P4 cells derived from the Nrl.GFP+ve neural retina. At 3 weeks posttransplantation the number of integrated, differentiated photoreceptor cells present in AAV-mediated neurotrophic factor-treated eyes was assessed and compared to control treated contralateral eyes. We show, firstly, that it is possible to manipulate the recipient retinal microenvironment via rAAV-mediated gene transfer with respect to these developmentally relevant growth factors. Moreover, when combined with cell transplantation, AAV-mediated expression of IGF1 led to significantly increased levels of cell integration, while overexpression of FGF2 had no significant effect on integrated cell number. Conversely, expression of CNTF led to a significant decrease in cell integration and an exacerbated glial response that led to glial scarring. Together, these findings demonstrate the importance of the extrinsic environment of the recipient retina for photoreceptor cell transplantation and show for the first time that it is possible to manipulate this environment using viral vectors to influence photoreceptor transplantation efficiency.
Keywords: Photoreceptor, Retina, Transplantation, Neurotrophic factors, Gene therapy, Stem cell
INTRODUCTION
Photoreceptor death is an irreversible process and represents one of the largest causes of untreatable blindness in the developed world. There are currently no effective treatments available to restore lost photoreceptors. Replacement of the lost cells by transplantation of healthy donor photoreceptor cells has been a long-standing goal. We have previously shown that rod photoreceptor transplantation is possible, provided that the donor cells are at an appropriate stage of development; they must be postmitotic rod precursor cells (45) and not proliferating progenitor or stem cells. Subretinally transplanted rod precursor cells, as identified by the expression of the rod-specific transcription factor, neural retina-specific leucine zipper protein (Nrl) (47,66), are able to integrate specifically within the outer nuclear layer (ONL) of the adult recipient retina and differentiate into functional and synaptically connected rod photoreceptors. This suggests that there are sufficient environmental cues present in the adult retina to permit photoreceptor precursor migration and the completion of differentiation, albeit at lower levels than that found in the developing retina at the time of rod photoreceptor genesis. However, the number of cells that integrate is markedly lower than the number transplanted (<0.4% in our earlier studies) (45,53,74).
Previous reports have shown that the integration of neural progenitors (brain- and retinal-derived cells) into the opossum (60–62) and rat (48) retina is significantly better when transplanted into immature recipients compared with adult recipients. The early postnatal opossum retina maintains an almost fetal-like environment, compared with the relatively mature postnatal murine retina. The determination and differentiation of rod photoreceptors is understood to result from the interaction of both intrinsic transcriptional complexes and extrinsic environmental factor-related signaling mechanisms (13,25, 42,75,76). We therefore considered the possibility that the integration of transplanted photoreceptors might be improved by manipulating the extrinsic microenvironment of the host retina toward a more developmental-like state.
The proliferation and differentiation of photoreceptors is profoundly affected by a number of secreted factors (38). Here, we examined three factors known to be important for photoreceptor development and neuroprotection: insulin-like growth factor 1 (IGF1), fibroblast growth factor 2 (FGF2), and ciliary neurotrophic factor (CNTF). Systemically present, IGF1 is mainly produced by the liver in response to pituitary growth hormone. However, a number of other tissues can also produce IGF1 locally, including the CNS (6). IGF1 and its receptor IGF1-R are widely expressed in the CNS (5,56). Consistent with a role in mammalian retinal developmental, IGF1 expression is highest in the postnatal retina compared with the adult retina (2,10). Studies involving transgenic mice lacking a downstream component of the insulin and IGF1 signaling cascades, irs2, have highlighted the importance of these pathways in postnatal retinal development, particularly in promoting the maturation and survival of photoreceptors immediately after birth (78).
Like IGF1, FGF2, and CNTF are also present during retinal development and have been shown to affect photoreceptor differentiation (21,27,34,35,82). FGF2 has been reported to have different effects depending upon the developmental stage of the cells to which it is applied. In embryonic cells isolated from E15–E18 rat retinae, FGF2 induced an increase in proliferation that declined with age, although it had little effect on photoreceptor fate as assessed by the number of rhodopsin-positive cells (38,41,82). Conversely, when applied to cultures derived from P0 retina, FGF2 led to an increase in the proportion of rhodopsin-positive cells, an effect that again declined with maturation (27). It also reportedly promotes photoreceptor survival in vitro (50). In the Xenopus retina, overexpression of FGF2 led to increased numbers of rod photoreceptors at the expense of cone photoreceptors (52) and appears to play a similar role in the macaque retina (16). FGF2 has also been reported to induce the migration of oligodendrocyte precursor cells in optic nerve myelination (7). Thus, FGF2 signaling appears to play a role in retinal cell proliferation, migration, and photoreceptor fate specification.
We have previously shown that the efficiency of transplantation declines with maturation of the donor cell population (45). CNTF plays a number of complex roles both in development and in the degenerating retina. Recent studies have shown that CNTF acts transiently to suppress photoreceptor differentiation and rhodopsin expression by the activation of the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathways (26,57). Earlier studies showed that CNTF suppressed rod differentiation at the expense of bipolar cell differentiation (21). CNTF is also upregulated in the degenerating retina (72,79), the ultimate recipient retina for cell transplantation therapy, and has been reported to have both neuroprotective (29,31) and inhibitory effects on photoreceptor survival and function (58). Thus, it is important to assess its impact on transplanted photoreceptor differentiation, integration, and survival and whether it enhances or impedes photoreceptor transplantation.
Here we assessed the effects of these three developmentally regulated neurotrophic factors on rod photoreceptor precursor cell transplantation and integration. IGF1, FGF2, and CNTF were overexpressed in recipient retinae by the use of adeno-associated viral vectors (serotype 2; AAV2/2) delivered to the inner retina prior to cell transplantation. We found that the overexpression of these growth factors influences the integration of transplanted photoreceptor precursors. This demonstrates that the extrinsic environment of the host retina is an important consideration for photoreceptor transplantation, as well as the intrinsic developmental stage of the donor cell population. It also provides proof of principle that it is possible to manipulate the recipient retinal environment via AAV-mediated gene transfer to improve transplanted photoreceptor integration and/or survival.
MATERIALS AND METHODS
Animals
C57Bl/6J and Nrl.gfp+/+ mice were maintained in the animal facility at University College London. All experiments have been conducted in accordance with the Policies on the Use of Animals and Humans in Neuroscience Research. Adult mice were 6–15 weeks old and kept on a standard 12-h light–dark cycle.
Viral Vector Production and Testing
The neurotrophic factor constructs all contained cytomegalovirus promoters (CMV) driving the expression of the relevant murine neurotrophin transgenes. A secretion signal was added to the CNTF transgene as previously described (44). The reporter construct used as the control vector contained a chicken β-actin promoter (CBA) driving a red fluorescent protein (RFP) coding domain. Constructs were subcloned by relevant restriction enzyme digestion (Promega, Southampton, UK) and T4 DNA ligation (New England BioLabs, Wilbury, UK), prior to competent cell transformation. A construct containing the cDNA coding region of mouse fibroblast growth factor 2 (FGF2) was purchased from Genscript (New Jersey, USA) and was used to subclone the FGF2 construct. Recombinant adeno-associated virus particles were produced by transient transfection of baby hamster kidney (BHK) cells with the vector constructs described above and the replicating herpes simplex virus (HSV) amplicon pHAV7.3, as previously described (4). Each batch of recombinant AAV (rAAV) produced was titered by dot-blot assay to give the number of vector genome copies per milliliter of virus. For transfection, 293T cells were incubated for 4 h with a mixture of peptide 6, construct DNA, and Lipofectin reagent (all Invitrogen, Paisley, UK). For transduction, cultured 293T cells were incubated with rAAV and wild-type adenovirus for 48 h. Only wild-type adenovirus was added to control wells. The culture medium from AAV2/2 CMV.IGF1, AAV2/2 CMV.FGF2, and AAV2/2 CMV.CNTF transduced cells was collected and tested for the presence of secreted IGF1, FGF2, or CNTF protein by sandwich ELISA, respectively (Table 1). No IGF1, FGF2, or CNTF secretion was detected from AAV2/2 CBA.RFP transduced cells, untransduced cells, or the cell culture medium.
Table 1.
In vitro Testing of Neurotrophic Factor Viral Vectors
| IGF1 | FGF2 | CNTF | |
|---|---|---|---|
| pg/ml | 1.31 ± 0.05 | 44.5 ± 2.5 | 101.8 ± 0.5 |
| N | 4 | 4 | 4 |
Levels of secreted insulin-like growth factor (IGF1), fibroblast growth factor (FGF2), and ciliary neurotrophic factor (CNTF) protein in the culture media from adeno-associated virus vector serotype 2 cytomegalovirus.IGF1 (AAV2/2 CMV.IGF1), AAV2/2 CMV.FGF2, and AAV2/2 CMV.CNTF transduced cells, as assessed by sandwich ELISA. Mean ± SEM. N = number of independent wells.
IGF1 and FGF2 concentrations were detected by Quantikine® kits following the manufacturer’s instructions (R&D Systems, Abington, UK), and CNTF concentrations were measured using R&D Systems’ recommended protocol and individual reagents: monoclonal anti-rat CNTF capture antibody (clone 34726, MAB5571), biotinylated anti-rat CNTF detection antibody (BAF557), and recombinant rat CNTF for standards (557-NT/CF, R&D Systems, UK).
Administration of Viral Vectors and Functional Analysis
Injections were performed in 6–8-week-old C57Bl/6J mice, as previously described (44). Mice were anesthetised with a single IP injection of 0.15 ml of a mixture of Dormitor (1 mg/ml medetomidine hydrochloride, Pfizer Pharmaceuticals, Kent, UK), ketamine (100 mg/ml, Fort Dodge Animal Health, Southampton, UK), and water in the ratio of 1:0.6:84. The pupils were dilated using drops of tropicamide (1%) and eyes kept moist using Viscotears™ (both Rigcharm Pharmacy, London, UK). Surgery was performed under direct retinoscopy through an operating microscope. An 8-mm, 34-gauge hypodermic needle (Hamilton, Switzerland) was inserted through the sclera at the inferior pars plana and positioned towards the superior retina in the vitreal cavity. Virus suspension (1 μl) was injected intravitreally per eye. Intravitreal injections were used to administer the virus as they are minimally invasive, can be targeted to the region of interest, and are known to permit efficient transduction of the nearby neural retina. Electroretinographs (ERGs) from treated mice were recorded in a standardized fashion 6–7 weeks post-rAAV injection. All animals were dark adapted overnight (16 h) prior to recording and anesthetized, as above. Ganzfeld ERGs were obtained as described previously (8,63). Briefly, single flash recordings from both eyes were simultaneously obtained at light intensities increasing from 0.1 millicandelas (mcds)/m2 to 3,000 mcds/m2. Ten responses per intensity level were averaged with an interstimulus interval of 5 s (0.1, 1, 10, 100 mcds/m2) or 5 responses per intensity with a 17-s interval (1,000 and 3,000 mcds/m2) to try and minimize the dark-adapted conditioning flash effect. The b-wave (a-wave through to b-wave peak) amplitude at 100 mcds/m2 was used for statistical analysis. The b-wave values of the treated (right) eye were paired with the untreated contralateral (left) eye. This method controls for the interanimal and test–retest variance that may be present in rodent ERGs.
Dissociation and Transplantation of Retinal Cells
Dissociated cells were prepared from postnatal day (P) 3–5 Nrl.gfp+/+ mice, in which green fluorescent protein (GFP) expression is driven by the promoter of Nrl, a transcription factor specific for postmitotic rod precursors that persists in adult rods (1,47), as described previously (45). Briefly, neural retinae were dissociated using a papain-based kit (Worthington Biochemical, Lorne Laboratories, UK) and single cells were resuspended at a concentration of 400,000 cells/μl in sterile Earle’s balanced salt solution (EBSS) and DNase (0.05%), immediately prior to injection. Animals were anesthetized and surgery was performed under direct ophthalmoscopy, as above. A hypodermic needle was loaded with cells (1 μl) before inserting tangentially through the superior sclera into the subretinal space, between the retinal pigment epithelium (RPE) and the neural retina. Cell suspensions were injected slowly to produce a standard and reproducible retinal detachment in the superior hemisphere. The needle was withdrawn, leading to a self-sealing of the wound tunnel.
Histology and Immunohistochemistry
Mice were sacrificed ~3 weeks after transplantation and the eyes fixed in 4% paraformaldehyde (with the exception of those used for CNTF staining, which required a protocol involving no fixation) before cryoprotecting in 20% sucrose and embedding in OCT (RA Lamb, Eastbourne, UK). Cryosections were cut (18 μm thick) and all sections were collected for analysis. For immunohistochemistry, sections were air dried and rinsed with Tris-buffered saline (TBS) before being pre-blocked in TBS containing normal goat serum (5%) and bovine serum albumin (1%) at room temperature (RT), and incubated with primary antibody overnight at 4°C. After washing, sections were incubated with secondary antibody for 2 h at RT, washed, and counterstained with Hoechst 33342 (Sigma-Aldrich, Gillingham, UK). Negative controls omitted the primary antibody. Primary antibodies used are all highly cited and tested and included rabbit anti-IGF1 (1:500), sheep anti-CNTF (1:500), rabbit anti-active caspase 3 (1:20), rat anti-F4/80 (1:250) (all Abcam, Cambridge, UK), rabbit anti-glial fibrillary acidic protein (GFAP; 1:200) (Dako, Ely, UK), and mouse anti-FGF2 (1:100) (Upstate Biotech, Milton Keynes, UK). Secondary antibodies included anti-mouse Alexa 546, anti-mouse Alexa 633, anti-rabbit Alexa 546, anti-rabbit Alexa 594, anti-rabbit Alexa 633, and anti-sheep Alexa 546 (all 1:500; Molecular Probes Inc.), as appropriate. Finally, the nuclear dye Hoechst 33342 (10 μM; Sigma-Aldrich) was applied for 10 min at room temperature, followed by three washes with PBS prior to mounting with citifluor AF-1 (Electron Microscopy Science) and cover-slipping.
Retinal sections were viewed on a confocal microscope (Zeiss LSM510 or Leica SPE). GFP-positive cells were located using epifluorescence illumination before taking a series of x–y optical sections, approximately 0.5 μm apart, throughout the depth of the section. Individual x–y scans were built into a stack to give an x–y projection image. LSM image browser software was used to compile and export the captured images.
Cell Counts
The average number of integrated cells per eye was determined as described previously (53,74). Briefly, cells were considered to be integrated if the whole cell body was correctly located within the ONL and at least one of the following was visible: spherule synapse, inner/outer processes, and/or inner/outer segments. The average number of integrated cells per eye was determined by counting all the integrated Nrl.GFP+ve cells in alternate serial sections through each eye. This was doubled to give an estimate of the mean number of integrated cells per eye. To account for variability resulting from interanimal variation, the ratio of the number of cells in the treated eye compared with the contralateral control eye was also calculated for each individual mouse. This revealed the average fold difference in the number of integrated transplanted photoreceptors for each animal as a result of the relevant treatment. Cell counts for individual eyes were excluded from the analysis if there were cells in the vitreous, indicative of accidental intravitreal transplantation of the cells, or there was no cell mass present in the subretinal space and surgical notes indicated excessive reflux of the cells transplanted into that eye. For caspase 3 cell counts entire coronal retinal sections were quantified for the presence of caspase 3-positive cells in the neural retina. Six or more alternate sections encompassing the superior retina were quantified per eye (IGF treated and control) for each animal.
Statistical Analysis
All means are presented ±SEM, unless otherwise stated; N, number of animals, unless otherwise stated; n, number of eyes or sections examined, where appropriate. The statistical test used to compare cell counts in contralateral eyes was a two-tailed paired t-test with a significance threshold of p < 0.05. The statistical program used for the analysis of all data was GraphPad Instat 3 (GraphPad Software, Inc, La Jolla, USA), with the majority of graphs prepared in Microsoft Excel. *p < 0.05, **p < 0.01, ***p < 0.001.
RESULTS
Neurotrophic Factors Present in the Developing Postnatal Retina
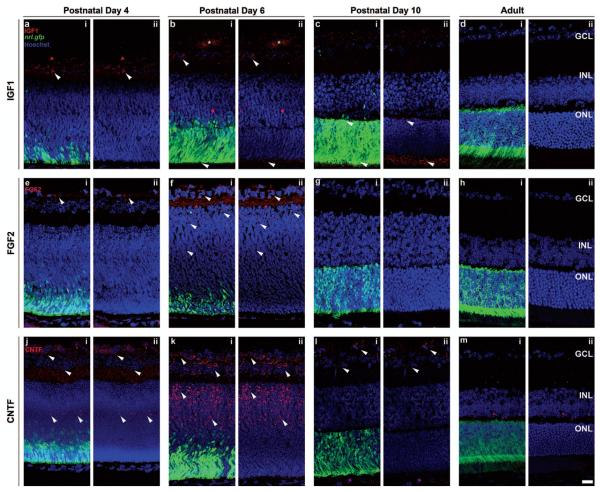
We first sought to establish the presence and localization in the early postnatal retina of three neurotrophic factors previously reported to play a role in photoreceptor development and maturation, IGF1, FGF2, and CNTF (Fig. 1). We assessed this in the Nrl.gfp mouse, to provide a comparison with rod photoreceptor development over the same period. Nrl is a transcription factor essential for the acquisition and maintenance of rod photoreceptor fate (47,66) and in the Nrl.gfp mouse, GFP labels all postmitotic rod photoreceptors (1). At P4, the first few Nrl.gfp+ve (green) postmitotic rod precursors could be seen accumulating at the outer edge of the retina (Fig. 1a,i, e,i, j,i). At this time, expression of IGF1 and FGF2 was observed at the inner edge of the retina (Fig. 1a, e; white arrowheads; red) while CNTF was present in a few cells at the center of the neuroblastic layer which extended processes, as well as the inner retina (Fig. 1j; white arrowheads; red). By P6, the presumptive ONL was seen, with greater numbers of Nrl.gfp+ve rod precursors (green) present at the outer edge (Fig. 1b,i, f,i, k,i). Inner segment formation (Fig. 1b,i; lower white arrowhead) was observed and IGF1 expression was present both in this region (Fig. 1b,ii; lower white arrowhead; red) and in the developing hyaloid vessels in the vitreous (Fig. 1b; asterisk), as well as being maintained at the inner edge of the retina (Fig. 1b; upper white arrowhead; red). FGF2 expression was also still present at the inner edge of the retina and in the neuroblastic layer (Fig. 1f; white arrowheads; red). CNTF was present throughout the inner retina adjacent to the presumptive ONL (Fig. 1k; white arrowheads; red). By P10, the ONL is distinct and densely populated (Figure 1c.i, g.i, l.i; green). IGF1 expression was much reduced in the inner retina, although still present in the outer plexiform layer (Fig. 1c,ii; upper white arrowhead; red) and the developing inner segments (Fig. 1c,ii; lower white arrowhead; red). Little or no FGF2 expression was seen in the retina by this stage (Fig. 1g; red) and CNTF expression was restricted to the innermost edge of the retina only (Fig. 1l; white arrowheads; red). Finally, the adult eye shows no expression of any of the three factors examined (Fig. 1d, h, m; red). These results demonstrate the presence of IGF1, FGF2, and CNTF in the developing postnatal mouse retina at the stage of rod photoreceptor precursor birth, migration, maturation, and synaptogenesis but not in the adult retina.
Figure 1.
Localization of neurotrophic factors expressed in the developing and adult retina. Single confocal images of retinal sections from postnatal day 4 (a, e, j), 6 (b, f, k), 10 (c, g, l), or adult (d, h, m) neural retina-specific leucine zipper protein-green fluorescent protein (Nrl.gfp) mice. Sequential staining for insulin-like growth factor (IGF1) (a, b, c, d; red), fibroblast growth factor 2 (FGF2) (e, f, g, h; red), and ciliary neurotrophic factor (CNTF) (j, k, l, m; red) to examine the presence and localization of these neurotrophins (i, ii; red; white arrowheads) at the time of rod photoreceptor (i; Nrl.gfp, green) differentiation and integration. Nuclei were counterstained with Hoechst 33342 (blue). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars: 50 μm.
Neurotrophic Factor Overexpression In Vivo by AAV Viral Vectors
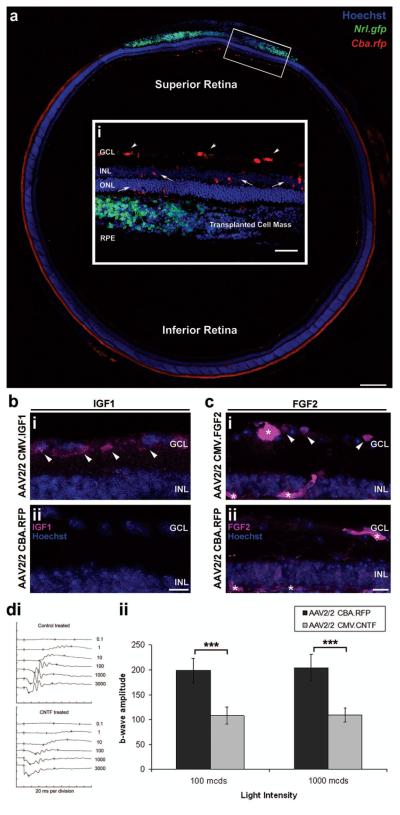
We next sought to determine whether or not it is possible to manipulate the recipient adult retinal environment to overexpress these neurotrophic factors (Fig. 2). AAV2/2 viral vectors (2 × 1011 particles/ml) encoding either the control RFP or the neurotrophic factor IGF1, FGF2, or CNTF transgenes were administered by intravitreal injection. By targeting the superior retina with the intravitreal injection of rAAV it was possible to transduce the prospective site of cell transplantation, the superior retina, to greater levels than the rest of the retina. This can be seen in the control AAV2/2 CBA.RFP virus-treated eyes, by the expression of rfp in transduced ganglion and inner retinal cells of the superior retina (Fig. 2a,i; red). The cell mass can be seen in the subretinal space of the superior retina, with Nrl.gfp+ve cells present (Fig. 2a,i; green). The cell types transduced by intravitreal administration of AAV2/2 viral particles are shown in the magnified inset (Figure 2a,i; red), and include ganglion cells (white arrowheads), inner retinal cells, and occasionally photoreceptors (white arrows). By targeting the administration of AAV2/2 viral vectors in this way it is possible to efficiently transduce the prospective area of cell transplantation, and therefore ensure maximum transgene expression at this site.
Figure 2.
In vivo investigation of neurotrophic factor expression by AAV2/2 viral vectors. Montage of confocal images (a) and a projection confocal image (a,i), from a coronal section of an adeno-associated virus vector serotype 2 chicken β-actin promoter red fluorescent protein (AAV2/2 Cba.rfp)-treated wild-type retina. (a) The control AAV2/2 CBA.RFP virus was intravitreally injected via a needle inserted through the sclera, at the inferior pars plana, and directed towards the superior retina. This resulted in the targeted transduction of inner retinal cells of the superior retina (a; red cells), at the site of cell transplantation (a,i,; Nrl.gfp; green cell mass). (a,i) The highlighted region is magnified to show the cell types transduced by the intravitreal injection of AAV2/2 viral vectors (inset i; red cells). Ganglion cells (i; white arrowheads), inner retinal neurons, and occasionally photoreceptors (i; white arrows) are transduced by the intravitreal administration of AAV2/2 viral vectors. Scale bar: 200 μm, inset 100 μm. (b) Projection confocal images of retinal sections from eyes that had been transduced with either AAV2/2 CMV.IGF1 (CMV = cytomegalovirus) (b,i) or the control AAV2/2 CBA.RFP (b,ii) viral vectors, were stained for IGF1. Increased IGF1 protein was only seen in the AAV2/2 CMV.IGF1-treated retina, at the ganglion cell layer (b,i; pink; white arrowheads). Scale bar: 20 μm. (c) Projection confocal images of retinal sections from eyes that had been transduced with either AAV2/2 CMV.FGF2 (c,i) or AAV2/2 CBA.RFP (c,ii) viral vectors were stained for FGF2. Increased FGF2 protein was observed in the GCL (c,i; pink; white arrowheads) of the AAV2/2 CMV.FGF2-treated retina only. However, nonspecific staining of the blood vessels (c,i,ii; pink; asterisks) was present in both. Scale bar: 20 μm. Nuclei were counterstained with Hoechst 33342 (blue). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. (d,i) Electroretinograph traces showing a representative scotopic intensity series for both AAV2/2-treated eyes. (d,ii) Histogram demonstrating significant differences in b-wave amplitudes at both 100 and 1,000 mcds/m2 in AAV2/2 CMV.CNTF-treated eyes (mean amplitudes ± SEM; ***p < 0.001, paired t-test; N = 6).
To establish that administration of AAV2/2 viral vectors to the adult retina resulted in increased expression of the relevant neurotrophic factors, immunohistochemistry was performed on retinal sections. As seen in uninjected adult wild-type retinae (Fig. 1d, h), little or no IGF1 or FGF2 staining was observed in any of the AAV2/2 CBA.RFP-treated retinae examined (N = 4) (Fig. 2b,ii, c,ii). In contrast, at 9–10 weeks postinjection increased IGF1 staining was consistently observed in AAV2/2 CMV.IGF1-treated retinae, with the majority of expression seen in and around the ganglion cells of the superior retina (Fig. 2b,i; white arrowheads). The pattern of IGF1 expression seen in AAV2/2 CMV.IGF1-treated retinae was comparable with that seen in P4–6 developing retinae (Fig. 2b.i, Fig. 1a, b; red), the time at which Nrl.gfp+ve precursor cells migrate to their mature locations in the developing retina and the developmental stage from which the donor precursor cells are isolated for transplantation. Staining for FGF2 demonstrated expression in the GCL of AAV2/2 CMV.FGF2-treated retinae (Fig. 2c,i; white arrowheads). Some FGF2 expression was also observed in Müller cell bodies of the inner nuclear layer (INL) in both AAV2/2 CMV.FGF2 and control AAV2/2 CBA.RFP-treated retinae. It was not possible to stain for CNTF. However, the presence of significantly increased levels of CNTF is known to suppress the scotopic electroretinogram (ERG) response (8,46,63). We therefore examined the scotopic ERG response of AAV2/2 CMV.CNTF-treated eyes, compared with CBA.RFP-treated contralateral control eyes to ascertain the secretion of CNTF (Fig. 2d). Significantly reduced b-wave amplitudes were observed in the CMV.CNTF-treated eyes, compared with CBA.RFP-treated contralateral controls (p < 0.001, paired t-test, N = 6) (Fig. 2d,i,ii), an effect characteristic of high levels of secreted CNTF in adult eyes (8,46) and suggests that increased secretion of CNTF is present in CMV. CNTF-treated eyes only. By contrast, overexpression of IGF1 or FGF2 had no discernible effects on the scotopic ERG response (data not shown).
The Effects of IGF1 Overexpression on Transplanted Photoreceptor Precursors
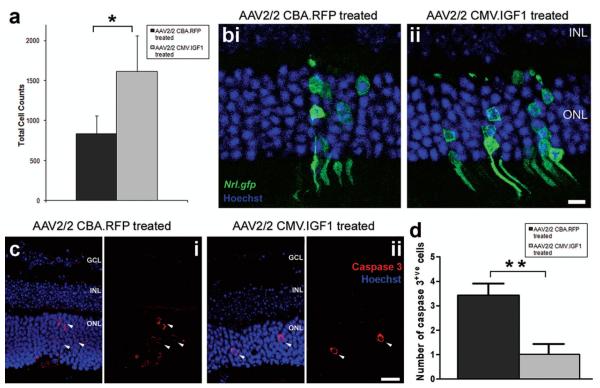
We next examined what impact increased IGF1 expression in the adult recipient retina had on the number of photoreceptor precursor cells integrating following subretinal transplantation (Fig. 3). AAV2/2 CMV. IGF1 was administered by intravitreal injection targeted towards the superior retina. At 6–7 weeks post-rAAV injection, dissociated retinal cells from P3–5 Nrl.gfp+/+ mice were transplanted to the superior subretinal space. Mice were sacrificed 3–4 weeks posttransplantation and the number of integrated photoreceptor precursor cells quantified. A significant increase in photoreceptor precursor cell integration was observed in the AAV2/2 CMV.IGF1-treated eyes, compared to the contralateral, AAV2/2 CBA.RFP-treated control eyes (Fig. 3a). Integrated photoreceptor number in the IGF-1-treated eyes was double that seen in the control eyes (1,614 ± 446 vs. 833 ± 226 cells, respectively; p < 0.05, paired t-test, N = 7) (Fig. 3a). To reduce interanimal variation, the ratio of integrated cell counts was calculated for each pair and averaged, giving a mean fold change of 2.6 ± 0.8. Normal photoreceptor morphology was observed in all eyes, with the usual pattern of clustered integration around the transplantation site (Fig. 3b,i, ii), as previously seen (53,74). The levels of integration seen in control AAV2/2 CBA.RFP-treated retinae were very similar to those we and others have previously reported for transplantation into naive adult wild-type recipients (45,53,73,74). This demonstrates that transduction of the host retina with AAV2/2 by intravitreal injection has no detrimental effect on cell integration and also confirms that the enhancement of precursor cell integration in AAV2/2 CMV.IGF1-treated retinae was not due to AAV2/2 transduction but instead was related to the increased levels of IGF1 in the adult retina. It is worth noting that the increase in the levels of IGF1 noted here following AAV2/2 CMV.IGF1 administration are relatively modest. It will be of interest to determine whether or not higher levels of expression increase, or indeed potentially decrease, the improvement seen further.
Figure 3.
The effects of IGF1 overexpression on transplanted photoreceptor precursors and the recipient retina. (a) Histogram showing the total cell counts for AAV2/2-treated eyes. Significant enhancement of cell integration was observed for the AAV2/2 CMV.IGF1-treated eyes (mean ± SEM; *p < 0.05, paired t-test; N = 7). (b) Projection confocal images of retinal sections from eyes that have been transduced with either AAV2/2 CBA.RFP (b,i) or AAV2/2 CMV.IGF1 (b,ii), and received a subretinal cell transplantation. Integrated cells are present in both retinas (b,i,ii; Nrl.gfp; green). Scale bar: 10 μm. (c) Projection confocal images of retinal sections from eyes that have been transduced with either AAV2/2 CBA.RFP (c,i) or AAV2/2 CMV.IGF1 (c,ii), and stained for the apoptotic cell marker caspase 3 (red). Greater numbers of apoptotic cells (red; white arrowheads) were present in the control treated retina (c,i) compared to the IGF-treated retina (c,ii). Nuclei were counterstained with Hoechst 33342 (blue). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar: 20 μm. (d) Histogram demonstrating a significant difference in the average number of caspase 3-positive cells per retinal section in the AAV2/2-treated eyes (cells/section ± SEM; **p < 0.01, paired t-test; N = 7).
As IGF1 signaling is known to inhibit caspase 3 activation and subsequent neuronal apoptosis (3,28), we considered the possibility that the increased integrated cell number may be due to a reduction in apoptosis of these cells following transplantation. The number of caspase 3-positive apoptotic cells present in the ONL was quantified per section (n > 6) for IGF1-treated and control-treated eyes. In each case, apoptotic cells were observed only around the site of cell transplantation (Fig. 3c,i, ii, white arrowheads). However, apoptotic cells were present in significantly higher numbers in the control-treated retinae, compared to IGF1-treated retinae, the latter only rarely containing apoptotic cells (3 ± 1 vs. 1 ± 1 cells/section; p < 0.01, paired t-test; N = 7) (Fig. 3d). Thus, the levels of IGF1 resulting from AAV2/2 CMV.IGF1 administration appear to be sufficient to reduce photoreceptor cell death in the recipient ONL retina and may also increase the survival of the integrated transplanted photoreceptor precursors.
The Effects of FGF2 Overexpression on Transplanted Photoreceptor Precursors
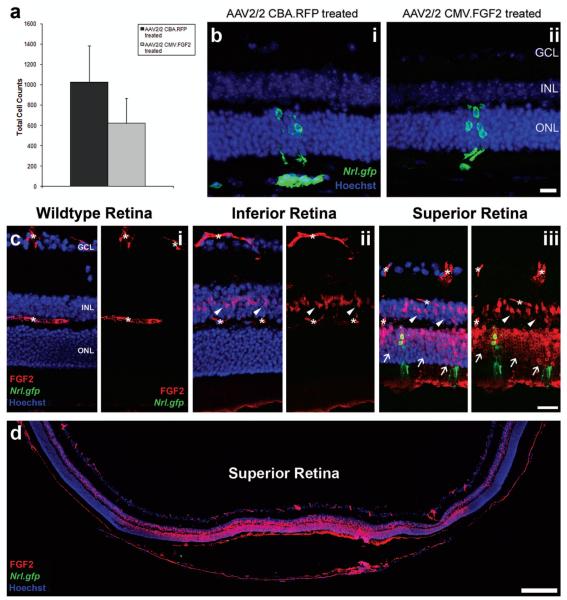
We examined the effect that increased FGF2 expression had on photoreceptor precursor cell integration into the adult retina (Fig. 4). The total number of integrated rod photoreceptors in FGF2-treated retinae was slightly reduced, compared with the control treated (Fig. 4a), although not significantly so (621 ± 247 vs. 1,024 ± 358 cells/eye, respectively; p > 0.05, paired t-test, N = 4). To reduce interanimal variation the ratio of integrated cell counts was calculated for each pair and averaged to give a mean relative ratio of 0.6 ± 0.3, showing little difference between control and treated eyes. Normal photoreceptor morphology was observed in both groups (Fig. 4b,i, ii; green).
Figure 4.
The effects of FGF2 overexpression following retinal cell transplantation. (a) Histogram showing the total cell counts for AAV2/2-treated eyes. No significant difference in cell integration was observed for the AAV2/2 CMV.FGF2-treated eyes (mean ± SEM; *p > 0.05, paired t-test, N = 4). (b) Projection confocal images of retinal sections from eyes that have been transduced with either AAV2/2 CBA.RFP (b,i) or AAV2/2 CMV.FGF2 (b,ii), and received a subretinal cell transplantation. Integrated cells are present in both retinas (b,i, ii; Nrl.gfp; green). Scale bar: 20 μm. (c) Projection confocal images of retinal sections from a wild-type eye and transplanted eyes, respectively. No detectable FGF2 was present in the untreated wild-type retina (c,i; red); asterisks denote nonspecific blood vessel staining. Increased FGF2 was observed in Müller cell bodies in the inferior and superior hemispheres of a normal transplanted eye (c,ii, iii; white arrowheads). Integrated cells were present in the superior retina (c,iii; Nrl.gfp; green), the site of cell transplantation, as well as increased levels of FGF2 in the ONL (c,iii; red; white arrows). Scale bar: 50 μm. (d) Montage of single confocal images showing the increased levels of FGF2 (red) at the site of cell transplantation, the superior hemisphere. Nuclei were counterstained with Hoechst 33342 (blue). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar: 200 μm.
Retinal detachment, degeneration, and injury are all known to upregulate the expression of FGF2 in the mouse retina (11). Therefore, to establish the difference in FGF2 expression in both AAV2/2-treated groups in comparison with untreated retinal cell transplants, FGF2 immunohistochemistry was performed. As shown previously (Fig. 1h), no FGF2 staining was observed throughout the retina in nonprocedured wild-type mice, the only staining seen being nonspecific secondary staining of the blood vessels (Fig. 4c,i; asterisks). The inferior retina of a transplanted eye without ectopic FGF2 expression showed FGF2 staining of Müller cell bodies in the inner nuclear layer only (Fig. 4c,ii; red; white arrowheads). In contrast, the superior retina, the site of cell transplantation, exhibited very strong FGF2 expression (Fig. 4c,iii, red). Expression was seen throughout the INL, including Müller cell bodies, and the ONL (Fig. 4c,iii; red; white arrowheads and white arrows, respectively). Integrated rod photoreceptors could also be seen in the superior ONL (Fig. 4c,iii; green). Similar FGF2 staining was also observed in both AAV2/2.CMV.FGF2-treated and AAV2/2 CBA.RFP-treated control eyes and in eyes receiving sham subretinal detachments of PBS alone (data not shown).
By examining the whole eyecup, we found that the FGF2 expression in the ONL was confined to the area of retinal detachment (Fig. 4d; red). However, FGF2 expression in Müller cell bodies, present in the INL, was observed in the entire coronal retinal section. The increased endogenous FGF2 staining seen around the site of surgery in the superior retina was significant, in keeping with previous observations in retinal injury models (10,80). Indeed, using this method of detection, the endogenous levels of FGF2 appeared to be far greater than the transgenic FGF2 expressed in the inner retina following rAAV transduction (compare FGF2 staining in Fig. 2c,i and 4c,iii). This may explain the lack of any effect observed in the AAV2/2 CMV.FGF2-treated eyes, in terms of integrated photoreceptor precursor cell numbers.
The Effects of CNTF Overexpression on Transplanted Photoreceptor Precursor Integration
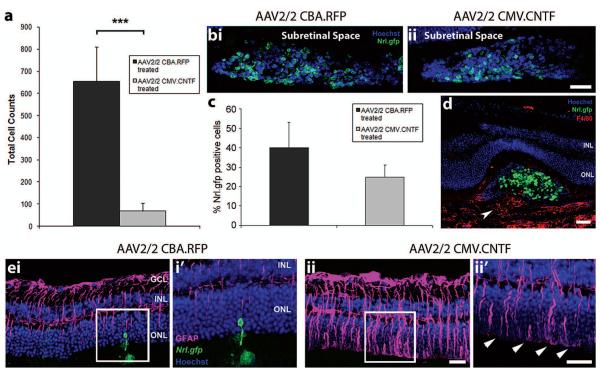
Finally, we investigated the effects of CNTF on photoreceptor precursor cell integration in the adult retina (Fig. 5). Transplanted photoreceptor precursor integration was severely reduced in the AAV2/2 CMV.CNTF-treated eyes, compared with AAV2/2 CBA.RFP contralateral control eyes (69 ± 35 vs. 655 ± 154 cells/eye, respectively; p < 0.001, paired t-test, N = 9) (Fig. 5a). The mean ratio of integrated cell counts in CNTF-treated versus control treated eyes was 0.1 ± 0.03, showing a substantial loss of integrated cells in the AAV2/2 CMV-.CNTF-treated eyes.
Figure 5.
The effects of secreted CNTF on transplanted photoreceptor precursors and the recipient neural retina. (a) Histogram showing the total cell counts for AAV2/2-treated eyes. A significant decrease in cell integration was observed for the AAV2/2 CMV.CNTF-treated eyes, compared to the AAV2/2 CBA.RFP-treated controls (mean ± SEM; ***p < 0.001 paired t-test; N = 9). (b,i, ii) Projection confocal images of transplanted cell masses (Nrl.gfp; green) in AAV2/2-treated eyes. (c) Histogram demonstrating the percentage of Nrl.gfp-positive cells (green) in the transplanted cell masses of AAV2/2-treated eyes (mean ± SEM; p > 0.05, paired t-test; N = 6). (d) Confocal image showing the infiltration of macrophages (F4/80, red; white arrowhead) in an AAV2/2 CMV.CNTF-treated retina. (e) Projection confocal images of retinal sections from eyes that have been transduced with AAV2/2 CBA.RFP (e,i) or AAV2/2 CMV.CNTF (e,ii), and received a subretinal cell transplantation (i′, ii′ show a single confocal image of the highlighted region). Integrated cells are present in the AAV2/2 CBA.RFP-treated retina only (ei, i′; Nrl.gfp; green). Increased GFAP was observed in the AAV2/2 CMV.CNTF-treated retina (e,ii, ii′; pink), compared to the control treated (e,i, i′). Increased staining of the glial processes at the OLM (e,ii′, white arrowheads) was present in the CNTF-treated and not the control-treated retina. Nuclei were counterstained with Hoechst 33342 (blue). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars: 50 μm.
To ensure that the reduction in photoreceptor cell integration was not due to any CNTF-induced loss of donor cells, the percentage of Nrl.gfp+ve cells present in the subretinal space was assessed in both control and treated eyes. Nrl.gfp+ve cells were observed in the subretinal space of all examined from both cohorts (Fig. 5b,i, ii; green). We observed a small but nonsignificant reduction in the percentage of Nrl.gfp+ve cells in the cell masses of CNTF-treated eyes (p > 0.05, paired t-test, N = 6).
We have previously demonstrated that increased macrophage presence is negatively correlated with integrated photoreceptor number (73). As CNTF has been shown to be a chemoattractant to macrophages in vitro (12), it is possible that an increase in macrophage infiltration could have impaired photoreceptor cell integration. To investigate this, retinal sections were stained with the macrophage marker F4/80 and the extent of macrophage infiltration was examined. While some macrophage infiltration was present in CNTF-treated eyes (Fig. 5d; red; white arrowhead), it was not notably increased compared with untransduced and AAV2/2 CBA.RFP-treated retinae.
The potentially detrimental effect of gliosis on photoreceptor precursor cell integration was indicated previously in the 12-week-old Crb1rd8/rd8 mouse (53). As CNTF has been shown to cause an upregulation in the expression of GFAP in the retina (39), the extent of gliosis was examined by immunohistochemistry in CNTF-and control-treated retinae (Fig. 5e,i, ii). We observed a few GFAP+ve Müller glial processes in control AAV2/2 CBA.RFP-treated retina, a pattern of staining also typical of untransduced recipient eyes around the site of transplantation (Fig. 5e,i, i′; pink). However, GFAP expression was significantly increased in AAV2/2 CMV.CNTF-treated retinae (Fig. 5e,ii, ii′; pink). Furthermore, glial hypertrophy, seen as GFAP+ve processes extending along the outer edge of the retina between the ONL and the inner segments, was frequently observed (Fig. 5e,ii′; pink; white arrowheads). These features are typical of glial scaring (39,40) and are likely to be a major factor in the reduction of precursor cell integration seen in CNTF-treated retinae.
DISCUSSION
Repair of the central nervous system by cell transplantation has been a long-standing goal for a number of degenerative disorders. This strategy requires the transplanted cell population to migrate and integrate efficiently within the target tissue. The extrinsic environment is well known to play an important role in determining the success or otherwise of this integration (43,64). Here, we have examined the potential for manipulating the recipient retinal environment by gene transfer in order to improve transplanted photoreceptor precursor cell integration and survival. Ectopic overexpression of IGF1, but not FGF2, in the adult wild-type recipient retina led to significantly improved numbers of Nrl.gfp+ve precursor cells integrating following transplantation into the subretinal space. Conversely, the upregulation of CNTF significantly impaired integration, potentially due to the induction of increased reactive gliosis. Therefore, modifying extrinsic signaling within the recipient retina can both positively and negatively modulate photoreceptor precursor cell integration into the adult retina, and the extrinsic recipient retinal microenvironment represents an important consideration for photoreceptor transplantation strategies.
We have previously shown that postmitotic photoreceptor precursor cells derived from the early postnatal retina are optimal donors for transplantation (45). IGF1 is expressed at higher levels in the developing postnatal retina compared with the adult (5,10) and the IGF1/insulin receptor pathway is important for both retinal cell survival and photoreceptor maturation (14,68,78). Although IGF1 levels are reduced in the adult, retinal neurons appear to retain the ability to respond to IGF1/insulin receptor signaling. Studies in the rds mouse have shown increased photoreceptor survival in the presence of increased proinsulin production (17), while retinal ganglion cell survival is improved in models of optic nerve transection when combined with intravitreal administration of IGF1 (17,32). The increased number of integrated rod photoreceptors observed in the AAV2/2 CMV.IGF1-treated eyes described here could be due either to IGF1 acting to increase the survival of donor cells (either before or after integration) or by augmenting the frequency of precursor cell integration itself. We consider the former more likely because barriers such as the OLM still remain in the host retina, which have previously been shown to limit the number of photoreceptor precursor cells that are able to integrate into the host ONL (53,74). IGF1 acts via the activation of the antiapoptotic phosphatidylinositol 3-kinase (PI3-K)/Akt pathway and inhibition of caspase 3 in the maintenance of retinal ganglion cells following optic nerve transection (32). Sustained PI3-K activation in transgenic mice has been reported to increase the survival of differentiated photoreceptors in the postnatal retina (55). It is possible that the enhanced survival of migrating and integrating transplanted photoreceptor cells occurs by a similar mechanism. Consistent with this, we found a reduction in active caspase 3+ve, apoptotic cells in the ONL of AAV2/2 IGF1-treated retinae compared with untreated retinae. In keeping with this idea, recent work in the retina has shown that XIAP (X-linked inhibitor of apoptosis protein), which inhibits apoptosis via the inhibition of caspases, including caspase 3, can reduce transplanted photoreceptor cell death in the rd9 model of retinal degeneration (77).
Another potential consequence of IGF1 upregulation might be the improved or strengthened synaptic connectivity of the transplanted cells. Newly born neurons, including photoreceptors, are vulnerable to pruning and apoptosis if appropriate synaptic connections with downstream targets are not formed or maintained (65,67). We observed IGF1 expression in the outer plexiform layer around the time of photoreceptor synaptogenesis in the postnatal retina. Overexpression of IGF1 has previously been shown to promote synaptogenesis in the hippocampus during postnatal development (51). IGF1 has also be associated with the upregulation of brain-derived neurotrophic factor (BDNF), an important modulator of synaptic plasticity in the adult brain, after mechanical injury and with exercise-induced cognitive function (15,19,30). A recent study investigating the molecular pathways involved in the increased development of visual acuity responses induced by environmental enrichment demonstrated that intraocular injection of IGF1 increased BDNF expression in the retina (36). Moreover, both IGF1 and BDNF were required to mediate the increased maturation of visual acuity observed in rats reared in enriched environments. Although beyond the scope of this study, it is possible that the increased expression of IGF1 in the current study could mediate the subsequent upregulation of BDNF in the adult retina, and that a combination of these factors might improve transplanted photoreceptor synaptic connectivity and, subsequently, survival.
FGF2 has been implicated in retinal neurogenesis (23,59), migration (7), and photoreceptor survival (29, 50). Mice with a targeted disruption of FGF receptors 1 and 2 (FGFR1 & 2) undergo a progressive loss of photoreceptors (9), suggesting that FGF2 may act as a survival factor for photoreceptors. Therefore, we considered FGF2 to be of interest in terms of the migration, integration, and survival of transplanted photoreceptor precursor cells. However, we observed no improvement in cell integration following the over expression of FGF2 in the recipient retina, compared with control transduced or untransduced retinae. Our assessment of FGF2 expression indicates that the cell transplantation procedure itself, which involves a substantial if temporary retinal detachment, leads to significant upregulation of FGF2, probably greater than that achieved by AAV transduction. In the adult mouse retina, mechanical injury results in the upregulation of FGF2 expression, which is maximal at 2–4 days posttrauma. Both injury- and light stress-related upregulation of FGF2 have been shown to protect photoreceptor cells from further light-induced degeneration in the adult mouse (49). Similarly, AAV-mediated upregulation of FGF2 has been shown to reduce photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa (37). Further work blocking FGF signaling will be required to determine the role FGF2 plays in the integration and survival of photoreceptors transplanted into the normal, untransduced retina.
CNTF has previously been shown to inhibit rod photoreceptor differentiation in the developing retina (20,81) and here we found photoreceptor precursor cell integration to be markedly reduced in AAV2/2 CMV. CNTF-treated eyes compared with controls. CNTF/LIF signaling has been shown to block the expression of the rod photoreceptor transcription factors Nrl and Crx (22), but the reduction in integrated cells did not appear to be due to a downregulation of Nrl, as the percentage of Nrl.gfp+ve cells in the transplanted cell mass was not significantly reduced compared with that of controls. We cannot rule out the possibility that the GFP protein remains in the absence of sustained Nrl expression, although this is unlikely.
Expression of the α-subunit of the CNTF receptor, CNTFRα, by photoreceptors is thought to be minimal, although the literature is complicated, possibly due to the fact that CNTFRα can act as a soluble mediator for CNTF signaling (18,24,35,70). Interestingly, a recent study has shown that CNTFRα is expressed in immature cells of the ONL, but is downregulated with the onset of rhodopsin expression (26). This may explain how CNTF signaling might affect immature photoreceptor precursor cells and not directly affect adult photoreceptors of the mammalian retina.
CNTF has been shown to be act as a chemoattractant for blood-derived macrophages (12). We have previously found that high macrophage presence is associated with poor integration (73). However, the extent of macrophage infiltration observed in CNTF-treated retinae was very similar to that seen in control eyes, suggesting that it was not a major factor in terms of precursor cell integration.
The most likely cause of reduced cell integration in the CNTF-treated retinae is glial scarring. In the adult retina, CNTF has been shown to exert its stress-related neuroprotective effects indirectly via the activation of Müller gial cells (54,69). Others have demonstrated the upregulation of GFAP, a marker of glial cell activation, in Müller glial cells following injection of recombinant CNTF, an effect mediated by the JAK/STAT signaling pathway (54,71). The ability of cytokines to induce GFAP expression varies, and CNTF has been shown to be the most potent compared with FGF2 and LIF (71). We observed very marked increases in GFAP expression; little GFAP was observed in control AAV2/2 CBA.RFP-treated retinae, demonstrating that neither the mechanical trauma of the intravitreal viral injection nor that of the subretinal cell transplantation were sufficient to elicit significant sustained glial cell activation in wild-type eyes. Chen and colleagues demonstrated that transplanted cell integration was significantly higher in mice in which GFAP and vimentin was knocked out (33). In keeping with this, we have observed an inverse correlation between the degree of glial scarring and the number of integrated photoreceptors (A. Barber and R. Pearson, unpublished observations), indicating that the glial scar presents a restrictive barrier that inhibits photoreceptor precursor cells from migrating into the ONL.
Here, we show that it is possible to manipulate the recipient retinal environment via rAAV-mediated gene transfer with respect to developmentally regulated neurotrophic factors. When combined with cell transplantation, IGF1 led to significantly increased levels of cell integration, while CNTF resulted in unwanted effects in both host and donor cells. It may be that different levels, both higher and lower, of these factors may have different effects and it will be of interest to establish a dose response, particularly for IGF1. Taken together, these findings demonstrate the importance of the extrinsic environment of the host retina for successful photoreceptor cell transplantation.
We have previously shown that a number of manipulations can improve the numbers of transplanted photoreceptor precursors that go on to integrate following transplantation, including transient disruption of the outer limiting membrane (OLM) (53,74). Here, we tested the impact of manipulating the levels of IGF1, CNTF, and FGF2 in isolation in normal wild-type mice to permit assessment of a single variable. It is likely that a combination of factors will be required to adequately modify the retinal environment to promote optimal cell integration. These may include manipulation of the OLM, endogenous growth factor levels, and potentially even changes to the donor cell population itself. It is also important to consider that the degenerating retinal environment will present a very different and potentially hostile environment to donor cells compared with that of the wild-type retina. Thus, it will be important to determine the impact of degeneration upon cell integration but also whether manipulations of this environment such as those described here could be used to enhance transplanted photoreceptor precursor cell integration.
ACKNOWLEDGMENTS
This work was supported by grants from the Wellcome Trust (082217), Medical Research Council UK (G03000341), The Health Foundation and Royal College of Surgeons. R.A.P is a Royal Society University Research Fellow; A.G.C is a Wellcome Trust Ph.D. student; R.E.M was funded by a Health Foundation Clinician Scientist Fellowship. J.C.S. is supported by Great Ormond Street Hospital Children’s Charity. R.R.A and R.E.M. were partially funded by the Department of Health’s National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital. Funding to pay the Open Access Charge was provided by the Wellcome Trust. We would like to thank A. Barber and N. Gent for technical assistance and the Department of Genetics for helpful discussions on the work.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Akimoto M, Cheng H, Zhu D, Brzezinski JA, Khanna R, Filippova E, Oh EC, Jing Y, Linares JL, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc. Natl. Acad. Sci. USA. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach MA, Shen-Orr Z, Lowe WL, Jr., Roberts CT, Jr., LeRoith D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res. Mol. Brain Res. 1991;10:43–48. doi: 10.1016/0169-328x(91)90054-2. [DOI] [PubMed] [Google Scholar]
- 3.Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- 4.Barker SE, Broderick CA, Robbie SJ, Duran Y, Natkunarajah M, Buch P, Balaggan KS, MacLaren RE, Bainbridge JW, Smith AJ, Ali RR. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J. Gene Med. 2009;11:486–497. doi: 10.1002/jgm.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondy C, Werner H, Roberts CT, Jr., LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: Comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- 6.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Bribian A, Barallobre MJ, Soussi-Yanicostas N, de Castro F. Anosmin-1 modulates the FGF-2-dependent migration of oligodendrocyte precursors in the developing optic nerve. Mol. Cell. Neurosci. 2006;33:2–14. doi: 10.1016/j.mcn.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Buch PK, MacLaren RE, Duran Y, Balaggan KS, MacNeil A, Schlichtenbrede FC, Smith AJ, Ali RR. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol. Ther. 2006;14:700–709. doi: 10.1016/j.ymthe.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Campochiaro PA, Chang M, Ohsato M, Vinores SA, Nie Z, Hjelmeland L, Mansukhani A, Basilico C, Zack DJ. Retinal degeneration in transgenic mice with photoreceptor-specific expression of a dominant-negative fibroblast growth factor receptor. J. Neurosci. 1996;16:1679–1688. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao W, Li F, Steinberg RH, Lavail MM. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina. Exp. Eye Res. 2001;72:591–604. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- 11.Cao W, Wen R, Li F, Lavail MM, Steinberg RH. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp. Eye Res. 1997;65:241–248. doi: 10.1006/exer.1997.0328. [DOI] [PubMed] [Google Scholar]
- 12.Cen LP, Luo JM, Zhang CW, Fan YM, Song Y, So KF, Van RN, Pang CP, Lam DS, Cui Q. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:4257–4266. doi: 10.1167/iovs.06-0791. [DOI] [PubMed] [Google Scholar]
- 13.Cepko CL. The patterning and onset of opsin expression in vertebrate retinae. Curr. Opin. Neurobiol. 1996;6:542–546. doi: 10.1016/s0959-4388(96)80062-6. [DOI] [PubMed] [Google Scholar]
- 14.Chavarria T, Valenciano AI, Mayordomo R, Egea J, Comella JX, Hallbook F, de Pablo F, de La Rosa EJ. Differential, age-dependent MEK-ERK and PI3K-Akt activation by insulin acting as a survival factor during embryonic retinal development. Dev. Neurobiol. 2007;67:1777–1788. doi: 10.1002/dneu.20554. [DOI] [PubMed] [Google Scholar]
- 15.Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007;25:118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- 16.Cornish EE, Madigan MC, Natoli R, Hales A, Hendrickson AE, Provis JM. Gradients of cone differentiation and FGF expression during development of the foveal depression in macaque retina. Vis. Neurosci. 2005;22:447–459. doi: 10.1017/S0952523805224069. [DOI] [PubMed] [Google Scholar]
- 17.Corrochano S, Barhoum R, Boya P, Arroba AI, Rodriguez-Muela N, Gomez-Vicente V, Bosch F, de Pablo F, de La Villa P, de La Rosa EJ. Attenuation of vision loss and delay in apoptosis of photoreceptors induced by proinsulin in a mouse model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2008;49:4188–4194. doi: 10.1167/iovs.08-2182. [DOI] [PubMed] [Google Scholar]
- 18.Davis S, Aldrich TH, Ip NY, Stahl N, Scherer S, Farruggella T, DiStefano PS, Curtis R, Panayotatos N, Gascan H, Chevalier S, Yancopoulos GD. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993;259:1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur. J. Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 20.Ezzeddine ZD, Yang X, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 21.Gao H, Hollyfield JG. Basic fibroblast growth factor in retinal development: Differential levels of bFGF expression and content in normal and retinal degeneration (rd) mutant mice. Dev. Biol. 1995;169:168–184. doi: 10.1006/dbio.1995.1135. [DOI] [PubMed] [Google Scholar]
- 22.Graham DR, Overbeek PA, Ash JD. Leukemia inhibitory factor blocks expression of Crx and Nrl transcription factors to inhibit photoreceptor differentiation. Invest. Ophthalmol. Vis. Sci. 2005;46:2601–2610. doi: 10.1167/iovs.05-0129. [DOI] [PubMed] [Google Scholar]
- 23.Gremo F, Presta M. Role of fibroblast growth factor-2 in human brain: A focus on development. Int. J. Dev. Neurosci. 2000;18:271–279. doi: 10.1016/s0736-5748(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 24.Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J. Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris WA. Cellular diversification in the vertebrate retina. Curr. Opin. Genet. Dev. 1997;7:651–658. doi: 10.1016/s0959-437x(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 26.Hertle D, Schleichert M, Steup A, Kirsch M, Hofmann HD. Regulation of cytokine signaling components in developing rat retina correlates with transient inhibition of rod differentiation by CNTF. Cell Tissue Res. 2008;334:7–16. doi: 10.1007/s00441-008-0651-3. [DOI] [PubMed] [Google Scholar]
- 27.Hicks D, Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J. Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodge RD, D’Ercole AJ, O’Kusky JR. Insulin-like growth factor-I (IGF-I) inhibits neuronal apoptosis in the developing cerebral cortex in vivo. Int. J. Dev. Neurosci. 2007;25:233–241. doi: 10.1016/j.ijdevneu.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joly S, Pernet V, Chemtob S, Di Polo A, Lachapelle P. Neuroprotection in the juvenile rat model of light-induced retinopathy: Evidence suggesting a role for FGF-2 and CNTF. Invest. Ophthalmol. Vis. Sci. 2007;48:2311–2320. doi: 10.1167/iovs.06-1205. [DOI] [PubMed] [Google Scholar]
- 30.Kazanis I, Giannakopoulou M, Philippidis H, Stylianopoulou F. Alterations in IGF-I, BDNF and NT-3 levels following experimental brain trauma and the effect of IGF-I administration. Exp. Neurol. 2004;186:221–234. doi: 10.1016/j.expneurol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Kent TL, Glybina IV, Abrams GW, Iezzi R. Chronic intravitreous infusion of ciliary neurotrophic factor modulates electrical retinal stimulation thresholds in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2008;49:372–379. doi: 10.1167/iovs.07-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kermer P, Klocker N, Labes M, Bahr M. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J. Neurosci. 2000;20:2–8. [PubMed] [Google Scholar]
- 33.Kinouchi R, Takeda M, Yang L, Wilhelmsson U, Lundkvist A, Pekny M, Chen DF. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 2003;6:863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- 34.Kirsch M, Fuhrmann S, Wiese A, Hofmann HD. CNTF exerts opposite effects on in vitro development of rat and chick photoreceptors. Neuroreport. 1996;7:697–700. doi: 10.1097/00001756-199602290-00004. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch M, Lee MY, Meyer V, Wiese A, Hofmann HD. Evidence for multiple, local functions of ciliary neurotrophic factor (CNTF) in retinal development: Expression of CNTF and its receptors and in vitro effects on target cells. J. Neurochem. 1997;68:979–990. doi: 10.1046/j.1471-4159.1997.68030979.x. [DOI] [PubMed] [Google Scholar]
- 36.Landi S, Ciucci F, Maffei L, Berardi N, Cenni MC. Setting the pace for retinal development: Environmental enrichment acts through insulin-like growth factor 1 and brain-derived neurotrophic factor. J. Neurosci. 2009;29:10809–10819. doi: 10.1523/JNEUROSCI.1857-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau D, McGee LH, Zhou S, Rendahl KG, Manning WC, Escobedo JA, Flannery JG. Retinal degeneration is slowed in transgenic rats by AAV-mediated delivery of FGF-2. Invest. Ophthalmol. Vis. Sci. 2000;41:3622–3633. [PubMed] [Google Scholar]
- 38.Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. Cell. Mol. Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. Int. Rev. Cytol. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- 40.Lewis GP, Matsumoto B, Fisher SK. Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest. Ophthalmol. Vis. Sci. 1995;36:2404–2416. [PubMed] [Google Scholar]
- 41.Lillien L, Cepko C. Control of proliferation in the retina: Temporal changes in responsiveness to FGF and TGF alpha. Development. 1992;115:253–266. doi: 10.1242/dev.115.1.253. [DOI] [PubMed] [Google Scholar]
- 42.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: Lessons from the retina. Nat. Rev. Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 43.Macklis JD. Transplanted neocortical neurons migrate selectively into regions of neuronal degeneration produced by chromophore-targeted laser photolysis. J. Neurosci. 1993;13:3848–3863. doi: 10.1523/JNEUROSCI.13-09-03848.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLaren RE, Buch PK, Smith AJ, Balaggan KS, MacNeil A, Taylor JS, Osborne NN, Ali RR. CNTF gene transfer protects ganglion cells in rat retinae undergoing focal injury and branch vessel occlusion. Exp. Eye Res. 2006;83:1118–1127. doi: 10.1016/j.exer.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 45.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 46.McGill TJ, Prusky GT, Douglas RM, Yasumura D, Matthes MT, Nune G, Donohue-Rolfe K, Yang H, Niculescu D, Hauswirth WW, Girman SV, Lund RD, Duncan JL, Lavail MM. Intraocular CNTF reduces vision in normal rats in a dose-dependent manner. Invest. Ophthalmol. Vis. Sci. 2007;48:5756–5766. doi: 10.1167/iovs.07-0054. [DOI] [PubMed] [Google Scholar]
- 47.Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat. Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 48.Mellough CB, Cui Q, Spalding KL, Symons NA, Pollett MA, Snyder EY, Macklis JD, Harvey AR. Fate of multipotent neural precursor cells transplanted into mouse retina selectively depleted of retinal ganglion cells. Exp. Neurol. 2004;186:6–19. doi: 10.1016/j.expneurol.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 49.O’Driscoll C, O’Connor J, O’Brien CJ, Cotter TG. Basic fibroblast growth factor-induced protection from light damage in the mouse retina in vivo. J. Neurochem. 2008;105:524–536. doi: 10.1111/j.1471-4159.2007.05189.x. [DOI] [PubMed] [Google Scholar]
- 50.O’Driscoll C, Wallace D, Cotter TG. bFGF promotes photoreceptor cell survival in vitro by PKA-mediated inactivation of glycogen synthase kinase 3beta and CREB-dependent Bcl-2 up-regulation. J. Neurochem. 2007;103:860–870. doi: 10.1111/j.1471-4159.2007.04827.x. [DOI] [PubMed] [Google Scholar]
- 51.O’Kusky JR, Ye P, D’Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel A, McFarlane S. Overexpression of FGF-2 alters cell fate specification in the developing retina of Xenopus laevis. Dev. Biol. 2000;222:170–180. doi: 10.1006/dbio.2000.9695. [DOI] [PubMed] [Google Scholar]
- 53.Pearson RA, Barber AC, West EL, MacLaren RE, Duran Y, Bainbridge JW, Sowden JC, Ali RR. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19:487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J. Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pimentel B, Rodriguez-Borlado L, Hernandez C, Carrera AC. A Role for phosphoinositide 3-kinase in the control of cell division and survival during retinal development. Dev. Biol. 2002;247:295–306. doi: 10.1006/dbio.2002.0703. [DOI] [PubMed] [Google Scholar]
- 56.Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 57.Rhee KD, Goureau O, Chen S, Yang XJ. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J. Neurosci. 2004;24:9779–9788. doi: 10.1523/JNEUROSCI.1785-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhee KD, Ruiz A, Duncan JL, Hauswirth WW, Lavail MM, Bok D, Yang XJ. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2007;48:1389–1400. doi: 10.1167/iovs.06-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell C. The roles of hedgehogs and fibroblast growth factors in eye development and retinal cell rescue. Vision Res. 2003;43:899–912. doi: 10.1016/s0042-6989(02)00416-9. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi DS, Van Hoffelen SJ, Grozdanic SD, Kwon YH, Kardon RH, Young MJ. Neural progenitor cell transplants into the developing and mature central nervous system. Ann. NY Acad. Sci. 2005;1049:118–134. doi: 10.1196/annals.1334.012. [DOI] [PubMed] [Google Scholar]
- 61.Sakaguchi DS, Van Hoffelen SJ, Theusch E, Parker E, Orasky J, Harper MM, Benediktsson A, Young MJ. Transplantation of neural progenitor cells into the developing retina of the Brazilian opossum: An in vivo system for studying stem/progenitor cell plasticity. Dev. Neurosci. 2004;26:336–345. doi: 10.1159/000082275. [DOI] [PubMed] [Google Scholar]
- 62.Sakaguchi DS, Van Hoffelen SJ, Young MJ. Differentiation and morphological integration of neural progenitor cells transplanted into the developing mammalian eye. Ann. NY Acad. Sci. 2003;995:127–139. doi: 10.1111/j.1749-6632.2003.tb03216.x. [DOI] [PubMed] [Google Scholar]
- 63.Schlichtenbrede FC, MacNeil A, Bainbridge JW, Tschernutter M, Thrasher AJ, Smith AJ, Ali RR. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 64.Sheen VL, Macklis JD. Targeted neocortical cell death in adult mice guides migration and differentiation of transplanted embryonic neurons. J. Neurosci. 1995;15:8378–8392. doi: 10.1523/JNEUROSCI.15-12-08378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherrard RM, Bower AJ. Role of afferents in the development and cell survival of the vertebrate nervous system. Clin. Exp. Pharmacol. Physiol. 1998;25:487–495. doi: 10.1111/j.1440-1681.1998.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 66.Swain PK, Hicks D, Mears AJ, Apel IJ, Smith JE, John SK, Hendrickson A, Milam AH, Swaroop A. Multiple phosphorylated isoforms of NRL are expressed in rod photoreceptors. J. Biol. Chem. 2001;276:36824–36830. doi: 10.1074/jbc.M105855200. [DOI] [PubMed] [Google Scholar]
- 67.Valenciano AI, Boya P, de La Rosa EJ. Early neural cell death: Numbers and cues from the developing neuroretina. Int. J. Dev. Biol. 2009;53:1515–1528. doi: 10.1387/ijdb.072446av. [DOI] [PubMed] [Google Scholar]
- 68.Valenciano AI, Corrochano S, de Pablo F, de La Villa P, de La Rosa EJ. Proinsulin/insulin is synthesized locally and prevents caspase- and cathepsin-mediated cell death in the embryonic mouse retina. J. Neurochem. 2006;99:524–536. doi: 10.1111/j.1471-4159.2006.04043.x. [DOI] [PubMed] [Google Scholar]
- 69.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest. Ophthalmol. Vis. Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 70.Wahlin KJ, Lim L, Grice EA, Campochiaro PA, Zack DJ, Adler R. A method for analysis of gene expression in isolated mouse photoreceptor and Muller cells. Mol. Vis. 2004;10:366–375. [PubMed] [Google Scholar]
- 71.Wang Y, Smith SB, Ogilvie JM, McCool DJ, Sarthy V. Ciliary neurotrophic factor induces glial fibrillary acidic protein in retinal Muller cells through the JAK/STAT signal transduction pathway. Curr. Eye Res. 2002;24:305–312. doi: 10.1076/ceyr.24.4.305.8408. [DOI] [PubMed] [Google Scholar]
- 72.Wen R, Cheng T, Song Y, Matthes MT, Yasumura D, Lavail MM, Steinberg RH. Continuous exposure to bright light upregulates bFGF and CNTF expression in the rat retina. Curr. Eye Res. 1998;17:494–500. doi: 10.1076/ceyr.17.5.494.5186. [DOI] [PubMed] [Google Scholar]
- 73.West EL, Pearson RA, Barker SE, Luhmann UF, MacLaren RE, Barber AC, Duran Y, Smith AJ, Sowden JC, Ali RR. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28:1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp. Eye Res. 2008;86:601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams RW, Goldowitz D. Lineage versus environment in embryonic retina: A revisionist perspective. Trends Neurosci. 1992;15:368–373. doi: 10.1016/0166-2236(92)90181-7. [DOI] [PubMed] [Google Scholar]
- 76.Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin. Cell Dev. Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao J, Feathers K, Khanna H, Thompson D, Tsilfidis C, Hauswirth WW, Heckenlively JR, Swaroop A, Zacks DN. XIAP therapy increases survival of transplanted rod precursors in a degenerating host retina. Invest. Ophthalmol. Vis. Sci. 2011;52:1567–1572. doi: 10.1167/iovs.10-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi X, Schubert M, Peachey NS, Suzuma K, Burks DJ, Kushner JA, Suzuma I, Cahill C, Flint CL, Dow MA, Leshan RL, King GL, White MF. Insulin receptor substrate 2 is essential for maturation and survival of photoreceptor cells. J. Neurosci. 2005;25:1240–1248. doi: 10.1523/JNEUROSCI.3664-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu DY, Cringle S, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 80.Zaverucha-do-Valle C, Gubert F, Bargas-Rega M, Coronel JL, Mesentier-Louro LA, Mencalha A, Abdelhay E, Santiago MF, Mendez-Otero R. Bonemarrow mononuclear cells increase retinal ganglion-cell survival and axon regeneration in the adult rat. Cell Transplant. 2011;20:391–406. doi: 10.3727/096368910X524764. [DOI] [PubMed] [Google Scholar]
- 81.Zhang SS, Wei J, Qin H, Zhang L, Xie B, Hui P, Deisseroth A, Barnstable CJ, Fu XY. STAT3-mediated signaling in the determination of rod photoreceptor cell fate in mouse retina. Invest. Ophthalmol. Vis. Sci. 2004;45:2407–2412. doi: 10.1167/iovs.04-0003. [DOI] [PubMed] [Google Scholar]
- 82.Zhao S, Barnstable CJ. Differential effects of bFGF on development of the rat retina. Brain Res. 1996;723:169–176. doi: 10.1016/0006-8993(96)00237-5. [DOI] [PubMed] [Google Scholar]