Abstract
Escalation of drug self-administration is a consequence of extended drug access and is thought to be specifically related to addiction, but few studies have investigated whether intake of non-drug reinforcers may also escalate with extended-access. The goal of these studies was to determine the effects of limited and extended-access to food reinforcers on behavioral and pharmacological endpoints in mice. In distinct groups, responding on a lever was maintained by liquid reinforcement, or nose-poke responses were maintained by sucrose pellets or liquid food in sessions lasting 1 hour (limited-access) or 10 hours (extended-access). The reinforcing strength of each food, as well as reinforcer-associated cues, was tested before and after extended-access using a progressive ratio (PR) schedule, and locomotor activity in response to novelty and increasing doses of cocaine was assessed in an open field setting in all animals after access to food reinforcers. Escalation of lever-pressing behavior reinforced by liquid food, but not nose-poke behavior reinforced by liquid food or sucrose pellets, was observed across successive extended-access sessions. A concomitant increase in the reinforcing strength of liquid food and its associated cues was apparent in mice that escalated their responding, but not in mice that did not escalate. Finally, extended reinforcer access leading to behavioral escalation was accompanied by an increased sensitivity to the psychostimulant effects of cocaine compared to limited-access. These findings indicate that behavioral escalation can develop as a consequence of extended-access to a non-drug reinforcer, although both the nature of the reinforcer (liquid versus solid food) and the topography of the operant response (lever versus nose-poke) modulate its development. These data also suggest that some of the behavioral and pharmacological corrolaries of behavioral escalation observed following extended-access to drug self-administration may not be due to drug exposure, but rather, may result from basic behavioral processes which underlie operant responding maintained by appetitive stimuli.
Keywords: Addiction, Animal models, Motivation, Sensitization, Cocaine, Reinforcement
Introduction
Extended-access to drug reinforcers can result in an “escalation” of drug intake (reviewed by Zernig et al., 2007), as was first demonstrated by Ahmed and Koob (1998) in rats allowed to intravenously self-administer cocaine for either 1 or 6 hr. The major finding of this report was that rats restricted to 1 hr access to cocaine maintained a stable level of drug self-administration over successive sessions, while animals allowed extended-access (6 hr) to cocaine progressively escalated their drug intakes (from their own baseline) over this experimental duration. This phenomenon has now been replicated in multiple laboratories and species with several other drugs of abuse, including heroin (Ahmed et al., 2000), methamphetamine (Kitamura et al., 2006) and phencyclidine (Carroll et al., 2005). The escalation procedure is generally thought to model “compulsive drug intake,” “increased motivation for drug,” or “loss of control” over drug self-administration, all of which are thought to be critical signs of the transition from controlled drug use to addiction. The increasing popularity of the Ahmed and Koob escalation model is highlighted by the fact that their initial finding has been cited well over 200 times since its publication, and its adoption by other researchers has allowed for a reasonably thorough exploration of the behavioral and neural consequences of escalated drug intake (e.g. Ferrario et al., 2005; Ahmed and Cador, 2006). Surprisingly, however, the specificity of the model has not been extensively tested using non-drug reinforcers. Because of this, addiction-based interpretations of behavioral and neural data derived from the escalation model must be regarded as tenuous at this time.
It is a well known tenet of operant conditioning that behaviors which result in the delivery of palatable foods will increase in frequency – this is a specific example of the general phenomenon of positive reinforcement (c.f., Ferster and Culbertson, 1982). In the drug studies outlined above, drug infusions function as positive reinforcers, and instrumental responses in animal subjects are maintained by drug delivery in sessions of either short (typically 1 hr) or long (anywhere between 6 and 11 hr) duration. While many investigators have studied within-session changes in patterns of food-reinforced responding (e.g. DeMarse et al., 1998; McSweeney and Murphy, 2000), studies designed to investigate whether extended-access to food reinforcers would lead to escalated intake across sessions have not been previously reported. However, similar experiments have been performed using non-operant techniques. In one such study, escalated sugar intake with bingeing in the first hour of daily access was engendered following one month of daily 12 hr food deprivation and 12 hr access to sugar and chow in rats (Colantuoni et al., 2001). Moreover, mice repeatedly exposed to palatable food in a specific environmental context displayed progressive and persistent increases in locomotor activity in that context, as well as “cross-sensitization” to the stimulant effects of some drugs of abuse (LeMerrer and Stephens, 2006). These data resemble the development of behavioral sensitization to repeated intermittent exposure to drugs of abuse such as cocaine, and suggest that the non-contingent delivery of drug and non-drug appetitive stimuli may result in similar behavioral changes. This interpreation implies a common mechanism underlying both food- and drug-induced behavioral sensitization.
The reinforcing strength of self-administered drugs seems to change as a consequence of behavioral escalation, but the data in this regard are often conflicting. Some previous studies utilizing the Ahmed and Koob escalation procedure with cocaine self-administration have demonstrated increased response rates under progressive ratio (PR) schedules following behavioral escalation, but no change in breakpoint (e.g., Liu et al., 2005). Other studies have quantified increases in maximal breakpoints maintained by high unit doses of cocaine with no corresponding change in sensitivity to lower doses (e.g., Morgan et al., 2005). Still other studies have reported “vertical” shifts in the entire dose-effect function for cocaine under a PR schedule following escalation of intake, suggesting an increased sensitivity to all drug doses including saline (e.g., Paterson and Markou, 2003). Finally, other studies have demonstrated dose-dependent escalation of cocaine self-administration (Roberts et al., 2007).
We thus studied the effects of limited and extended-access to non-drug reinforcers on food-maintained behavior, PR performance, responding during extinction, and sensitivity to the locomotor stimulant effects of various doses of cocaine. To assess the behavioral specificity of these effects, liquid or solid food reinforcers were used to maintain behavior on either a response lever or a nose-poke device. Although previous studies utilizing drug self-administration in mice have demonstrated remarkably similar dose-effect functions generated with lever-press or nose-poke operant responses (Caine et al., 1999; David et al., 2001), the topography of the operant response can profoundly influence the behavioral effects of psychostimulants in other settings (Graeff and De Oliveira, 1975). Thus, the operant response and the particular food reinforcer maintaining behavior were parametrically varied across groups.
Materials and Methods
Animals
Drug and experimentally naïve male Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, MA) weighing approximately 25 g at the start of behavioral training served as subjects. Mice were housed in a temperature- and humidity-controlled colony room at the Yerkes Center, where lights were set to a 12 h light / dark cycle. Animals had free access to standard rodent chow and water in the home cage. To facilitate food-reinforced responding, access to chow was suspended for 3 h prior to the start of each experimental session. Animals were not used in experiments until at least 2 days after arrival in the laboratory. All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health, and all experimental protocols were approved by the Emory University IACUC.
Procedures
Food-maintained responding
Each experimental group contained 6 male Swiss Webster mice, tested 7 days per week in small animal test chambers (Med-Associates Model ENV-008) housed in larger lightproof Malaguard sound attenuating cubicles (Med-Associates Model ENV-022M). Test chambers were bisected to allow for the simultaneous testing of two animals at a time (one on each side), doubling the number of experimental chambers within the same amount of physical space. Animals tested simultaneously in the same experimental chamber cohabitated within the same home cage during periods when they were not being tested, and were separated from each other within the chamber by an opaque polycarbonate wall during experimental sessions. Malaguard cubicles were equipped with an exhaust fan to provide ventilation of the experimental space. The right side of each test chamber contained a house light, two retractable response levers, a spout through which liquid reinforcement was delivered, and a red stimulus light which illuminated with reinforcer delivery. The downward force required to depress response levers varied from chamber to chamber, but was approximately 0.245 N (or 25 g; mean = 25.67 g, SEM = 2.76 g). The left side of each test chamber contained a house light, two illuminated nose-poke apertures, a food receptacle that received 45 mg sucrose pellets (later replaced with a liquid spout) following completion of the appropriate response requirement, and a green stimulus light which illuminated with reinforcer delivery. Thus, animals assigned to the lever groups were always reinforced with vanilla-flavored liquid, while animals assigned to nose-poke groups were reinforced with either sucrose pellets or vanilla-flavored liquid, depending on the particular study. Sucrose pellets and liquid reinforcement were stored according to manufacturer recommendations and were adequately labeled with regards to contents and expiration date.
Prior to each experimental session, mice were transported from the colony room to the lab, weighed, and placed in operant chambers. A 10 min start delay was imposed at the beginning of each session, and during this time all stimulus lights were extinguished, levers were retracted, and nose-poke apertures were inactive. Sessions formally began with the illumination of the house light, extension of the levers into the experimental space, and illumination of the nose-poke aperture stimulus lights. Reinforcers were delivered following the emission of the appropriate response on the active device under a fixed ratio 1 (FR1) schedule using the MED-PC version IV behavioral programming application in daily 1 hr (limited-access) or 10 h (extended-access) sessions. Liquid reinforcement consisted of a non-dairy vanilla flavored liquid food (Coffee-Mate, diluted 1:1 with tap water) delivered by a 3.33 RPM syringe pump (Med-Associates model PHM-100). Pumps were loaded with 12 ml syringes containing the liquid reinforcer, and were calibrated to deliver 0.2 ml over 10 sec. Thus, the 2 sec pump operation here used to reinforce lever-pressing drove 0.04 ml of fluid through the spouts. This small amount, coupled with the viscosity of the vanilla solution, formed a small drop which would cling to the end of the spout and allow the mouse time to move from the lever to the spout to collect it. In studies in which sucrose pellets were used to reinforce nose-poke behavior, 45 mg sucrose pellets (Noyes Precision Pellets, PJFSC-0045) were delivered by rotary type dispensers (Med-Associates model ENV-203) mounted outside the chamber. Delivery of both liquid and sucrose pellet reoinforcers was followed by a 20 sec timeout period during which all stimulus lights were extinguished and operant responding had no programmed consequences. Total responses on the active and inactive manipulanda were quantified, as was the number of reinforcers delivered. In all cases, the right (“active”) manipulanda was reinforced while the left (“inactive”) response device had no programmed consequences. All mice were allowed 24 days of limited-access (1 hr sessions) under the FR1 schedule. On the 25th day, the PR and extinction tests described below were conducted. Upon completion of those tests, limited-access sessions were resumed, or extended-access (10 h sessions) was initiated and maintained for 40 sessions. With the exception of session duration, extended-access sessions were procedurally identical to limited-access sessions. The PR and extinction tests described below were repeated after 40 sessions of limited or extended-access.
Progressive ratio responding
To assess the reinforcing strength of the food reinforcers, as well as their associated cues, before and after extended-access, a PR schedule was used. Briefly, PR schedules require the subject to emit an increasing number of responses for each successive food reinforcer; the response requirement at which animals cease responding is termed the breakpoint and taken as an index of the reinforcing strength of the stimulus maintaining the behavior (see Stafford et al., 1998). Here we reinforced the first lever-press of every session, and then incremented the response requirement by 2 with each subsequent reinforcer delivery. In this manner, the work requirement for each successive reinforcer increased by 2 responses (1, 3, 5, 7, etc.) throughout the session. As with the limited and extended-access sessions described above, a 20 sec post-reinforcer timeout was imposed after each reinforcer delivery. Interspersed during the 6 days of PR trials were 3 extinction tests. During these extinction tests, identical PR procedures were imposed, however, the reinforcer delivery devices were empty. Thus, completion of the appropriate work requirement resulted in all of the stimuli associated with reinforcer delivery (stimulus light change, sound of the food dispenser or syringe driver turning, imposition of the 20 sec timeout, etc.), but no food was presented. In all cases, sessions ended when 1 h elapsed during which time the response requirement was not met. The breakpoint achieved, as well as overall number of reinforcers delivered were calculated at the end of each session. Breakpoints during reinforced trials and during extinction trials were determined in triplicate for all subjects, and the order of reinforced and extinction PR tests was fixed for all groups as: reinforcer, extinction, reinforcer, extinction, extinction, reinforcer. Thus, after the 24 limited-access training sessions, all mice were tested in six daily PR sessions where responding was maintained with the same reinforcer used in operant training, or where the reinforcer was omitted. These initial determinations of the reinforcing strength of the sucrose pellets and liquid food, as well as their associated cues, were then compared with a redetermination after the final 40 sessions of either continued limited-access or extended-access.
Locomotor activity
The day after completion of the second regimen of PR sessions, mice were transported to an adjacent behavioral testing room where locomotor activity studies in response to various doses of cocaine were conducted. A group of experimentally-naïve mice lacking any prior operant training were also tested. Locomotor activity was measured in photocell cages (Omnitech Electronics; Columbus OH) measuring 40 × 40 × 30 cm. Each cage had 32 photocells (16 front to back and 16 side to side) positioned 2.5 cm off the cage floor. Each cage was isolated in a stainless steel box equipped with a ventilated fan, 10 W light bulb and a peep hole to observe the subjects. The distance traveled (in cm) was calculated by measuring the consecutive breaks of adjacent photocell beams, while repetitive interruptions of the same beam were tracked as behavioral stereotypy. Operation of the photocell cages and data collection was done by an interfaced computer. On the first day of testing, mice were injected with saline, immediately placed into the experimental chambers, and tested for 2 hr. As mice had no prior experience with injection or the locomotor apparatus, this first day provided a measure of locomotor reactivity to novelty. Subsequent studies were then conducted each day with ascending doses (1, 3, 10, 30 and 56 mg/kg) of cocaine in order to generate a dose-effect function for all groups of mice.
Data analysis
Graphical presentation of all data depicts mean ± SEM. Between-group differences in food-maintained behavior, PR performance, extinction responding, and dose-response functions for cocaine-induced locomotor activity and stereotypy were analyzed using a one way repeated measures analysis of variance (ANOVA, F values), or, when normality tests failed, with Friedman’s repeated measures ANOVA on ranks (χ2 values). Post hoc tests on all pairwise multiple comparisons were performed after the method of Holm-Sidak (t values), or, when normality tests failed, after the method of Student-Newman-Keuls (q values). All statistical tests were performed using commercially available software, and significance was judged at P<0.05.
Drugs
Cocaine HCl was supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC), was dissolved in 0.9% physiological saline, and administered intraperitoneally (IP) at a volume of 1.0 ml/100g.
Results
Food-maintained responding
During the 24 limited-access training sessions, nose-poke behavior maintained by sucrose pellets was rapidly acquired and stably maintained (Figure 1A, white background) by all animals. When session length was extended to 10 hr, the number of reinforcers earned increased by approximately 2-fold, and showed no trend of increasing further over the next 20 extended-access sessions (Figure 1A, grey background.) A similar pattern was observed with the number of responses emitted during these sessions (data not shown). A water bottle was added to these chambers 21 days into the extended-access period (indicated by the striped bars in Figure 1A), but this did not lead to any further increases in reinforcers earned or responses emitted over the remaining 19 days of extended-access, although subjects did drink significant amounts of the available water. Since no progressive increases in either reinforcers earned or responses emitted were obtained as a function of extended-access in these animals, a constant limited-access control group for these subjects was not established.
Figure 1.
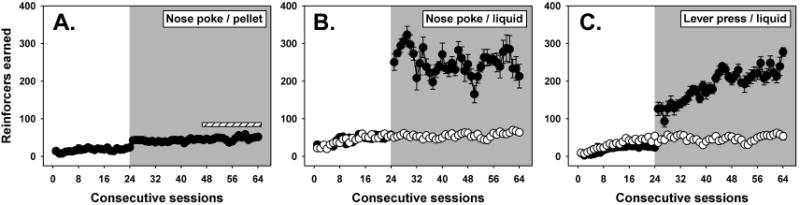
Mean reinforcers earned in sessions where nose-poke responses were reinforced with sucrose pellets (A) or liquid food (B), or where lever-press responses were reinforced with liquid food (C). All groups initially acquired food-maintained behavior during 24 1 h limited-access sessions (white backgrounds), then were randomly assigned to experimental groups (shaded backgrounds) receiving either 40 additional 1 h limited-access sessions (open symbols) or 40 sessions of 10 h extended-access (filled symbols). The striped bar above sessions 45 through 64 in panel A indicates ad libitum non-contingent water availability within the experimental chamber. Each point represents the mean reinforcers earned per session (± SEM) from groups where N=6, and any points without error bars indicate instances in which the SEM is encompassed by the data point.
Nose-poke responses reinforced with liquid food were acquired over a few sessions and then maintained with minor variability during the limited-access training period (Figure 1B, white background.) Mice that remained in limited-access conditions continued to sustain a steady behavioral baseline across these 40 sessions, both in terms of reinforcers earned (Figure 1B, open symbols on the grey background) and responses emitted. In contrast, mice that transitioned to 10 h extended-access displayed an immediate increase in reinforcers earned (Figure 1B, filled symbols on the grey background) and total session responses. However, over subsequent sessions, responding was variable from day to day, fluctuating between 4- and 7-fold higher than that of limited-access controls. Importantly, no trend towards a progressive increase in food-maintained behavior was apparent from reinforcer data or from response data.
Mice trained to lever-press using liquid reinforcement (Figure 1C, white background) acquired this behavior at rates comparable to those of the nose-poke groups (compare points on the white backgrounds of Figures 1A, 1B and 1C). Once acquired, responding was stably maintained across consecutive limited-access training sessions, and no systematic changes in reinforcers earned (Figure 1C, open symbols on grey background) or repsonses were observed in mice remaining under limited-access conditions for the duration of these studies. Interestingly, extending session length to 10 h resulted in a progressive escalation of food-maintained behavior across consecutive sessions as compared to limited-access controls, both in terms of reinforcers obtained (Figure 1C, filled symbols on grey background) and number of responses emitted per session. The progressive increase in reinforcers earned was statistically significant across all 40 sessions of extended access (F=10.255, P<0.001), and by the end of the extended-access period, the number of reinforcers earned had increased by 7-fold. A similar pattern of results was obtained with total session responses, where a progressive increase over consecutive sessions was observed which was elevated by approximately 10-fold over baseline by the end of this phase of the study.
Progressive ratio responding
Sucrose pellets supported an average breakpoint of approximately 40 nose-pokes after 24 sessions of limited-access, and this breakpoint remained unchanged when redetermined after 40 sessions of extended-access (filled bars in Figure 2A). In PR sessions conducted in extinction (i.e., where the food dispenser was not loaded), a breakpoint approximately half that supported when the sucrose reinforcers were delivered was maintained (open bars in Figure 2A). Again, this breakpoint did not significantly change after 40 sessions of extended-access. A similar pattern of data was obtained when total session responses (including timeout responses) were considered (data not shown). It is important to note that the breakpoints supported by the sucrose pellets were similar to those maintained by the liquid food, suggesting that the reinforcing strengths of these two stimuli were comparable across the operant responses here used.
Figure 2.
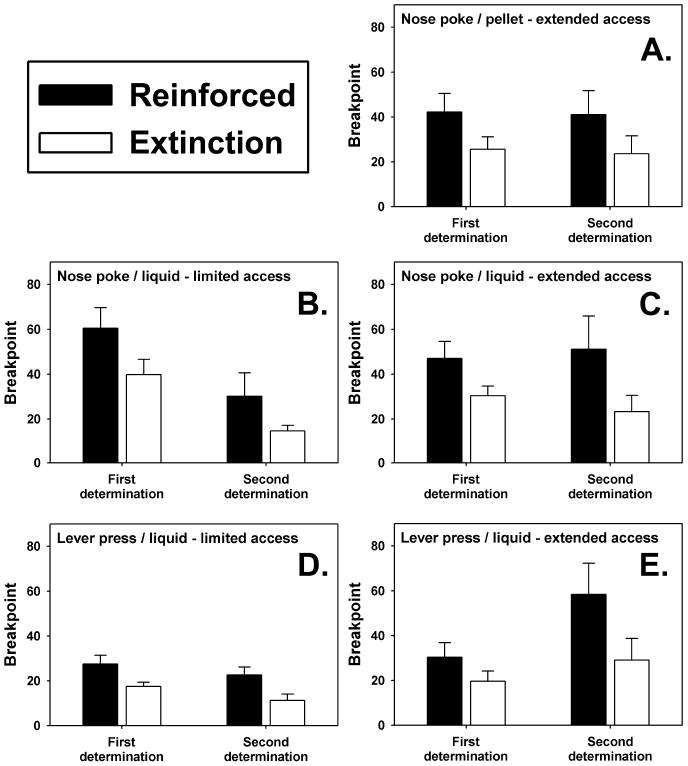
Progressive ratio breakpoints engendered by food reinforcers (filled bars) or obtained under extinction conditions (open bars). The first determination occurred after 24 sessions of limited-access for all groups, while the second determination occurred after either 40 more sessions of limited-access (left panels) or 40 sessions of extended-access (right panels). Nose-poke responses were reinforced with sucrose pellets (A) or liquid food (B and C), and lever-press responses were reinforced with liquid food (D and E). Each bar represents the mean breakpoint achieved (± SEM) from groups where N=6.
In mice trained to nose-poke using a liquid reinforcer and maintained under limited-access conditions, the initial breakpoint supported by the liquid food after 24 sessions of limited-access was approximately 60, but decreased, non-significantly, by approximately half after 40 subsequent sessions of limited-access (filled bars in Figure 2B). A similar pattern was observed in this group during extinction sessions where the syringe driver was not loaded. In these extinction sessions, breakpoint significantly dropped by about half between the first and second determinations (open bars in Figure 2B, q= 5.338, P<0.05). Response output for this group of mice also showed a reduction from the first to second PR regimen. Interestingly, exposure to 40 sessions of extended-access to the liquid reinforcer after the initial PR sessions attenuated these reductions in breakpoints and overall response output. Indeed, breakpoints maintained by the liquid reinforcer (filled bars in Figure 2C), and those measured in extinction (open bars in Figure 2C) were unchanged across the two individual determinations. With regards to total responses emitted, there was a non-significant trend towards increased responding in reinforced PR sessions, but responses emitted in PR sessions conducted in extinction did not differ as a consequence of extended-access.
Breakpoints determined in mice trained to lever-press using a liquid reinforcer (Figures 2D and 2E) were generally less than those quantified in mice making nose-poke responses, although breakpoints derived from reinforced PR sessions were always higher than those measured in extinction. After 24 sessions of limited-access, the liquid reinforcer maintained breakpoints of approximately 30 (filled bars in Figures 2D and 2E), and this remained unaffected when redetermined after 40 additional sessions of limited-access (filled bars in Figure 2D). Similarly, breakpoints obtained under extinction conditions did not differ from one determination to the next (open bars in Figure 2D). An analogous set of results was obtained when total session responses (including timeout responses) were considered. In contrast, mice allowed 40 sessions of extended-access after the initial PR sessions exhibited a significant increase in breakpoint maintained by the liquid reinforcer (filled bars in Figure 2E, q=7.000, P<0.05). A non-significant trend towards increased breakpoint during extinction sessions was also observed (open bars in Figure 2E), accompanied by a robust increase in the number of responses emitted during reinforced PR sessions and a smaller increase in responses emitted under extinction conditions.
Locomotor activity
Locomotor activity was measured in an open field setting on the day following completiojn of the second regimen of PR sessions. Cocaine induced dose-dependent increases in ambulatory activity (Figure 3A, F=14.242, P<0.001) and behavioral stereotypy (Figure 3B) in subjects with a history of emitting nose-poke responses reinforced with liquid food. No statistically significant differences in locomotor reactivity to saline injection and the novel environment of the locomotor apparatus as a function of extended reinforcer access were detected (see SAL points in all panels of Figure 3), and the sensitivity to the effects of cocaine on motor behavior and stereotypy were not significantly different for the limited-access (open symbols in Figures 3A and 3B) and extended-access groups (filled symbols in Figures 3A and 3B).
Figure 3.
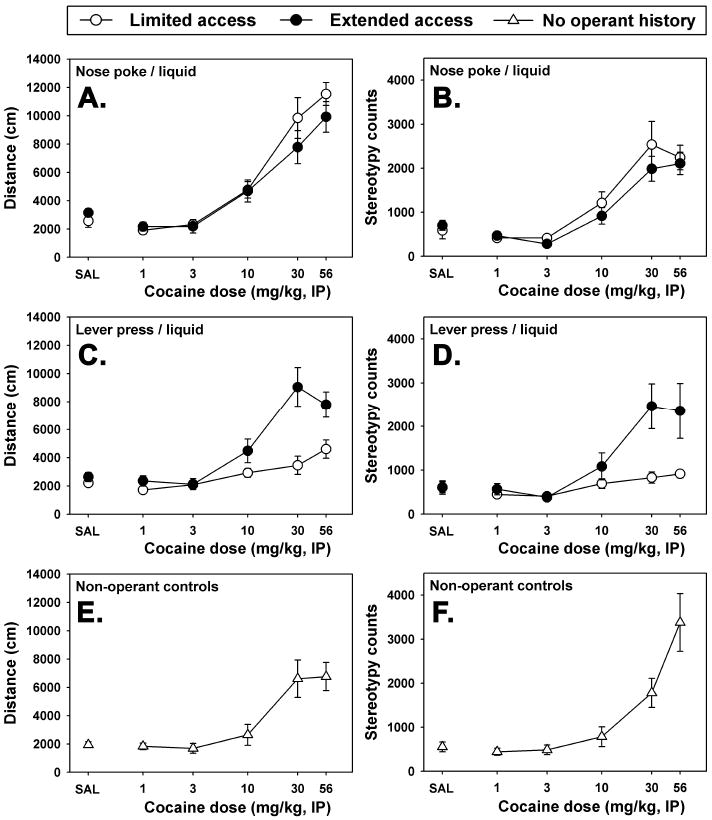
Effects of saline and cocaine on ambulatory activity (A, C and E) and stereotypy (B, D and F) in mice previously reinforced with liquid food following nose-poke behavior (A and B) or lever-press responses (C and D) in limited-access (open circles) or extended-access (filled circles) sessions, or in experimentally-naïve control mice without any operant training (E and F). Each point represents the mean locomotor response (± SEM) from groups where N=6, and any points without error bars indicate instances in which the SEM is encompassed by the data point.
Mice with a history of lever-pressing reinforced with liquid food also exhibited statistically significant dose-dependent hyperlocomotion (Figure 3C, F=12.342, P<0.001) and stereotypy (Figure 3D, F=5.156, P<0.001) in response to cocaine injection. As with the nose-poke subjects, no differences in locomotor reactivity to saline injection and the novel environment of the locomotor apparatus as a function of extended reinforcer access were apparent in mice trained to respond on a lever. Importantly, mice allowed extended-access to liquid food reinforced lever-pressing behavior exhibited profoundly more ambulatory activity (filled symbols in Figure 3C) and stereotypy (filled symbols in Figure 3D) in response to the highest doses of cocaine, as compared to their limited-access controls (open symbols in Figures 3C and 3D). These differences were statistically significant at cocaine doses of 30 mg/kg (t=5.394, P<0.001 for distance traveled; t=3.829, P<0.001 for stereotypy) and 56 mg/kg (t=3.379, P=0.002 for distance traveled; t=3.418, P<0.001 for stereotypy).
The effects of saline injection and the novelty of the locomotor chambers in mice with no prior experimental history were similar to those observed in all mice with previous operant training (compare SAL points in all panels of Figure 3.) Similarly, cocaine elicited statistically significant dose-dependent increases in locomotor activity (Figure 3E, F=7.426, P<0.001) and stereotypy (Figure 3F, χ2=13.714, P=0.018) in these control animals. Distinct from mice with previous operant training, 56 mg/kg cocaine (the highest dose tested) in the non-operant control animals induced significantly more stereotyped behavior than did 30 mg/kg cocaine (q=3.464, P<0.05). In contrast, the dose-effect curves for cocaine-elicited stereotypy in mice with previous operant training all tended to approximate asymptotic functions.
Discussion
The major findings of this research are two fold. First, under conditions where the operant response was a nose-poke, extended-access to both a liquid and solid food did not result in an escalation of reinforced behavior and reinforcer consuption and did not cross-generalize to locomotor activity induced by cocaine. Secondly, consistent with previous research using drug reinforcers under lever operant conditions (i.e., Ahmed and Koob, 1998; Ahmed et al., 2000; Carroll et al., 2005; Kitamura et al., 2006), extended-access to a liquid food under lever operant conditions resulted in an an escalation of reinforced behavior and reinforcer consumption. Furthermore, again congruent with previous studies using drugs as reinforcers (Ferrario et al., 2005), animals expressing this escalated pattern of reinforced behavior also emitted more “seeking” behaviors (previosly-reinforced behaviors emitted in extinction conditions) than those animals subjected to limited-access only, or those animals maintained under extended-access conditions which did not lead to behavioral escalation (see below). Similarly, as previously shown with drug reinforcers (Paterson and Markou, 2003; Morgan et al., 2005; Roberts et al., 2007), behavioral escalation led to increased “motivation” to work for the liquid food reinforcer, as indicated by the capacity of the liquid food to maintain higher breakpoints under the progressive ratio schedule. Finally, still consistent with studies using drugs as reinforcers (Ferrario et al., 2005), we observed that behavioral escalation elicited by extended-access to liquid food rendered mice more sensitive to the psychostimulant effects of cocaine compared to limited-access mice. This pattern of results suggests that the phenomenon of behavioral escalation can be engendered using non-drug reinforcers, and that once established, similar behavioral and pharmacological concommitants are also observed. This in turn seems to imply that the post-escalation consequences reported in studies using drug reinforcers (such as increases in drug “seeking”, higher “motivation” for the drug, and increased sensitivity to acute drug effects such as locomotor stimulation) are not due to drug exposure per se, but rather, are the result of basic behavioral processes which underlie operant responding, specifically lever pressing, maintained by appetitive stimuli.
It is important to note that the nature of the non-drug reinforcer may modulate development of behavioral escalation. Although sucrose pellets and the liquid reinforcer maintained similar breakpoints in these studies, extended-access to the sucrose pellets under the nose-poke operant condition did not lead to a progressive increase in consumption or contingent responding. One unfortunate caveat of the present studies is that we do not have a group of mice lever pressing under the sucrose pellet condition. Furthermore, the reinforcing strength of the stimulus maintaining behavior may not be a critical factor in establishing behavioral escalation. Rather, it seems likely that issues related to satiety are critical in this regard. Hodos and Kalman (1963) reported that larger unit volumes of a liquid reinforcer depressed breakpoints in rats (as compared to smaller unit volumes), but that this effect could be overcome by increasing the step value of the PR schedule. The authors concluded that the decline in performance with the larger volumes of liquid was due to progressive satiation across the session. Although the absolute mass of the reinforcers here used were similar (45 mg sucrose pellets vs. 40 mg of liquid reinforcement), it seems reasonable to suggest that satiation would develop to solid sucrose pellets much more rapidly than to a comparable amount of the liquid reinforcer, as physiological mechanisms which detect caloric intake are reportedly less sensitive to liquid foods (reviewed in Almiron-Roig et al., 2003). Similarly, differences in bite effort and oral processing time result in smaller bite sizes and lower total intake of solid foods as compared to liquid foods (de Wijk et al., 2008). Therefore, despite the comparable reinforcing strengths of these two appetitive stimuli, mice may have simply been able to consume more of the liquid reinforcer than the sucrose reinforcer, on a per unit basis, resulting in a higher “ceiling” toward which to escalate.
The present studies also suggest that the topography of the operant response may also temper the development of behavioral escalation. In the present studies, lever-press and nose-poke behaviors were similarly entrained and maintained by the same liquid reinforcer under limited-access conditions. However, implementation of extended-access conditions resulted in marked differences in reinforcer consumption depending on the topography of the operant response, and such differences were apparent on the very first day of extended-access. In this regard, mice working on a nose-poke device immediately increased their reinforcer intake by approximately 4-fold, despite a 10-fold increase in the amount of time the reinforcer was available. In contrast, subjects responding on levers showed a smaller initial increase immediately upon transition to extended-access conditions. However, subjects responding on levers displayed the behavioral escalation phenotype by the end of the 40 extended-access sessions resulting in an approximate 12-fold increase in reinforcer intake. One obvious difference between the two operant response devices which might partially explain this discrepency is the force requirement necessary to register a response. While the levers used in these studies required a downward force of approximately 0.245 N (25 g), the nose-poke operants had no moving parts to act upon and required only that the animal position its head within its aperture to break a photobeam. Relatively few studies have manipulated force requirements for operant responding, but these studies have demonstrated that, as compared to lower force requirements, responses requiring more force support lower response rates, extinguish more rapidly, may elicit escape or avoidance behavior, and are less prefered by subjects (discussed in Alling and Poling, 1995). Thus, it may be the case that increased work effort (as was here required in the lever-press group) is critical to the expression of behavioral escalation. In fact, to the best of our knowledge, there is not a published study demonstrating behavioral escalation of drug self-administration on a nose-poke operant. Future studies involving higher FR values might then be expected to produce more dramatic behavioral escalation, and a systematic evaluation of the effects of various force requirements on behavioral escalation would seem warranted.
Mice responding on levers progressively increased their consumption of the liquid reinforcer when allowed extended-access, and at the end of the study were earning approximately as many reinforcers as were mice responding on nose-poke devices. Interestingly, mice responding on levers emitted the same number of responses as did mice working on nose-poke devices on the first day of extended-access. For nose-poke mice, the number of responses emitted remained relatively constant across consecutive extended-access sessions, but a clear pattern of behavioral escalation was obtained in mice responding on levers. This difference is due to a greater degree of responses emitted during post-reinforcement timeout periods by mice working on response levers, which may be analagous to the increased “seeking” behaviors previously reported in the context of escalation of drug self-administration (Rogers et al., 2008). Although dose-effect curves for drug self-administration do not differ when mice are trained to emit lever-press or nose-poke responses to receive drug infusions (Caine et al., 1999; David et al., 2001), it is probably not surprising that response topography may interact with other reinforcers resulting in distinct patterns of behavior. Thus, one alternative explanation for mice not displaying behavioral escalation under the nose-poke operant could be due to a “celilng” effect in which the behavioral escalation occurred within the first extended-access session instead of across extended-access sessions as demonstrated in the lever operant group.
Previous research has amply demonstrated that rodents which exhibit increased activity in novel environments also manifest an increased sensitivity to the locomotor-activating effects of numerous drugs of abuse, including amphetamine, morphine, cocaine and caffeine (c.f., Brabant et al., 2005). However, motor activity on the first day of exposure to the activity chambers (which included a saline injection) was remarkably similar for all groups of mice presently studied. Indeed, mice lacking previous operant training – or even any previous handling – were no more or less active in the activity chambers than were the subjects with previous operant histories. Thus, the differences in sensitivity to the stimulant effects of cocaine uncovered by the present studies is not likely attributable to novelty-induced locomotor responsivity. Comparing the locomotor effects of cocaine in mice with a history of responding on a nose-poke device or a lever to that of mice with no prior operant training, however, does reveal intruiging differences. Although the effects of saline injection and the novel environment of the locomotor apparatus were similar in all groups, it is clear that a history of reinforced lever-pressing behavior under limited-access conditions decreases sensitivity to the locomotor stimulant effects of cocaine. The attenuated ambulatory activity observed in these animals was not accompanied by increased stereotypy – indeed, stereotypy counts were markedly lower in mice with a history of lever-press behavior under limited-access conditions than in any other group tested, including the non-operant controls. Reasons for this decreased sensitivity to the locomotor stimulant effects of cocaine in the limited-access groups are not apparent and require further studies to elucidate behavioral mechanisms.
In conclusion, the studies presently reported were designed to provide a critical set of control data in order to allow for a more specific interpretation of the results commonly obtained in drug self-administration escalation paradigms. As this model gains traction in behavioral pharmacology and neuroscience research, it becomes all the more imperative to determine whether or not the results of such experiments truly reflect important addiction-related phenomena, or whether similar findings can also be achieved in the absence of drug administration. Our observation of behavioral escalation as a result of extended-access to a liquid reinforcer under a lever operant condition is similar to the transition from “controlled” to “uncontrolled” drug intake often obtained in drug self-administration experiments employing extended-access sessions. That our results with liquid food reinforcement mirror those of previous drug studies challenges the continued reliance on the escalation model as a specific marker of drug addiction. Based upon the results of the experiments in this report, we would suggest that behavioral escalation may be an important but non-specific marker of compulsive behaviors, which may occur across multiple addiction-like phenotypes. It may be the case that, as with drugs of abuse and palatable foods, extended access to other positive reinforcers (such as electrical brain stimulation, access to a sexually receptive partner, etc.) might also elicit behavioral escalation. Further studies designed to assess behavioral escalation in the context of other reinforcers would seem warranted.
Acknowledgments
Expert animal husbandry services were provided by the animal care staff at the Yerkes National Primate Research Center. Portions of this manuscript were presented at the 2006 annual meeting of the International Study Group Investigating Drugs as Reinforcers. These studies were funded by US PHS Grants DA020645, RR020146, and by the Yerkes Base Grant RR00165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–21. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31(3):563–71. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Alling K, Poling A. The effects of differing response-force requirements on fixed-ratio responding of rats. J Exp Anal Behav. 1995;63(3):331–46. doi: 10.1901/jeab.1995.63-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almiron-Roig E, Chen Y, Drewnowski A. Liquid calories and the failure of satiety: how good is the evidence? Obes Rev. 2003;4(4):201–12. doi: 10.1046/j.1467-789x.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Tirelli E. Evidence that the relations between novelty-induced activity, locomotor stimulation and place preference induced by cocaine qualitatively depend upon the dose: a multiple regression analysis in inbred C57BL/6J mice. Behav Brain Res. 2005;158(2):201–10. doi: 10.1016/j.bbr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology. 1999;147(1):22–4. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology. 2005;180(3):414–26. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and muopioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- David V, Polis I, McDonald J, Gold LH. Intravenous self-administration of heroin/cocaine combinations (speedball) using nose-poke or lever-press operant responding in mice. Behav Pharmacol. 2001;12(1):25–34. doi: 10.1097/00008877-200102000-00003. [DOI] [PubMed] [Google Scholar]
- DeMarse TB, Killeen PR, Baker D. Satiation, capacity, and within-session responding. J Exp Anal Behav. 1999;72:407–423. doi: 10.1901/jeab.1999.72-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wijk RA, Zijlstra N, Mars M, de Graaf C, Prinz JF. The effects of food viscosity on bite size, bite effort and food intake. Physiol Behav. 2008;95(3):527–32. doi: 10.1016/j.physbeh.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137(1-2):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58(9):751–9. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Culbertson SA. Behavior principles. 3. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Graeff FG, De Oliveira L. Influence of response topography on the effect of apomorphine and amphetamine on operant behavior of pigeons. Psychopharmacologia. 1975;41(2):127–32. doi: 10.1007/BF00421069. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav. 1963;6:387–92. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186(1):48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- LeMerrer J, Stephens DM. Food-induced sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26(27):7163–7171. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20(6):1647–54. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology. 2005;179(3):644–51. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Murphy ES. Criticisms of the satiety hypothesis as an explanation for within-session decreases in responding. J Exp Anal Behav. 2000;74:347–361. doi: 10.1901/jeab.2000.74-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DC. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology. 2005;178(2-3):309–16. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14(17):2229–32. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1614–24. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199(4):615–24. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear DJ, Katz JL. Cocaine and food as reinforcers: effects of reinforcer magnitude and response requirement under second-order fixed-ratio and progressive-ratio schedules. J Exp Anal Behav. 1991;56(2):261–75. doi: 10.1901/jeab.1991.56-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139(3):169–84. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80(2-3):65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]


