Abstract
The surface of the oral plaque bacterium Streptococcus cristatus is decorated with a lateral tuft of fibrils. The fibrillar tuft functions in the adhesion of S. cristatus to heterologous bacterial species in the plaque biofilm. The tuft typically consists of a densely packed fringe of shorter fibrils 238 ± 19 nm long with longer, less abundant fibrils 403 ± 66 nm long projecting through the fringe of short fibrils. The two types of fibrils in the tufts of S. cristatus have been refractory to biochemical separation, complicating their characterization. A hexadecane partition assay was used to enrich for subpopulations of S. cristatus CR311 (type strain NCTC 12479) having distinct fibrillar morphotypes. Negative staining in the TEM revealed that cells of a hydrophobic subpopulation of S. cristatus (CR311var1) carried only the long fibrils (395 ± 32 nm). A hydrophilic subpopulation of S. cristatus (CR311var3) consisted of mixed morphotypes having no fibrils or remnant short fibrils (223 ± 49 nm). No long fibrils were observed on any cells in the CR311var3 subpopulation. The CR311var3 morphotype, unlike the wild-type strain and CR311var1, was not able to form corncobs with either Corynebacterium matruchotii or Fusobacterium nucleatum. Variant CR311var3 did not express the novel gene srpA, which encodes a high molecular weight (321,882 Da) serine-rich protein, SrpA. The SrpA protein contains two extensive repeat motifs of 17 and 71 amino acids and a gram-positive cell wall anchor consensus sequence (LPNTG). The unusual properties of SrpA most closely resemble those of Fap1, the fimbrial-associated adhesin protein of Streptococcus parasanguis. The association of long fibrils, high surface hydrophobicity, ability to form corncob formations, and expression of the srpA gene suggest that SrpA is a long fibril protein in S. cristatus.
Keywords: corncobs, fibrils, SrpA, Streptococcus cristatus, tufts
The surfaces of oral streptococci control the adhesive interactions that determine the specific ecologic niche of these bacteria within the oral cavity. It is believed that many of these interactions are associated with appendages that decorate the bacterial surface. Two classes of these structures, fibrils and fimbriae, have been described on different streptococcal species (11). The majority of the streptococci carry short fibrils (40–400 nm), which are thin clumped structures that may be peritrichous or localized in tufts (8). A few streptococcal strains carry peritrichous fimbriae, with a width of 3–5 nm, which are longer (1–3 μm) and more flexible than fibrils.
The structure and function of one peritrichous fibril component on Streptococcus gordonii DL1 has been studied by McNab et al. (17), who showed that the sparse, peritrichous fibrils (61 nm long) are encoded by the cshA gene (18). The CshA polypeptide (259 kDa) contains 2508 amino acid residues and is apparently not glycosylated. The protein comprises a 41-residue amino-terminal signal sequence, a nonrepetitive region, and an extensive 101 amino acid sequence that repeats 13 times. A consensus carboxy-terminal cell wall anchor domain is responsible for covalent attachment of the fibrils to the peptidoglycan. The CshA polypeptide appears to be the structural and functional component of the fibrils. CshA is also associated with adhesion to fibronectin (19), other oral bacteria and mucosal surfaces of the mouse oral cavity (20). Recent studies have shown that the CshA-like fibrillar protein also occurs on the surfaces of other members of the mitis group of oral streptococci (6).
Fimbrial appendages have been found on Streptococcus parasanguis and Streptococcus salivarius. In the former species these peritrichous appendages contain a 200 kDa protein called Fap1 (30). It has been suggested that this protein is a structural subunit of one type of fimbriae produced by this organism. The Fap1 protein is glycosylated (3, 26) and every second amino acid residue is a serine. The protein contains 2552 amino acids, including a 50-amino acid N-terminal leader peptide, the consensus cell wall anchor sequence at the carboxy-terminus, and 1000 repeats of the sequence E/V/I-S (31). There appears to be a link between the fimbriae and adherence to salivary proteins (7).
The fibrils of S. salivarius are also peritrichous and consist of a glycoprotein assembled into flexible appendages 1.0 μm long and 3–5 nm wide (28, 29). In this glycoprotein the N-terminal unit contains the repeating motif A/X/T-E-Q/φ, where X represents a modified amino acid residue and φ, a blank cycle (16). This fimbrial glycoprotein is assembled into a filamentous structure that could not be dissociated. The common theme among these streptococcal appendages is the relatively large size of their constituent protein, the presence of a number of novel repeat sequences in the protein, and their relative resistance to dissociation. In addition, at least two types of these proteins appear to be heavily glycosylated.
Our studies have focused on Streptococcus cristatus. This species was formerly known as S. crista (9, 27) and is a member of the mitisoralis group of streptococci found in oral biofilms (22). In contrast to the peritrichous appendages on S. parasanguis and S. salivarius, the fibrils on S. cristatus are organized in localized tufts on the cell surface (8). We have shown in previous studies that the fibrillar tufts mediate binding of S. cristatus to several heterologous bacterial species, including the gram-positive aerobe Corynebacterium matruchotii (14) and the gram-negative anaerobe Fusobacterium nucleatum (15). This binding results in the formation of a unique bicellular arrangement called a ‘corncob’ (22). These bicellular units are prominent in mature dental biofilms and are believed to constitute the mechanism responsible for ‘fixing’ the nonstreptococcal species to the plaque biofilm.
The fibrillar tufts of strains of S. cristatus appear to be complex organizations of two lengths of fibrils (10), teichoic acid (22) and polysaccharide (12). S. cristatus CR311 (type strain NCTC 12479) (9) represents a typical strain in which the tufts consist of a densely packed fringe of fibrils projecting approximately 250 nm from the cell surface (short tuft fibrils) and longer, sparser fibrils projecting through the short fibrils to a distance of 400 nm (long tuft fibrils). The long tuft fibrils tend to clump together towards the proximal end and separate or ‘splay’ out at the ends of the clumps. These fibrils are also often more prominent at the edges of the tufts (8). In this report a hexadecane partition assay was used to enrich for subpopulations of S. cristatus with a distinct tuft ultrastructure (tuft morphotypes). We examined the structure and properties of the surfaces of these subpopulation variants, establishing a relationship between the fibrils and the expression of a gene that encodes for an unusual, large molecular weight cell surface protein.
Material and methods
Bacterial strains and culturing conditions
S. cristatus CR311 was originally isolated from a human periodontal abscess (8) and has been designated the type strain for this species (NCTC 124790) (9). S. cristatus CR311var1 and S. cristatus CR311var3 were isolated after serial enrichment in a hexadecane partition assay (2). S. cristatus CC5A was isolated from human dental plaque corncobs by Mouton et al. (22) and has been used as a prototype strain in studies of corncob formation (14, 15).
The CR311 strains were grown in tryptone soy broth containing 0.1% yeast extract (TSBY) or on tryptone soya agar containing 0.1% yeast extract (TSOY) in a CO2 atmosphere at 37°C overnight. S. cristatus CC5A was grown in brain heart infusion (BHI; BD, Sparks, MD) or Todd-Hewett (TH; BD, Sparks, MD) broth in an atmosphere containing 5% CO2 at 37°C. C. matruchotii NCTC 14266 was grown in BHI broth for 48 h with gentle shaking at 37°C. F. nucleatum ATCC 10953 was grown in BHI supplemented with 0.2% yeast extract (BD, Sparks, MD), 0.05 liter cysteine (Fischer Scientific, Fairlawn, NJ) and 0.5% sodium bicarbonate under anaerobic conditions (Gas Pack; BBL Microbiology Systems, Cockeysville, MD) at 37°C. All strains used in this study are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or Plasmid | Description | Source/Reference |
|---|---|---|
| Strains | ||
| S. cristatus CR311 | wild-type strain; type strain; fibrillar tufts | Laboratory collection |
| S. cristatus CC5A | wild-type strain; fibrillar tufts | Laboratory collection |
| S. cristatus Psha | wild-type strain; fibrillar tufts | Laboratory collection |
| S. cristatus Pshb | wild-type strain; fibrillar tufts | Laboratory collection |
| S. cristatus CR3 | wild-type strain; fibrillar tufts | Laboratory collection |
| S. cristatus CR311var1 | hydrophobic subpopulation of CR311 | This study |
| S. cristatus CR311var3 | hydrophilic subpopulation of CR311 | This study |
| S. oralis CN3410 | wild-type strain; fibrillar tufts | Obtained from H. Jenkinson (13) |
| S. parasanguis FW213 | fimbriate strain; expresses fap1 | Obtained from P. Fives-Taylor (7) |
| C. matruchotii NCTC 14266 | corncob partner | Laboratory collection |
| F. nucleatum ATCC 10953 | corncob partner | Laboratory collection |
| Escherichia coli JM109 | host strain for all plasmids | Promega |
| Plasmids | ||
| pB15-23 | pCR-Script SK+ contains 21 kb SalI fragment from S. cristatus B15 locus (Ampr) | (5) |
| pB15-18 | subclone of pB15-23 contains 10.9 kp EcoRI—Sau3A fragment (Ampr) | This study |
| pB15-2.2 | subclone of pB15-18 contains 2249 bp EcoRI – HindIII fragment cloned into pGem-T (Ampr) | (5) |
| pB15-1 | subclone of pB15-18 contains 1017 bp HindIII fragment (Ampr) | This study |
| pB15-8 | subclone of pB15-18 contains 7622 bp HindIII—Sau3A fragment (Ampr) | This study |
| Zap Express™ | lambda cloning vector | Stratagene |
| pBK-CMV | phagemid vector derived from ZAP Express™ (Kanr) | Stratagene |
| pY-1 | contains a fragment, with an additional 3895 bp adjacent to the Sau3A end of pB15-23; cloned into pBK-CMV (Kanr) | This study |
| pW-2 | contains a fragment with an additional 550 bp adjacent to pY-1 cloned into pBK-CMV (Kanr) | This study |
Hexadecane enrichment assay
For the basic hexadecane assay (24) cells were grown overnight in TSB broth in a static culture, washed in Sorenson’s phosphate buffer (pH 7.2) and the optical density adjusted to 0.5 at 440 nm. Cell suspensions (3 ml) were partitioned with 200 μl hexadecane (BDH chemicals). A hydrophilic variant S. cristatus CR311var3 was isolated from the buffer phase by growth on BHI blood agar. The hydrophobic variant S. cristatus CR311var1 was obtained from the hexadecane phase. The bacterial surface ultrastructure of the sub-populations was then examined by electron microscopy of negatively stained preparations.
Electron microscopy
Specimens were prepared for electron microscopy on carbon coated formvar grids (400 mesh) in a Bio-Rad E6200 Turbo Coater (Hercules, CA). All grids were made hydrophilic in a plasma glow unit (Plasma Barrel Etcher PT7150, Fisons, Loughborough, UK). The bacteria were negatively stained using 1 or 2% methylamine tungstate (8). The grids were photographed using a Phillips Fei Technai Biotwin TEM. To determine the average lengths of the tuft fibrils, negative images were printed in TIF format at actual negative size with the nm scale bar. Measurements were taken from the bacterial surface (delineated by the dark area).
Corncob assay
S. cristatus wild-type and mutants C. matruchotii and F. nucleatum strains were grown for 18 h and 48 h in BHI broth, and were washed and suspended in cold 0.15 M sodium chloride solution for the corncob assay. The assay was performed as described by Lancy et al. (14, 15). Corncob preparations were viewed by light microscopy (phase contrast) at 100 × magnification using oil emersion with the semiphotomat set to darkfield and 100 ASA.
srpA gene sequence
A portion of the insert of plasmid pB15-23 (5) containing sequence downstream from the previously described ABC (adenosine triphosphate-binding-cassette) transport locus of S. cristatus CC5A (GenBank Accession Number U96166), was sub-cloned into three new plasmids, designated pB15-2.2, pB15-1 and pB15-8, to facilitate sequencing of this region (Table 1). The insert DNA from pB15-2.2 and pB15-1 was sequenced by the primer walking method. In order to sequence the relatively large repetitive insert of pB15-8, a set of nested deletions was made using the Exo-Size™ deletion kit (NEB, Beverly, MA). To obtain the sequence adjacent to the pB15-23 DNA insert, S. cristatus CC5A genomic DNA was digested to completion with BamHI and SacI and was ligated to similarly digested ZAP Express™ vector (Stratagene, La Jolla, CA). This library was then screened with a probe sequence made using a Bam HI-Sau3AI fragment derived from the 3′-end of pB15-8. A positive plaque yielded the phagemid pY-1, which contained an additional 3895 bp of sequence downstream from pB15-8. To obtain the sequence adjacent to the pY-1 insert, S. cristatus CC5A genomic DNA was digested to completion with Bam HI and Hind III and ligated to similarly digested ZAP Express™ vector. This Zap library was screened with a Hind III-Sac I DNA fragment derived from the 3′-end of pY-1. A positive plaque yielded phagemid pW-2, which was used to obtain an additional 550 bp of sequence. Automated cycle sequencing reactions were conducted by the Genetics Core Facility at the University of Pennsylvania using an Applied Biosystems, Inc. 373 A sequencer with Dye Primer chemistry (Foster City, CA). The srpA gene sequence was deposited in GenBank under Accession Number U96166.
Northern and Southern blotting
For Northern blots, RNA was obtained from S. cristatus CC5A using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNA 10 μg was loaded onto a 1% agarose gel containing 1 × MOPS and 6% formaldehyde as described (25). Southern blots were prepared and run as described previously (4). Genomic DNA from S. cristatus strains CC5A, Psha, Pshb, CR3, S. parasanguis FW213, Streptococcus oralis CN3410 and F. nucleatum ATCC 10953 was isolated as described (4) and digested with HindIII. Probe DNA was made by amplifying the 5′-end of srpA from strain CC5A using the PCR primers SRP003 (5′-CCGGATCCTCTCCAGAAACAAGTGGTCGT-3′) and SRP004 (5′-CCGAAT TCTAGTGGTTCAGGGGAAGGAA-3′). Probe DNA was labeled with α[32P]dATP (6000 Ci/mmol; Amersham, Piscataway, NJ) using a random primer labeling kit (Boehringer Mannheim Biochemicals, Indianapolis, IN).
Polymerase chain reaction (PCR)
DNA primers SRP003 and SRP004 were used to amplify the 5′-end of srpA from S. cristatus strains. Approximately 2 μg of genomic DNA was used as the template. Conditions were 2 min at 94°C followed by 30 cycles of 1 min at 94°C, 2 min at 60°C and 3 min at 72°C. Amplified products were examined on an 8% agarose gel stained with ethidium bromide. Molecular size was estimated by comparison with a commercial 100 bp ladder.
DNA fingerprinting
Genomic DNA was digested with EcoRI and the fragments separated on an 8% agarose gel. DNA bands were visualized by staining with ethidium bromide.
Protein analysis
Proteins were examined on 8% SDS-polyacrylamide gels as described by Correia et al. (4). Samples were prepared by boiling whole washed cells in solubilizing solution for 10 min. Some samples were treated with 1 mg/ml of trypsin (Sigma, St. Louis, MO) for 1 h at 37°C prior to gel electrophoresis. Protein bands were visualized by staining with Coomassie Brilliant Blue. Molecular size markers were from Bio-Rad.
Statistics
Fibril length was determined from measurements made on 10–20 cells per strain or variant. The Student t-test was used to compare the mean lengths of fibrils.
Results
Isolation of hydrophobicity mutants of S. cristatus and characterization of surface fibrils
Based on the possibility that the long fibrils in the tufts of S. cristatus give the bacterial surface its predominantly hydrophobic character (10), a hexadecane partitioning method was used to enrich for subpopulations that lacked the longer tuft fibrils (2). When wild-type strain S. cristatus CR311 was partitioned between hexadecane and buffer, hydrophobic and hydrophilic subpopulations were recovered from each phase, respectively. The bacteria recovered from the hexadecane partitioning assay were fingerprinted by comparing genomic DNA restriction endonuclease fragments and total soluble protein patterns (data not shown).
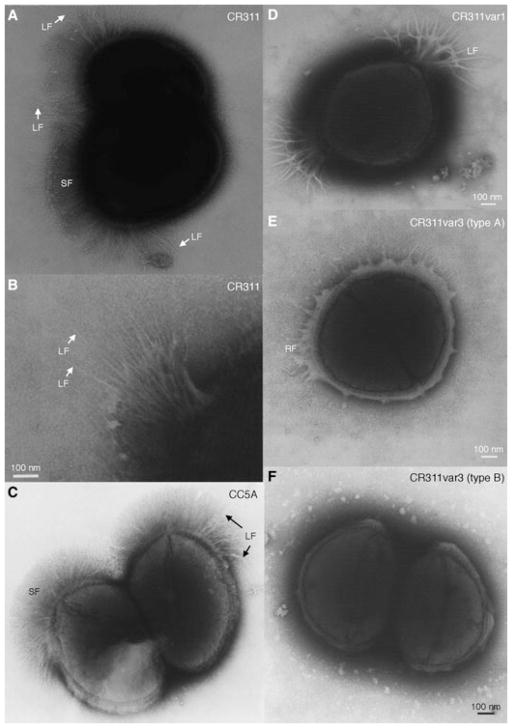
Examination of the S. cristatus subpopulations, recovered from the hexadecane partitioning assay, in the TEM revealed alterations in the surface fibril composition. As shown in a negatively stained preparation in Fig. 1A, the parent strain CR311 carries short fibrils arrayed within the tufts. The average length of the short fibrils is 238 ± 18.9 nm. The long fibrils are also distributed throughout the tufts but clusters can often be seen located in the tuft farthest away from the septum (Fig. 1B). The long fibrils average 403 ± 68.7 nm in length. They were sparser than the short fibrils and projected through the more densely packed short fibrils. Even though the cells were washed, the fibrils sometimes had associated extraneous electron transparent material (Fig. 1B). A negative stain of S. cristatus CC5A shows the same pattern of long and short tuft fibrils (Fig. 1C), indicating that this general fibril arrangement is characteristic of the species.
Fig. 1.
Negatively stained preparations of wild-type and fibrillar tuft variants of S. cristatus. A) Wild-type CR311 showing the organization of the short (SF) and long (LF) fibrils. B) Enlargement of the long fibrils in the fibrillar tufts of CR311. C) Wild-type CC5A showing both short and long fibrils. D) CR311var1 showing only the long fibrils that splay out at the ends. E) CR311var3 (type A) showing the remnant tuft fibrils (RF) only. F) CR311var3 (type B) having no fibrils. Magnification bar = 100 nm.
A hydrophobic variant (CR311var1) that only carried long fibrils was isolated from the hexadecane phase (Fig. 1D). These fibrils average 395 ± 32.4 nm in length (Table 2). The length of the fibrils on CR311var1 was not statistically significantly different from the length of the long fibrils on the wild-type strain. The long fibrils clumped during staining to give a branched appearance but the ends of the long fibrils consisted of very fine fibrils.
Table 2.
Average lengths of tuft fibrils on S. cristatus CR311 and fibril variants and surface hydrophobicity
| Strain | Average length of short fibrils (nm) | Average length of long fibrils (nm) | Average length of remnant fibrils (nm) | Percent hydrophobicity |
|---|---|---|---|---|
| CR311 | 238 ± 18.9 | 403 ± 68.7 | 91.6 ± 4.1 | |
| CR311var1 | 395 ± 32.4 | 92.9 ± 7.1 | ||
| CR311var3 | 223 ± 48.8 | 55.7 ± 21.9 |
One hydrophilic variant (CR311var3) was isolated from cells partitioning into the buffer phase and lacked both the short and long fibrils. However, examination of overnight cultures of CR311var3 revealed four distinct morphologic types. Most of the cells showed sparse, thin, lateral fibrils that did not appear to be like the lateral tufts that project from cell surface crenations (CR311var3 type A). These cells lacked the long tuft fibrils but had sparse remnants of the lateral short fibrils in association with surface crenations in the position of the tuft (Fig. 1E). Other members of the population (CR311var3 type B) had no polar fibrils and showed no obvious surface crenation (Fig. 1F). A small percentage of the cells in the population appeared to have possible remnants of short tuft lateral fibrils in combination with fine polar fibrils or only the fine fibrils (data not shown). The remnant lateral short fibrils average 223 ± 48.8 nm in length. They are not statistically significantly different from the short tuft fibrils on the wild-type strain. Thus, the fibril phenotype of CR311var3 appears to be variable. In cultures of this variant there are typically 40% cells with the remnant lateral fibrils (Fig. 1E), 37% cells having no fibrils (Fig. 1F), 14% cells with sparse fine polar fibrils and 9% cells with fine polar and remnant lateral fibrils.
Surface properties of the fibril variants
Both S. cristatus CR311 and CR311var1 formed corncobs with C. matruchotii (Fig. 2). In contrast, S. cristatus CR311var3 did not form corncobs with C. matruchotii. Similar results were obtained in coaggregation experiments with F. nucleatum (data not shown). The data obtained with the S. cristatus CR311var3 mutant confirmed that the long fibrils are required for this species to form corncobs. Electron microscopy in previous studies showed that members of this species bind to C. matruchotii and F. nucleatum through the tuft fibrils (14, 15). Table 2 compares the hydrophobicity of the wild-type strain CR311 with that of the two structural variants. The results are from an average of six replicate experiments carried out for each strain. The results confirm that strains that have the long fibrils (CR311 and CR311var1) are significantly more hydrophobic than CR311var3 strains, which carried only remnants of short tuft fibrils.
Fig. 2.
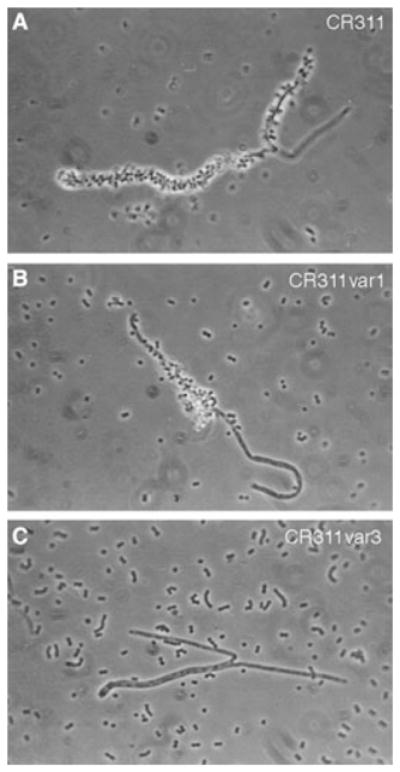
Phase contrast microscopy of corncob formation between S. cristatus fibril variants and C. matruchotii. A) Wild-type CR311. B) CR311var1. C) CR311var3. Magnification × 100.
Correlation between the loss of short fibrils and expression of a novel serine-rich protein in S. cristatus
A previously characterized transposon insertion mutant, S. cristatus CC5A-B15, exhibited a 70% reduction in binding to F. nucleatum relative to the wild-type strain CC5A (4). The transposon insertion was mapped to an ABC transport locus comprising four open reading frames (ORF) (5). The fibrillar tufts of this mutant have a disordered, irregular appearance, in contrast to the relatively ordered arrangement of the fibrils on the wild-type strain. The short fibrils could not be clearly discerned on the mutant.
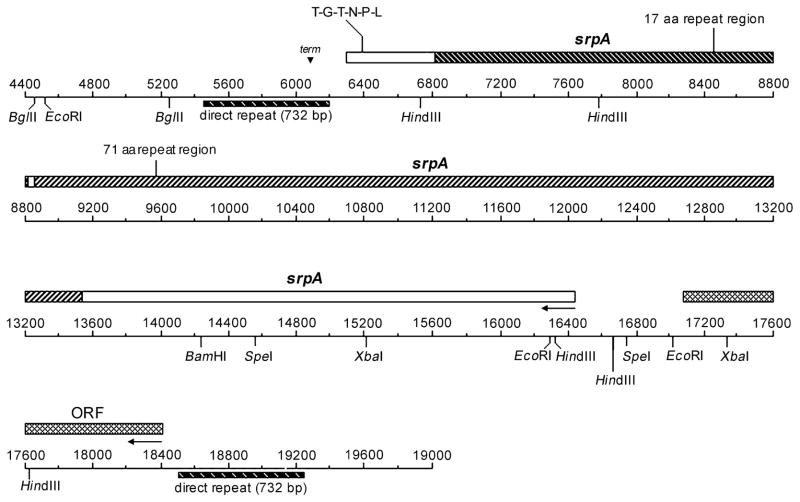
Additional sequence obtained downstream of the CC5A-B15 Tn916 insertion locus revealed an unusually large ORF of 10,146 bp (Fig. 3). This ORF (positions 6301–16446; [for numbering refer to Accession Number U96166]) is encoded on the opposite strand from the ABC cassette operon that was previously shown to affect interbacterial binding. This large ORF is designated srpA for the potential serine-rich protein it encodes. The srpA ORF is flanked by direct repeats of 732 bp, which themselves show homology to a transposase encoded by Tn5382 from Enterococcus faecalis (GenBank Accession Number AF173641). Abutting DR1a (positions 6207–6248) and 3′ to the srpA coding sequence is a prokaryotic factor-independent terminator as determined by the method of Brendel & Trifonov (1). An ORF of 1329 bp that does not show a similarity with any genes in the nonredundant databases was found upstream of srpA and within the boundaries marked by the direct repeats.
Fig. 3.
Physical map of the region of the S. cristatus CC5A genome containing the srpA ORF. The map shows 14.6 kb of DNA immediately downstream from the binding-deficient locus of S. cristatus CC5A-B15 sequenced previously (5). Two ORFs are depicted by filled bars above the scale. The ORFs reside within a region bounded by a 732 bp direct repeat. Arrows show the direction of transcription. The scale is in bp and numbering continues from the upstream sequence previously published. The complete contiguous 19,841 bp sequence is in the GenBank file (Accession Number U96166).
Transcription of srpA in S. cristatus CC5A was examined by Northern blotting (Fig. 4A). An approximately 11.0 kb transcript was detected, indicating that srpA was monocistronic. A lower molecular weight hybridization positive band was also detected and most likely represents transcript degradation due to the exceptionally large size of the mRNA for the SrpA protein. Southern blot analysis showed that DNA sequence homologous to the srpA sequence of strain CC5A could be detected in a number of tufted S. cristatus strains including PshA, Pshb and CR3 as well as in a tufted strain of S. oralis (CN3410) (Fig. 4B). No homology was detected with DNA from S. parasanguis FW213 that contains the fap1 gene.
Fig. 4.
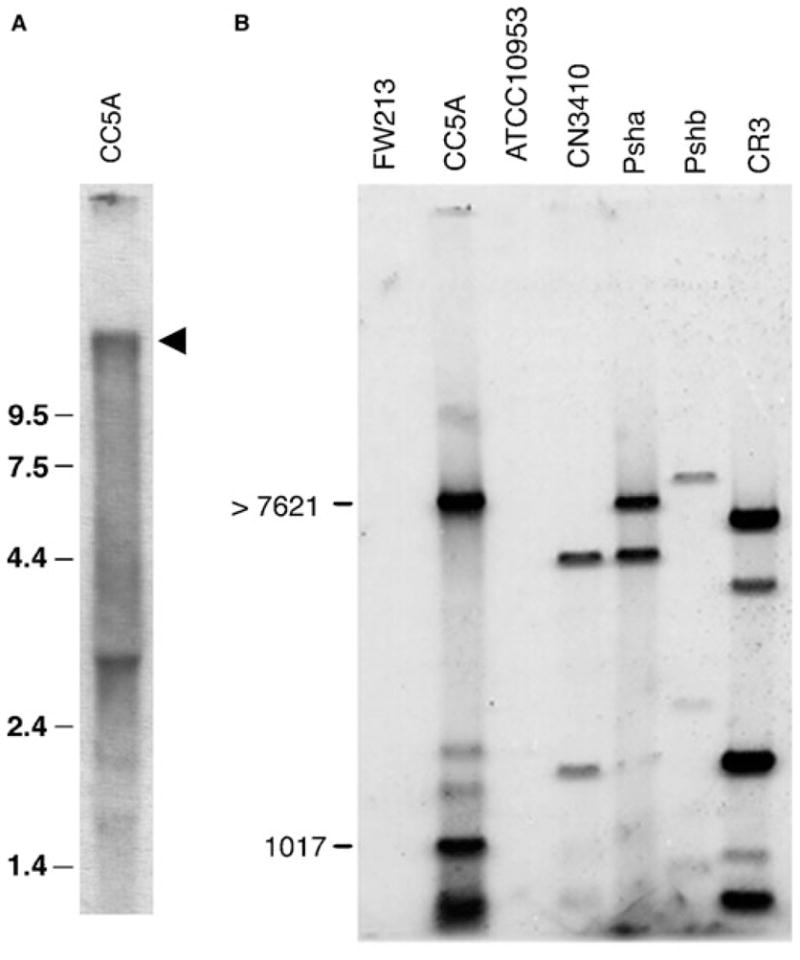
Genetic analysis of the srpA ORF. A) Northern blot showing the srpA transcript from S. cristatus CC5A. Total RNA from wild-type CC5A was hybridized with a DNA sequence amplified by PCR from the 5′-end of the srpA gene. Molecular size standards are shown in kb. The arrow marks the position of a 11 kb hybridization positive band. B) Southern blot of the distribution of srpA DNA in various Streptococcus strains. Total genomic DNA was obtained from S. cristatus strains CC5A, CR3, Psha and Pshb; S. oralis CN3410; S. parasanguis FW213; and F. nucleatum ATCC 10953. The DNA samples were digested to completion with HindIII and the fragments hybridized with a fragment of DNA amplified from the 5′-end of the CC5A srpA ORF. The positions of molecular size markers are shown in bp.
The srpA ORF potentially encodes a protein (SrpA) of 321,882 Da (Fig. 5) that comprises 3382 amino acids, 1346 of which (39.8%) are serines. The serines alternate with nonserine residues (with the exception of a recurring NNQN motif) over approximately 75% of the protein. The serine repeat sequence has three distinct patterns. Closest to the carboxyl terminal is a 17-amino acid unit (SASTSMSNVSASISAS[E, V, Q, A, I]), repeated 35 times (not consecutively as there are some truncated repeats) containing the infrequently used amino acid methionine. The amino acid in the 17th position of this repeat is variable. Adjacent to the methionine-type repeat is a 71-amino acid unit that is repeated 22 times. The order of serines in this repeat is disrupted by the polar amino acids NNQN and is a characteristic feature of the larger serine repeat unit. Some of the NNQN-type repeat units contain insertions or deletions within the repeat pattern. Some of the alternating serine residues appear in regions with no obvious pattern. One of these regions comprises the amino-terminus of the protein. A standard signal peptide sequence (23) is found at the amino-terminus. At the carboxy-terminus, SrpA has the gram-positive cell wall (peptidoglycan) anchor consensus sequence LPNTG preceding a typical hydrophobic domain and charged tail found in gram-positive surface proteins (23). Codon usage for srpA was not unusual relative to previously sequenced S. cristatus ORFs.
Fig. 5.
Schematic representation of the SrpA protein from S. cristatus CC5A. Organization of the predominant features of the protein are shown. Theoretical glycosylation and phosphorylation sites were determined by computer analysis. Amino acid sequences of the repeat regions and carboxy terminal features of gram-positive wall-anchored proteins were deduced from the nucleotide sequence deposited in GenBank. Sites of amino acid variation within the repetitive sequences are bound by parentheses. Brackets indicate where deletions occur in some of the repeats.
A possible connection between the putative cell wall associated SrpA protein, fibrillar tuft, and corncob formation prompted us to look for srpA DNA in S. cristatus corncob-deficient mutants. PCR was used to determine whether srpA DNA could be detected in variants CR311var1 and CR311var3 (Fig. 6). PCR primers designed to amplify the 5′-end of the srpA ORF were used to avoid the extensive repeat sequence throughout most of the ORF. Amplified product of the expected size (820 bp) was obtained with template DNA from the wild-type strain CR311 and the short fibril-deficient variant CR311var1. However, no PCR product was obtained with DNA from the long fibril-deficient variant CR311var3, indicating that the srpA gene was not present or was extensively altered in S. cristatus CR311var3.
Fig. 6.
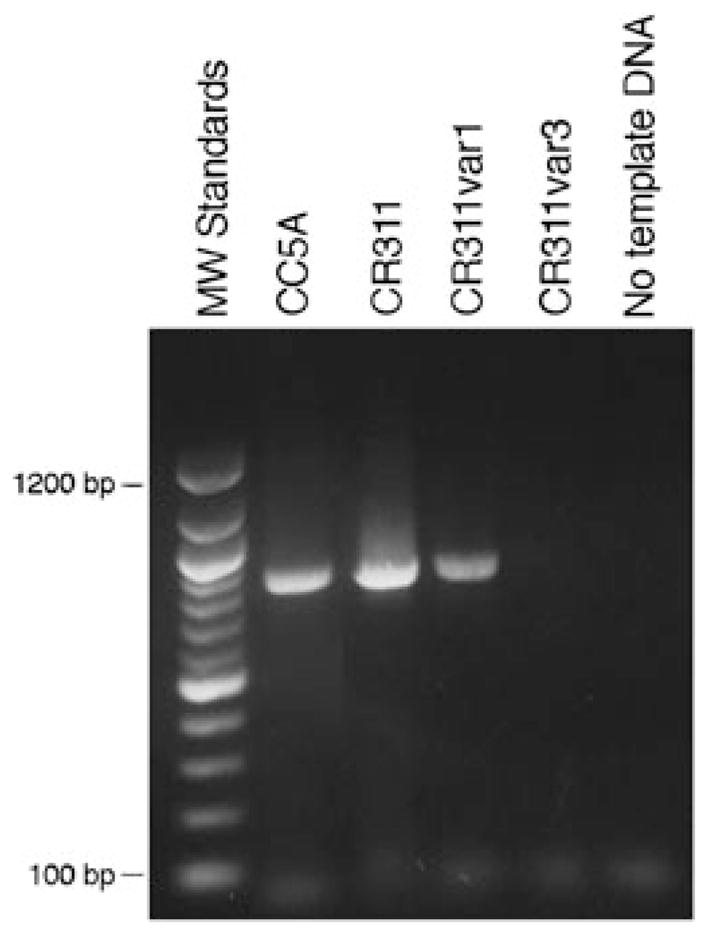
PCR analysis of srpA gene sequences in wild-type and fibril variants of CR311. Total DNA from CC5A, CR311, CR311var1 and CR311var3 was amplified in PCR using primers made against the 5′-end of the srpA gene. PCR products were separated on an agarose gel and stained with ethidium bromide. A 100 bp DNA ladder was used as molecular size markers.
Total protein from the wild-type and variant strains of CR311 was examined on 8% SDS-polyacrylamide gels for the presence of polypeptide bands of exceptionally high apparent molecular weight. A single, high molecular weight polypeptide band was detected in extracts from S. cristatus CC5A and CR311 (Fig. 7). This polypeptide was absent in total protein preparations from the long fibril-deficient mutant CR311var3. A tryptic digest of the high molecular weight CR311 polypeptide contained a single 230 kDa peptide as predicted from the deduced amino acid sequence (data not shown). This relative resistance to trypsin was expected based on the extensive serine-rich repeats in the amino acid sequence of SrpA.
Fig. 7.

SDS-polyacrylamide gel analysis of high molecular weight proteins in wild-type and fibril variants of CR311. Proteins from whole cell lysates of CC5A, CR311 and CR311var3 were separated on an 8% SDS-polyacrylamide gel and detected by staining with Coomassie Blue. Molecular weight markers are in kDa. The arrow marks the position of the putative SrpA protein.
Discussion
Mouton et al. (22) made the original observation that S. cristatus contained characteristic tufts of fimbriae (now called fibrils), which were proposed to function in adhesion to C. matruchotii. These studies influenced numerous investigations, leading to the conclusion that streptococcal species carrying these fibrils play a prominent role in the interactions among diverse bacteria in the dental biofilm. Studying the structure and composition of these fibrils may offer some unique insights into the ecology of the plaque biofilm.
Electron microscopy of negative stained preparations revealed that S. cristatus has two types of fibrils, long (ca. 400 nm) and short (ca. 240 nm), within the fibril tuft (2, 8). It had been difficult to segregate these two types of fibrils, by either biochemical or genetic methods, to determine function. The ability of the long fibrils to impart a hydrophobic character to the surface of the streptococci was previously shown by hexadecane partitioning and the binding of colloidal gold particles to the tips of the fibrils (10). This apparent relationship between surface hydrophobicity and fibril composition was exploited in the present study to enrich for variants in the wild-type population that contained the different fibril arrangements. We found that fibril expression is a highly variable phenotype (summarized in Fig. 8). In general, all of the cells in cultures of the wild-type strain CR311 carry tufts of fibrils with no fibril-less (type B) cells or cells with fine polar fibrils (type D) detectable. The fine polar fibrils are very difficult to detect and it is possible that they may only have been present on a few cells in the wild-type population before hexadecane enrichment was carried out. Cultures of the hydrophobic variant CR311var1 contain only long fibrils and no type B cells. However, cultures of the hydrophilic variant CR311var3 exhibited a mixed morphologic phenotype such that approximately equal numbers of cells carried remnant short fibrils (type A) or no fibrils (type B) (ca. 40% in each category). The length of the remnant short fibrils was not statistically different from that of the short fibrils present on strain CR311. Variant CR311var3 can be considered to be devoid of long fibrils. Although short fibrils were detected on CR311var3 they were very sparse, indicating that the majority of dense short fibrils in the tuft had been lost. The recovery of phenotypically distinct morphotypes suggests that there may be more than one structural gene for fibrils in S. cristatus. The multiplicity of fimbrial (fibril) genes among different species of oral streptococci, including S. parasanguis, S. gordonii and S. salivarius, supports this complexity of fibril structure and function. It is possible that this bacterium may undergo phase variation.
Fig. 8.
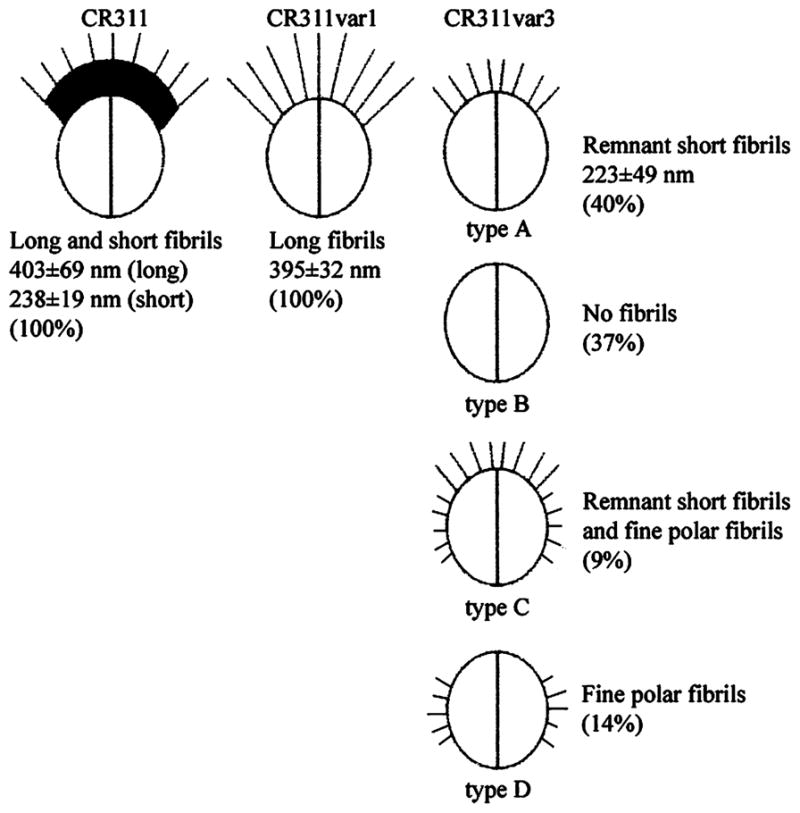
Population distribution of the various S. cristatus CR311 fibrillar tuft morphological variants. Percent values represent the relative distribution of each fibril type in stationary phase cultures.
Physical segregation of these morphotypes allowed us to examine fibril function. The long fibrils appear to be important for corncob formation. Both S. cristatus CR311 and CR311var1 adhered to other oral bacteria and formed indistinguishable corncobs. Both the quantity of the corncobs and the density of streptococci attached to C. matruchotii were similar in each case. This suggests that the loss of the short fibrils does not adversely affect binding of S. cristatus to C. matruchotii. In contrast, attachment of CR311var3 to C. matruchotii was significantly reduced relative to that observed with the wild-type strain. Thus, it appears that the loss of the long fibrils is linked to the loss of corncob formation.
Our studies provide evidence that a novel gene for a high molecular weight serine-rich protein is associated with the long fibrils, and therefore corncob formation, in S. cristatus. According to best estimates this SrpA protein is greater than 300 kDa. Its unusually large apparent molecular weight hampered its characterization even on low percentage SDS-polyacrylamide gels. The SrpA protein is marked by two relatively long stretches of amino acids that repeat multiple times. Also, almost half of the protein comprises serine. The SrpA protein is physically similar but neither genetically nor serologically related to the Fap1 fimbrial-associated protein of S. parasanguis (31). There is no similarity between either the nucleotide or the deduced amino acid sequences of Fap1 and SrpA. Fap1 is a bit smaller than SrpA (2552 vs. 3382 amino acids), contains two extensive amino acid repeat regions, and its amino acid composition is 43% serine. Even more fascinating is that most of the serine residues in SrpA are arranged in dipeptides as in Fap1. Both proteins contain the gram-positive cell wall anchor motif LPNTG. Similar, but not identical, properties have been reported for other very large streptococcal surface proteins such as CshA (18–21) and S. salivarius cell wall associated proteins (17, 30), which appear to be involved in colonization. The physical similarities between SrpA and these other surface appendage-associated proteins suggest a similarity in function such that it is likely that the srpA gene also codes for a fibril-related protein. It is known that protease treatment of the streptococci results in removal of the long fibrils and correspondingly reduced cell surface hydrophobicity, corncob formation and the other properties that appear to be associated with these structures (13). A relationship between the srpA gene and the long fibrils is also supported by PCR analysis, which showed that a homologous sequence could not be amplified from DNA from the fibril-deficient variant CR311var3. Furthermore, the SrpA protein was not detected in whole cell lysates of this variant, indicating that the gene is not expressed. The loss of the gene or gene expression, accompanied by the absence of the long fibrils, reduction in hydrophobicity, inability to form corncobs with C. matruchotii and loss of coaggregation with F. nucleatum, further strengthens this relationship between the long fibrils and the SrpA protein. It is possible that the loss of srpA expression indirectly affects transport or assembly of the long fibrils. However, the putative repeat structure and exceptionally large size of the SrpA protein is more consistent with a structural role for the protein.
The putative structure of the SrpA protein supports the presence of N-linked glycosylation sites. However, we do not have direct evidence that the native SrpA protein is modified with carbohydrate. Previous studies have shown that the surface of S. cristatus is decorated with a Ruthenium red-staining material that may be polysaccharide (12). Based on the finding that polysaccharide is present in the fibrillar tufts, it has been speculated that the fibrils of S. cristatus CR311 are composed of a glycoprotein (10, 13). This idea would also be consistent with reports that the Fap1 protein of S. parasanguis (3, 26) and the fibril elements of S. salivarius (16) are modified with carbohydrate.
Thus, the similarity of our data with S. cristatus to those of other studies (17, 18, 28–30) strengthens the possibility that these relatively large streptococcal surface proteins, with extensive repeat structure and adhesion associated properties, represent a family of fibril/fimbrial proteins important for oral biofilm formation. The present study was performed using planktonic phase bacteria grown under nutrient-rich conditions. It is important now to extend these studies using bacteria grown under biofilm-forming conditions to examine the expression of the srpA gene and fibril formation.
Acknowledgments
The able technical assistance of Tamara Allen and Alla Volgina is gratefully acknowledged. We thank P. Fives-Taylor and H. Jenkinson for supplying bacteria.
This study was supported by Public Health Service grant R01 DE03180 (J.D.).
References
- 1.Brendel V, Trifonov EN. A computer algorithm for testing potential prokaryotic terminators. Nucl Acids Res. 1984;12:4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busscher HJ, Handley PS, Rouxhet P, Hesketh GLM, van der Mei HC. The relationship between structural and physicochemical surface properties of tufted Streptococcus sanguis strains. In: Mozes N, Busscher HJ, Handley PS, Busscher HJ, Rouxhet PG, editors. Microbial Cell Surface Analysis, Structural and Physicochemical Methods. Weinheim; Wiley-VCH: 1991. pp. 319–337. [Google Scholar]
- 3.Chen Q, Wu H, Fives-Taylor PM. Construction of a novel transposon mutagenesis system useful in the isolation of Streptococcus parasanguis mutants defective in Fap1 glycosylation. Infect Immun. 2002;70:6534–6540. doi: 10.1128/IAI.70.12.6534-6540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correia FF, DiRienzo JM, Lamont RJ, Anderman C, McKay TL, Rosan B. Insertional inactivation of binding determinants of Streptococcus crista CC5A using Tn916. Oral Microbiol Immun. 1995;44:287–291. doi: 10.1111/j.1399-302x.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correia FF, Lamont RJ, Bayer ME, Rosan B, DiRienzo JM. Cloning and sequencing of a mutated locus that affects fimbrial tuft organization and corncob formation in Streptococcus crista CC5A. Int J Oral Biol. 1997;22:241–248. [Google Scholar]
- 6.Elliot D, Harrison E, Handley PS, Ford SK, Jaffray E, Mordan N, McNab R. Prevalence of Csh-like fibrillar surface proteins among mitis group oral streptococci. Oral Microbiol Immunol. 2003;18:114–120. doi: 10.1034/j.1399-302x.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 7.Froeliger EH, Fives-Taylor P. Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect Immun. 2001;69:2512–2519. doi: 10.1128/IAI.69.4.2512-2519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handley P, Carter PL, Wyatt JE, Hesketh LM. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect Immun. 1985;47:217–227. doi: 10.1128/iai.47.1.217-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handley P, Coykendall A, Beighton D, Hardie JM, Whiley RA. Streptococcus crista sp. nov. a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int J Syst Bacteriol. 1991;41:543–547. doi: 10.1099/00207713-41-4-543. [DOI] [PubMed] [Google Scholar]
- 10.Handley PA, Hesketh LM, Moumena RA. Charged and hydrophobic groups are localized in the short and long tuft fibrils of Streptococcus sanguis strains. Biofouling. 1991;4:105–111. [Google Scholar]
- 11.Handley PS, McNab R, Jenkinson HF. Dental plaque revisited: oral biofilms in health and disease. In: Newman HN, Wilson M, editors. Adhesive, Surface Structures on Oral Bacteria, Bioline. 1999. pp. 145–170. [Google Scholar]
- 12.Hesketh LM, Wyatt JE, Handley PS. Effect of protease on cell surface structure, hydrophobicity and adhesion of tufted strains of Streptococcus sanguis biotypes I and II. Microbios. 1987;50:131–145. [PubMed] [Google Scholar]
- 13.Jameson MW, Jenkinson HF, Parnell K, Handley PS. Polypeptides associated with tufts of cell-surface fibrils in an oral Streptococcus. Microbiology. 1995;141:2729–2738. doi: 10.1099/13500872-141-10-2729. [DOI] [PubMed] [Google Scholar]
- 14.Lancy P, Jr, Appelbaum B, Holt SC, Rosan B. Quantitative in vitro assay for ‘corncob’ formation. Infect Immun. 1980;29:663–670. doi: 10.1128/iai.29.2.663-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancy P, Jr, DiRienzo JM, Appelbaum B, Rosan B, Holt SC. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect Immun. 1983;40:303–309. doi: 10.1128/iai.40.1.303-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L’Èvesque C, Vadeboncoeur C, Chandad F, Frenette M. Streptococcus salivarius fimbriae are composed of a glycoprotein containing a repeated motif assembled into a filamentous nondissociable structure. J Bacteriol. 2001;183:2724–2732. doi: 10.1128/JB.183.9.2724-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNab R, Forbes H, Handley PS, Loach DM, Tannock GW, Jenkinson HF. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J Bacteriol. 1999;181:3087–3095. doi: 10.1128/jb.181.10.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNab R, Jenkinson HF. Gene disruption identifies a 290 kDa cell-surface polypeptide conferring hydrophobicity and coaggregation properties in Streptococcus gordonii. Mol Microbiol. 1992;6:2939–2949. doi: 10.1111/j.1365-2958.1992.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 19.McNab R, Holmes AR, Clarke JM, Tannock GW, Jenkinson HF. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect Immun. 1996;64:4204–4210. doi: 10.1128/iai.64.10.4204-4210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNab R, Jenkinson HF, Loach DM, Tannock GW. Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol Microbiol. 1994;14:743–754. doi: 10.1111/j.1365-2958.1994.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 21.Mouton C, Reynolds HS, Gasiecki EA, Genco RJ. In vitro adhesion of tufted oral streptococci to Bacterionema matruchotii. Curr Microbiol. 1979;3:181–186. [Google Scholar]
- 22.Mouton C, Reynolds HS, Genco RJ. Characterization of tufted streptococci isolated from ‘corn cob’ configuration of human plaque. Infect Immun. 1980;27:235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarre WW, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 25.Sambrook J, Fritch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Stephenson AE, Wu H, Novak J, Tomana M, Mintz K, Fives-Taylor P. The Fap1 fimbrial adhesin is a glycoprotein. antibodies specific for the glycan moiety block the adhesion of Streptococcus parasanguis in an in vitro tooth model. Mol Microbiol. 2002;43:147–157. doi: 10.1046/j.1365-2958.2002.02725.x. [DOI] [PubMed] [Google Scholar]
- 27.Truper HG, de’Clari L. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) in ‘apposition’. Int J Syst Bacteriol. 1997;47:908. [Google Scholar]
- 28.Weerkamp AH, Handley PS, Barrs A, Slot JW. Negative staining and immunoelectron microscopy of adhesion deficient mutants of Streptococcus salivarius reveal that the adhesive protein antigens are separate classes of cell surface fibril. J Bacteriol. 1986;165:746–755. doi: 10.1128/jb.165.3.746-755.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weerkamp AH, van der Mei HC, Liem RS. Structural properties of fibrillar proteins isolated from the cell surface and cytoplasm of Streptococcus salivarius K+ cells and nonadhesive mutants. J Bacteriol. 1986;165:756–762. doi: 10.1128/jb.165.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Mintz KP, Ladha M, Fives-Taylor PM. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol. 1998;28:487–500. doi: 10.1046/j.1365-2958.1998.00805.x. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Fives-Tayor PM. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol Microbiol. 1999;34:1070–1081. doi: 10.1046/j.1365-2958.1999.01670.x. [DOI] [PubMed] [Google Scholar]