Abstract
Objectives
The aim of this study was to assess the impact of extreme (class III) obesity (body mass index [BMI] ≥40 kg/m2) on care and outcomes in patients with ST-segment elevation myocardial infarction (STEMI).
Background
Although its prevalence is increasing rapidly, little is known about the impact of extreme obesity on STEMI presentation, treatments, complication rates, and outcomes.
Methods
The relationship between BMI and baseline characteristics, treatment patterns, and risk-adjusted in-hospital outcomes was quantified for 50,149 patients with STEMI from the National Cardiovascular Data Registry (NCDR) ACTION Registry–GWTG.
Results
The proportions of patients with STEMI by BMI category were as follows: underweight (BMI <18.5 kg/m2) 1.6%, normal weight (18.5 kg/m2 ≤BMI <25 kg/m2) 23.5%, overweight (25 kg/m2 ≤BMI <30 kg/m2) 38.7%, class I obese (30 kg/m2 ≤ BMI <35 kg/m2) 22.4%, class II obese (35 kg/m2 ≤ BMI <40 kg/m2) 8.7%, and class III obese 5.1%. Extreme obesity was associated with younger age at STEMI presentation (median age 55 years for class III obese vs. 66 years for normal weight); a higher prevalence of diabetes, hypertension, and dyslipidemia; a lower prevalence of smoking; and less extensive coronary artery disease and higher left ventricular ejection fraction. Process-of-care measures were similar across BMI categories, including the extremely obese. Using class I obesity as the referent, risk-adjusted in-hospital mortality rates were significantly higher only for class III obese patients (adjusted odds ratio: 1.64; 95% confidence interval: 1.32 to 2.03).
Conclusions
Patients with extreme obesity present with STEMI at younger ages and have less extensive coronary artery disease, better left ventricular systolic function, and similar processes and quality of care. Despite these advantages, extreme obesity remains independently associated with higher in-hospital mortality.
Keywords: extreme obesity, obesity, outcomes, quality of care, STEMI
The prevalence of obesity, defined according to National Heart, Lung, and Blood Institute criteria (1) as a body mass index (BMI) ≥30 kg/m2, has more than doubled over the past 3 decades (2) and currently affects 1 in 3 U.S. adults (3). Obesity is strongly associated with cardiovascular risk factors such as diabetes, hypertension, and dyslipidemia. In addition, obese patients have an increased burden of coronary artery disease and a higher incidence of acute coronary syndromes (4–6). Despite the adverse association between obesity and incident cardiovascular disease, a paradoxical survival benefit after myocardial infarction (MI) has been attributed to obesity (7–13). Prior studies, however, have included relatively few subjects with extreme obesity (class III, BMI ≥40 kg/m2). The relationships between obesity and non–ST-segment elevation myocardial infarction (NSTEMI) presentation, processes of care, and outcomes have been described previously (14). However, little is known about the relationship between obesity, particularly extreme obesity, and care and outcomes in ST-segment elevation MI (STEMI).
Patient demographics, presentation, and treatments differ notably between STEMI and NSTEMI populations. It is possible that extreme obesity may affect logistical issues such as STEMI diagnosis, cardiac catheterization laboratory table weight limits, problems with vascular access, and appropriate dosing of anticoagulant therapies. In addition, higher complication rates from interventional and medical therapies in obese patients, whether actual or perceived, may substantively affect risk/benefit calculations and alter management. To better clarify the impact of extreme obesity on STEMI care and outcomes, we analyzed the association between BMI categories and baseline characteristics, treatment, and in-hospital outcomes for 50,149 patients with STEMI from the National Cardiovascular Data Registry (NCDR) Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry–Get With The Guidelines (GWTG) (15–17).
Methods
Data collection
The ACTION Registry–GWTG, created by a merger of the American College of Cardiology Foundation’s NCDR ACTION Registry and the American Heart Association’s GWTG program, collects and reports data for patients with STEMI and NSTEMI from 360 participating centers nationwide. Data abstraction was performed retrospectively by trained data collectors via review of medical records. Demographic and clinical information, clinical presentation, medical therapies and associated contraindications, use and timing of cardiac procedures, laboratory results, and in-hospital outcomes were recorded using standardized definitions; details of data collection have been reported previously (15–18).
Study population
The present study is based on the 51,980 subjects enrolled in the registry between January 1, 2007, and June 30, 2009, who were diagnosed with STEMI (defined as the clinical presentation of acute MI plus 1 of the following: new or presumed new ST-segment elevation, new left bundle branch block, or isolated posterior MI). We excluded 1,831 subjects who did not have BMI data available. This resulted in a cohort of 50,149 subjects from 344 centers for the BMI category prevalence estimates. For analyses of processes of care and outcomes, we further excluded the 820 subjects in the underweight category, defined by National Heart, Lung, and Blood Institute criteria as BMI ≤18.5 kg/m2, because of the small proportion of subjects in this category (1.6%) and the potential impact of confounding by comorbid conditions not captured in the registry, which could prevent proper characterization of the true relationship between BMI and in-hospital course. Therefore, all analyses in the present study, except BMI category prevalence estimates, are based on the 49,329 patients with STEMI and BMI >18.5 kg/m2.
Exposure variable
BMI was calculated on the basis of height and weight recorded by treating physicians at the time of STEMI presentation and divided into clinically relevant categories on the basis of National Heart, Lung, and Blood Institute criteria (1): underweight (BMI <18.5 kg/m2), normal weight (18.5 kg/m2 ≤BMI <25 kg/m2), overweight (25 kg/m2 ≤ BMI <30 kg/m2), class I obese (30 kg/m2 ≤BMI <35 kg/m2), class II obese (35 kg/m2 ≤BMI <40 kg/m2), and class III obese (BMI ≥40 kg/m2). The primary outcome of the present study was all-cause mortality. Secondary outcomes included rate and type of reperfusion, time to percutaneous coronary intervention (PCI), and rates of reinfarction, congestive heart failure (HF), cardiogenic shock, stroke, major bleeding, and red blood cell transfusion unrelated to coronary artery bypass graft surgery, which have been defined previously (15–17).
Statistical analysis
Demographics, clinical presentation, medical therapies, use and timing of cardiac procedures, laboratory results, and in-hospital outcomes were compared across BMI categories. To evaluate the relationship between in-hospital outcomes and BMI categories, the logistic generalized estimating equations method with exchangeable working correlation matrix was used to account for within-hospital correlation of responses. Variables used for in-hospital mortality adjustment were from the validated ACTION Registry–GWTG in-hospital mortality model (19): age, prior peripheral artery disease, systolic blood pressure on presentation, heart rate on presentation, HF or shock on admission (HF only, shock only or HF with shock, none), electrocardiographic findings (STEMI, ST-segment changes vs. no ST-segment changes), initial troponin ratio, and initial serum creatinine. ST-segment changes included ST-segment depressions or transient ST-segment elevations, and no ST-segment changes included T-wave inversions and no electrocardiographic changes. Variables used for major bleeding adjustment were from the validated ACTION Registry–GWTG in-hospital major bleeding model (17): female sex, age, diabetes, prior peripheral artery disease, body weight (excluded from the model for the present study), home warfarin therapy, heart rate on presentation, systolic blood pressure on presentation (≤130, 130 to 160, or ≥160 mm Hg), HF on presentation (HF only, shock only or HF with shock, none), electrocardiographic findings (STEMI, ST-segment changes vs. no ST-segment changes), initial serum creatinine, and initial hemoglobin. NSTEMI was dropped from both models because patients with NSTEMI were excluded from this analysis. Subjects in the class I obesity category were used as the referent group for the analyses of clinical outcomes; this group was selected a priori as the referent because of the previously well-described “U-shaped” relationship between BMI and outcomes in patients with a broad spectrum of existing cardiovascular disease (20) as well as the results of prior work examining NSTEMI and BMI in the ACTION Registry (14). Adjusted associations were displayed as odds ratios and 95% confidence intervals. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Values of p <0.05 were considered statistically significant.
Results
The proportions of patients with STEMI by BMI category were as follows: underweight 1.6%, normal weight 23.5%, overweight 38.7%, class I obese 22.4%, class II obese 8.7%, and class III obese 5.1%. Class III obese patients with STEMI were more than a decade younger than their normal-weight counterparts (Table 1). Class III obese patients with STEMI were more likely to be women and of self-reported African American race/ethnicity compared with all other weight categories. Overall, 29.5% of patients were women, compared with 42.6% of the extremely obese patients. Overall, 7.4% of the cohort was of African American race/ethnicity, compared with 11.4% of the class III obese patients. Taken together, these trends resulted in an increase in the prevalence of African American race/ethnicity from 6.2% of normal-weight women to 15.3% of class III obese women. Smoking decreased, while other traditional cardiac risk factors increased across increasing categories of obesity. Class III obese patients were almost 3-fold more likely to have diabetes mellitus than their normal-weight counterparts. Hemoglobin and low-density lipoprotein cholesterol were higher while high-density lipo-protein was lower for class III obesity compared with normal weight (Table 2). The extent of coronary disease, in terms of number of vessels affected, and the likelihood of having moderate to severe left ventricular (LV) systolic dysfunction also decreased progressively across increasing BMI categories (Table 2).
Table 1.
Demographics, Medical History, and Home Medications Stratified by BMI Category
| Variable | BMI Category (kg/m2)
|
||||
|---|---|---|---|---|---|
| Normal Weight (18.5 to <25)
|
Overweight (25 to <30)
|
Class I Obese (30 to <35)
|
Class II Obese (35 to <40)
|
Class III Obese (≥40)
|
|
| (n = 11,780) | (n = 19,391) | (n = 11,234) | (n = 4,376) | (n = 2,548) | |
| Demographics | |||||
| BMI (kg/m2) | 23.1 (21.7–24.1) | 27.4 (26.3–28.7) | 32.0 (30.9–33.3) | 36.9 (35.9–38.1) | 43.4 (41.4–47.2) |
| Age (yrs) | 66 (55–78) | 60 (52–71) | 58 (50–67) | 56 (49–65) | 55 (48–64) |
| Female | 4,446 (37.7%) | 4,671 (24.1%) | 2,962 (26.4%) | 1,371 (31.3%) | 1,094 (42.9%) |
| Race/ethnicity | |||||
| Women (n = 14,544) | |||||
| Caucasian | 3,878 (87.7%) | 3,962 (85.4%) | 2,453 (83.7%) | 1,123 (82.4%) | 876 (80.7%) |
| Black | 273 (6.2%) | 423 (9.1%) | 327 (11.2%) | 173 (12.7%) | 166 (15.3%) |
| Asian | 92 (2.1%) | 46 (1.0%) | 26 (0.9%) | 5 (0.4%) | 1 (0.1%) |
| Hispanic | 104 (2.4%) | 142 (3.1%) | 69 (2.4%) | 39 (2.9%) | 26 (2.4%) |
| Other | 73 (1.7%) | 68 (1.5%) | 56 (1.9%) | 23 (1.7%) | 17 (1.6%) |
| Men (n = 34,785) | |||||
| Caucasian | 6,034 (82.8%) | 12,584 (86.1%) | 7,163 (87.2%) | 2,607 (87.3%) | 1,256 (86.8%) |
| Black | 604 (8.3%) | 877 (6%) | 508 (6.2%) | 195 (6.5%) | 125 (8.6%) |
| Asian | 221 (3.0%) | 215 (1.5%) | 53 (0.6%) | 17 (0.6%) | 5 (0.3%) |
| Hispanic | 270 (3.7%) | 618 (4.2%) | 347 (4.2%) | 115 (3.9%) | 46 (3.2%) |
| Other | 162 (2.2%) | 317 (2.2%) | 143 (1.7%) | 51 (1.7%) | 15 (1.0%) |
|
| |||||
| Medical history | |||||
| Current/recent smoker | 5,299 (45.0%) | 8,355 (43.1%) | 4,764 (42.4%) | 1,872 (42.8%) | 1,051 (41.2%) |
| Hypertension | 6,698 (56.9%) | 11,343 (58.5%) | 7,282 (64.8%) | 3,032 (69.3%) | 1,881 (73.8%) |
| Dyslipidemia | 5,109 (43.4%) | 9,647 (49.7%) | 6,063 (54.0%) | 2,457 (56.1%) | 1,427 (56.0%) |
| Diabetes mellitus | 1,749 (14.8%) | 3,788 (19.5%) | 3,049 (27.1%) | 1,538 (35.1%) | 1,110 (43.6%) |
| Prior MI | 2,265 (19.2%) | 3,602 (18.6%) | 2,203 (19.6%) | 915 (20.9%) | 530 (20.8%) |
| Prior PCI | 2,140 (18.2%) | 3,638 (18.8%) | 2,289 (20.4%) | 972 (22.2%) | 545 (21.4%) |
| Prior CABG | 824 (7.0%) | 1,373 (7.1%) | 800 (7.1%) | 309 (7.1%) | 141 (5.5%) |
| Atrial fibrillation or flutter | 254 (5.1%) | 287 (3.5%) | 166 (3.4%) | 78 (4.2%) | 38 (3.2%) |
| Prior stroke | 781 (6.6%) | 879 (4.5%) | 473 (4.2%) | 176 (4.0%) | 107 (4.2%) |
| Peripheral artery disease | 908 (7.7%) | 1,007 (5.2%) | 507 (4.5%) | 174 (4.0%) | 141 (5.5%) |
| Prior CHF | 726 (6.2%) | 780 (4.0%) | 506 (4.5%) | 230 (5.3%) | 192 (7.5%) |
|
| |||||
| Home medications | |||||
| Aspirin | 3,877 (32.9%) | 6,542 (33.7%) | 3,882 (34.6%) | 1,555 (35.5%) | 876 (34.4%) |
| Clopidogrel | 1,122 (9.5%) | 1,643 (8.5%) | 1,025 (9.1%) | 444 (10.1%) | 288 (11.3%) |
| Beta-blockers | 3,178 (27.0%) | 5,031 (25.9%) | 3,151 (28.0%) | 1,366 (31.2%) | 841 (33.0%) |
| ACE inhibitors | 2,261 (19.2%) | 3,950 (20.4%) | 2,577 (22.9%) | 1,140 (26.1%) | 706 (27.7%) |
| Angiotensin receptor blockers | 798 (6.8%) | 1,430 (7.4%) | 1,050 (9.3%) | 470 (10.7%) | 305 (12.0%) |
| Aldosterone-blocking agents | 126 (1.1%) | 158 (0.8%) | 125 (1.1%) | 59 (1.3%) | 48 (1.9%) |
| Statins | 3,091 (26.2%) | 5,658 (29.2%) | 3,511 (31.3%) | 1,466 (33.5%) | 879 (34.5%) |
Values are median (interquartile range) or n (%).
ACE = angiotensin-converting enzyme; BMI = body mass index; CABG = coronary artery bypass graft surgery; CHF = congestive heart failure; MI = myocardial infarction; PCI = percutaneous coronary intervention.
Table 2.
Presentation Characteristics, Reperfusion Strategy and Medical Therapy, Discharge Medications, Counseling, and Referrals Stratified by BMI Category
| Variable | BMI Category (kg/m2)
|
||||
|---|---|---|---|---|---|
| Normal Weight (18.5 to <25)
|
Overweight (25 to <30)
|
Class I Obese (30 to <35)
|
Class II Obese (35 to <40)
|
Class III Obese (≥40)
|
|
| (n = 11,780) | (n = 19,391) | (n = 11,234) | (n = 4,376) | (n = 2,548) | |
| Initial laboratory values | |||||
| Hemoglobin (g/dl) | 13.8 (12.5–15.0) | 14.4 (13.2–15.4) | 14.5 (13.3–15.6) | 14.5 (13.3–15.6) | 14.3 (13.1–15.5) |
| Total cholesterol (mg/dl) | 161 (134–190) | 168 (141–198) | 172 (143–201) | 173 (145–203) | 171 (145–202) |
| HDL cholesterol (mg/dl) | 39 (32–48) | 35 (30–43) | 34 (29–41) | 33 (28–40) | 34 (29–41) |
| LDL cholesterol (mg/dl) | 97 (74–123) | 103 (79.0–129.6) | 104 (79–130) | 104 (79–130) | 103 (79–130) |
| Triglycerides (mg/dl) | 97 (68–142) | 121 (84–177) | 140 (96–208) | 148 (101–224) | 144 (100–218) |
| Serum creatinine (mg/dl) | 1.1 (0.9–1.2) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.2) | 1.1 (0.8–1.2) |
|
| |||||
| Presentation characteristics | |||||
| HF | 1,594 (13.5%) | 2,127 (11.0%) | 1,173 (10.4%) | 506 (11.6%) | 280 (11.0%) |
| Shock | 839 (7.1%) | 1,151 (5.9%) | 579 (5.2%) | 241 (5.5%) | 141 (5.5%) |
| Heart rate (beats/min) | 77 (64–93) | 77 (64–91) | 79 (66–93) | 80 (68–95) | 82 (70–96) |
| SBP (mm Hg) | 134 (113–154) | 138 (118–159) | 141 (120–160) | 142 (121–163) | 143 (122–164) |
| eGFR (ml/min/1.73 m2)* | 71.5 (56.0–88.1) | 72.9 (58.6–88.1) | 72.9 (58.9–87.7) | 73.7 (58.8–89.7) | 74.3 (58.9–91.8) |
| Number of diseased vessels† | |||||
| No significant disease | 338 (3.2%) | 432 (2.4%) | 214 (2.0%) | 107 (2.6%) | 72 (3.1%) |
| 1 | 3,819 (36.4%) | 6,782 (37.6%) | 4,113 (39.3%) | 1,605 (39.2%) | 1,029 (44.1%) |
| 2 | 3,323 (31.6%) | 5,765 (32%) | 3,305 (31.6%) | 1,339 (32.7%) | 699 (30.0%) |
| 3 | 2,983 (28.4%) | 4,978 (27.6%) | 2,781 (26.6%) | 1,036 (25.3%) | 522 (22.4%) |
| LV ejection fraction (%)‡ | |||||
| ≥50 | 4,629 (43.1%) | 8,645 (48.3%) | 5,093 (49.3%) | 2,062 (51.3%) | 1,171 (51.0%) |
| 40–50 | 2,789 (26.0%) | 4,743 (26.5%) | 2,722 (26.3%) | 1,019 (25.3%) | 632 (27.6%) |
| 25–40 | 2,476 (23.0%) | 3,552 (19.8%) | 1,991 (19.3%) | 764 (19.0%) | 377 (16.4%) |
| >25 | 751 (7.0%) | 839 (4.7%) | 447 (4.3%) | 146 (3.6%) | 86 (3.7%) |
|
| |||||
| Reperfusion strategy§ | |||||
| Overall reperfusion | 8,715 (92.3%) | 15,475 (93.5%) | 9,020 (93.9%) | 3,502 (93.5%) | 1,978 (93%) |
| Thrombolytic therapy | 1,212 (12.8%) | 2,311 (13.9%) | 1,356 (14%) | 554 (14.7%) | 304 (14.1%) |
| Primary PCI | 7,627 (80.8%) | 13,398 (80.9%) | 7,801 (81.2%) | 2,996 (80%) | 1,711 (80.4%) |
| Arrival to primary PCI (min)|| | 69 (53–87) | 68 (52–85) | 68 (53–86) | 69 (54–87) | 73 (58–90) |
| CABG | 766 (7.2%) | 1,388 (7.7%) | 815 (7.8%) | 291 (7.1%) | 147 (6.3%) |
| Arrival to CABG (h) | 41.0 (68.0–100.1) | 38.6 (52.0–90.9) | 38.1 (46.0–90.1) | 54.9 (69.0–120.5) | 45.9 (82.0–132.8) |
|
| |||||
| Medications within 24 h§ | |||||
| Aspirin | 11,253 (97.9%) | 18,721 (98.5%) | 10,880 (98.7%) | 4,232 (98.8%) | 2,436 (98.1%) |
| Clopidogrel | 9,471 (85.6%) | 16,318 (88.7%) | 9,487 (89%) | 3,687 (88.2%) | 2,108 (87.4%) |
| Beta-blocker | 9,263 (94.2%) | 16,027 (95.5%) | 9,581 (96.1%) | 3,747 (95.2%) | 2,170 (95.5%) |
| ACE inhibitors or ARBs | 5,347 (50.8%) | 9,945 (55.6%) | 6,209 (59.6%) | 2,435 (59.8%) | 1,435 (60.6%) |
| Aldosterone-blocking agents | 228 (2.0%) | 327 (1.8%) | 208 (1.9%) | 85 (2.0%) | 57 (2.3%) |
| Statins | 7,407 (66.3%) | 12,981 (69.9%) | 7,608 (70.5%) | 2,928 (69.6%) | 1,683 (68.9%) |
| Glycoprotein IIb/IIIa inhibitors | 7,532 (68.9%) | 13,495 (73.4%) | 8,035 (75.1%) | 3,092 (74.0%) | 1,737 (71.9%) |
| Heparin¶ | |||||
| None | 1,241 (10.7%) | 1,898 (9.9%) | 1,128 (10.1%) | 417 (9.6%) | 253 (10.0%) |
| Low–molecular weight | 1,138 (9.8%) | 1,683 (8.8%) | 1,014 (9.1%) | 435 (10.0%) | 218 (8.6%) |
| Unfractionated | 8,316 (71.9%) | 14,268 (74.4%) | 8,223 (74%) | 3,161 (72.9%) | 1,859 (73.7%) |
| Both | 822 (7.1%) | 1,268 (6.6%) | 721 (6.5%) | 305 (7.0%) | 178 (7.1%) |
| Bivalirudin¶ | 1,581 (13.7%) | 2,787 (14.5%) | 1,620 (14.6%) | 623 (14.4%) | 382 (15.1%) |
|
| |||||
| Discharge medications§ | |||||
| Aspirin | 9,813 (98.4%) | 16,909 (98.6%) | 9,865 (98.7%) | 3,837 (98.9%) | 2,157 (98.2%) |
| Clopidogrel | 8,830 (90.2%) | 15,542 (92.1%) | 9,105 (92.1%) | 3,498 (91.8%) | 1,995 (91.9%) |
| Warfarin | 830 (8.1%) | 1,267 (7.3%) | 725 (7.1%) | 285 (7.2%) | 147 (6.6%) |
| Beta-blockers | 9,343 (96.5%) | 16,372 (97.5%) | 9,612 (97.6%) | 3,742 (97.9%) | 2,113 (97.2%) |
| ACE inhibitors or ARBs | 2,045 (85%) | 3,094 (87.7%) | 1,788 (88.7%) | 645 (88.6%) | 336 (89.6%) |
| Aldosterone-blocking agents | 329 (8.2%) | 495 (7.4%) | 333 (7.5%) | 135 (7.1%) | 79 (6.6%) |
| Statins | 9,050 (90.8%) | 16,011 (93.7%) | 9,379 (93.8%) | 3,647 (94.2%) | 2,033 (91.9%) |
|
| |||||
| Discharge interventions§ | |||||
| Smoking cessation counseling | 4,639 (96.9%) | 7,522 (96.8%) | 4,316 (97.0%) | 1,683 (96.7%) | 926 (96.4%) |
| Diet modification counseling | 9,567 (93.6%) | 16,535 (94.7%) | 9,711 (95.2%) | 3,765 (95.2%) | 2,148 (95.6%) |
| Cardiac rehabilitation referral | 7,456 (79.9%) | 13,494 (81.4%) | 7,944 (81.9%) | 3,112 (82.4%) | 1,758 (82.6%) |
| Exercise counseling | 8,819 (86.9%) | 15,331 (88.1%) | 9,038 (89%) | 3,533 (89.8%) | 1,961 (87.7%) |
Values are median (interquartile range) or n (%).
Estimated using the MDRD (Modification of Diet in Renal Disease) formula. Excludes dialysis patients.
Among patients who underwent cardiac catheterization.
Among patients who had LV ejection fraction measured.
Among eligible patients.
Excludes transfer-in patients.
Any time during hospital stay.
ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HF = heart failure; LDL = low-density lipoprotein; SBP = systolic blood pressure; other abbreviations as in Table 1.
Reperfusion was attempted in more than 90% of patients across all BMI categories (Table 2). No differences were present in the proportion of patients receiving fibrinolytic therapy or PCI according to BMI, even among those with extreme obesity, with >80% of patients in all BMI groups receiving primary PCI. In-hospital use of evidence-based medical therapies was high overall and similar across BMI groups. Similarly, prescription of evidence-based therapies at hospital discharge, including aspirin, clopidogrel, beta-blockers, and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, was high and did not differ across BMI groups, including class III obesity (Table 2). Statin therapy was slightly less common at discharge among class III obese patients. Rates of counseling regarding smoking cessation, dietary modification and exercise, and referral to cardiac rehabilitation were similarly high across BMI categories, including class III obesity.
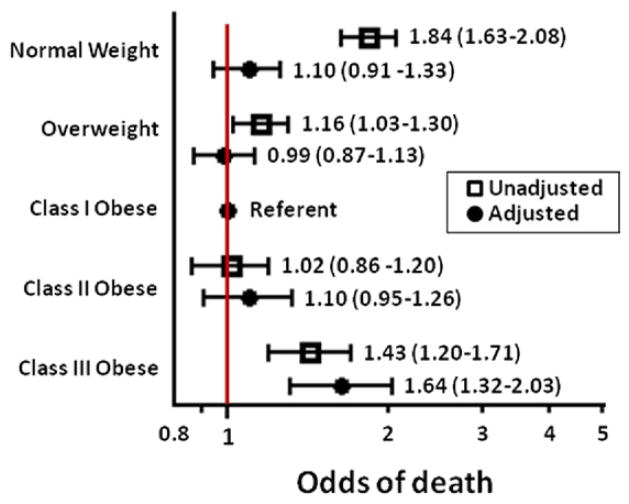
In unadjusted analyses, a U-shaped association with BMI categories was seen for both mortality (Fig. 1) and major bleeding (Fig. 2). Rates of adverse outcomes in general were highest among normal-weight patients, lower in overweight and mild to moderately obese patients, and then increased again in patients with class III obesity (Table 3). After multivariate adjustment, compared with class I obese patients, the adjusted odds of death were not significantly different for normal-weight, overweight, and class II obese patients. Odds of death were 64% higher for class III obese patients compared with class I obese patients (Fig. 1). In contrast, the adjusted odds of major bleeding were highest in normal-weight patients and did not differ significantly for class III compared with class I obese patients (Fig. 2).
Figure 1.
In-Hospital Mortality by BMI
Unadjusted and adjusted odds of in-hospital mortality across body mass index (BMI) categories, using class I obesity as the referent. After multivariate adjustment, extreme (class III) obesity was associated with increased in-hospital mortality (odds ratio: 1.64; 95% confidence interval: 1.32 to 2.03).
Figure 2.
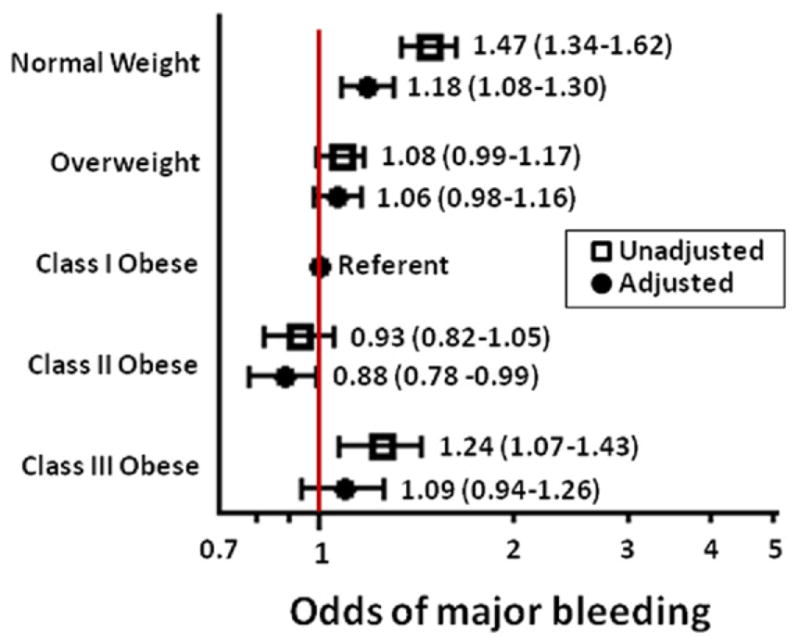
Major Bleeding by BMI
Unadjusted and adjusted 8 odds of major bleeding across body mass index (BMI) categories, using class I obesity as the referent. After multivariate adjustment, normal weight was associated with increased major bleeding (odds ratio: 1.18; 95% confidence interval: 1.08 to 1.30), while extreme (class III) obesity was not (odds ratio: 1.09; 95% confidence interval: 0.94 to 1.26).
Table 3.
In-Hospital Clinical Events Stratified by BMI
| Variable | BMI Category (kg/m2)
|
||||
|---|---|---|---|---|---|
| Normal Weight (18.5 to <25)
|
Overweight (25 to <30)
|
Class I Obese (30 to <35)
|
Class II Obese (35 to <40)
|
Class III Obese (≥40)
|
|
| (n = 11,780) | (n = 19,391) | (n = 11,234) | (n = 4,376) | (n = 2,548) | |
| Death | 868 (7.7%) | 919 (5.0%) | 461 (4.3%) | 182 (4.4%) | 147 (6.1%) |
|
| |||||
| Recurrent MI | 138 (1.2%) | 184 (1.0%) | 103 (1.0%) | 40 (1.0%) | 29 (1.2%) |
|
| |||||
| Death or MI | 960 (8.5%) | 1,058 (5.7%) | 540 (5.0%) | 211 (5.1%) | 171 (7.1%) |
|
| |||||
| Cardiogenic shock | 849 (7.5%) | 1,106 (6.0%) | 552 (5.1%) | 215 (5.2%) | 152 (6.3%) |
|
| |||||
| Congestive HF | 898 (8.0%) | 1,099 (5.9%) | 608 (5.7%) | 269 (6.5%) | 159 (6.6%) |
|
| |||||
| Stroke | 118 (1.0%) | 137 (0.7%) | 71 (0.7%) | 23 (0.6%) | 14 (0.6%) |
|
| |||||
| Major bleeding | 1,574 (14.0%) | 1,972 (10.6%) | 1,062 (9.9%) | 388 (9.3%) | 288 (11.9%) |
|
| |||||
| Non-CABG transfusion | 932 (8.9%) | 908 (5.3%) | 471 (4.8%) | 191 (5.0%) | 147 (6.5%) |
Discussion
In this contemporary analysis of the relationship between BMI and clinical characteristics, treatment patterns, and in-hospital outcomes for 50,149 patients with STEMI, we observed that: 1) three-fourths of patients with STEMI were overweight or obese; 2) class III obesity (BMI >40 kg/m2) now affects 1 in 20 patients with STEMI and is particularly common in African American women presenting with STEMI (more than 1 in 7); 3) the most obese patients with STEMI presented more than a decade younger than their normal-weight counterparts, with less extensive coronary artery disease and better LV systolic function despite a higher risk factor burden; 4) processes and quality-of-care measures did not differ in a clinically meaningful way for obese patients, including class III obese patients; and 5) despite similar processes and quality of care and a lower risk profile, class III obesity was associated with a notable increase in the risk for in-hospital mortality but not for major bleeding.
Obesity prevalence and presentation characteristics
The prevalence of obesity in patients presenting with STEMI was 36.2%, and that of overweight and obesity combined was 74.9%. The closest comparable national obesity prevalence data available, from the NHANES (National Health and Nutrition Examination Survey) in 2008 (21), show a lower prevalence of obesity (33.8%) and overweight and obesity combined (68.0%) in the general population of U.S. adults age ≥20 years. The prevalence of class III obesity (BMI ≥40 kg/m2) in this NHANES cohort was 5.7% overall, with the prevalence in women (7.2%) higher than in men (4.2%) and the highest prevalence seen among non-Hispanic black women (14.2%) (21). The higher obesity prevalence among patients with STEMI compared with the general population likely reflects the contribution of obesity to the pathogenesis of STEMI, which is mediated at least in part by increases in hypertension, diabetes, and dyslipidemia in obese individuals. Importantly, in the class III obese subgroup, the overrepresentation of African American and female patients and the younger age of these patients at presentation suggest the possibility that class III obesity may be contributing to premature MI in African American women. This is especially relevant in light of our finding that class III obesity was associated with early mortality after MI and suggests the possibility that increasing rates of class III obesity in African American women may contribute to worsening race-based and sex-based disparities in outcomes after STEMI (22).
Despite a higher prevalence of traditional cardiac risk factors, obese patients tended to have less extensive coronary disease and LV systolic dysfunction, likely because they presented with STEMI at much younger ages than their normal-weight counterparts; class III obese patients were on average more than a decade younger than normal-weight patients. Brain natriuretic peptide and N-terminal pro–brain natriuretic peptide decreased markedly across increasing BMI categories, disproportionately to underlying differences in LV function or clinical HF, most likely reflecting the known inverse relationship between higher body mass and lower natriuretic peptide concentrations (23).
Process of care
Contrary to our a priori hypothesis, we observed few meaningful differences in processes of care for obese patients, including those with class III obesity. For the subset undergoing primary PCI, no differences were noted regarding the administration of reperfusion therapy, with the exception of a slightly longer door-to-balloon time, a difference we believe is too small to be clinically meaningful. Moreover, the administration of concomitant anti-platelet and antithrombotic agents and the initiation of secondary prevention therapies such as statins and beta-blockers were similar across BMI categories. Prescription of guideline-indicated medications at discharge, dietary counseling, referral to cardiac rehabilitation, and counseling regarding exercise were also similar across weight categories, with obese patients receiving appropriate referrals as often as their normal-weight counterparts. These data are encouraging and suggest the absence of an obesity-related systematic bias in the delivery of STEMI care, even to class III obese patients.
Obesity and in-hospital mortality
The adjusted odds of death were lowest for class I obese patients and were not significantly different for normal-weight, overweight, or class II obese patients but were significantly higher for class III compared with class I obese patients. The observed increase in mortality in the class III obese patients, which persisted after multivariate adjustment, is of particular concern given the very rapid rise in class III obesity, far exceeding the overall increase in obesity prevalence in the U.S. population. From 1960 to 2004, national estimates of obesity prevalence as a whole increased from 13.3% (10.7% in men, 15.8% in women) to 32.9% (31.7% in men, 34.0% in women), a relative increase of almost 150%. Over the same time interval, however, the population prevalence of class III obesity increased from 0.9% (0.3% in men, 1.4% in women) to 5.1% (3.0% in men, 7.3% in women), a relative increase of 460% (2).
The mechanism for this increase in mortality risk in class III obese patients presenting with STEMI is not known, as these patients are younger, have less extensive coronary disease, less LV systolic dysfunction, and higher estimated glomerular filtration rates, and receive similar care compared with less obese patients. Thus, class III obesity must either carry intrinsic hazard or must be accompanied by hidden comorbidities not captured by the registry. Obese patients have an increased total body blood volume, higher filling pressures, and increased sympathetic activation, which lead to increased stroke volume and heart rate; cardiac work is increased, and this may be accentuated at very elevated levels of adiposity seen in class III obese patients. Cardiac structural changes in class III obesity include markedly increased LV mass (20), known to be a risk factor for increased ventricular arrhythmias and sudden cardiac death. In addition, class III obesity has been described as an inflammatory and prothrombotic state (24), which may also contribute to adverse prognosis. Although published research does not reach a consensus on the effect of obesity on in-hospital course of critically ill patients (25–29) obese patients are more susceptible to comorbid respiratory complications such as aspiration pneumonia (30), pulmonary thromboembolism (31), or sleep apnea and obesity hypoventilation syndrome (31,32). In addition, there are practical difficulties in caring for class III obese patients who are critically ill that may affect the in-hospital outcomes reported here, such as prolonged immobility, difficulty obtaining venous access, or inability to perform indicated diagnostic or therapeutic procedures because of equipment weight restrictions (33).
It is of considerable interest that the higher unadjusted rates of adverse outcomes in the normal-BMI subgroup, compared with the overweight or mildly obese subgroups, disappeared after multivariate adjustment. This finding strongly suggests that the unadjusted association of lower mortality with overweight or mild obesity is explained by the effect of confounders in the normal-BMI group, such as older age, more extensive cardiac disease, or known or undiagnosed serious medical conditions. This raises the provocative hypothesis that the “obesity paradox” described in many cardiovascular disease states (11–14,20,34 –36) may be explained in whole or in part by residual confounding. Such an explanation has been widely believed to explain excess risk among underweight individuals with cardiovascular disease but has not been applied to normal-weight individuals. In other words, being normal weight in a contemporary population with cardiovascular disease is now so uncommon that it may reflect the presence of unmeasured serious comorbid conditions. As such, “protective” effects that have been attributed to overweight and moderate obesity in patients with cardiovascular disease may not actually exist and may simply reflect unmeasured confounding in normal-weight individuals. Moreover, because normal BMI may reflect unmeasured comorbidities, overweight or mild obesity likely represents the more appropriate referent body mass in the post-MI setting.
Obesity and in-hospital major bleeding
In contrast to the associations observed for in-hospital mortality, the risk for major bleeding was not significantly higher in the class III obese patients compared with the class I obese patients after adjusting for patient baseline characteristics. This finding suggests that factors contributing to increased mortality with obesity do not also contribute to higher bleeding rates and that bleeding-related complications are unlikely to explain the excess mortality in the class III obese. The lack of an unfavorable bleeding signal in the class III obese likely reflects the younger age and lower overall bleeding risk profile of the more obese patients, as well as the generally high quality of care applied to all patients, including the very obese. Excess dosing of anticoagulant and antiplatelet drugs likely occurs less often in more obese patients, which would potentially mitigate bleeding risks. In contrast, it is plausible that relative underdosing of anticoagulant and antiplatelet medications could contribute to the excess mortality described here, a hypothesis that merits further exploration with pharmacokinetic and pharmacodynamic studies targeted specifically at individuals with class III obesity.
Study limitations
The present study used registry data and thus could not account for confounders that are not captured in the database. Centers participating in the registry may differ systematically from facilities that do not, especially with regard to processes of care and attention to quality-of-care metrics. Patients in the registry are mostly white and more likely to be men, which may limit generalizability to other populations, especially African American and Hispanic women, who have substantially higher rates of obesity and are less well represented in the present study. Information on radial versus femoral access is not available, so we could not assess the possible role of radial access in lowering bleeding risks in class III obese patients. Data reported here can only establish the association between BMI and in-hospital outcomes; no long-term follow-up data are available.
Conclusions
In this large, multicenter cohort of 50,149 patients with STEMI, extreme obesity (BMI ≥40 kg/m2) was independently associated with in-hospital death compared with class I obesity after multivariate adjustment for potential confounding. This is true despite the fact that extremely obese patients present more than a decade younger, with less extensive coronary artery disease and better LV systolic function and with better renal function. In addition, processes and quality-of-care measures were generally excellent for class III obese patients, showing no evidence of systematic bias adversely affecting morbidly obese patients. This somewhat surprising finding likely reflects how ubiquitous obesity, even class III obesity, is in modern practice. The enigma of patients who are at lower a priori risk and receive similar care but nevertheless have worse outcomes mandates further attention and elucidation as the population prevalence of class III obesity continues to grow at a pace that far exceeds the overall rise in obesity.
Acknowledgments
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). ACTION Registry–GWTG is an initiative of the American College of Cardiology Foundation and the American Heart Association, with partnering support from the Society of Chest Pain Centers, the American College of Emergency Physicians, and the Society of Hospital Medicine. The registry is sponsored in part by the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership. The views expressed in this report represent those of the authors and do not necessarily represent the official views of the NCDR or its associated professional societies, identified at http://www.ncdr.com. Dr. Diercks has received research support from Beckman Coulter and Nanosphere; and consulting fees from Daichii Sankyo. Dr. Peterson has received grant support BMS/Sanofi, Lilly, and Johnson & Johnson. Dr. Roe has received research funding from Eli Lilly, Hoffmann-La Roche, Bristol-Myers Squibb, Novartis, the American College of Cardiology, and the American Heart Association; and consulting fees or honoraria from KAI Pharmaceuticals, Bristol-Myers Squibb, Sanofi-Aventis, Merck, Orexigen Therapeutics, Helsinn Pharmaceuticals, AstraZeneca, and Regeneron. Dr. de Lemos has received speaker honoraria from BMS/Sanofi-Aventis; and consulting income from AstraZeneca.
Abbreviations and Acronyms
- BMI
body mass index
- GWTG
Get With the Guidelines
- HF
heart failure
- LV
left ventricular
- MI
myocardial infarction
- NCDR
National Cardiovascular Data Registry
- NSTEMI
non–ST-segment elevation myocardial infarction
- PCI
percutaneous coronary intervention
- STEMI
ST-segment elevation myocardial infarction
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.National Heart, Lung, and Blood Institute Expert Panel. [Accessed April 3, 2011];Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Available at: http://www.nhlbi.nih.gov/guidelines/obesity.
- 2.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999 –2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Krauss RM AHA Nutrition Committee. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. Circulation. 1998;97:2099–100. doi: 10.1161/01.cir.97.21.2099. [DOI] [PubMed] [Google Scholar]
- 5.Wolk R, Berger P, Lennon RJ, Brilakis ES, Somers VK. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108:2206–11. doi: 10.1161/01.CIR.0000095270.85646.E8. [DOI] [PubMed] [Google Scholar]
- 6.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–27. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan RC, Heckbert SR, Furberg CD, Psaty BM. Predictors of subsequent coronary events, stroke, and death among survivors of first hospitalized myocardial infarction. J Clin Epidemiol. 2002;55:654– 64. doi: 10.1016/s0895-4356(02)00405-5. [DOI] [PubMed] [Google Scholar]
- 8.Hoit BD, Gilpin EA, Maisel AA, Henning H, Carlisle J, Ross J., Jr Influence of obesity on morbidity and mortality after acute myocardial infarction. Am Heart J. 1987;114:1334– 41. doi: 10.1016/0002-8703(87)90534-5. [DOI] [PubMed] [Google Scholar]
- 9.Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA. Obesity and the risk of death after acute myocardial infarction. Am Heart J. 2004;147:841– 6. doi: 10.1016/j.ahj.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Jimenez F, Jacobsen SJ, Reeder GS, Weston SA, Meverden RA, Roger VL. Prevalence and secular trends of excess body weight and impact on outcomes after myocardial infarction in the community. Chest. 2004;125:1205–12. doi: 10.1378/chest.125.4.1205. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122:1106–14. doi: 10.1016/j.amjmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Ades PA, Savage PD. The obesity paradox: perception vs knowledge. Mayo Clin Proc. 2010;85:112– 4. doi: 10.4065/mcp.2009.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diercks DB, Roe MT, Mulgund J, et al. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid Risk Stratification of Unstable angina patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140– 8. doi: 10.1016/j.ahj.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Peterson ED, Roe MT, Chen AY, et al. The NCDR ACTION Registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart. 2010;96:1798– 802. doi: 10.1136/hrt.2010.200261. [DOI] [PubMed] [Google Scholar]
- 16.Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254– 63. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Peterson ED, Roe MT, Rumsfeld JS, et al. A call to ACTION (Acute Coronary Treatment and Intervention Outcomes Network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–9. doi: 10.1161/CIRCOUTCOMES.108.847145. [DOI] [PubMed] [Google Scholar]
- 18.Hoekstra JW, Pollack CV, Jr, Roe MT, et al. Improving the care of patients with non-ST-elevation acute coronary syndromes in the emergency department: the CRUSADE initiative. Acad Emerg Med. 2002;9:1146–55. doi: 10.1111/j.1553-2712.2002.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 19.Chin CT, Chen AY, Wang TY, et al. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get With the Guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2010;161:113–22. doi: 10.1016/j.ahj.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235– 41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 22.Truman BI, Smith CK, Roy K, et al. [Accessed March 28, 2011];Rationale of regular reporting on health disparities and inequalities—United States. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/su6001a2.htm?s_cid=su6001a2_w. [PubMed]
- 23.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163– 8. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 24.Cottam DR, Mattar SG, Barinas-Mitchell E, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14:589–600. doi: 10.1381/096089204323093345. [DOI] [PubMed] [Google Scholar]
- 25.El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120:1989–97. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 26.Galanos AN, Pieper CF, Kussin PS, et al. for the SUPPORT Investigators. Relationship of body mass index to subsequent mortality among seriously ill hospitalized patients. Crit Care Med. 1997;25:1962–8. doi: 10.1097/00003246-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641– 4. doi: 10.1001/archinte.160.17.2641. [DOI] [PubMed] [Google Scholar]
- 28.Ray DE, Matchett SC, Baker K, Wasser T, Young MJ. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127:2125–31. doi: 10.1378/chest.127.6.2125. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123:1202–7. doi: 10.1378/chest.123.4.1202. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan RW, Bauer S, Wise L. Volume and pH of gastric juice in obese patients. Anesthesiology. 1975;43:686–9. doi: 10.1097/00000542-197512000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Kral JG. Morbid obesity and related health risks. Ann Intern Med. 1985;103:1043–7. doi: 10.7326/0003-4819-103-6-1043. [DOI] [PubMed] [Google Scholar]
- 32.Valencia-Flores M, Orea A, Castano VA, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res. 2000;8:262–9. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 33.Nasraway SA, Jr, Albert M, Donnelly AM, Ruthazer R, Shikora SA, Saltzman E. Morbid obesity is an independent determinant of death among surgical critically ill patients. Crit Care Med. 2006;34:964–70. doi: 10.1097/01.CCM.0000205758.18891.70. [DOI] [PubMed] [Google Scholar]
- 34.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74– 81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578– 84. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 36.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–70. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]