Summary
The vertebrate spine exhibits two striking characteristics. The first one is the periodic arrangement of its elements – the vertebrae – along the antero-posterior axis. This segmented organization is the result of somitogenesis, which takes place during organogenesis. The segmentation machinery involves a molecular oscillator – the segmentation clock – which delivers a periodic signal controlling somite production. During embryonic axis elongation, this signal is displaced posteriorly by a system of traveling signaling gradients – the wavefront – which depends on the Wnt, FGF and retinoic acid pathways. The other characteristic feature of the spine is the subdivision of groups of vertebrae into anatomical domains, such as the cervical, thoracic, lumbar, sacral and caudal regions. This axial regionalization is controlled by a set of transcription factors called Hox genes. Hox genes exhibit nested expression domains in the somites which reflect their linear arrangement along the chromosomes– a property termed colinearity. The colinear disposition of Hox genes expression domains provides a blueprint for the regionalization of the future vertebral territories of the spine. In amniotes, Hox genes are activated in the somite precursors of the epiblast in a temporal colinear sequence and they were proposed to control their progressive ingression into the nascent paraxial mesoderm. Consequently, the positioning of the expression domains of Hox genes along the antero-posterior axis is largely controlled by the timing of Hox activation during gastrulation. Positioning of the somitic Hox domains is subsequently refined through a cross talk with the segmentation machinery in the presomitic mesoderm. In this review, we focus on our current understanding of the embryonic mechanisms that establish vertebral identities during vertebrate development.
Introduction
The vertebrate spine is formed by the periodic series of vertebrae distributed along the antero-posterior (AP) body axis (Hirsinger et al., 2000). This repetitive pattern is established during somitogenesis, a process by which segmental structures called somites are produced in the embryo. In vertebrates, somites are epithelial blocks of mesodermal cells that contain the precursors of vertebrae, skeletal muscles and dorsal dermis (Hirsinger et al., 2000). They form as the tissue pinches off from the anterior tip of the presomitic mesoderm (PSM) in a rhythmic fashion. Somitogenesis begins anteriorly at the level immediately caudal to the otic vesicle (Hinsch and Hamilton, 1956; Huang et al., 1997) and proceeds posteriorly on both sides of the neural tube and notochord to the caudal tip of the embryo. Although the number of somites that forms in a given species is highly constrained, it varies widely between species, ranging from approximately 30 somite pairs in some fish to several hundred pairs in snakes (Gomez et al., 2008; Richardson et al., 1998). In amniotes, such as birds or mammals, the anterior-most somites contribute to the baso-occipital bone at the base of the skull (Christ et al., 2007; Couly et al., 1993; Noden, 1986); whereas, the more posterior somites form the vertebral column. The paraxial tissue anterior to the otic vesicle is called the cephalic or head mesoderm. It does not segment into somites and gives rise to bones and muscles of the head. Together with the somitic series, the cephalic mesoderm forms the paraxial mesoderm.
Although all newly formed somites exhibit very similar morphology (Figure 1a), they eventually differentiate into very distinct anatomical structures depending on their position along the AP axis (Figure 1b). The vertebrate spine is partitioned into domains that exhibit different identities, such as cervical, thoracic, lumbar, sacral and caudal. Within a given species, the number of each type of vertebrae – the vertebral formula – is usually fixed. The acquisition of vertebral identities is controlled by the Hox transcription factors (Krumlauf, 1994; Wellik, 2007), which are arranged in clusters in the genome (Duboule, 2007). Mammals have 39 Hox genes organized into four clusters, and birds share a very similar set of Hox genes (Richardson et al., 2007). In each cluster, the genes are arranged on the chromosome in a sequence that reflects the timing of their expression during embryogenesis (temporal colinearity) and the position of their expression domain along the AP axis (spatial colinearity) (Dolle et al., 1989; Gaunt et al., 1988; Graham et al., 1989). Thus, Hox genes exhibit nested expression domains in the vertebral precursors along the AP axis. The spatial colinearity of the expression domains of Hox genes results in a specific combination of genes to be expressed in each somite (Kessel and Gruss, 1991). This combination of Hox genes is involved in the control of the specification of vertebral identities ((Carapuco et al., 2005; Deschamps and van Nes, 2005; Kessel and Gruss, 1991; Wellik, 2007) and reference therein). However, whereas the expression domain of many Hox genes extends from the posterior end of the embryo to a defined anterior level in the somites, their action is essentially restricted to their anterior-most expression domain. Thus, the identity of a segment is controlled only by the posterior-most Hox genes that are expressed in this segment (Burke et al., 1995; Duboule and Morata, 1994). This property is termed posterior prevalence in vertebrates (Burke et al., 1995; Duboule and Morata, 1994).
Figure 1. Segmentation and regional patterning of somitic derivatives.
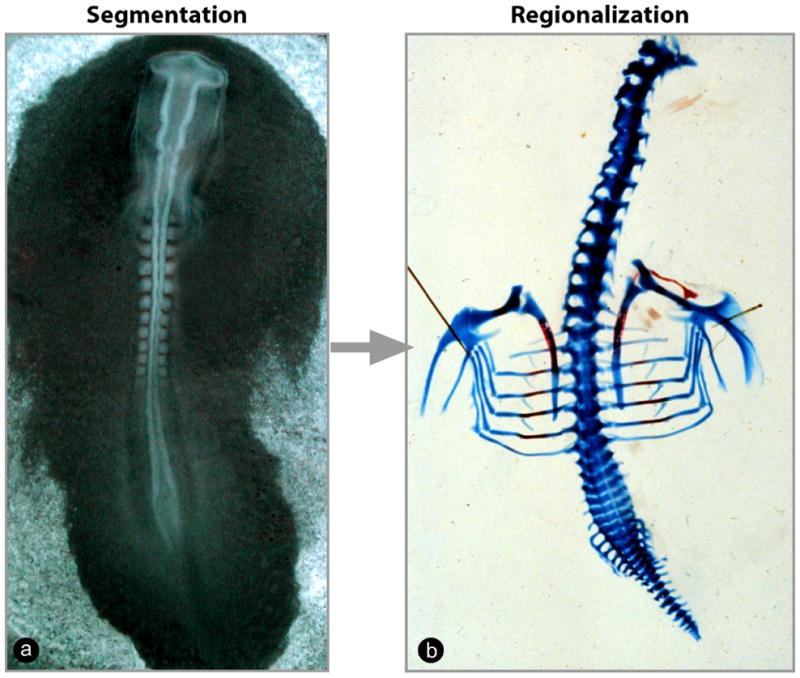
(a) Two-day-old chicken embryo in the process of forming the embryonic somites. (b) Skeletal preparation of a 10-day-old chicken embryo illustrating the different anatomical regions along the antero-posterior axis of the spine.
In the vertebral precursors of amniotes, Hox genes are first activated prior to gastrulation in the superficial epiblast in a colinear manner (Deschamps et al., 1999; Forlani et al., 2003; Gaunt and Strachan, 1994; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006). Hox genes were proposed to control the timing of ingression of mesodermal precursors in the primitive streak (Iimura and Pourquie, 2006). Thus, epiblast precursors expressing more 5′ genes ingress later than those expressing more 3′ genes and, hence, are positioned more posteriorly along the AP axis because of the progressive mode of cell deposition involved in the formation of the embryonic axis. This mechanism was proposed to be involved in the conversion of the temporal colinearity into the colinear or nested arrangement of the expression domain of these genes along the AP axis (Iimura and Pourquie, 2007). In this review, we will first discuss the establishment of the colinear Hox expression domains in the somites of amniote embryos.
Initial Hox gene activation in paraxial mesoderm precursors in the epiblast
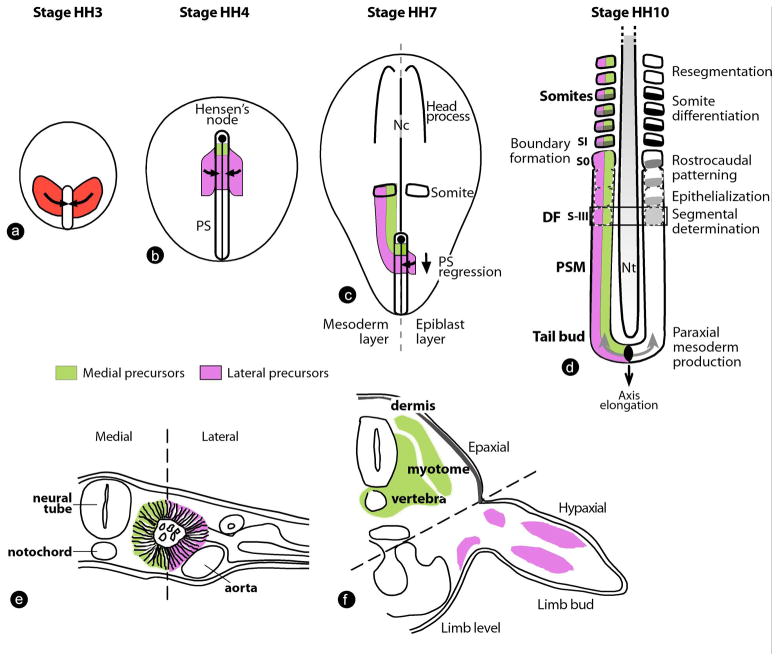
At the beginning of gastrulation, during the early stages of primitive streak formation, the presumptive territory of the paraxial mesoderm in the epiblast is located bilaterally to the forming primitive streak (Figure 2a) (Bortier and Vakaet, 1992; Hatada and Stern, 1994; Iimura et al., 2007; Lawson et al., 1991; Nicolet, 1971; Rosenquist, 1966; Schoenwolf et al., 1992; Tam and Beddington, 1987; Tam and Trainor, 1994; Waddington, 1952). During gastrulation, these domains converge toward the streak where they undergo an epithelium-to-mesenchyme transition, which allows their ingression to form the paraxial mesoderm (Hatada and Stern, 1994; Psychoyos and Stern, 1996; Schoenwolf et al., 1992; Selleck and Stern, 1991). The first paraxial mesoderm precursors to ingress form the head mesoderm (Lawson et al., 1991; Nicolet, 1971; Psychoyos and Stern, 1996). This tissue lies at the anterior tip of the embryo and does not express Hox genes (Iimura and Pourquie, 2006; Kuratani et al., 1997; Prince and Lumsden, 1994). After the primitive streak reaches a maximal size (Figure 2b), it begins to shrink and regress, and its anterior tip – which corresponds to the Spemann organizer of amniotes (called the Hensen’s node or the Node) – begins to move posteriorly (Spratt Jr., 1947)(Figure 2b–d). This regression movement lays in its wake the forming body axis as cells become progressively deposited by the regressing primitive streak. Fate maps of early chicken and mouse embryos show that paraxial mesoderm precursors are located in the anterior portion of the streak (medial precursors, green) and in the adjacent epiblast (lateral precursors, purple) (Figure 2b, c) (Forlani et al., 2003; Hatada and Stern, 1994; Iimura et al., 2007; Lawson et al., 1991; Nicolet, 1971; Psychoyos and Stern, 1996; Rosenquist, 1966; Schoenwolf et al., 1992; Selleck and Stern, 1991; Tam and Beddington, 1987; Tam and Trainor, 1994; Waddington, 1952).
Figure 2. Paraxial mesoderm formation and segmentation in the chicken embryo.
(a–d) Dorsal views of chicken embryos. (a) Gastrulating chicken embryo at stage 3 Hamburger and Hamilton (Stage HH3) (Hamburger and Hamilton, 1992). The presumptive territory of the paraxial mesoderm (red), which contains the precursors of vertebrae and skeletal muscles, converge toward the primitive streak. (b) Stage HH4 chicken embryo. At this stage, the PS has reached its maximal length. Presumptive territories of the paraxial mesoderm are located in the superficial epiblast just below Hensen’s node (medial precursor population, green) and in two symmetrical domains located on both sides of the PS (lateral precursor population, purple). These cells are ingressing (arrows) through the PS to form the paraxial mesoderm. (c) Stage HH7 chicken embryo. The PS and node have begun their posterior regression (arrow), leaving in their wake the embryonic axis comprising the head process anteriorly and the notochord (Nc) axially. Epiblast cells (purple) continue to ingress in the PS (arrow) and join the descendents of a population of resident stem cells located in the anterior primitive streak (green) to generate the paraxial mesoderm. The mesodermal layer is represented on the left side without the superficial epiblast. (d) Posterior region of a stage HH10 chicken embryo. Somitogenesis progresses posteriorly on both sides of the neural tube (Nt) in concert with axis elongation (arrow). Paraxial mesoderm cells are produced at the tail bud level and undergo a maturation process in the presomitic mesoderm (PSM), leading to the periodic formation of new pairs of somites. Segmental determination occurs at the level of the determination front (DF, black rectangle)). Presumptive somite nomenclature according to Pourquié and Tam (Pourquie and Tam, 2001).
(e–f) Transverse sections showing the fate of medial and lateral paraxial mesoderm precursors (only the right side of the embryos are shown). The hatched line separates the medial and the lateral domains. (e) Epithelial somite in a two-day-old chicken embryo. Medial (green) and lateral (purple) somitic cells are indicated according to their origins in panels b and c. (f) Differentiation of the somitic derivatives in a five-day-old chicken embryo at the limb level. Medial somitic cells (green) contribute to epaxial body structures, such as vertebra, muscle (myotome) and dermis; whereas, lateral somitic cells (purple) give rise to hypaxial muscles in the ventral body and limbs.
PS-primitive streak; Nc-notochord; Nt-neural tube; PSM-presomitic mesoderm
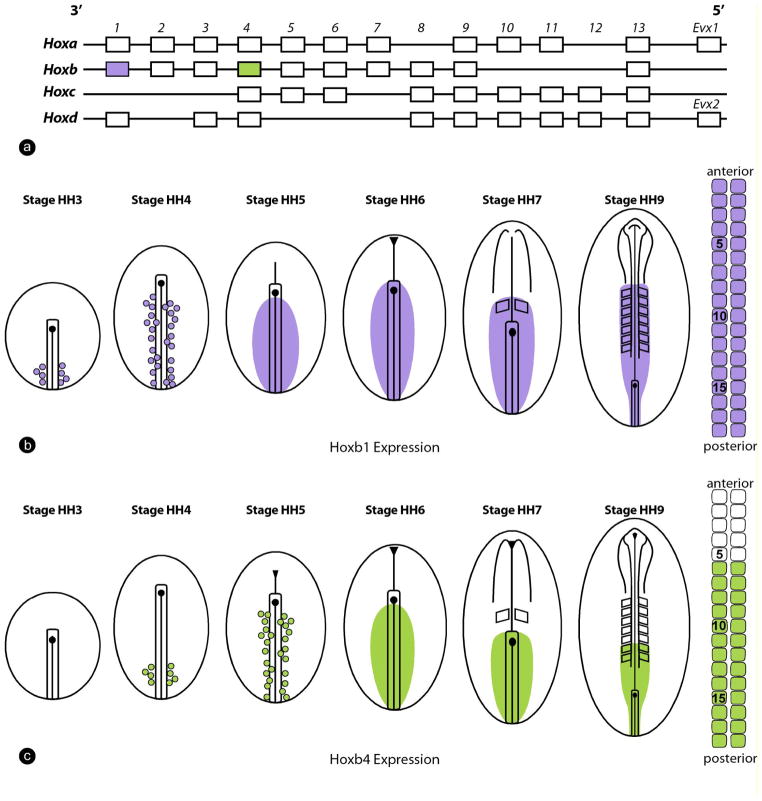
The onset of Hox activation in vertebral precursors has been examined in detail for several genes in chicken and mouse embryos (Figure 3a–c). Hox gene transcription is first initiated in the epiblast at the level of the posterior streak, a region that gives rise to mainly the lateral plate and extra-embryonic mesoderm (Figure. 3b, c) (Deschamps and Wijgerde, 1993; Forlani et al., 2003; Frohman et al., 1990; Gaunt et al., 1986; Gaunt and Strachan, 1994; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006). Hox gene expression initially appears as a salt-and-pepper pattern (Iimura and Pourquie, 2006) and progressively extends anteriorly in the epiblast along the primitive streak (Figure 3b, c) (Forlani et al., 2003; Gaunt and Strachan, 1994; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006). Then, expression of Hox genes progressively spreads to the surrounding cells, resulting in their expression in virtually all of the epiblast cells along the streak up to the node level (Deschamps et al., 1999; Forlani et al., 2003; Gaunt and Strachan, 1994; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006). Next, Hox gene expression subsequently spreads even further anterior to the node in the posterior neural plate (Deschamps et al., 1999; Forlani et al., 2003; Gaunt and Strachan, 1994; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006). This progressive mode of expression has been described as anterior propagation, forward spreading or rostral expansion (Deschamps and van Nes, 2005; Gaunt and Strachan, 1994).
Figure 3. Onset of Hox gene activation in the chicken embryo.
(a) Chicken Hox clusters showing the location of Hoxb1 (purple) and Hoxb4 (green). (b) Activation of Hoxb1 in the chicken embryo. Hoxb1 (purple) is first expressed in a salt-and-pepper pattern in the posterior part of the primitive streak at stage HH3 and its expression subsequently spreads anteriorly along the streak. At stage HH4, only precursors of the lateral somitic cells express the gene. Expression continues to spread until all cells of the epiblast adjacent to the streak, as well as cells entering the primitive streak, activate Hoxb1 at stage HH4. From this stage onward, Hoxb1 is expressed by the precursors of the medial and the lateral somites in the epiblast and the streak, and then this expression is maintained in the descendents of these cells entering the posterior presomitic mesoderm. While the embryonic axis forms, paraxial mesoderm cells continue to express Hoxb1, leading to the formation of the columns of Hoxb1-expressing somites. This strategy results in the position of the Hoxb1 expression domain in the paraxial mesoderm to be roughly defined by the timing of Hox activation in paraxial mesoderm precursors in the epiblast.
(c) Activation of Hoxb4 (green). Similar expression kinetics to that of Hoxb1 is observed, albeit starting a bit later in the posterior streak, at stage HH4. As a result, the presumptive territory of the paraxial mesoderm is reached later by Hoxb4 at stage HH5, after the cells that will form the anterior-most somites expressing Hoxb1 have been produced by the primitive streak. Consequently, the first cells expressing Hoxb4 will be positioned more posteriorly (at the level of somites 5–6) than the Hoxb1-expressing cells in the somitic series. In this way, the temporal colinear activation of Hox genes in the posterior primitive streak is translated into nested colinear domains along the somitic series. Expression domains of Hox genes in other tissues such the neural plate are not represented.
In chicken embryos, spreading occurs across an implanted glass barrier (Gaunt and Strachan, 1994). In contrast, in mouse embryos, cultures of isolated anterior primitive streak region taken prior to activation of Hoxb1 or Hoxb8 activate these genes if they are taken from embryos less than 12 hours before activation of these genes or if they are co-cultured with the posterior primitive streak region (Forlani et al., 2003). Therefore, Hox activation in the anterior primitive streak requires a signal from the posterior primitive streak. However, the necessary molecular events for the activation of a given Hox gene along the epiblast appear to have already taken place in the epiblast precursors at least 12 hours before the gene is actually detected, thus, potentially providing an explanation as to why the glass barrier does not inhibit the spreading in the chicken embryo. The molecular basis underlying this spreading of Hox expression is not understood. This anterior spreading movement of Hox expression continues for some time in the neural tissue until the level of the anterior boundary is reached; whereas in the paraxial mesoderm, no significant forward spreading is observed in the PSM after ingression of the descendents of the Hox-expressing epiblast cells (Forlani et al., 2003; Iimura and Pourquie, 2006). Consequently, boundaries in the paraxial mesoderm and neural tube are usually located at different AP levels, and the regulation of their positioning obeys different rules (Bel-Vialar et al., 2002). Here, we essentially discuss the positioning of boundaries in the mesoderm.
The first Hoxb gene to be expressed, Hoxb1, is activated early during gastrulation before complete extension of the primitive streak, and it follows the expression kinetics described above (Forlani et al., 2003; Frohman et al., 1990; Iimura and Pourquie, 2006) (Figure 3a–b). All Hoxb genes examined follow similar activation kinetics but their expression is initiated in the posterior primitive streak at progressively later times as one considers genes located more 5′ along the cluster (Forlani et al., 2003; Iimura and Pourquie, 2006) (Figure 3a–c). Thus, the activation of Hox genes in the epiblast follows a temporal colinear sequence (Forlani et al., 2003; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006). Soon after the expression domain of a given Hox gene reaches the node level, all cells in the paraxial mesoderm territory of the epiblast now express this gene (as well as the more 3′ genes). Descendents of the epiblast cells that express this Hox gene enter the posterior PSM at the primitive streak level and maintain expression of this gene. Thus, the anterior boundary of a given Hox gene in the paraxial mesoderm is roughly defined by the moment when the expression domain of this gene reaches the paraxial mesoderm territory of the epiblast (Forlani et al., 2003; Iimura and Pourquie, 2006). The temporal colinearity of Hox gene expression ensures that this territory is reached progressively later by successively more 5′ Hox genes. Hence, cells that express progressively more posterior Hox genes sequentially enter into the posterior PSM. Due to the posterior elongation of the embryo resulting from primitive streak regression, this leads to the establishment of the nested Hox expression domains along the AP axis.
Whereas the primitive streak contributes to the formation of the most anterior somites; more posteriorly, the paraxial mesoderm is formed from the tail bud (Figure 2d). In this structure, gastrulation movements, similar to those of the primitive streak, are still observed (Cambray and Wilson, 2002; Cambray and Wilson, 2007; Catala et al., 1995; Davis and Kirschner, 2000; Gont et al., 1993; Kanki and Ho, 1997; Knezevic et al., 1998; Pasteels, 1937; Stern, 2006). In the tail bud, precursors of the paraxial mesoderm are located immediately posterior to the region where the territory of the notochord and of the neural tube become fused (the chordo-neural hinge) (Cambray and Wilson, 2007; Charrier et al., 1999; McGrew et al., 2008). During axis elongation, newly ingressed paraxial mesoderm cells are deposited bilaterally to the chordo-neural hinge, resulting in the continuous formation of the two strips of paraxial mesoderm tissue. The sequence of Hox gene activation in the primitive streak appears to continue in the tail bud with the colinear activation of the posterior-most Abd-B-related genes (paralogs 10–13) (Izpisua-Belmonte et al., 1991). However, the cellular details of their expression kinetics in the tail bud have not been reported. Nevertheless, there are very significant differences in the timing of the activation of the 3′ and 5′ genes. Whereas the time between activation of Hoxb1 and Hoxb8 in the streak is very short in mouse and chicken embryos (~10 hours) (Forlani et al., 2003; Gaunt and Strachan, 1996; Iimura and Pourquie, 2006), the activation time between the paralogs 10–13 is much slower (Izpisua-Belmonte et al., 1991).
Recent observations support a conservation of the mode of mesoderm formation among vertebrates (Cambray and Wilson, 2007; Iimura et al., 2007; Solnica-Krezel, 2005). A Hox gene activation pattern similar to that reported in the paraxial mesoderm precursors in chicken and mouse embryos is observed in the Xenopus marginal zone (Wacker et al., 2004b). In the frog, temporal colinear activation of Hox genes is observed in the entire marginal zone, except in the organizer region. Colinear Hox gene activation proceeds normally in ultraviolet (UV) ventralized Xenopus embryos, suggesting that organizer signals are not required for the temporal colinearity of Hox activation during gastrulation (Wacker et al., 2004b). The organizer is required for the spreading of the colinear activation of Hox genes to the neural plate (Wacker et al., 2004b). These experiments in frogs confirm that the colinear activation of Hox genes is first initiated in the mesodermal precursors prior to their internalization and then spreads to the neural plate.
Molecular control of temporal colinearity
In vertebrates, Hox genes are arranged colinearly in intact clusters of ~100kb in length (Duboule, 2007). The striking conservation of the integrity of vertebrate Hox clusters has led to propose a functional link between the tight colinear arrangement of Hox genes in the genome and their temporal activation during embryogenesis (Iimura and Pourquie, 2007; Kmita and Duboule, 2003). Thus far, such a temporal colinearity has been observed only in species that display intact Hox clusters, suggesting that this process relies on some higher order properties of the organization of the clusters. Animals that show temporal colinearity also exhibit a progressive mode of axis formation and segmentation. For instance, short-germ band insects such as Tribolium or cephalochordates such as Amphioxus, which elongate and segment their body axis progressively, exhibit an intact Hox cluster (Putnam et al., 2008; Richards et al., 2008). In contrast, flies or ascidians, in which the Hox cluster is broken into several pieces, do not show temporal colinearity and form their axis and segments in a highly derived fashion (Dehal et al., 2002; Duboule, 2007; Seo et al., 2004). A segmentation machinery involving dynamic expression of the Notch pathway and a posterior Wnt gradient also appears to be a shared feature of animals showing a progressive mode of axis formation and segmentation (Angelini and Kaufman, 2005; Bolognesi et al., 2008; Chipman and Akam, 2008; McGregor et al., 2008; Stollewerk et al., 2003). Therefore, the progressive mode of segmentation strategy coupled to the temporal colinearity of Hox gene expression in the segment precursors might reflect an ancestral patterning strategy of Bilateria.
The molecular mechanism governing the sequential activation of Hox genes in the epiblast is unknown. In vertebrates, it has been proposed that temporal colinearity reflects the progressive opening of chromatin in a 3′-to-5′ direction, thus providing a temporally controlled access of Hox genes to the transcription machinery (Kmita and Duboule, 2003). This conclusion was initially suggested by relocation experiments of Hoxd9/LacZ and Hoxd11/LacZ transgenes at the 5′ extremity of the Hoxd complex (between Hoxd13 and Evx2). This resulted in a delayed activation of these transgenes in the tail bud, which behaved like Hoxd13 in the transgenic animals (van der Hoeven et al., 1996). Insertion of a Hoxd9/LacZ transgene upstream of the Hoxd cluster followed by a series of deletions identified a global regulatory element in the 5′ end of the Hoxd complex (Kondo and Duboule, 1999). This element is essential for the early colinear activation of the cluster. Together, these observations led to propose a silencing mechanism originating at the 5′ end of the cluster (Kmita and Duboul e, 2003). Progressive opening of the chromatin (or derepression) in a 3′-to-5′ direction would make genes progressively available for transcription. The sequential activation of genes along the Hoxb cluster was imaged in vivo in mouse embryonic stem (ES) cells (Chambeyron and Bickmore, 2004) and in the primitive streak of early mouse embryos (Chambeyron et al., 2005) using fluorescent in situ hybridization. Progressive chromatin decondensation was observed for the mouse Hoxb and Hoxd clusters in differentiating ES cells and in the primitive streak/tail bud of mouse embryos (Chambeyron et al., 2005; Morey et al., 2007). Chromatin decondensation, accompanied by successive looping out of the chromosomal territories (CT), is concomitant with gene transcription both in vitro and in the primitive streak context (Chambeyron et al., 2005; Morey et al., 2007). Although a functional link between the looping out of genes and transcription remains to be established, extrusion of a locus from the CT probably represents a poised state for transcription. However, no extrusion of the Hoxd genes CT was observed in the limb bud despite chromatin decondensation and transcription of the genes, indicating that both processes can occur also independently (Morey et al., 2007).
The colinearity has also been proposed to reflect the action of graded signaling pathways, such as retinoic acid (RA) and FGF (and their target genes Cdx), acting on cis-regulatory elements dispersed in the Hox clusters during gastrulation (Duboule, 1998). The sequence of the human genome shows an extremely low density of interspersed repeats (as Alu sequence) in the four Hox clusters, suggesting that cis-regulatory elements in each cluster are a strong selective constraint that cannot tolerate being interrupted by insertions (Lander et al., 2001). Several experimental configurations, in which cis-regulatory sequences within the cluster have been mutated, have been shown to alter the temporal colinearity of Hox gene activation during gastrulation (Gerard et al., 1996; Juan and Ruddle, 2003). In mutant mice, in which a Hoxb1/LacZ transgene was relocated in the 5′ end of the Hoxd cluster, not only was the transgene expressed early in the mesoderm but it also induced ectopic expression of Hoxd13 in the primitive streak in a pattern reminiscent of Hoxb1 (Kmita et al., 2000). In this transgenic mouse, ectopic chromatin decondensation and looping out of the CT resulting in a breaking of Hoxd colinearity are observed (Morey et al., 2008). Thus, the Hoxb1 locus contains cis-regulatory elements that can initiate local chromatin rearrangement, leading to gene activation. Ectopic expression of Hoxb1 and Hoxb2 in response to RA depends on whether the genes are within or outside the cluster (Roelen et al., 2002). A randomly integrated Hoxb1/LacZ transgene that contains RA-Response Elements (RARE) faithfully mimics the endogenous spatial expression pattern of Hoxb1; however, unlike the endogenous locus, it cannot be prematurely activated by short RA exposure (Roelen et al., 2002). While able to prematurely activate Hoxb1 and Hoxb2, RA is unlikely to control temporal colinearity because roughly normal activation of Hox genes is observed in mouse raldh2−/− mutants in which RA synthesis is blocked (Niederreither et al., 1999). Other candidates, such as Wnt, BMP and FGF signaling, have been proposed to play a role in the control of the onset of Hox gene activation; however, there is no conclusive evidence implicating them in the control of temporal colinearity thus far (Deschamps and van Nes, 2005). Therefore, the timing of activation of a Hox gene in the cluster depends on its position within the cluster and on local cis-regulatory sequences. Interaction between cis-regulatory sequences and the chromatin remodeling machinery might lead to control the progressive availability of Hox genes loci for transcription.
The temporal colinearity also involves a temporal regulation of transcription by global enhancers. In vertebrates, temporal and spatial transcriptional regulation of Hoxd genes in the early developing mouse limb was shown to depend on interactions between two opposite global regulatory regions located on both sides of the Hoxd cluster (Deschamps, 2007). Analysis of an inversion and a series of internal deletions in the Hoxd cluster showed that the posterior genes, now positioned more 3′ in the cluster due to the deletion, were activated earlier in the limb bud (Tarchini and Duboule, 2006; Zakany et al., 2004). This work identified an enhancer located in the 3′ region upstream of the cluster called Early Limb Control Region (ELCR). The ELCR controls progressive activation of genes in a 3′-to-5′ sequence, while a repressor element in the 5′ region controls the spatial restriction of Hox expression in the limb bud. Since the lateral plate mesoderm that forms the limb bud mesenchyme in which the ELCR is acting is derived from the epiblast, the colinear expression of Hoxd genes reported during the early phase of limb bud development likely reflects the earlier temporal colinearity observed in the mesodermal precursors in the epiblast described above. Examination of the same deletion mutants at an earlier stage should reveal whether Hox genes that are precociously activated in the limb bud are also precociously activated in the epiblast. This would indicate whether the ELCR enhancer is also operating at the trunk level to control temporal Hox activation in the epiblast. These observations suggest that the initial transcriptional availability of Hox genes depends both on their position within the cluster and on specific regulatory elements located outside of the cluster.
Converting temporal into spatial colinearity
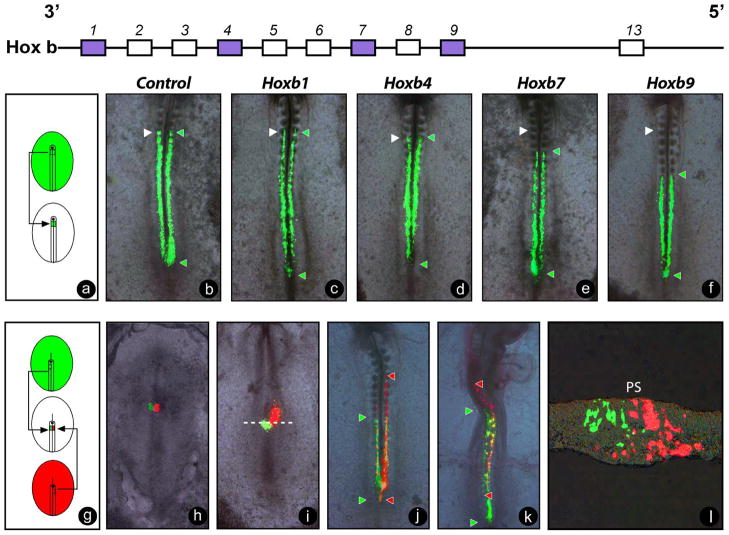
In vertebrates, the expression dynamics of Hox genes described above follows the gastrulation movements of cells from the epiblast to the mesoderm, suggesting that Hox genes could play a role in mesoderm formation through gastrulation. This hypothesis was tested in chicken embryos by electroporation of Hox-expressing constructs containing a fluorescent reporter in epiblast cells (Iimura and Pourquie, 2006). In this way, the fate of the descendants of Hox-overexpressing cells can be easily tracked in the embryo. Overexpression of Hoxb genes in the paraxial mesoderm territory in the epiblast at the gastrula stage resulted in colinear phenotypes in terms of AP distribution of Hox-expressing cells (Figure 4a–f). In other words, cells overexpressing more 3′ Hox genes contributed to more anterior portions of the somitic columns; whereas, cells overexpressing more 5′ Hox genes contributed to more caudal domains. When posterior Hox genes, such as Hoxb7 or Hoxb9, were expressed prior to their normal activation, the Hox-expressing cells retained their epithelial structure and remained in the epiblast longer than cells expressing either control GFP or anterior Hox genes, such as Hoxb1 and Hoxb4 (Figure 4g–l). A deletion mutant of the third helix of the Hoxb9 homeo-domain did not show this delay effect on epiblast ingression, suggesting that DNA binding is essential for this function. Similar results were observed in Xenopus, when the B1 blastomere was injected with Hoxb9 (XlhBox6) at the 32-cell stage (Niehrs and De Robertis, 1991). Involution of the expressing mesodermal cells appeared delayed compared to control cells. The injected cells changed their fate and became incorporated into more posterior territories in the embryo. Therefore, the different Hox genes appear to exert a graded (colinear) control over the properties involved in epiblast cell ingression, such as adhesion of epithelial cells and epithelium-to-mesenchyme transition. Consistently, in the developing limb bud mesenchyme, Hox genes elicit changes in homophilic cell-adhesion properties, possibly through controlling cell adhesion molecules, such as EphA7-receptor (Salsi and Zappavigna, 2006; Stadler et al., 2001; Yokouchi et al., 1995). Strikingly, a similar role for C. Elegans Hox genes in the control of cell migration has been demonstrated (Kenyon et al., 1997). In the worm, the QL neuroblast, which is located on the left side of the embryo, expresses the mab-5 Hox gene (AntP-AbdA homolog) and migrates posteriorly; whereas, the QR neuroblast located on the right side expresses the lin-39 (Pb-Scr homolog) and migrates anteriorly.
Figure 4. Hox genes control the timing of ingression of epiblast cells into the primitive streak.
(a) Schematic representation of the homotopic and homochronic grafts of fragments of the 80% level of the primitive streak electroporated with Hox-expressing constructs. (b–f) Contributions of the electroporated somitic precursors expressing control (b) or Hoxb1 (c), Hoxb4 (d), Hoxb7 (e), and Hoxb9. (f) Expression vectors driven by a ubiquitous CAGGS promoter with IRES2-ZsGreen were observed following a reincubation period of 16h. White arrowheads denote the 5th somite level, and green arrowheads denote the anterior limit of the clones overexpressing the constructs. Note the colinear distribution of the anterior limit of the green clones shifting more posteriorly as progressively more 5′ Hox genes are overexpressed.
(g) Schematic representation of homotopic and homochronic double graft of fragments of the 80% level of the primitive streak from embryos electroporated with Hoxb4-IRES2-DsRed and Hoxb9-IRES2-ZsGreen, respectively. The grafted embryo is shown just before reincubation (h), and 6h (i), 16h (j) and 40h (k) after reincubation. Green and red arrowheads denote the anterior and posterior extension of the descendants of the grafted labeled cells by each reporter along the AP axis. (h–k). The descendents of the cells expressing Hoxb4 (red) are located more anteriorly than those expressing Hoxb9 (j, k). (l) Transverse section of an embryo grafted as in (g) and incubated for 6h at the level indicated in (i) (white hatched line). Note that the cells expressing Hoxb4 (red) have entered the mesodermal layer; whereas, Hoxb9-expressiong cells (green) remain in the surface epiblast layer. Ventral views, anterior to the top. PS: primitive streak, panels h-k.
These data suggest that the sequential activation of Hox genes controls the timing of cell ingression during gastrulation. This mechanism has been proposed to control the translation of the early temporal colinearity into the colinear positioning of the Hox gene expression domains along the AP axis (Iimura and Pourquie, 2006).
Posterior prevalence is required for the establishment of spatial colinearity
In the model described above, the colinear activation of Hox genes in the epiblast controls the sequential ingression of mesodermal cells in the embryo (Iimura and Pourquie, 2006). Progressively more 5′ paralog genes are successively activated in the paraxial mesoderm territory in the epiblast. Activation of these genes begins in a salt-and-pepper fashion in cells that already express more anterior Hox genes (Figure 3). In the chicken embryo, when 5′ Hox genes are overexpressed in epiblast cells fated to contribute to more anterior territories, these cells alter their migratory properties, remain longer in the epiblast and contribute to more posterior derivatives (Figure 4). Furthermore, when a 3′ and a 5′ Hox gene are co-overexpressed in the same cells in the chicken embryo epiblast, the resulting phenotype is similar to that elicited when only the most-posterior gene is overexpressed (Iimura and Pourquie, 2006). Therefore, in the epiblast, the function of the 5′ Hox genes is dominant over that of the 3′ Hox genes. This well-established property of functional dominance of the posterior Hox genes is termed posterior prevalence in vertebrates (Duboule and Morata, 1994). In the absence of such a mechanism, one might expect that the temporal colinearity of Hox gene expression along the primitive streak would result in mixed populations of cells expressing consecutive Hox paralog genes to enter simultaneously the posterior PSM and to become located at similar AP levels. Thus, the control of Hox genes over epiblast cell ingression might provide a mechanism to ensure a separation of Hox gene expression domains by controlling the serial ingression of cells expressing progressively more 5′ Hox genes. This is particularly important for the segregation of cells in the anterior-most domain of expression of Hox genes, which is the domain where the genes exert their patterning role (Duboule and Morata, 1994). Hence, in this model, the posterior prevalence of Hox genes is required for translating the temporal colinearity of their activation in their spatial colinear expression along the somitic columns.
Posterior prevalence or phenotypic suppression was initially characterized in flies mutant for the Polycomb-gene extra-sex combs (esc) in which all Hox genes are expressed ubiquitously along the AP axis (Struhl, 1983). In these mutants, all segments acquire the identity of the posterior-most A8 segment, which is controlled by the most 5′ Hox gene Abd-B. Also, ubiquitous overexpression of Ubx in the fly embryo overrides the effect of Hox genes expressed more anteriorly, such as Sex comb reduced (Scr) but not that of more 5′ genes such as AntP (Gonzalez-Reyes and Morata, 1990). Such observations led to the idea that posterior Hox genes are functionally dominant over the anterior ones (Duboule and Morata, 1994). In mouse loss-of-function mutants for Hox genes, the actual vertebral phenotypes are usually restricted to a narrow window in the anterior-most expression domain of the mutated genes (Horan et al., 1995a; van den Akker et al., 2001; Wellik and Capecchi, 2003). These mutant mice usually exhibit a normal morphology posteriorly, where more posterior Hox genes are normally expressed but the mutated gene is lacking. In transgenic mice that overexpress Hox genes anterior to their normal domains, vertebrae that ectopically express the transgene undergo posterior homeotic transformations (Kessel et al., 1990; Lufkin et al., 1992). Thus, only the posterior-most Hox genes expressed in a somite play a functional role in specifying its future vertebral identity.
In vertebrates, the ancestral Hox gene cluster has been repeatedly duplicated, resulting in seven sets of Hox clusters in zebrafish and four sets in birds and mammals (Duboule, 2007). These duplications generated groups of paralog genes that share high similarities in sequence, expression pattern and function. Paralog swapping experiments in the mouse using a knock-in strategy, in which Hoxa3 was substituted by Hoxd3, or Hoxa1 by Hoxb1, show that these paralog genes carry out similar, although not fully identical functions (Greer et al., 2000; Tvrdik and Capecchi, 2006). Combinations of Hox4 paralog mutants show a dose-dependent increase in the number of affected vertebrae in mutants lacking more paralogs (Horan et al., 1995b). Furthermore, null mutants for all paralog genes in a group show a much more severe phenotype than mutants retaining one single, wild-type allele (McIntyre et al., 2007; van den Akker et al., 2001; Wellik and Capecchi, 2003).
Whereas the posterior region is normal in mouse compound mutants for complete paralog groups as predicted by posterior prevalence, the posterior part of the affected domain overlaps with the functional domain of the next paralog group (McIntyre et al., 2007; Wellik, 2007). Thus, the axial identity of a vertebra is not strictly controlled by the posterior-most Hox genes expressed in its precursors but requires input from adjacent paralogs. Therefore, a combination of adjacent paralog Hox genes is required for the specification of vertebral identities. Thus, posterior prevalence probably reflects a graded trend of functional dominance of posterior-over-anterior Hox genes, rather than an absolute functional suppression of Hox genes by genes located more 5′ in the clusters.
Experiments in the chicken embryo further support this idea. Following premature expression of a given Hoxb gene in the epiblast, cells do not end up exactly in the normal expression domain of this gene as one might have predicted in a strict interpretation of posterior prevalence (Iimura and Pourquie, 2006). For example, precocious expression of Hoxb9 in the epiblast leads the expressing cells to delay their ingression and become positioned at least five to six somites more posteriorly than control cells electroporated with a GFP vector (Figure 4). However, the anterior-most location of Hoxb9 overexpressing cells does not correspond to the normal boundary of Hoxb9 in the somites, which is located at the level of somites 22 to 23. In fact, cells electroporated with Hoxb9 in the epiblast before its normal activation, end up being located posterior to somite 11, hence, much more anterior than their endogenous expression domain. Varying the amount of a given Hox gene overexpressed in the epiblast by using different promoters does not alter the definitive positioning of the overexpressing cells along the AP axis. Thus, posterior prevalence appears to rely on qualitative rather than quantitative differences between Hoxgenes (Iimura and Pourquie, 2006).
The molecular mechanism underlying posterior prevalence remains unclear. Hox gene expression domains largely overlap along the body axis, indicating that posterior genes do not repress transcription of anterior genes. miRNA-mediated, post-transcriptional regulation of Hox gene expression has recently been proposed to play a role in the control of posterior prevalence (Yekta et al., 2008). Three miRNAs – miR-196a-1, miR-196a-2 and miR-196b – can direct cleavage of Hoxb8 mRNA in vitro through their complementary sequence to this gene (Yekta et al., 2004). Hoxb, Hoxc and Hoxa clusters, respectively, encode these miRNAs between Hox9 and Hox10 genes, at a very similar position to the miRNA mir-iab4 in the Drosophila HOM complex, which lies between AbdA and AbdB genes (Ronshaugen et al., 2005). These miRNAs are extensively conserved among vertebrates and potentially bind to the 3′UTR sequence of Hoxb8, Hoxc8, Hoxd8 and Hoxa7 (Yekta et al., 2008). These miRNAs were shown to be involved in targeting Hoxb8 3′UTR during posterior limb bud patterning, thus providing a safeguard mechanism against inappropriate Hox activity (Hornstein et al., 2005). Target sequences for these miRNAs are enriched in Hox genes predominantly located more 3′ than miR-196, consistent with their potential role in posterior prevalence (Ronshaugen et al., 2005; Yekta et al., 2008). Two other miRNA – miR10a and miR10b – were identified 5′ to the group 4 genes in the Hoxb and Hoxd complexes (Woltering and Durston, 2008). Blocking their function leads to extensive vertebral defects, including homeotic transformation.
Post-transcriptional mechanisms other than miRNAs are also likely to be involved in the control of posterior prevalence. Gain-of-function experiments in which Hox genes coding sequences were over-expressed from ubiquitous promoters in transgenic flies or mice show specific defects only in the region anterior to the normal expression domain of these genes (Gonzalez-Reyes and Morata, 1990; Kessel et al., 1990; Lufkin et al., 1992). Also, in the over-expression experiments in the chicken epiblast, the Hox expression constructs only contain the coding sequence driven by a ubiquitous chicken β-actin promoter (Iimura and Pourquie, 2006). Thus, in these experiments, the functional suppression is neither acting transcriptionally nor through the 3′UTR of Hox genes. This raises the possibility that the suppression of the anterior gene function by posterior genes occurs at the protein level.
Spatial dissociation of segmentation and Hox gene activation programs
The origin of the paraxial mesoderm during gastrulation can be traced to two distinct populations of precursors (Figure 2b, c) (Bellairs, 1986; Iimura et al., 2007; Nicolet, 1970; Rosenquist, 1966; Selleck and Stern, 1991). These two populations exhibit different origins and fates, and contribute to the future medial and lateral somitic compartments (Figure 2e). The medial compartment gives rise to the epaxial muscles, the vertebral column and the dermis of the back; whereas, the lateral compartment produces essentially the ribs and the hypaxial muscles that include intercostals and limb muscles (Olivera-Martinez et al., 2000; Ordahl and Le Douarin, 1992) (Figure 2e, f).
In the chicken embryo, medial somites derive from a population of precursors that exhibit a stem-cell-like behavior and are located in the anterior-most primitive streak and Hensen’s node, while the lateral somite precursors derive from the epiblast adjacent to the anterior primitive streak that continues to ingress during axis formation (Bellairs, 1986; Iimura et al., 2007; Selleck and Stern, 1991). A stem-cell-like population, located in the primitive streak and contributing to somites, has also been identified in the mouse (Cambray and Wilson, 2002; Eloy-Trinquet and Nicolas, 2002; McGrew et al., 2008; Nicolas et al., 1996). The self-renewal capacity of these stem cells has been revealed by lineage analysis using various cell-labeling strategies and by serial transplants in chicken and mouse embryos (Cambray and Wilson, 2002; Cambray and Wilson, 2007; McGrew et al., 2008; Nicolas et al., 1996; Selleck and Stern, 1991). This dual origin of paraxial mesoderm precursors is likely conserved across vertebrates (Iimura et al., 2007).
The distinction between medial and lateral precursors is important because the two populations are involved in different aspects of spine patterning. Microsurgically separating the medial from the lateral PSM results in a segmented medial PSM, while the lateral one remains unsegmented (Freitas et al., 2001). Furthermore, transplantation of the region containing the anterior primitive streak to different regions of the primitive streak of a stage-matched host shows that the descendents of the grafted cells form ectopic rows of somites, whereas, no such effect is observed with other epiblast regions (Schoenwolf, 1992). These lines of evidence suggest that the segmentation program is controlled by the medial cells precursors, which eventually give rise to most of the segmental derivatives of the body axis
The dynamics of the posterior-to-anterior waves of activation of Hox genes along the epiblast described earlier imply that the territory fated to give rise to the lateral portion of the somite in the epiblast first activates expression of Hox genes. Due to forward spreading, the stem-cell-containing territory located in the anterior streak will be reached by the Hox expression wave only later. Therefore, Hox activation (initiated in the posterior primitive streak by temporal colinearity) and segmentation (initiated in the anterior primitive streak) are spatially segregated.
A conserved feature of the tetrapod axis is the order of morphologically distinct regions along the AP axis level (e.g., cervical, thoracic, lumbar, sacral and caudal vertebrae). Yet, different numbers of segments contribute to various regions, resulting in the diversity of vertebral formulae between vertebrate species. For instance, mice have 14 rib-bearing thoracic vertebrae, while chickens have seven and corn snakes have 225. Mutation of an entire paralog series of Hox genes in the mouse does not affect the total number of segments in the mutants, suggesting that Hox genes are not directly involved in control of the total segment number (Wellik, 2007). While Hox gene expression has not been investigated in detail in snakes, the large expansion of the thoracic and caudal regions observed (225 thoracic and 65 caudal vertebrae) was shown to be associated with an acceleration of the segmentation clock relative to the axis growth (Gomez et al., 2008). This acceleration was proposed to lead to the formation of smaller segments, hence allowing the formation of more segments for the same amount of growth compared to mouse and chicken embryos. Such an uncoupling between the processes of segmentation and axial regionalization, is consistent with their spatial dissociation. It further suggests that their control is likely to be largely independent, hence allowing the huge diversity of vertebral formulae observed across vertebrates.
Definitive positioning of Hox gene boundaries in the somites
The precise timing of Hox gene activation serves a critical function in defining the final spatial colinear distribution of Hox gene expression domains along the body axis, which ultimately determines axial identity of the different vertebrae (Forlani et al., 2003; Juan and Ruddle, 2003; Wacker et al., 2004a). Importantly, however, this initial blueprint does not entirely match the definitive Hox gene expression (Forlani et al., 2003). For instance, in the mouse embryo, Hoxb8 expression is initiated at the head-fold stage (day 7.5 dpc) in cells located in the anterior primitive streak (Forlani et al., 2003) and the most anterior localization of descendents of these cells is found to be mainly at the level of somites 6/7(Forlani et al., 2003). However, the final expression boundary of Hoxb8 will be more posterior, at the level of somites 10/11. Thus, the position of the definitive Hox gene expression boundaries is not solely imposed by the colinear initiation phase, but in addition, requires modification of Hox gene expression domains after cells exit the primitive streak and before they are incorporated into a somite, hence, while located in the PSM.
Cells of the posterior PSM experience transcriptional oscillations of the cyclic genes that are driven by the segmentation clock (Figure 5a) (Dequeant et al., 2008). Signaling components, including many negative feedback inhibitors of the Notch, Wnt and Fgf pathways, are involved in the segmentation clock and have been proposed to control the periodic activation of the system. The segmentation clock oscillator generates a complex periodic pulse of signaling in the PSM, which was proposed to set the rhythm of somite production. Molecular evidence for the existence of such an oscillator has now been obtained in the chicken, mouse, frog and zebrafish, suggesting that it is a conserved feature of vertebrates ((Dequeant et al., 2008) and references therein). Posterior-to-anterior gradients of Fgf and Wnt signaling within the PSM were shown to define a signaling threshold where PSM cells become competent to respond to this periodic clock signal. Due to axis elongation, this threshold (termed the determination front or wavefront) constantly regresses posteriorly while maintaining its relative position in the PSM at the same level. This displacement controls the spacing of the clock response along the AP axis and the conversion of the clock pulse into the spatial periodicity of somites (Dequeant et al., 2008). Therefore, the size of a somite is determined by the distance traveled by the wavefront during one period of the segmentation clock oscillation. Reciprocal inhibitory interactions between RA produced by the anterior PSM and FGF produced by the posterior PSM provide a fine-tuning mechanism involved in refining the positioning of the determination front (Diez del Corral et al., 2003; Moreno and Kintner, 2004). In addition to the important function of controlling differentiation and defining the somite-forming unit, the gradient system of Wnt/Fgf and RA signaling is also involved in the control of Hox gene expression in the PSM.
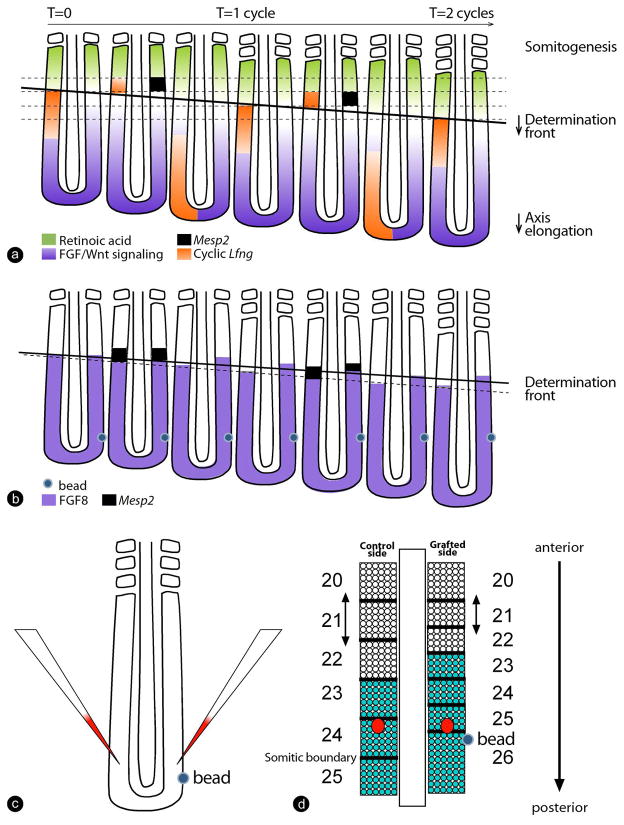
Figure 5. Interaction between Hox patterning and the segmentation machinery.
(a) The Clock and Wavefront model for somitic segment determination.
Antagonistic gradients of FGF/Wnt signaling (purple) and retinoic acid signaling (green) position the determination front (thick, black line). The periodic wave of cyclic gene expression reflecting the segmentation clock signal is shown in orange (represented on the left side only). As the embryo extends posteriorly, the determination front moves caudally. Cells that pass the determination front are exposed to the periodic clock signal, initiating the segmentation program and activating expression of gened, such as Mesp2 (black squares, represented on the right side only), in a stripe that prefigures the future segment. This establishes the segmental pattern of the presumptive somites.
(b) The graft of an Fgf8 bead in the posterior part of the embryo leads to an anterior extension of the FGF gradient (purple), corresponding to a slowing down of the determination front regression. As a result, less competent cells pass the determination front during one oscillation of the clock, leading to a smaller segment. Ultimately, this segment will form a smaller somite compared to the control side (shown in d).
(c,d) Labeling cells at the same axial position with DiI in embryos grafted with a Fgf8 bead (c) shows that cells remain at the same axial position but that the position of the somite boundaries is changed on the grafted side due to the formation of smaller somites (Dubrulle et al., 2001). (d) As a result, cells from the same axial level become incorporated into differently numbered somites. Strikingly, the expression of Hox genes (here, Hoxb9, green) is maintained at the appropriate somitic boundary rather than at the appropriate axial level, providing evidence for a cross talk between the segmentation machinery and Hox patterning. Red spots show the DiI labeled cells marked in c. Dorsal views, anterior to the top.
Local modification of the FGF signaling gradient by grafting a bead of Fgf8 in the posterior PSM was shown to result in the formation of smaller somites anterior to the bead (Dubrulle et al., 2001) (Figure 5b). This effect was interpreted as a slowing down of the determination front progression (due to the local FGF concentration increase). As a result, less competent cells are released at the front level during one period of the clock oscillation, hence, resulting in smaller somite formation (Figure 5b). Remarkably, the position of the Hoxb9 and Hoxa10 anterior boundary at the level of these smaller somites become located more anteriorly on the grafted side, remaining at the appropriate somitic level (Alvares et al., 2003; Dubrulle et al., 2001). In other words, the Hox gene expression boundary remains located at the appropriate somite number (somite 23 for Hoxb9) rather than at the absolute axial position (as defined by the position of the Hox boundary on the unoperated contralateral side) (Figure 5c, d). Cells that ectopically alter their Hox expression in response to the bead graft also alter their axial properties. For instance, in response to ectopic Hoxa10 expression triggered by the Fgf8 bead graft, cells begin to activate the muscle migratory program downstream of Lbx1 (Alvares et al., 2003; Dubrulle et al., 2001). Thus, in these experiments, a gain of function of FGF signaling in the posterior PSM results in a posterior transformation of cells in the smaller somites. Therefore, Hox gene expression in the PSM remains plastic and can still be altered by manipulating the segmentation machinery (Dubrulle et al., 2001; Zakany et al., 2001). Additional evidence indicates that Notch signaling, which shows oscillating activity in the posterior PSM (Huppert et al., 2005; Morimoto et al., 2005), likewise is involved in the control of Hox gene expression in the PSM (Cordes et al., 2004).
Various perturbations of FGF and RA signaling have been shown to cause changes in Hox gene expression and homeotic transformations in mouse embryos. The effect of gain- or loss-of-function mutations of the FGF pathway on Hox gene expression is consistent with the results of the FGF bead graft experiments described above. For instance, Fgfr1 gain-of-function mutations introduced into the mouse embryo led to posterior homeotic transformation; whereas, hypomorphic mutations led to anterior transformation (Partanen et al., 1998). FGF signaling and RA signaling were shown to exert mutually antagonistic actions in the PSM (Diez del Corral et al., 2003; Moreno and Kintner, 2004; Vermot and Pourquie, 2005). Hence, a RA gain of function leads to an FGF loss of function in the PSM. The modulation of RA signaling levels in developing mouse embryos causes homeotic transformations which, depending on the time of treatment, causes vertebrae to acquire either more anterior or more posterior identities (Kessel and Gruss, 1991). RA treatment of mice at 8.5 dpc causes a posterior shift of 5′ Hox genes, consistent with the anterior homeotic transformations observed in the FGF hypomorphic mutants. In contrast, the same experiment at gastrulation stage (7.0 dpc) induces an anterior shift of 3′ Hox genes (Kessel and Gruss, 1991), consistent with their ability to precociously activate Hoxb1 in the epiblast (Marshall et al., 1992). These important functions of RA signaling in specifying vertebral axial identity and the control of Hox genes were also confirmed through genetic deletion of RA signaling components (Lohnes et al., 1994).
Finally, Wnt signaling has also been connected directly to the process of axial specification. In Wnt3a mutant mouse embryos (Ikeya and Takada, 2001) or in Wnt3a hypomorphic embryos vestigial tail (vt), homeotic transformations and corresponding changes in Hox gene expression are observed (Greco et al., 1996). In these mutants, Fgf8 expression is downregulated in the PSM (Aulehla et al., 2003), suggesting that the effect on Hox gene expression could result from the downregulation of FGF signaling in the PSM. The caudal transcription factors (Cdx) are targets of the Wnt, FGF and RA pathways, which play a conserved role in Hox regulation during AP axis formation (Chawengsaksophak et al., 2004; Copf et al., 2004; Houle et al., 2003a; Pilon et al., 2007; van den Akker et al., 2002). In mice, Cdx gene expression (Cdx1, 2 and 4) is initiated at the primitive streak stage and later, is restricted to the posterior part of the embryo (Bel-Vialar et al., 2002; Deschamps and van Nes, 2005; Houle et al., 2000; Houle et al., 2003b; Ikeya and Takada, 2001; Isaacs et al., 1994; Lohnes, 2003; Pilon et al., 2006; Pilon et al., 2007; Pownall et al., 1996; Prinos et al., 2001). Cdx transcription factors act by regulating a subset of Hox genes in a dose-dependent manner (van den Akker et al., 2002). Together, these data suggest that the FGF, Wnt and RA pathways control the expression of Hox gene domains in the PSM.
Several other factors involved in the regulation of Hox genes and in the patterning of the axial skeleton have been identified. In the mouse, the TGF-β family secreted factor, Gdf11 (which is expressed in the tail bud) acts upstream of Hox genes. Mice mutant for Gdf11 show homeotic transformation of the vertebral column and tail truncation, correlating with disruption of Hox expression domains (McPherron et al., 1999; Nakashima et al., 1999). These patterning defects are partially phenocopied in mice mutant for the Gdf11 pro-protein convertase Pcsk5, specifically expressed in the PSM, as well as in mutants for the TGF-β type I receptor (Alk5) and type II receptors (ActRIIA/B [Acvr2/b]) (Andersson et al., 2006; Essalmani et al., 2008; Oh and Li, 1997; Oh et al., 2002; Rancourt and Rancourt, 1997; Szumska et al., 2008). How and if these genes interact with the pathways described above remain to be investigated.
Positioning of Hox gene boundaries in the forming segments
After passing through the determination front, competent cells that receive the clock signal simultaneously activate the transcription factor Mesp2, which defines the future segmental domain and positions the future somitic boundary (Figure 5a, black) (Dequeant et al., 2008; Morimoto et al., 2005). Based on mathematical modeling, it was proposed that the system of opposing FGF and RA gradients defines a bistability window in the PSM, where cells can adopt either of two distinct steady states: FGF-dominant or RA-dominant (Goldbeter et al., 2007). Cells in the FGF-dominant state can abruptly switch to the RA-dominant stable state, enabling a group of cells that will form the future segment to be exposed simultaneously to RA signaling. This switch was proposed to be triggered by the periodic signaling pulse delivered by the segmentation clock.
The resulting segmental prepattern, which appears as bilateral stripes of Mesp2 located immediately anterior to the determination front, provides the blueprint for the morphological segments. The morphological boundaries form when the epithelial somite separates from the anterior tip of the PSM, and subsequently, somitic tissue begins to differentiate as directed by the surrounding tissues (Hirsinger et al., 2000). During this step, somites subdivide into the ventral sclerotome (which contains the skeletal precursors) and the dorsal dermomyotome (which contributes to the myotome and dermatome, giving rise to the skeletal muscles of the body and the dorsal dermis, respectively).
Mesp2 also plays a key role in establishing the rostrocaudal polarity of future somites (Takahashi et al., 2000). This rostrocaudal subdivision of somites imposes the segmental patterning of the nervous system by restricting migration of neural crest cells and axonal guidance of motor neurons within the rostral half of the somite (Bronner-Fraser, 2000). This rostrocaudal subdivision is also critical for the formation of vertebrae, which develop when the caudal half of the skeletal precursors (the sclerotome) of one somite fuses to the rostral half of the sclerotome of the following somite during a process called resegmentation (Christ et al., 2007).
The spatial regulation of Hoxd genes in the anterior PSM is controlled by the segmentation machinery (Zakany et al., 2001). Replacing the entire Hoxd cluster with a LacZ reporter showed periodic stripes of gene expression in the anterior PSM, suggesting that a global enhancer outside of the cluster is responsible for the segmental expression in the PSM. Strikingly, Hoxd1 is expressed in the same striped domain as Mesp2 but slightly later in time, suggesting that Hoxd1 activation might lie downstream of Mesp2. Subsequently, Hoxd1 expression, like Mesp2, becomes restricted to the anterior compartment of the forming somite. A similar expression pattern is observed for other Hox genes (e.g., Hoxd3). This regulatory expression is under the influence of the Notch pathway, since the striped expression is lost in the mouse Rbpjk-null mutants in which Notch signaling is abolished. The role of Hox genes during later stages of somite patterning remains to be investigated.
Conclusion: Determination of the axial fate of vertebral precursors
Due to posterior prevalence, only the anterior expression domain of Hox genes appears to be functionally relevant for patterning the vertebral column. Thus, the positioning of the anterior boundaries of Hox expression domains is of key importance for the establishment of axial identity along the vertebrate spine. Data discussed in this review indicate that the positioning of Hox anterior boundaries is essentially defined by the timing of their activation during gastrulation. The importance of the precise temporal activation of Hox genes is illustrated by mutations that change the timing of Hox activation in mouse embryos (Juan and Ruddle, 2003). Mutation of the cis-regulatory region controlling Hoxc8 expression, which causes an initial delay in expression, affects skeletal patterning by largely phenocopying the Hoxc8-null mutant, even if the normal somitic expression appeared to be recovered at later stages (Juan and Ruddle, 2003).
Grafting experiments in the chicken embryo demonstrated that Hox expression and axial identity of vertebrae are already fixed in the PSM (Kieny et al., 1972; Nowicki and Burke, 2000). Furthermore, overexpression of Hox genes driven by a PSM-specific promoter in transgenic mice leads to severe skeletal patterning defects; whereas, overexpression from a promoter driving expression in somites does not elicit such phenotypes (Carapuco et al., 2005). Thus, the appropriate expression of Hox genes early in the development of paraxial mesoderm is critical for the establishment of the future vertebral identity.
In fact, Hox expression appears to be fixed extremely early in the paraxial mesoderm precursors soon after their initial activation at the epiblast stage (Iimura and Pourquie, 2006). Heterochronic transplantation of epiblast cells in the chicken embryo showed that groups of transplanted cells maintain their endogenous Hox expression schedule. Strikingly, however, in grafts of very small fragments that contain the paraxial mesoderm precursors or in isolated cells that detach from the graft, the original Hox gene expression is not maintained, and cells adapt to their new location ((McGrew et al., 2008), OP and TI unpublished observations). Such a situation is also observed in the nervous system (Trainor and Krumlauf, 2000), suggesting that whereas the axial fate appears to be determined very early at the tissue level, small group of cells can be reprogrammed.
Together, theses experiments suggest that the axial identity of paraxial mesoderm precursors is sealed very early in the differentiation of this lineage, possibly even before their ingression during gastrulation, in response to Hox gene expression. Therefore, although the position of the anterior boundaries of Hox expression domains somehow becomes subsequently refined in the PSM resulting in their definitive positioning along the AP axis, the timing of Hox gene activation during gastrulation largely controls vertebral identity.
Acknowledgments
The current work was supported by the Stowers Institute for Medical Research and NIH grant R01 HD043158 to OP, and by the grant from the Japanese Ministry of Education, Global Center of Excellence (GCOE) Program, “International Research Center for Molecular Science in Tooth and Bone Diseases” to TI. We thank Pourquié lab members for critical reading of the manuscript and S. Esteban for artwork. OP is a Howard Hughes Medical Institute Investigator.
References
- Alvares LE, Schubert FR, Thorpe C, Mootoosamy RC, Cheng L, Parkyn G, Lumsden A, Dietrich S. Intrinsic, Hox-dependent cues determine the fate of skeletal muscle precursors. Dev Cell. 2003;5:379–90. doi: 10.1016/s1534-5807(03)00263-6. [DOI] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Ibanez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7:831–7. doi: 10.1038/sj.embor.7400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC. Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev Biol. 2005;283:409–23. doi: 10.1016/j.ydbio.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S, Itasaki N, Krumlauf R. Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development. 2002;129:5103–15. doi: 10.1242/dev.129.22.5103. [DOI] [PubMed] [Google Scholar]
- Bellairs R. The primitive streak. Anat Embryol(Berl) 1986;174:1–14. doi: 10.1007/BF00318331. [DOI] [PubMed] [Google Scholar]
- Bolognesi R, Farzana L, Fischer TD, Brown SJ. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol. 2008;18:1624–9. doi: 10.1016/j.cub.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortier H, Vakaet LC. Fate mapping the neural plate and the intraembryonic mesoblast in the upper layer of the chicken blastoderm with xenografting and time-lapse videography. Dev Suppl. 1992:93–7. [PubMed] [Google Scholar]
- Bronner-Fraser M. Rostrocaudal differences within the somites confer segmental pattern to trunk neural crest migration. Curr Top Dev Biol. 2000;47:279–96. doi: 10.1016/s0070-2153(08)60728-0. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–46. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129:4855–66. doi: 10.1242/dev.129.20.4855. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–40. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- Carapuco M, Novoa A, Bobola N, Mallo M. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005;19:2116–21. doi: 10.1101/gad.338705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala M, Teillet MA, Le Douarin NM. Organization and development of the tail bud analyzed with the quail- chick chimaera system. Mech Dev. 1995;51:51–65. doi: 10.1016/0925-4773(95)00350-a. [DOI] [PubMed] [Google Scholar]
- Chambeyron S, Bickmore WA. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004;18:1119–30. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambeyron S, Da Silva NR, Lawson KA, Bickmore WA. Nuclear re-organisation of the Hoxb complex during mouse embryonic development. Development. 2005;132:2215–23. doi: 10.1242/dev.01813. [DOI] [PubMed] [Google Scholar]
- Charrier JB, Teillet MA, Lapointe F, Le Douarin NM. Defining subregions of Hensen’s node essential for caudalward movement, midline development and cell survival. Development. 1999;126:4771–83. doi: 10.1242/dev.126.21.4771. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A. 2004;101:7641–5. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman AD, Akam M. The segmentation cascade in the centipede Strigamia maritima: involvement of the Notch pathway and pair-rule gene homologues. Dev Biol. 2008;319:160–9. doi: 10.1016/j.ydbio.2008.02.038. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M. Amniote somite derivatives. Dev Dyn. 2007 doi: 10.1002/dvdy.21189. [DOI] [PubMed] [Google Scholar]
- Copf T, Schroder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci U S A. 2004;101:17711–5. doi: 10.1073/pnas.0407327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes R, Schuster-Gossler K, Serth K, Gossler A. Specification of vertebral identity is coupled to Notch signalling and the segmentation clock. Development. 2004;131:1221–33. doi: 10.1242/dev.01030. [DOI] [PubMed] [Google Scholar]
- Couly G, Coltey P, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Dev Suppl. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Davis RL, Kirschner MW. The fate of cells in the tailbud of Xenopus laevis. Development. 2000;127:255–67. doi: 10.1242/dev.127.2.255. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K, Boore J, Branno M, Chin-Bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee BI, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shin-i T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–67. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Dequeant ML, Ahnert S, Edelsbrunner H, Fink TM, Glynn EF, Hattem G, Kudlicki A, Mileyko Y, Morton J, Mushegian AR, Pachter L, Rowicka M, Shiu A, Sturmfels B, Pourquie O. Comparison of pattern detection methods in microarray time series of the segmentation clock. PLoS ONE. 2008;3:e2856. doi: 10.1371/journal.pone.0002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J. Ancestral and recently recruited global control of the Hox genes in development. Curr Opin Genet Dev. 2007;17:422–7. doi: 10.1016/j.gde.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Deschamps J, van den Akker E, Forlani S, De Graaff W, Oosterveen T, Roelen B, Roelfsema J. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol. 1999;43:635–50. [PubMed] [Google Scholar]
- Deschamps J, van Nes J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development. 2005;132:2931–42. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- Deschamps J, Wijgerde M. Two phases in the establishment of HOX expression domains. Dev Biol. 1993;156:473–80. doi: 10.1006/dbio.1993.1093. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dolle P, Izpisua-Belmonte JC, Falkenstein H, Renucci A, Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989;342:767–72. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Duboule D. Vertebrate hox gene regulation: clustering and/or colinearity? Curr Opin Genet Dev. 1998;8:514–8. doi: 10.1016/s0959-437x(98)80004-x. [DOI] [PubMed] [Google Scholar]
- Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–60. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- Duboule D, Morata G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994;10:358–64. doi: 10.1016/0168-9525(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–32. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Eloy-Trinquet S, Nicolas JF. Cell coherence during production of the presomitic mesoderm and somitogenesis in the mouse embryo. Development. 2002;129:3609–19. doi: 10.1242/dev.129.15.3609. [DOI] [PubMed] [Google Scholar]
- Essalmani R, Zaid A, Marcinkiewicz J, Chamberland A, Pasquato A, Seidah NG, Prat A. In vivo functions of the proprotein convertase PC5/6 during mouse development: Gdf11 is a likely substrate. Proc Natl Acad Sci U S A. 2008;105:5750–5. doi: 10.1073/pnas.0709428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–19. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Freitas C, Rodrigues S, Charrier JB, Teillet MA, Palmeirim I. Evidence for medial/lateral specification and positional information within the presomitic mesoderm. Development. 2001;128:5139–47. doi: 10.1242/dev.128.24.5139. [DOI] [PubMed] [Google Scholar]
- Frohman MA, Boyle M, Martin GR. Isolation of the mouse Hox-2.9 gene; analysis of embryonic expression suggests that positional information along the anterior-posterior axis is specified by mesoderm. Development. 1990;110:589–607. doi: 10.1242/dev.110.2.589. [DOI] [PubMed] [Google Scholar]
- Gaunt SJ, Miller JR, Powell DJ, Duboule D. Homoeobox gene expression in mouse embryos varies with position by the primitive streak stage. Nature. 1986;324:662–4. doi: 10.1038/324662a0. [DOI] [PubMed] [Google Scholar]
- Gaunt SJ, Sharpe PT, Duboule D. Spatially restricted domains of homeo-gene transcripts in mouse embryos: relation to a segmented body plan. Development. 1988;104:169–179. [Google Scholar]
- Gaunt SJ, Strachan L. Forward spreading in the establishment of a vertebrate Hox expression boundary: the expression domain separates into anterior and posterior zones, and the spread occurs across implanted glass barriers. Dev Dyn. 1994;199:229–40. doi: 10.1002/aja.1001990307. [DOI] [PubMed] [Google Scholar]
- Gaunt SJ, Strachan L. Temporal colinearity in expression of anterior Hox genes in developing chick embryos. Dev Dyn. 1996;207:270–80. doi: 10.1002/(SICI)1097-0177(199611)207:3<270::AID-AJA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gerard M, Chen JY, Gronemeyer H, Chambon P, Duboule D, Zakany J. In vivo targeted mutagenesis of a regulatory element required for positioning the Hoxd-11 and Hoxd-10 expression boundaries. Genes Dev. 1996;10:2326–34. doi: 10.1101/gad.10.18.2326. [DOI] [PubMed] [Google Scholar]
- Goldbeter A, Gonze D, Pourquie O. Sharp developmental thresholds defined through bistability by antagonistic gradients of retinoic acid and FGF signaling. Dev Dyn. 2007;236:1495–508. doi: 10.1002/dvdy.21193. [DOI] [PubMed] [Google Scholar]
- Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquie O. Control of segment number in vertebrate embryos. Nature. 2008 doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- Gont LK, Steinbeisser H, Blumberg B, de Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, Morata G. The developmental effect of overexpressing a Ubx product in Drosophila embryos is dependent on its interactions with other homeotic products. Cell. 1990;61:515–22. doi: 10.1016/0092-8674(90)90533-k. [DOI] [PubMed] [Google Scholar]
- Graham A, Papalopulu N, Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–78. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–24. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Greer JM, Puetz J, Thomas KR, Capecchi MR. Maintenance of functional equivalence during paralogous Hox gene evolution. Nature. 2000;403:661–5. doi: 10.1038/35001077. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo (1951) Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hatada Y, Stern CD. A fate map of the epiblast of the early chick embryo. Development. 1994;120:2879–2889. doi: 10.1242/dev.120.10.2879. [DOI] [PubMed] [Google Scholar]
- Hinsch CW, Hamilton HL. The developmental fate of the first somite in the chick embryo. Anatomical Record. 1956;125:225–246. doi: 10.1002/ar.1091250206. [DOI] [PubMed] [Google Scholar]
- Hirsinger E, Jouve C, Dubrulle J, Pourquie O. Somite formation and patterning. Int Rev Cytol. 2000;198:1–65. doi: 10.1016/s0074-7696(00)98002-1. [DOI] [PubMed] [Google Scholar]
- Horan GS, Kovacs EN, Behringer RR, Featherstone MS. Mutations in paralogous Hox genes result in overlapping homeotic transformations of the axial skeleton: evidence for unique and redundant function. Dev Biol. 1995a;169:359–72. doi: 10.1006/dbio.1995.1150. [DOI] [PubMed] [Google Scholar]
- Horan GS, Ramirez-Solis R, Featherstone MS, Wolgemuth DJ, Bradley A, Behringer RR. Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 1995b;9:1667–77. doi: 10.1101/gad.9.13.1667. [DOI] [PubMed] [Google Scholar]
- Hornstein E, Mansfield JH, Yekta S, Hu JK, Harfe BD, McManus MT, Baskerville S, Bartel DP, Tabin CJ. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature. 2005;438:671–4. doi: 10.1038/nature04138. [DOI] [PubMed] [Google Scholar]
- Houle M, Allan D, Lohnes D, Lufkin T. Advances in Developmental Biology and Biochemistry. Vol. 13. Elsevier; 2003a. Cdx homeodomain proteins in vertebral patterning; pp. 69–105. [Google Scholar]
- Houle M, Prinos P, Iulianella A, Bouchard N, Lohnes D. Retinoic acid regulation of Cdx1: an indirect mechanism for retinoids and vertebral specification. Mol Cell Biol. 2000;20:6579–86. doi: 10.1128/mcb.20.17.6579-6586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle M, Sylvestre JR, Lohnes D. Retinoic acid regulates a subset of Cdx1 function in vivo. Development. 2003b;130:6555–67. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Ordahl CP, Christ B. The fate of the first avian somite. Anat Embryol(Berl) 1997;195:435–449. doi: 10.1007/s004290050063. [DOI] [PubMed] [Google Scholar]
- Huppert SS, Ilagan MX, De Strooper B, Kopan R. Analysis of Notch function in presomitic mesoderm suggests a gamma-secretase-independent role for presenilins in somite differentiation. Dev Cell. 2005;8:677–88. doi: 10.1016/j.devcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442:568–71. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- Iimura T, Pourquie O. Hox genes in time and space during vertebrate body formation. Development, growth & differentiation. 2007;49:265–75. doi: 10.1111/j.1440-169X.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Iimura T, Yang X, Weijer CJ, Pourquie O. Dual mode of paraxial mesoderm formation during chick gastrulation. Proc Natl Acad Sci U S A. 2007;104:2744–9. doi: 10.1073/pnas.0610997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. Embo J. 1994;13:4469–81. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisua-Belmonte JC, Falkenstein H, Dolle P, Renucci A, Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. Embo J. 1991;10:2279–89. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan AH, Ruddle FH. Enhancer timing of Hox gene expression: deletion of the endogenous Hoxc8 early enhancer. Development. 2003;130:4823–34. doi: 10.1242/dev.00672. [DOI] [PubMed] [Google Scholar]
- Kanki JP, Ho RK. The development of the posterior body in zebrafish. Development. 1997;124:881–93. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ, Austin J, Costa M, Cowing DW, Harris JM, Honigberg L, Hunter CP, Maloof JN, Muller-Immergluck MM, Salser SJ, Waring DA, Wang BB, Wrischnik LA. The dance of the Hox genes: patterning the anteroposterior body axis of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1997;62:293–305. [PubMed] [Google Scholar]
- Kessel M, Balling R, Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990;61:301–8. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kieny M, Mauger A, Sengel P. Early regionalization of somitic mesoderm as studied by the development of axial skeleton of the chick embryo. Dev Biol. 1972;28:142–161. doi: 10.1016/0012-1606(72)90133-9. [DOI] [PubMed] [Google Scholar]
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–3. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- Kmita M, van Der Hoeven F, Zakany J, Krumlauf R, Duboule D. Mechanisms of Hox gene colinearity: transposition of the anterior Hoxb1 gene into the posterior HoxD complex. Genes Dev. 2000;14:198–211. [PMC free article] [PubMed] [Google Scholar]
- Knezevic V, De Santo R, Mackem S. Continuing organizer function during chick tail development. Development. 1998;125:1791–801. doi: 10.1242/dev.125.10.1791. [DOI] [PubMed] [Google Scholar]
- Kondo T, Duboule D. Breaking colinearity in the mouse HoxD complex. Cell. 1999;97:407–17. doi: 10.1016/s0092-8674(00)80749-7. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Matsuo I, Aizawa S. Developmental patterning and evolution of the mammalian viscerocranium: genetic insights into comparative morphology. Dev Dyn. 1997;209:139–55. doi: 10.1002/(SICI)1097-0177(199706)209:2<139::AID-AJA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Lohnes D. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. Bioessays. 2003;25:971–80. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–48. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Mark M, Hart CP, Dolle P, LeMeur M, Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992;359:835–41. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Marshall H, Nonchev S, Sham MH, Muchamore I, Lumsden A, Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992;360:737–41. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- McGregor AP, Pechmann M, Schwager EE, Feitosa NM, Kruck S, Aranda M, Damen WG. Wnt8 is required for growth-zone establishment and development of opisthosomal segments in a spider. Curr Biol. 2008;18:1619–23. doi: 10.1016/j.cub.2008.08.045. [DOI] [PubMed] [Google Scholar]
- McGrew MJ, Sherman A, Lillico SG, Ellard FM, Radcliffe PA, Gilhooley HJ, Mitrophanous KA, Cambray N, Wilson V, Sang H. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–99. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM. Hox patterning of the vertebrate rib cage. Development. 2007;134:2981–9. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–4. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- Moreno TA, Kintner C. Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev Cell. 2004;6:205–18. doi: 10.1016/s1534-5807(04)00026-7. [DOI] [PubMed] [Google Scholar]
- Morey C, Da Silva NR, Kmita M, Duboule D, Bickmore WA. Ectopic nuclear reorganisation driven by a Hoxb1 transgene transposed into Hoxd. J Cell Sci. 2008;121:571–7. doi: 10.1242/jcs.023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey C, Da Silva NR, Perry P, Bickmore WA. Nuclear reorganisation and chromatin decondensation are conserved, but distinct, mechanisms linked to Hox gene activation. Development. 2007;134:909–19. doi: 10.1242/dev.02779. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Takahashi Y, Endo M, Saga Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature. 2005;435:354–9. doi: 10.1038/nature03591. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFbeta superfamily during mouse embryogenesis. Mech Dev. 1999;80:185–9. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- Nicolas JF, Mathis L, Bonnerot C, Saurin W. Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development. 1996;122:2933–2946. doi: 10.1242/dev.122.9.2933. [DOI] [PubMed] [Google Scholar]
- Nicolet G. Analyse autoradiographique de la localisation des differentes ebauches presomptives dans la ligne primitive de l’embryon de poulet. J Embryol Exp Morph. 1970;23:79–108. [PubMed] [Google Scholar]
- Nicolet G. Avian gastrulation. Adv Morphog. 1971;9:231–62. doi: 10.1016/b978-0-12-028609-6.50010-8. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–8. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niehrs C, De Robertis EM. Ectopic expression of a homeobox gene changes cell fate in Xenopus embryos in a position-specific manner. EMBO J. 1991;10:3621–3629. doi: 10.1002/j.1460-2075.1991.tb04928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. Patterning of avian craniofacial muscles. Dev Biol. 1986;116:347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Nowicki JL, Burke AC. Hox genes and morphological identity: axial versus lateral patterning in the vertebrate mesoderm. Development. 2000;127:4265–75. doi: 10.1242/dev.127.19.4265. [DOI] [PubMed] [Google Scholar]
- Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–26. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002;16:2749–54. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera-Martinez I, Coltey M, Dhouailly D, Pourquie O. Mediolateral somitic origin of ribs and dermis determined by quail-chick chimeras. Development. 2000;127:4611–7. doi: 10.1242/dev.127.21.4611. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Partanen J, Schwartz L, Rossant J. Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes Dev. 1998;12:2332–44. doi: 10.1101/gad.12.15.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteels J. Etudes sur la gastrulation des Vertébrés méroblastiques. III. Oiseaux. IV. Conclusions générales. Arch Biol. 1937;48:381–488. [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, Lohnes D. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Savory JG, Lohnes D. Wnt signaling is a key mediator of Cdx1 expression in vivo. Development. 2007;134:2315–23. doi: 10.1242/dev.001206. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Tam PP. A nomenclature for prospective somites and phases of cyclic gene expression in the presomitic mesoderm. Dev Cell. 2001;1:619–20. doi: 10.1016/s1534-5807(01)00082-x. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Tucker AS, Slack JM, Isaacs HV. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development. 1996;122:3881–92. doi: 10.1242/dev.122.12.3881. [DOI] [PubMed] [Google Scholar]
- Prince V, Lumsden A. Hoxa-2 expression in normal and transposed rhombomeres: independent regulation in the neural tube and neural crest. Development. 1994;120:911–23. doi: 10.1242/dev.120.4.911. [DOI] [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D. Multiple pathways governing Cdx1 expression during murine development. Dev Biol. 2001;239:257–69. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- Psychoyos D, Stern CD. Fates and migratory routes of primitive streak cells in the chick embryo. Development. 1996;122:1523–1534. doi: 10.1242/dev.122.5.1523. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu JK, Benito-Gutierrez EL, Dubchak I, Garcia-Fernandez J, Gibson-Brown JJ, Grigoriev IV, Horton AC, de Jong PJ, Jurka J, Kapitonov VV, Kohara Y, Kuroki Y, Lindquist E, Lucas S, Osoegawa K, Pennacchio LA, Salamov AA, Satou Y, Sauka-Spengler T, Schmutz J, Shin IT, Toyoda A, Bronner-Fraser M, Fujiyama A, Holland LZ, Holland PW, Satoh N, Rokhsar DS. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–71. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- Rancourt SL, Rancourt DE. Murine subtilisin-like proteinase SPC6 is expressed during embryonic implantation, somitogenesis, and skeletal formation. Developmental genetics. 1997;21:75–81. doi: 10.1002/(SICI)1520-6408(1997)21:1<75::AID-DVG9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Beeman RW, Brown SJ, Bucher G, Friedrich M, Grimmelikhuijzen CJ, Klingler M, Lorenzen M, Richards S, Roth S, Schroder R, Tautz D, Zdobnov EM, Muzny D, Gibbs RA, Weinstock GM, Attaway T, Bell S, Buhay CJ, Chandrabose MN, Chavez D, Clerk-Blankenburg KP, Cree A, Dao M, Davis C, Chacko J, Dinh H, Dugan-Rocha S, Fowler G, Garner TT, Garnes J, Gnirke A, Hawes A, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Jackson L, Kovar C, Kowis A, Lee S, Lewis LR, Margolis J, Morgan M, Nazareth LV, Nguyen N, Okwuonu G, Parker D, Richards S, Ruiz SJ, Santibanez J, Savard J, Scherer SE, Schneider B, Sodergren E, Tautz D, Vattahil S, Villasana D, White CS, Wright R, Park Y, Beeman RW, Lord J, Oppert B, Lorenzen M, Brown S, Wang L, Savard J, Tautz D, Richards S, Weinstock G, Gibbs RA, Liu Y, Worley K, Weinstock G, Elsik CG, Reese JT, Elhaik E, Landan G, Graur D, Arensburger P, Atkinson P, Beeman RW, Beidler J, Brown SJ, Demuth JP, Drury DW, Du YZ, Fujiwara H, Lorenzen M, Maselli V, Osanai M, Park Y, Robertson HM, Tu Z, Wang JJ, Wang S, Richards S, Song H, Zhang L, Sodergren E, Werner D, Stanke M, Morgenstern B, Solovyev V, Kosarev P, Brown G, Chen HC, Ermolaeva O, Hlavina W, Kapustin Y, Kiryutin B, Kitts P, Maglott D, Pruitt K, Sapojnikov V, Souvorov A, Mackey AJ, Waterhouse RM, Wyder S, Zdobnov EM, Zdobnov EM, Wyder S, Kriventseva EV, Kadowaki T, Bork P, Aranda M, Bao R, Beermann A, Berns N, Bolognesi R, Bonneton F, Bopp D, Brown SJ, Bucher G, Butts T, Chaumot A, Denell RE, Ferrier DE, Friedrich M, Gordon CM, Jindra M, Klingler M, Lan Q, Lattorff HM, Laudet V, von Levetsow C, Liu Z, Lutz R, Lynch JA, da Fonseca RN, Posnien N, Reuter R, Roth S, Savard J, Schinko JB, Schmitt C, Schoppmeier M, Schroder R, Shippy TD, Simonnet F, Marques-Souza H, Tautz D, Tomoyasu Y, Trauner J, Van der Zee M, Vervoort M, Wittkopp N, Wimmer EA, Yang X, Jones AK, Sattelle DB, Ebert PR, Nelson D, Scott JG, Beeman RW, Muthukrishnan S, Kramer KJ, Arakane Y, Beeman RW, Zhu Q, Hogenkamp D, Dixit R, Oppert B, Jiang H, Zou Z, Marshall J, Elpidina E, Vinokurov K, Oppert C, Zou Z, Evans J, Lu Z, Zhao P, Sumathipala N, Altincicek B, Vilcinskas A, Williams M, Hultmark D, Hetru C, Jiang H, Grimmelikhuijzen CJ, Hauser F, Cazzamali G, Williamson M, Park Y, Li B, Tanaka Y, Predel R, Neupert S, Schachtner J, Verleyen P, Raible F, Bork P, Friedrich M, Walden KK, Robertson HM, Angeli S, Foret S, Bucher G, Schuetz S, Maleszka R, Wimmer EA, Beeman RW, Lorenzen M, Tomoyasu Y, Miller SC, Grossmann D, Bucher G. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–55. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Allen SP, Wright GM, Raynaud A, Hanken J. Somite number and vertebrate evolution. Development. 1998;125:151–160. doi: 10.1242/dev.125.2.151. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Crooijmans RP, Groenen MA. Sequencing and genomic annotation of the chicken (Gallus gallus) Hox clusters, and mapping of evolutionarily conserved regions. Cytogenetic and genome research. 2007;117:110–9. doi: 10.1159/000103171. [DOI] [PubMed] [Google Scholar]
- Roelen BA, de Graaff W, Forlani S, Deschamps J. Hox cluster polarity in early transcriptional availability: a high order regulatory level of clustered Hox genes in the mouse. Mech Dev. 2002;119:81–90. doi: 10.1016/s0925-4773(02)00329-5. [DOI] [PubMed] [Google Scholar]
- Ronshaugen M, Biemar F, Piel J, Levine M, Lai EC. The Drosophila microRNA iab-4 causes a dominant homeotic transformation of halteres to wings. Genes Dev. 2005;19:2947–52. doi: 10.1101/gad.1372505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist GC. A radioautographic study of labeled grafts in the chick blastoderm. Development from primitive-streak stages to stage 12. Contr Embryol Carnegie Inst Wash. 1966;38:71–110. [Google Scholar]
- Salsi V, Zappavigna V. Hoxd13 and Hoxa13 directly control the expression of the EphA7 Ephrin tyrosine kinase receptor in developing limbs. J Biol Chem. 2006;281:1992–9. doi: 10.1074/jbc.M510900200. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC. Morphological and mapping studies of the paranodal and postnodal levels of the neural plate during chick neurulation. Anat Rec. 1992;233:281–90. doi: 10.1002/ar.1092330211. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Garcia-Martinez V, Dias MS. Mesoderm movement and fate during avian gastrulation and neurulation. Dev Dyn. 1992;193:235–248. doi: 10.1002/aja.1001930304. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Stern CD. Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development. 1991;112:615–626. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- Seo HC, Edvardsen RB, Maeland AD, Bjordal M, Jensen MF, Hansen A, Flaat M, Weissenbach J, Lehrach H, Wincker P, Reinhardt R, Chourrout D. Hox cluster disintegration with persistent anteroposterior order of expression in Oikopleura dioica. Nature. 2004;431:67–71. doi: 10.1038/nature02709. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–28. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Spratt NT., Jr Regression and shortening of the primitive streak in the explanted chick blastoderm. J Exp Zool. 1947;104:69–100. doi: 10.1002/jez.1401040105. [DOI] [PubMed] [Google Scholar]
- Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128:4177–88. doi: 10.1242/dev.128.21.4177. [DOI] [PubMed] [Google Scholar]
- Stern CD. Evolution of the mechanisms that establish the embryonic axes. Curr Opin Genet Dev. 2006;16:413–8. doi: 10.1016/j.gde.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Stollewerk A, Schoppmeier M, Damen WG. Involvement of Notch and Delta genes in spider segmentation. Nature. 2003;423:863–5. doi: 10.1038/nature01682. [DOI] [PubMed] [Google Scholar]
- Struhl G. Role of the esc+ gene product in ensuring the selective expression of segment-specific homeotic genes in Drosophila. J Embryol Exp Morphol. 1983;76:297–331. [PubMed] [Google Scholar]
- Szumska D, Pieles G, Essalmani R, Bilski M, Mesnard D, Kaur K, Franklyn A, El Omari K, Jefferis J, Bentham J, Taylor JM, Schneider JE, Arnold SJ, Johnson P, Tymowska-Lalanne Z, Stammers D, Clarke K, Neubauer S, Morris A, Brown SD, Shaw-Smith C, Cama A, Capra V, Ragoussis J, Constam D, Seidah NG, Prat A, Bhattacharya S. VACTERL/caudal regression/Currarino syndrome-like malformations in mice with mutation in the proprotein convertase Pcsk5. Genes Dev. 2008;22:1465–77. doi: 10.1101/gad.479408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Koizumi K, Takagi A, Kitajima S, Inoue T, Koseki H, Saga Y. Mesp2 initiates somite segmentation through the Notch signalling pathway. Nat Genet. 2000;25:390–6. doi: 10.1038/78062. [DOI] [PubMed] [Google Scholar]
- Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99:109–26. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- Tam PP, Trainor PA. Specification and segmentation of the paraxial mesoderm. Anat Embryol(Berl) 1994;189:275–305. doi: 10.1007/BF00190586. [DOI] [PubMed] [Google Scholar]
- Tarchini B, Duboule D. Control of Hoxd genes’ collinearity during early limb development. Dev Cell. 2006;10:93–103. doi: 10.1016/j.devcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Trainor P, Krumlauf R. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat Cell Biol. 2000;2:96–102. doi: 10.1038/35000051. [DOI] [PubMed] [Google Scholar]
- Tvrdik P, Capecchi MR. Reversal of Hox1 gene subfunctionalization in the mouse. Dev Cell. 2006;11:239–50. doi: 10.1016/j.devcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- van den Akker E, Forlani S, Chawengsaksophak K, de Graaff W, Beck F, Meyer BI, Deschamps J. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–93. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- van den Akker E, Fromental-Ramain C, de Graaff W, Le Mouellic H, Brulet P, Chambon P, Deschamps J. Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development. 2001;128:1911–21. doi: 10.1242/dev.128.10.1911. [DOI] [PubMed] [Google Scholar]
- van der Hoeven F, Zakany J, Duboule D. Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell. 1996;85:1025–35. doi: 10.1016/s0092-8674(00)81303-3. [DOI] [PubMed] [Google Scholar]
- Vermot J, Pourquie O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature. 2005;435:215–20. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- Wacker SA, Jansen HJ, McNulty CL, Houtzager E, Durston AJ. Timed interactions between the Hox expressing non-organiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev Biol. 2004a;268:207–19. doi: 10.1016/j.ydbio.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Wacker SA, McNulty CL, Durston AJ. The initiation of Hox gene expression in Xenopus laevis is controlled by Brachyury and BMP-4. Dev Biol. 2004b;266:123–37. doi: 10.1016/j.ydbio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The epigenetics of birds. Cambridge at the University Press; Cambridge: 1952. [Google Scholar]
- Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236:2454–63. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–7. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Durston AJ. MiR-10 represses HoxB1a and HoxB3a in zebrafish. PLoS ONE. 2008;3:e1396. doi: 10.1371/journal.pone.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–6. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Yekta S, Tabin CJ, Bartel DP. MicroRNAs in the Hox network: an apparent link to posterior prevalence. Nat Rev Genet. 2008;9:789–96. doi: 10.1038/nrg2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi Y, Nakazato S, Yamamoto M, Goto Y, Kameda T, Iba H, Kuroiwa A. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995;9:2509–22. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- Zakany J, Kmita M, Alarcon P, de la Pompa JL, Duboule D. Localized and transient transcription of Hox genes suggests a link between patterning and the segmentation clock. Cell. 2001;106:207–17. doi: 10.1016/s0092-8674(01)00436-6. [DOI] [PubMed] [Google Scholar]
- Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–72. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]