Abstract
Motion-induced artefacts in magnetic resonance spectroscopic imaging are much harder to recognize than in imaging experiments and can therefore lead to erroneous interpretation. A method for prospective motion correction based on an optical tracking system has recently been proposed and has already been successfully applied to single voxel spectroscopy. In this work, the utility of prospective motion correction in combination with retrospective phase correction is evaluated for spectroscopic imaging in the human brain. Retrospective phase correction, based on the interleaved reference scan method, is used to correct for motion-induced frequency shifts and ensure correct phasing of the spectra across the whole spectroscopic imaging slice. It is demonstrated that the presented correction methodology can reduce motion-induced degradation of spectroscopic imaging data.
Keywords: spectroscopic imaging, SI, prospective motion correction, interleaved reference scan
INTRODUCTION
Magnetic resonance spectroscopy (MRS) is an invaluable tool for the investigation of brain pathologies such as tumours and neurodegenerative diseases. Due to its robustness, single voxel spectroscopy (SVS) is currently the method of choice in most clinical brain studies. However, for the investigation of complex and heterogeneous brain lesions, metabolic information with a spatial resolution as provided by spectroscopic imaging (SI) methods is desired (1). Besides, SI also facilitates brain examinations involving a contralateral comparison such as in epilepsy studies. Due to their long scan times, SI experiments are particularly susceptible to motion-induced artefacts. Unlike in imaging, these artefacts cannot be easily recognized and thus may lead to false diagnoses in clinical scans.
A widely-used approach for head motion detection is the interleaved acquisition of navigator echoes, which can be used for real-time motion correction either in k-space or in image space. Several k-space methods based on orbital (2,3), spherical (4) and cloverleaf navigators (5) have been proposed. However, navigator-based correction methods always represent a tradeoff between precision, scan time penalty and robustness. Motion tracking in image space allows for masking areas such as jaw or neck, which may corrupt the rigid-body motion estimates of the skull. Thesen et al. (6) suggested a self-navigated image-based technique called Prospective Acquisition CorrEction (PACE), which provides accurate 3D rigid-body motion estimates without modification of the original EPI sequence. Due to its robustness, PACE is widely used, especially for EPI measurements, but correction is performed on a slow volume-by-volume basis and the method is not applicable for spectroscopy scans. An alternative image-based approach for prospective motion correction called PROMO is based on multiple spiral navigators and extended Kalman filtering (7). Navigator acquisition, motion estimation and feedback require approximately 500 ms, which is problematic for most imaging applications, but acceptable for typical spectroscopy scans. The method has already been successfully applied and validated for single-voxel spectroscopy, using a long repetition time (TR = 3 s) and exploiting the dead-time period after each acquisition interval for the PROMO navigator module (8). Hess et al. recently proposed a three-dimensional multishot EPI navigator with a total duration of 928 ms for simultaneous real-time motion and B0 correction in single voxel spectroscopy(9).
Alternatively, prospective motion correction can be performed with an external optical motion tracking system, thus avoiding the scan time penalty inherent to all navigator-based methods. Various camera-based techniques have recently been proposed (10–14). The approach suggested by Zaitsev et al. uses two infrared cameras for motion detection and feeds the object motion information back to the sequence where it is used to update the slice geometry in real time after each repetition (14). Furthermore, if excessive motion is detected over a TR period, the acquired data can be rejected and the acquisition of the corresponding average or encoding step is repeated. The method has already been successfully applied to functional MRI (15) as well as to single-voxel spectroscopy in the human brain (16).
Motion-induced changes of the static field distribution can impair the quality of the shim and may thus increase the linewidths in the acquired spectra. Motion-induced frequency shifts in regions with an inhomogeneous susceptibility distribution can be corrected with the interleaved reference scan (IRS) method, originally proposed by Thiel et al. (17). This method contains the interleaved acquisition of an additional water reference signal after each repetition and uses this reference signal for retrospective phase correction of the actual spectral signal on a point-by-point basis. The method has recently been validated on a clinical 3T MR system for the correction of heating-induced frequency drifts in SVS (18) and was successfully combined with motion tracking, where it was applied to the correction of motion-induced shifts in SVS (16).
This work combines prospective motion correction with IRS-based retrospective phase correction in SI of the human brain. The feasibility of the proposed correction method is shown in phantom and in one subject in vivo. Furthermore, the in vivo data are quantitatively evaluated with a LCModel analysis.
MATERIALS AND METHODS
Prospective motion correction and retrospective phase correction were implemented with a PRESS-based SI sequence on a Magnetom Trio 3T system (Siemens Healthcare, Germany). The in vitro measurements were performed with a 12-channel head coil for signal reception while the in vivo data were acquired with a 32-channel head coil.
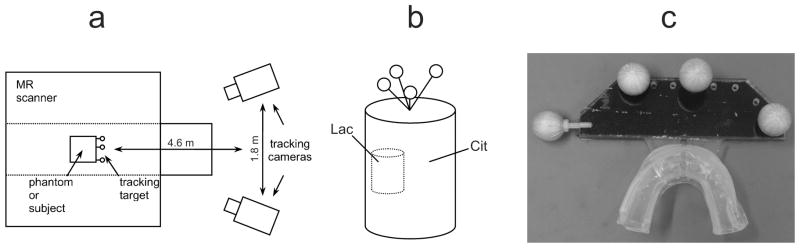
The stereoscopic tracking system cameras (ARTrack3, Advanced Realtime Tracking GmbH, Germany) were positioned outside the bore of the scanner and reported positions of dedicated tracking targets for in vitro as well as in vivo experiments (Fig. 1a). A rigid target equipped with retro-reflective spheres was used for phantom experiments (Fig. 1b) while the tracking target for in vivo experiments consisted of retro-reflective spheres attached to a mouth piece, which was individually fitted to the subject (Fig. 1c). The tracking system reported target motion in all three translational and three rotational degrees of freedom with a quoted positional accuracy of 0.1 mm (RMS) and an update rate of 60 Hz. An IRS sequence block as well as the real-time position update was incorporated into the product PRESS (point-resolved spectroscopy) sequence (Fig. 2). The PRESS volume, the SI FOV and the outer volume suppression (OVS) slabs are updated at the end of every TR period. Motion correction without a real-time update of the OVS slabs would not make sense since, due to the unfavorable point spread function in SI experiments, imperfect suppression of subcutaneous fat would severely impair even the spectra in the middle of the brain. The coordinate update was configured to take place within less than 5 ms prior to the OVS block. In principle, the coordinate update does not imply any TR penalty.
FIG. 1.
a) The optical tracking system setup using two infrared cameras for motion detection. b) Cylindrical phantom with attached tracking target, as used for the in vitro experiments. c) Mouthpiece with retroreflective spheres, as used for the in vivo experiments.
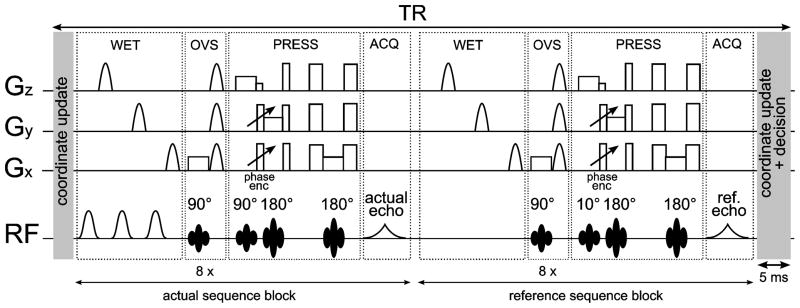
FIG. 2.
SI-PRESS sequence with water suppression enhanced through T1 effects (WET) and OVS (eight slabs) for subcutaneous fat suppression, including a coordinate update using the motion tracking data and an IRS sequence block. After the actual acquisition an additional water reference spectrum is acquired without water suppression pulses and using a small excitation angle (10°), but with an otherwise identical sequence block. Since a minimal TR was used for the experiments, the second coordinate update module was merged with the first one from the subsequent average.
The IRS sequence was implemented to acquire an additional reference signal without water suppression after each repetition as previously proposed by Lange et al. (18) and as shown in Fig. 2. Retrospective phase correction using the water reference data was performed before coil combination for each acquired time-domain signal on a point-by-point basis according to Klose (19). Thus also higher-order phase distortions can be corrected with this method. This enables not only the correction of eddy current distortions, but also of motion-induced frequency shifts, which correspond to linear phase distortions in the time domain data. The phase correction was implemented in the online reconstruction code, writing out the original SI data processed with the standard reconstruction, the water reference data and the IRS-corrected SI data.
The SI sequence with IRS was validated in a phantom experiment where the same acquired SI data sets from multiple receive channels underwent three different post-processing modes prior to channel combination: a) no phase correction, b) zero-order phase correction based on the first point of the FID (standard reconstruction) and c) IRS phase correction.
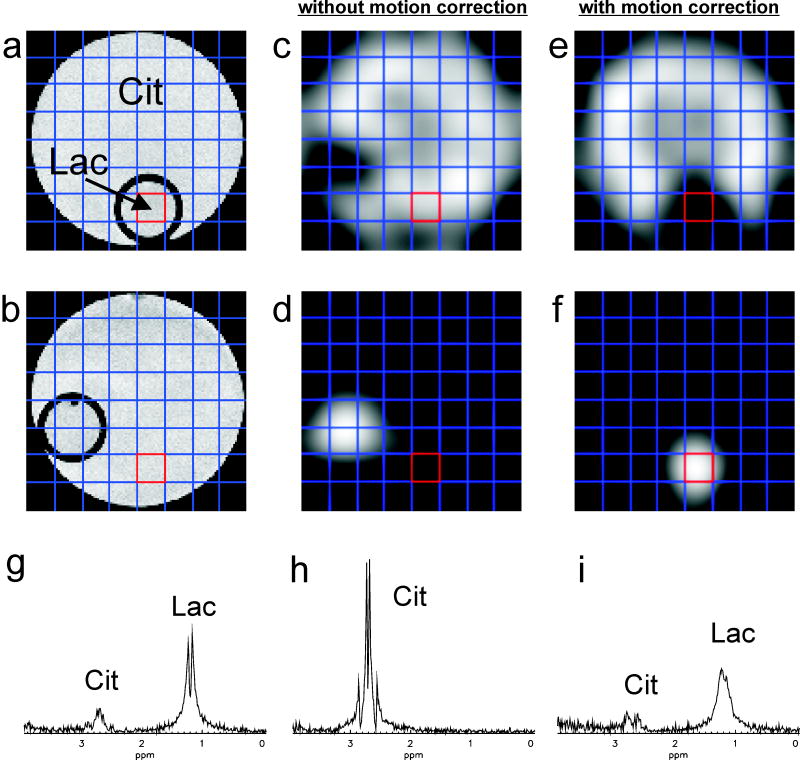
For validation purposes, SI data sets (8 × 8) were acquired from a large cylindrical phantom bottle containing citrate (Cit) solution with a small cylindrical phantom containing lactate (Lac) solution attached to its wall (Fig. 1b). A long rod attached to the base of the phantom bottle was used to rotate it inside the scanner bore during the scan without affecting the shim. The phantom was rotated by approximately 90° after the first phase encoding step of the scan. Data sets with and without motion correction were acquired and metabolite maps of Cit and Lac were created by peak integration in the respective chemical shift range and subsequent Fourier interpolation.
In vivo 2D SI data were acquired from a transverse slice through the brain of a healthy subject, who gave written informed consent prior to participating in the experiment (Fig. 3). A typical SI protocol (TR = 2.5 s, TE = 30 ms, FOV = 20 cm, SI resolution = 16 × 16, acquisition bandwidth = 1500 Hz, OVS with 8 slabs) with a total scan duration of 11 min was used.
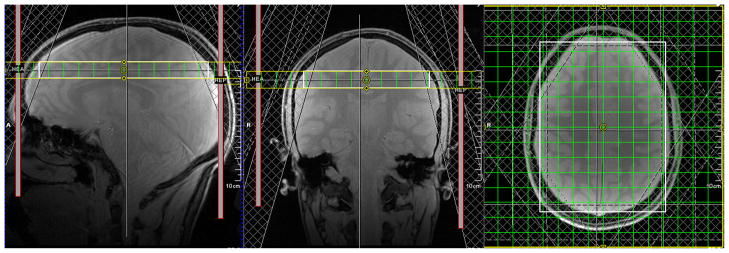
FIG. 3.
Experimental setup for the in vivo experiments: A transverse SI slice (thickness = 15 mm, FOV = 20 cm, resolution = 16 × 16) was placed above the ventricles in the brain of a healthy subject. Eight OVS slabs were used for suppression of subcutaneous lipid signal.
The SI experiment was planned on T1-weighted gradient-echo scout images, which were also acquired using motion correction. Interscan ‘position locking’ corrected for subject motion between the acquisition of the scout images and the spectroscopy measurement by updating the positions of the PRESS volume, the SI field of view and the OVS slabs with respect to the first scout scan in all subsequent scans. This is to ensure that the SI experiment is performed with the geometrical setup as planned on the scout images. At the start of the experiment, one SI data set was acquired without intentional subject motion. Keeping the shim settings of the first scan, the experiment was repeated twice with subject motion, once with and once without motion correction. Starting from the centre position, the subject was asked to rotate his head sideways once per minute during the scan, back and forth between two previously defined positions (left and right). Acquisitions with motion above a certain threshold (2 mm translation and 3° rotation per TR) were rejected and automatically repeated, which increased the scan duration by about 30 s. All tracking data and real-time decisions were logged to file for further processing.
The acquired SI data were analyzed with LCModel and the concentration ratio of N-acetylaspartate (NAA) to creatine (Cr), the Cramer-Rao lower bound (CRLB) of NAA, the full width at half maximum (FWHM) and the signal-to-noise ratio (SNR) as determined by LCModel were mapped for the whole SI slice.
RESULTS
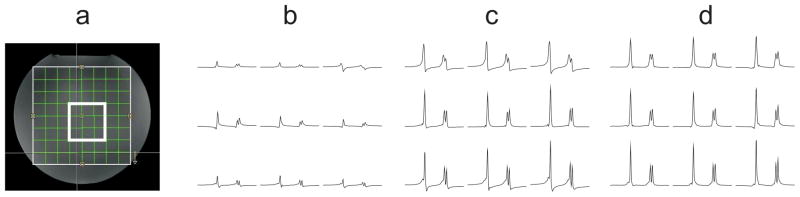
Figure 4 shows the SI spectra obtained with different post-processing modes. When no phase correction is applied prior to channel combination, most of the signal is lost through non-coherent addition (Fig. 4b). With zero-order phase correction as applied in the standard scanner reconstruction, this signal loss can be prevented, but the combined SI spectra are slightly out of phase with respect to each other (Fig. 4c). When IRS phase correction was applied instead of zero-order phase correction, SI data with in-phase spectra were obtained (Fig. 4d).
FIG. 4.
SI phantom experiment with different phase correction modes in the reconstruction: a) setup with the bold white box indicating the region of interest (3×3), b) no phase correction, c) zero-order phase correction using the first point of the time-domain signal, d) phase correction with IRS signal.
Figure 5 shows the results of the phantom experiments where the phantom was rotated by approximately 90° immediately after the start of the scan, once without and once with motion correction. The metabolite maps for Cit (Figs. 5c and 5e) and Lac (Figs 5d and 5f), created through peak integration and Fourier interpolation from the acquired SI data, demonstrate that spatial encoding is correctly updated in the measurement with motion correction, while the uncorrected experiment yields metabolite maps corresponding to the rotated phantom. Spectra from the voxel initially situated in the small Lac phantom are shown for an experiment without motion (Fig. 5g), with uncorrected motion (Fig. 5h) and with corrected motion (Fig. 5i). The experiment without motion yielded a spectrum with a large Lac doublet and with a small Cit resonance arising from signal contamination through the point spread function. While the experiment with uncorrected motion produces a pure Cit spectrum, the experiment with corrected motion yields a spectrum with a Lac/Cit peak ratio similar to the one without motion. However, the line width is heavily impaired by the strong motion due to the associated deterioration of the shim, and the lactate doublet is no longer clearly resolved. Integration of the spectra in the respective chemical shift ranges of Cit and Lac yielded an increase of the Cit signal by 1.8 % and a decrease of the Lac signal by 1.5 % in the motion-corrected experiment (i) compared to the experiment without motion (g) for the voxel of interest located in the small Lac phantom.
FIG. 5.
Spectroscopic imaging results for a phantom experiment with a rotation from a) to b) at the beginning of the scan: without motion correction (middle column), with motion correction (right column). Metabolite maps for Cit (c, e) and Lac (d, f) are shown for both cases. Spectra from the voxel initially situated in the compartment of the phantom containing Lac are shown for an experiment without motion (g), with uncorrected motion (h) and with corrected motion (i). The same vertical scaling was used for the representation of the three spectra.
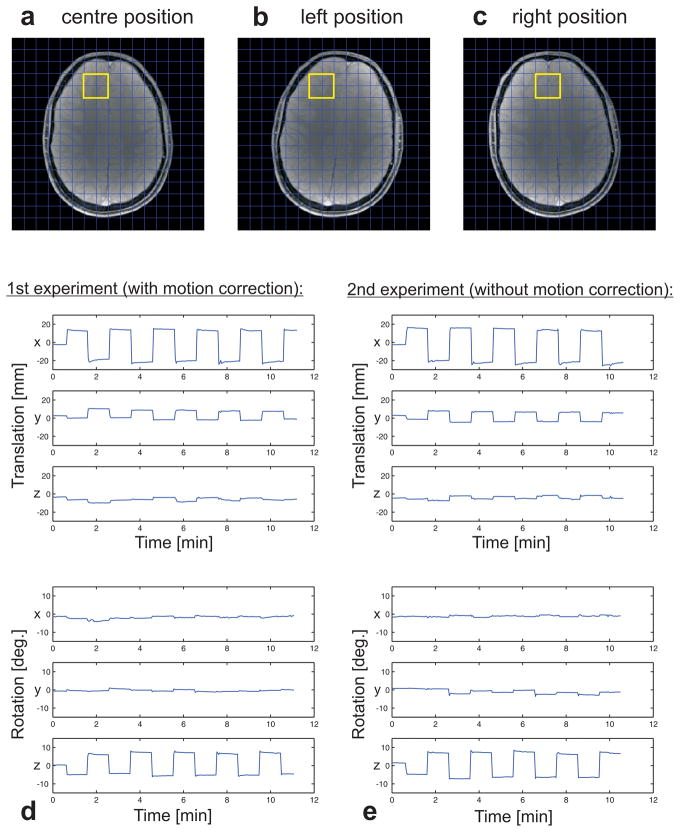
Images of the three head positions during the in vivo experiments are presented in Fig. 6, along with the tracking data for the three translational as well as the three rotational degrees of freedom. The tracking data show very similar motion patterns for the two experiments (with and without motion correction), and therefore a comparison of the spectral results is legitimate. The yellow box indicates the region of interest in the frontal cortex, for which the spectral results are presented in Fig. 7.
FIG. 6.
Motion pattern for the in vivo experiments: The scan preparation was done for the central position (a). During the scan, the subject was asked to tilt his head sideways once per minute, back and forth between two previously defined positions (b and c). The same experiment was performed twice, once with and once without motion correction. The tracking data recorded by the infrared cameras during the motion-corrected (d) as well as the uncorrected (e) scan are plotted for the three translational and three rotational degrees of freedom.
FIG. 7.
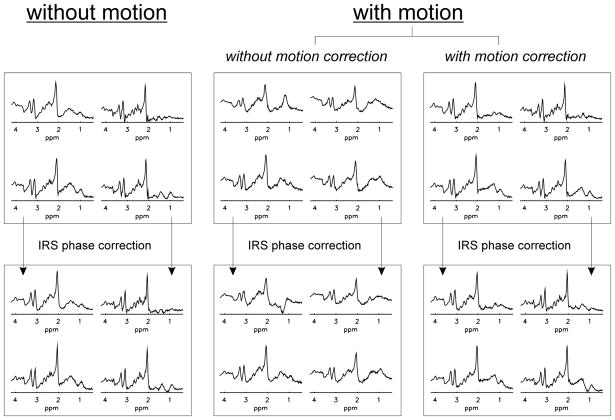
Results from the in vivo experiments: Clippings (2×2) from the yellow box indicated in Fig. 6 are shown for experiments without motion, uncorrected motion and corrected motion, respectively. Retrospective phase correction with the interleaved reference scan was applied to all acquired data sets.
In Fig. 7 the baseline SI data without deliberate motion (left column) as well as the motion-affected data acquired without (middle column) and with motion correction (right column) are shown. The corresponding IRS-corrected spectra are presented at the bottom. The spectra acquired without motion correction show line broadening and considerable lipid contamination compared to the baseline scan and IRS phase correction alone does not improve the spectral quality. In contrast, the spectral quality is almost thoroughly recovered in the motion-corrected spectra. Additional IRS phase deconvolution further improved the spectral quality by correcting for phase distortions and line broadening caused by motion-induced frequency shifts. Motion correction in combination with IRS phase correction yielded a SI data set with a spectral quality comparable to the results obtained without deliberate subject motion. The greatest improvement was found for the spectra in the frontal cortex, which was most affected by motion.
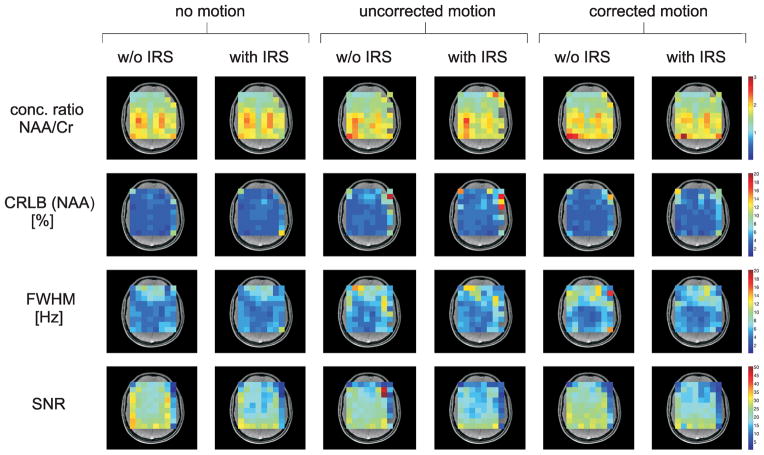
The results from the LCModel quantification are shown in Fig. 8. The NAA/Cr concentration ratio, the CRLB of NAA, the FWHM and the SNR are mapped for all voxels inside the PRESS volume. The row of voxels in the occipital cortex was excluded from the analysis since it was strongly impaired by chemical shift displacement. Voxels that could not be processed with LCModel due to excessively poor spectral quality are not mapped either. The quantitative results reveal that for most voxels the measured NAA/Cr concentration ratio in the motion-corrected spectra only deviates by a few percent from the ratio in the no-motion data. The IRS phase correction does not significantly distort the concentration ratios either. In the data without IRS correction, the FWHM analysis does not show an overall improvement of the line-widths in the motion-corrected spectra compared to the spectra with uncorrected motion. However, while there is hardly any improvement through IRS phase correction in the spectra with uncorrected motion, the line-widths can be reduced with IRS in the motion-corrected spectra. Motion correction in combination with IRS phase correction yields spectra with line-widths very similar to the no-motion case for most voxels. However, it has to be noted that IRS correction gives rise to an apparent loss in SNR and also a slight increase in the CRLBs.
FIG. 8.
Results from the LCModel quantification of the in vivo experiment: The color-coded maps show the NAA/Cr concentration ratio, the CRLB of NAA, the FWHM and the SNR for the volume of interest as determined by LCModel. Voxels that could not be processed with LCModel due to excessively poor spectral quality are not mapped. The NAA/Cr concentration ratio is only mapped for those voxels where CRLB (NAA) < 20 % and CRLB (Cr) < 20 %. Only CRLBs smaller than 20 % are mapped.
DISCUSSION
The results presented demonstrate the feasibility of prospective motion correction for SI experiments. Compared to purely retrospective correction techniques, this method not only mitigates motion-induced degradation of the spectral quality, but also locks the spatial origin of the recorded data and the position of the OVS slabs throughout the scan. Because of smaller voxel sizes, SI experiments are typically more sensitive to motion than SVS experiments and therefore benefit more from motion correction. Due to the low concentration of brain metabolites compared to fat, and the coarse spatial resolution giving rise to an unfavorable point spread function, OVS plays a pivotal role in SI experiments. Therefore, a real-time update of the OVS slabs is crucial, particularly for quantitative studies. Since the SI protocol, in particular the OVS geometry, needs to be adjusted for every subject individually, more than 20 minutes can pass between the acquisition of the scout images and the start of the SI scan, which makes the method particularly prone to inter-scan motion. A suboptimal experimental setup, however, can result in a strongly impaired spectral quality. Furthermore, the assignment of metabolic features to anatomical structures can be affected by motion between the SI measurement and the acquisition of the reference image for data analysis, potentially giving rise to erroneous interpretation of the SI data in clinical scans. Therefore, inter-scan position locking is a key feature for SI experiments with motion correction.
The rejection threshold of 2 mm of translation and 3° of rotation is rather high and could be readily reduced at the expense of increased scan times. The high threshold ensured that data rejection only occurred during deliberate head motion. Thus, for the two in vivo experiments (with and without motion correction), motion occurred at approximately the same stages of k-space sampling, yielding comparable results. For MRS experiments a rejection threshold of 1 mm translation or even less would be appropriate, as was shown for SVS (16). In our implementation, a coordinate update is performed once per TR, but an additional parameter update in between the actual and the reference scan module would be feasible. However, the rejection decision should be taken on the basis of motion over a whole TR since for good IRS phase correction the actual signal and the reference signal have to be acquired not only from the same voxel, but also under identical shim conditions.
Properly phased spectra are a prerequisite for robust post-processing and quantification of SI data. Motion-induced frequency shifts can be corrected retrospectively, using the IRS method. As an additional benefit, IRS phase correction yields SI data with in-phase spectra, which is a prerequisite for robust post-processing and quantification. The observed line-width degradation in the phantom experiment with a 90° rotation (Fig. 5) can be explained with the B0 heterogeneity of the phantom bottle introduced by the small lactate phantom, which was not made of special susceptibility-matched material. The results show that motion-induced shim changes can strongly impair the spectral line-widths even though it has to be noted that such strong motion does not reflect typical experimental in vivo conditions.
As was shown in this work, motion-correction and position locking in between scans preserve the NAA/Cr concentration ratio within acceptable limits. Furthermore, the quantitative results suggest that IRS phase correction reduces the line-widths in the motion-corrected data, yielding a spectral quality which is comparable to the no-motion data. Interestingly, despite the line-width improvement, a SNR reduction is observed for IRS correction in all three data sets. It has to be noted that the WET water suppression does not work very well for SI experiments and that IRS phase correction exacerbates baseline distortions in the noisy parts of the spectra arising from bad water suppression since phase correction with a water reference signal corrects phase distortions of the water signal even more efficiently than for the metabolites of interest. As LCModel only models the baseline for the spectral region of interest between 0.2 and 4.0 ppm, baseline distortions outside this region will inevitably give rise to an overestimation of the noise and thus an underestimation of the SNR. This noise overestimation is also reflected by the CRLBs, which scale with the SNR and therefore show a slight decrease in the IRS-corrected data sets. The large SNR observed in two voxels of the SI data set with uncorrected motion is due to the fact that the spectra were impaired by strong lipid contamination. IRS correction of these voxels gave rise to artifacts in the noisy regions of the spectrum, thus decreasing the SNR.
The in vivo experiments were performed with an overall motion amplitude of about 40 mm translation and 15° rotation. A much stronger motion amplitude is usually restricted by the size of the head coil, especially when the subject wears headphones. However, for strong rotations or forward tilting of the head some of the tracking targets can be obscured by the subject’s body. Even though motion tracking with three out of four tracking targets is still feasible, jumping from a setup with four markers to a setup of three markers during the scan gives rise to tracking inconsistencies and therefore to artifacts in the image.
Although the tracking system used in this work was reported to have a sub-millimetre accuracy (14), it should be noted that systems based on infrared cameras show temperature-induced drifts in the tracking data. These drifts are negligible on the time-scale of most imaging experiments, but they can affect spectroscopy examinations where there is often more than half an hour between the acquisition of the scout images and the end of the spectroscopy scan. For the in vivo experiment performed in this work, thermal drifts gave rise to an additional inaccuracy on the order of 1 mm. Further tracking inaccuracies may arise from the non-ideal fixation of the tracking targets via a mouth piece.
When doing motion correction in measurements with phased array coils, changes in sensitivity have to be taken into account. After signal combination, strong motion can therefore give rise to data inconsistencies in k-space, which translate into residual motion artifacts in the reconstructed image. In SI datasets with a much lower spatial resolution compared to imaging such artifacts are not a major issue. However, they could be corrected with an augmented generalized SENSE reconstruction as proposed by Bammer et al. for parallel imaging with motion correction (20).
It has to be conceded that the optical tracking system used in this work is sufficient for a feasibility study, but too cumbersome for routine clinical applications. In particular, the mouth piece causes significant subject discomfort and can give rise to tracking inaccuracies well beyond the specified precision of the system. An approach based on retro-grate reflectors, which can be mounted on goggles, is presumably the method of choice in a more clinical setting (11). Alternative tracking systems using in-bore cameras have recently been proposed (10,13). In-bore tracking ensures higher accuracy and visibility of the tracking targets at all times. Furthermore, instead of optically tracked spheres, small extracorporeal micro-coils can be used for motion detection (12). With these points in mind, it should be noted that the motion correction methodology used in this work is readily compatible with three tracking modalities available at our institution and can easily be adapted to other tracking hardware.
For strongly motion-affected measurements in inhomogeneous regions (e.g. close to the nasal cavities) a real-time shim update would be desirable. Dynamic shimming, at least up to first order, could be achieved with an interleaved acquisition of 3D field maps (7,9), shim navigators (21,22) or an extended version of SENSE shimming (23). Hess et al. proposed EPI-based navigators for simultaneous real-time motion and B0 correction and demonstrated the advantage of a first-order shim update in SVS experiments (9). A correction of second-order field changes would be desirable, but is beyond the capabilities of currently available clinical MR systems. Fortunately, the shim setting in SI experiments is inherently less sensitive to displacement than in SVS experiments. For SVS, the shim is very local, potentially leading to large field distortions outside the selected voxel, which can strongly impair the quality of motion-corrected experiments. For SI experiments, however, the shim volume usually comprises major parts of the SI slice, which tends to give rise to smaller in-plane shim corrections. Therefore the shim in SI experiments is only mildly affected by in-plane subject motion while being quite sensitive to motion along the slice normal where the size of the shim volume is comparable to SVS experiments.
In this work, subject motion mainly consisted of an axial rotation and a translation along the x direction, constituting in-plane motion for the performed SI experiment. Besides, the slice was located in the distal part of the brain, above the ventricles and far away from the nasal cavities. Therefore the motion-induced field changes were relatively benign and gave rise to only minor shim distortions. Prospective motion correction has recently been performed for a SI experiment with a very similar setup, but with stronger through-plane motion by a deliberate forward tilt of the head (24). In contrast to the in vivo experiment performed in this work, the resulting SI data were strongly corrupted by shim degradation, in particular in the frontal cortex, concluding that for the correction of large through-plane motion dynamic shimming is mandatory.
In conclusion, optical motion tracking in combination with IRS phase correction is a useful method for improving the spectral quality in SI brain experiments, which are typically much longer than imaging or SVS scans and are therefore more affected by subject motion or frequency drifts arising from scanner instabilities. Thus, in clinical studies with uncooperative patients, erroneous results on the basis of motion-degraded SI data can be reduced.
Acknowledgments
The authors thank Dr. Uwe Bottcher for assistance with sequence implementation and Michael Herbst for his assistance with the ART tracking system.
This work was supported in part through a cooperative project with Siemens Healthcare (Erlangen, Germany), in part through the INUMAC project (grant #01EQ0605) and in part through NIH/NIDA (1R01 DA021146).
References
- 1.Burtscher IM, Holtas S. Proton MR spectroscopy in clinical routine. J Magn Reson Imaging. 2001;13(4):560–567. doi: 10.1002/jmri.1079. [DOI] [PubMed] [Google Scholar]
- 2.Fu ZW, Wang Y, Grimm RC, Rossman PJ, Felmlee JP, Riederer SJ, Ehman RL. Orbital navigator echoes for motion measurements in magnetic resonance imaging. Magn Reson Med. 1995;34(5):746–753. doi: 10.1002/mrm.1910340514. [DOI] [PubMed] [Google Scholar]
- 3.Ward HA, Riederer SJ, Grimm RC, Ehman RL, Felmlee JP, Jack CR., Jr Prospective multiaxial motion correction for fMRI. Magn Reson Med. 2000;43(3):459–469. doi: 10.1002/(sici)1522-2594(200003)43:3<459::aid-mrm19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Welch EB, Manduca A, Grimm RC, Ward HA, Jack CR., Jr Spherical navigator echoes for full 3D rigid body motion measurement in MRI. Magn Reson Med. 2002;47(1):32–41. doi: 10.1002/mrm.10012. [DOI] [PubMed] [Google Scholar]
- 5.van der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn Reson Med. 2006;56(5):1019–1032. doi: 10.1002/mrm.21038. [DOI] [PubMed] [Google Scholar]
- 6.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, Kuperman J, Dale A. PROMO: Real-Time Prospective Motion Correction in MRI Using Image-Based Tracking. Magn Reson Med. 2010;63(1):91–105. doi: 10.1002/mrm.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating B, Deng W, Roddey JC, White N, Dale A, Stenger VA, Ernst T. Prospective motion correction for single-voxel (1)H MR spectroscopy. Magn Reson Med. 2010;64(3):672–679. doi: 10.1002/mrm.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess AT, Tisdall MD, Andronesi OC, Meintjes EM, van der Kouwe AJW. Real-Time Motion and B0 Corrected Single Voxel Spectroscopy Using Volumetric Navigators. Magn Reson Med. 2011;66(2):314–323. doi: 10.1002/mrm.22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aksoy M, Newbould R, Straka M, Holdsworth S, Skare S, Santos J, Bammer R. A real time optical motion correction system using a single camera and 2D marker. Intl Soc Mag Reson Med Toronto. 2008:3120. [Google Scholar]
- 11.Andrews-Shigaki BC, Armstrong BS, Zaitsev M, Ernst T. Prospective motion correction for magnetic resonance spectroscopy using single camera Retro-Grate reflector optical tracking. J Magn Reson Imaging. 2011;33(2):498–504. doi: 10.1002/jmri.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krueger S, Schaeffter T, Weiss S, Nehrke K, Rozijn T, Boernert P. Intl Soc Mag Reson Med. Vol. 14. Seattle: 2006. Prospective Intra-Image Compensation for Non-Periodic Rigid Body Motion Using Active Markers; p. 3196. [Google Scholar]
- 13.Qin L, van Gelderen P, Derbyshire JA, Jin FH, Lee J, de Zwart JA, Tao Y, Duyn JH. Prospective Head-Movement Correction for High-Resolution MRI Using an In-Bore Optical Tracking System. Magn Reson Med. 2009;62(4):924–934. doi: 10.1002/mrm.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaitsev M, Dold C, Sakas G, Hennig J, Speck O. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. NeuroImage. 2006;31(3):1038–1050. doi: 10.1016/j.neuroimage.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Speck O, Hennig J, Zaitsev M. Prospective real-time slice-by-slice motion correction for fMRI in freely moving subjects. Magma. 2006;19(2):55–61. doi: 10.1007/s10334-006-0027-1. [DOI] [PubMed] [Google Scholar]
- 16.Zaitsev M, Speck O, Hennig J, Buchert M. Single-voxel MRS with prospective motion correction and retrospective frequency correction. NMR Biomed. 2010;23(3):325–332. doi: 10.1002/nbm.1469. [DOI] [PubMed] [Google Scholar]
- 17.Thiel T, Czisch M, Elbel GK, Hennig J. Phase coherent averaging in magnetic resonance spectroscopy using interleaved navigator scans: compensation of motion artifacts and magnetic field instabilities. Magn Reson Med. 2002;47(6):1077–1082. doi: 10.1002/mrm.10174. [DOI] [PubMed] [Google Scholar]
- 18.Lange T, Zaitsev M, Buechert M. Correction of frequency drifts induced by gradient heating in 1H spectra using interleaved reference spectroscopy. J Magn Reson Imaging. 2011;33(3):748–754. doi: 10.1002/jmri.22471. [DOI] [PubMed] [Google Scholar]
- 19.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14(1):26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 20.Bammer R, Aksoy M, Liu CL. Augmented generalized SENSE reconstruction to correct for rigid body motion. Magn Reson Med. 2007;57(1):90–102. doi: 10.1002/mrm.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Splitthoff DN, Hennig J, Zaitsev M. Assessment of signal variations in EPI, induced by breathing and hardware instabilities. Proceedings 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; Toronto. 2008. p. 2476. [Google Scholar]
- 22.Ward HA, Riederer SJ, Jack CR., Jr Real-time autoshimming for echo planar timecourse imaging. Magn Reson Med. 2002;48(5):771–780. doi: 10.1002/mrm.10259. [DOI] [PubMed] [Google Scholar]
- 23.Splitthoff DN, Zaitsev M. SENSE Shimming (SSH): A Fast Approach for Determining B-0 Field Inhomogeneities Using Sensitivity Coding. Magn Reson Med. 2009;62(5):1319–1325. doi: 10.1002/mrm.22083. [DOI] [PubMed] [Google Scholar]
- 24.Lange L, Splitthoff DN, Zaitsev M, Maclaren J. Characterisation of Motion-Induced Field Distortions in Spectroscopic Imaging With Prospective Motion Correction Proceedings of the 19th Annual Meeting of ISMRM; Montreal, Canada. 2011. p. 2682. [Google Scholar]