Abstract
Bacteria can develop resistance to antibiotics, but less is known about their ability to increase resistance to chemical disinfectants. This study randomly sampled three AERs in the USA using aldehydes for endoscope disinfection. Bacterial contamination was found post-disinfection in all AERs and some mycobacteria isolated demonstrated significant resistance to glutaraldehyde and OPA disinfectants. Bacteria can survive aldehyde-based disinfection and may pose a cross-contamination risk to patients.
Introduction
Antibiotic resistance is widespread, but bacteria can also develop tolerance to some biocides used for disinfection1. Such increases in biocide MICs have little significance to the clinical use of disinfectants. Resistance leading to disinfectant failure has, however, been reported in mycobacteria to glutaraldehyde disinfectants2–5. These have been linked to patient infections, particularly a device-associated outbreak with a Mycobacterium massiliense strain that survives disinfection with 2% glutaraldehyde 5. It is not known if such strains are widespread or if similar resistance could develop against other high-level disinfectants, in particular ortho-phthaldehyde (OPA). This study investigated the potential for resistance development in automated endoscope reprocessors (AERs) employing aldehydes disinfectants. Bacterial survival was investigated post-disinfection and any isolates examined for disinfectant sensitivity.
Methods
AERs at three clinical sites in the United States using aldehyde disinfectants were sampled immediately following reprocessing cycles. All AERs were correctly maintained and in full operation at the time of sampling. Facilities followed recommended guidelines and manufacturer’s instructions for flexible endoscope reprocessing. Two sites used ~2.5% glutaraldehyde (at 25°C and 35°C respectively) and one 0.55% OPA (at 20°C). Rinsing post-disinfection was with water filtered through bacteria-retentive filters associated with the AERs. AERs samples included final rinse water (200 mL) and surface samples (swabbing at 10 sites) previously contacted with the disinfectant. Rinse water samples were filtered (through 0.45μm filters) and plated; swabs were directly streaked into agar. Samples were incubated on Tryptic Soy Agar (TSA) at 30 °C for 7 days for mesophilic bacteria, and Middlebrook 7H11 agar at 30°C for 14 days for atypical mycobacteria. Isolates from TSA plates were subcultured in Tryptic Soy Broth (TSB) at 30°C for 7 days and from 7H11 in enriched Middlebrook 7H9 broth at 30°C for 14 days. Isolates were identified by Sherlock® Systems (MIDI Inc).
Disinfectant-resistance was investigated for all isolates, including Mycobacterium gordonae ATCC 14470 as a representative atyptical mycobacteria control. 1mL of subcultured organism was mixed with 9 mL of each disinfectant at the designated test temperature. Disinfectants included 2.5% glutaraldehyde at 25 °C (Cidex®; Johnson & Johnson), 0.55% OPA at 20°C (Cidex®; Johnson & Johnson), peracetic acid at 50 °C (1200 mg/L; Reliance® DG, STERIS) and hydrogen peroxide at 20°C (2%; Resert XL HLD, STERIS). Over time, 1mL samples were taken, added to 9 ml neutralizer (Letheen broth with 1% sodium thiosulfate), filtered through 0.45μm filters and quantified by plate counting on TSA or 7H11 agar at 30 °C for 7 or 14 days respectively. Neutralization was validated for each disinfectant tested and tests were performed in triplicate.
Results
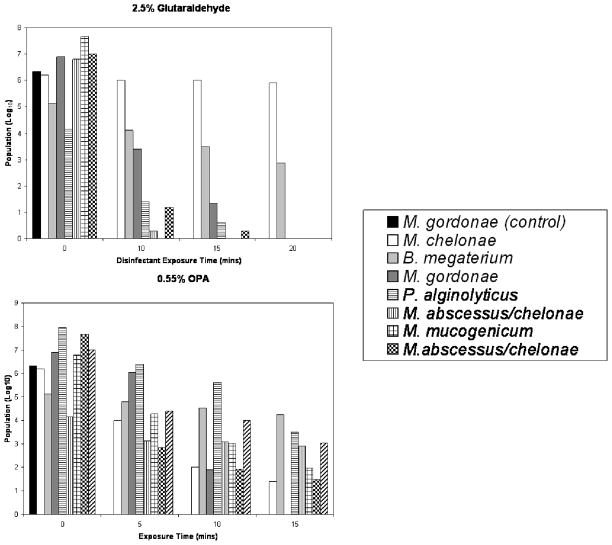
Bacterial contamination was shown in all washer-disinfectors sampled post- disinfection. Subcultures were speciated and included Mycobacterium and Methylobacterium species. All isolates where analyzed for their resistance profiles to disinfection (only strains with increased resistance are shown in Figure 1). For the first facility (using glutaraldehyde), all isolates (but one) were sensitive to all disinfectants tested, suggesting they may have been associated with biofilm or from post-disinfection rinsing. A Bacillus megaterium isolate tested under non-sporing conditions (investigated by direct microscopic analysis) showed unusual resistance to aldehydes but not other disinfectants. The second facility, employing OPA, demonstrated one strain (M. gordonae) with increased resistance to glutaraldehyde and OPA and one (M. avium) with resistance to OPA (Figure 1). A further strain (Paenibacillus alginolyticus) showed marked tolerance to aldehydes. These results suggest the ability of these strains to survive the disinfection process. All strains were rapidly inactivated by oxidizing agent disinfectants. The third facility (glutaraldehyde) demonstrated both sensitive and resistant (e.g., Mycobacterium abscessus/chelonae) strains to aldehydes (Figure 1), but all strains were sensitive to oxidizing agents.
Figure 1.
Sensitivity of isolates to aldehyde disinfection (average log reduction shown). Note, only one control (M. gordonae) and some of the strains showing significant resistance are shown; culture collection strains of the other bacteria species identified where tested and did not show greater resistance to disinfection than the M. gordonae control strain.
Discussion
Mycobacteria have been reported to survive glutaraldehyde high level disinfection2–4, including strains that caused hospital-acquired infections5. Genetic and biochemical analysis implicated decreased porin availability on the surface of cells as the main resistance mechanism7. Resistance does not appear to be due to porin influx/efflux mechanisms, but the lack of protein at the cell surface as a major target for aldehydes1. The ability to survive standard aldehyde disinfection processes is clinically significant, but other implications of the resistance mechanism(s) include cross-resistance to antibiotics7 and increase virulence8. This study investigated the development of resistance profiles in strains cultured from AERs using aldehydes for the disinfection of flexible endoscopes in the United States. Bacterial contamination was observed post-disinfection, but many isolates were sensitive to the disinfectants tested. Rinse water contamination and/or biofilm development in the AER were suspected in these cases. Other isolates (in particular mycobacteria, a Bacillus and Paenibacillus strain) had high level resistance to aldehydes, in particular OPA. This suggests that some isolates could tolerate aldehyde-based disinfection with clinical implications. The mechanisms of resistance in these isolates are currently under investigation, but preliminary results suggest similar mechanisms to those previously described7. In conclusion, mycobacteria can develop significant resistance to aldehyde-based high level disinfectants, including in AERs and may pose a cross-contamination risk to patients.
References
- 1.McDonnell G. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance. Washington DC: ASM Press; 2007. [Google Scholar]
- 2.Griffiths PA, Babb JR, Bradley CR, Fraise AP. Glutaraldehyde resistant Mycobacterium chelonae from endoscope washer disinfectors. J Appl Microbiol. 1997;82:519–526. doi: 10.1046/j.1365-2672.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 3.Nomura K, Ogawa M, Miyamoto H, Muratani T, Taniguchi H. Antibiotic susceptibility of glutaraldehyde-tolerant Mycobacterium chelonae from bronchoscope washing machines. Am J Infect Control. 2004;32(4):185–188. doi: 10.1016/j.ajic.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 4.van Klingeren B, Pullen W. Glutaraldehyde resistant mycobacteria from endoscope washers. J Hosp Infect. 1993;25(2):147–149. doi: 10.1016/0195-6701(93)90107-b. [DOI] [PubMed] [Google Scholar]
- 5.Duarte RS, Lourenço MC, de Fonseca LS, Leão SC, de Amorim EL, et al. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J Clin Microbiol. 2009;47(7):2149–2155. doi: 10.1128/JCM.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay WG, Leanord AT, Williams CL. Water, water everywhere nor any a sterile drop to rinse your endoscope. J Hosp Infect. 2002;51(4):256–261. doi: 10.1053/jhin.2002.1235. [DOI] [PubMed] [Google Scholar]
- 7.Svetlíková Z, Skovierová H, Niederweis M, Gaillard JL, McDonnell G, Jackson M. Role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob Agents Chemother. 2009;53(9):4015–4018. doi: 10.1128/AAC.00590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang S, Gibbs S, Henao-Tamayo M, Shanley CA, McDonnell G, Duarte RS, Ordway DJ, Jackson M. Increased Virulence of an Epidemic Strain of Mycobacterium massiliense in Mice. PLoS One. 2011;6(9):e24726. doi: 10.1371/journal.pone.0024726. [DOI] [PMC free article] [PubMed] [Google Scholar]