Abstract
Objective
To evaluate the comparative cost-effectiveness of interventions to improve adherence to evidence-based medications among postmyocardial infarction (MI) patients.
Data Sources/Study Setting
Cost-effectiveness analysis.
Study Design
We developed a Markov model simulating a hypothetical cohort of 65-year-old post-MI patients who were prescribed secondary prevention medications. We evaluated mailed education, disease management, polypill use, and combinations of these interventions. The analysis was performed from a societal perspective over a lifetime horizon. The main outcome was an incremental cost-effectiveness ratio (ICER) as measured by cost per quality-adjusted life year (QALY) gained.
Data Collection/Extraction Methods
Model inputs were extracted from published literature.
Principal Findings
Compared with usual care, only mailed education had both improved health outcomes and reduced spending. Mailed education plus disease management, disease management, polypill use, polypill use plus mailed education, and polypill use plus disease management cost were $74,600, $69,200, $133,000, $113,000, and $142,900 per QALY gained, respectively. In an incremental analysis, only mailed education had an ICER of less than $100,000 per QALY and was therefore the optimal strategy. Polypill use, particularly when combined with mailed education, could be cost effective, and potentially cost saving if its price decreased to less than $100 per month.
Conclusions
Mailed education and a polypill, once available, may be the cost-saving strategies for improving post-MI medication adherence.
Keywords: Cost-effectiveness, adherence, myocardial infarction
Long-term adherence to secondary prevention medications after myocardial infarction (MI) is extremely poor (Choudhry et al. 2008a). For example, less than half of post-MI patients are adherent to their prescribed β-blocker or statin (Benner et al. 2002; Kramer et al. 2006). Poor adherence has substantial clinical and economic consequences (Rasmussen, Chong, and Alter 2007; Ho et al. 2008; Jakevicius, Li, and Tu 2008). Fortunately, even small improvements in adherence to evidence-based post-MI therapies can significantly reduce rates of major vascular events (Choudhry et al. 2011a). Thus, efforts to promote adherence are gathering increasing attention from patients, providers, and payers (World Health Organization 2003; Agency for Healthcare Research and Quality 2011).
A variety of interventions that could effectively improve adherence to chronic preventive medications have been identified and evaluated in clinical trials (Peterson, Takiya, and Finley 2003; Kripalani, Yao, and Haynes 2007; Haynes et al. 2008). Successful strategies include those that educate and motivate patients by providing educational materials (i.e., informational interventions), reduce the complexity of medication regimens or provide reminders (i.e., behavioral interventions), and apply multidisciplinary approaches with several features of the preceding strategies (i.e., complex interventions). Although complex interventions are generally believed to be more effective than simple ones, little is known about the potential trade-off between their increased administrative costs and the cost saving that may result due to improvements in adherence. Accordingly, we used decision analytic techniques to estimate the long-term clinical impact and cost-effectiveness of interventions intended to improve adherence with secondary preventive medications among post-MI patients.
Methods
We developed a Markov model to evaluate the incremental costs and quality-adjusted life expectancy that would result from different strategies to improve adherence to post-MI secondary prevention medications (Figure 1). We simulated the prognosis of a hypothetical cohort of patients 65 years of age who were discharged alive after MI and were prescribed all four classes of guideline-recommended post-MI therapy: aspirin, a β-blocker, an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), and a statin. We did not explicitly model the use of clopidogrel because our analysis focused on the use of drugs intended for lifelong use and the appropriate length of treatment with antiplatelet agents other than aspirin remains in debate (Kushner et al. 2009). Patients were followed as they transitioned in 3-month cycles through a series of health states over the course of their lifetimes. In each cycle, patients could remain well, have a reinfarction or stroke, be hospitalized for congestive heart failure (CHF) with the potential of dying of these conditions, or die of other causes. We assumed a societal perspective, lifetime horizon, and a discount rate of 3 percent per year for both health benefits and costs (Gold et al. 1996). The analysis was performed using TreeAge Pro Suite 2011 software (TreeAge Software, Williamstown, MA, USA).
Figure 1.
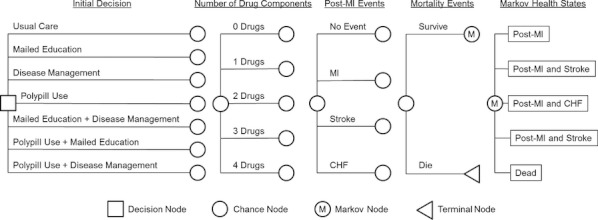
Markov Model Structure
Note. At the decision node patients were assigned to one of the seven adherent interventions. Proportions of patients adherent to different numbers of drug components of combination pharmacotherapy were determined as shown in Appendix Table 1. Then patients entered the model with the post-myocardial infarction (MI) state. Every 3 months, patients were at risk for recurrent MI, stroke, or hospitalization for congestive heart failure (CHF) with the potential of dying of these conditions. Throughout the patients' lifetime, all patients were at risk for death from causes unrelated to MI, stroke, or CHF.
Interventions
Interventions were categorized by different targets for adherence improvement: informational, behavioral, or complex (Peterson, Takiya, and Finley 2003; Kripalani, Yao, and Haynes 2007; Haynes et al. 2008). From a recent systematic review of interventions to improve cardiovascular medication adherence, we selected clinical trials that (1) evaluated U.S. patients who had the established diagnosis of MI, stroke, or CHF and were prescribed multiple cardiovascular drugs; (2) had at least 3 months of follow-up; and (3) presented data regarding intervention costs (Cutrona et al. 2010). We identified one informational intervention (i.e., mailed education) (Smith et al. 2008) and one complex intervention (i.e., disease management) (Murray et al. 2007). On the basis of a clinical trial reported by Smith and colleagues, we assumed that the mailed education group received biannual mailings that described the reason to use secondary prevention medications following MI, the risk of not taking them, information on adverse effects, and the importance of remembering to refill prescriptions. On the basis of a clinical trial reported by Murray and colleagues, we assumed that the disease management group received ongoing care from a specially trained pharmacist, including medication review, assessment of patients' knowledge and skills, electronic drug monitoring, and communications with patients' primary care physicians. We also evaluated a stepped approach, including one with mailed education followed by disease management for patients who were not adherent to all four components of combination pharmacotherapy. Although we identified no behavioral interventions, we included interventions to reduce the complexity of self-administration of multiple medications (i.e., polypill) (Connor, Rafter, and Rodgers 2004), which is currently being evaluated by clinical trials in post-MI patients (http://ClinicalTrials.gov 2011; Sanz et al. 2011). We assumed that the polypill group was prescribed a single pill that included all four-drug components of combination pharmacotherapy (e.g., aspirin, a β-blocker, an ACEI or ARB, and a statin) in the same formulation (Wise 2005; Gaziano, Opie, and Weinstein 2006; Lonn et al. 2010; Muntner et al. 2011). We evaluated combined interventions (i.e., polypill use plus mailed education or disease management). Because a polypill is not currently available in the United States, we also conducted a secondary analysis that only included usual care, mailed education, and disease management. All the interventions were compared with usual care, defined as the absence of adherence interventions. We assumed that all the interventions were continued without crossover until patients died.
Model Inputs
The model parameters are summarized in Table 1 and are described in greater detail below.
Table 1.
Base-Case Assumptions
| Parameter | Value | Sensitivity Range Tested | Reference |
|---|---|---|---|
| Age at the diagnosis of MI | 65 | 55–75% | Roger et al. (2011) |
| Intervention effectiveness, relative increase in proportion of patients fully adherent | |||
| Mailed education | 6% | 3–9% | Smith et al. (2008) |
| Disease management | 16% | 9–25% | Murray et al. (2007) |
| Polypill use | 28% | 0–60% | Bangalore et al. (2007) |
| Annual post-MI event rate in patients receiving no therapy† | |||
| MI | 16% | ±25% of the base-case rates | Choudhry et al. (2008b) |
| Stroke | 1% | ±25% of the base-case rates | Choudhry et al. (2008b) |
| CHF | 3% | ±25% of the base-case rates | Choudhry et al. (2008b) |
| Relative risk reduction in post-MI events by number of drug components | See footnotes* | Choudhry et al. (2008b), Dargie (2001), Danchin et al. (2006), Gaziano, Opie, and Weinstein (2006) | |
| MI | |||
| 1 drug | 23% | ||
| 2 drugs | 40% | ||
| 3 drugs | 54% | ||
| 4 drugs | 65% | ||
| Stroke | |||
| 1 drug | 17% | ||
| 2 drugs | 31% | ||
| 3 drugs | 39% | ||
| 4 drugs | 53% | ||
| CHF | |||
| 1 drug | 10% | ||
| 2 drugs | 18% | ||
| 3 drugs | 27% | ||
| 4 drugs | 35% | ||
| Effectiveness of a polypill compared with those of its components administered separately | 100% | See footnotes* | Assumed |
| Event-related mortality† | |||
| MI | 6% | ±25% of the base-case rates | Choudhry et al. (2008b) |
| Stroke | 4% | ±25% of the base-case rates | Choudhry et al. (2008b) |
| CHF | 3% | ±25% of the base-case rates | Choudhry et al. (2008b) |
| Baseline utility† | 0.83 | ±25% of the base-case disutility | Hanmer et al. (2006) |
| Utility multiplier | |||
| MI (<3 months of onset; ≥3 months of onset) | 0.77; 0.85 | ±25% of the base-case disutility | Shrive et al. (2005) |
| Stroke (<3 months of onset; ≥3 months of onset) | 0.60; 0.65 | ±25% of the base-case disutility | Gore et al. (1995), Gunz et al. (2000), Xie et al. (2006) |
| CHF (<3 months of onset; ≥3 months of onset) | 0.57; 0.84 | ±25% of the base-case disutility | Nichol et al. (2004) |
| Monthly drug cost | |||
| 1 drug component of combination pharmacotherapy | ±25% of the base-case costs | Choudhry et al. (2011a), Drugstore.com (2012) | |
| 2012 | $29 | ||
| 2013 | $25 | ||
| 2014 | $22 | ||
| 2017 | $20 | ||
| 2018 | $17 | ||
| Polypills | $200 | See footnotes* | Drugstore.com (2012) |
| Intervention cost, per year | |||
| Mailed education | $10 | See footnotes* | Smith et al. (2008) |
| Disease management | $357 | See footnotes* | Murray et al. (2007) |
| Post-MI event cost | |||
| MI | $17,941 | ±25% of the base-case costs | Choudhry et al. (2008b) |
| Stroke | $13,456 | ±25% of the base-case costs | Choudhry et al. (2008b) |
| CHF | $10,989 | ±25% of the base-case costs | Choudhry et al. (2008b) |
| Subsequent care cost, per year | |||
| MI | $2,808 | ±25% of the base-case costs | Choudhry et al. (2008b) |
| Stroke | $16,188 | ±25% of the base-case costs | Choudhry et al. (2008b) |
| CHF | $4,052 | ±25% of the base-case costs | Choudhry et al. (2008b) |
| Discount rate | 3% | 0–6% | Gold et al. (1996) |
Varied ranges in threshold analyses assuming a cost-effectiveness threshold at $100,000 per QALY gained.
Varied by year. Values presented are for a 65-year-old patient (or post-MI year 0).
CHD, coronary heart disease; CHF, congestive heart failure; MI, myocardial infarction.
Current and Expected Medication Adherence
The proportions of patients taking each possible combinations of β-blockers, ACEI/ARB, and statins in the usual care group were obtained from the placebo arm of a recent prospective randomized controlled trial data (Choudhry et al. 2011a). We used the use of aspirin based on self-reported data from patients with coronary artery disease (Newby et al. 2006). We assumed that the relative increase in adherence from mailed education was 6 percent based on a clinical trial to enhance adherence to β-blockers in post-MI patients (Smith et al. 2008). We assumed that the relative increase in adherence from disease management was 16 percent based on a clinical trial to enhance adherence to cardiovascular medications in CHF outpatients (Murray et al. 2007). We conservatively assumed that the effectiveness of disease management for patients who did not respond to mailed education was 50 percent of that of disease management for all medication takers. Because mailed education and disease management could be more effective in patients who were prescribed multiple cardiovascular drugs than those who were prescribed a single drug, we conservatively assumed that the effectiveness of each intervention was 50 percent of the base-case estimate when combined with polypill use. We estimated that the relative increase in adherence from polypill use was 28 percent based on a meta-analysis of fixed-dose combination regimen in patients with chronic illnesses (Bangalore et al. 2007). The impact of each intervention was applied to all patients regardless of how many drugs to which they were adherent at baseline. For example, incremental adherence improvement from mailed education was applied equally across patients currently taking no drugs, aspirin alone, β-blocker alone, ACEI/ARB alone, aspirin and β-blocker, aspirin and ACEI/ARB, and all three (aspirin, β-blocker, and ACEI/ARB), such that average adherence to all four drugs increased by 6 percent, which was the observed impact on Smith et al.'s trial. In sensitivity analyses, we applied the best and the worst estimates to evaluate the impact of each intervention on adherence.
Post-MI Event Rates
We estimated the incidence of post-MI events based on claims for all Medicare beneficiaries discharged from a hospital with a primary diagnosis of MI (Choudhry et al. 2008b) and calibrated these results to population-based observational data (Roger et al. 2011). We used previously published methods to estimate the impact of greater adherence on post-MI event rates (Dargie 2001; Danchin et al. 2006; Gaziano, Opie, and Weinstein 2006; Choudhry et al. 2008b). Because events rates vary as a function of the number of medications to which a patient is adherent, we calculated the impact of taking 0, 1, 2, 3, and all 4 components of combination pharmacotherapy by multiplying relative risk reductions in each component compared with placebo. We used averaged risk reductions for all possible combinations of 1, 2, and 3 drugs in our analysis because patients who were not fully adherent may take any combinations of the components of combination pharmacotherapy and because each combination has a different efficacy. We conservatively assumed that aspirin and statins did not reduce the risk of CHF hospitalization. Because the use of multiplicative model to estimate treatment effects has been debated, we varied the effect of combination pharmacotherapy extensively in our sensitivity analyses. In the base case, we assumed that the effectiveness of a polypill was equivalent to that of the standard four-drug regimen of combination pharmacotherapy. Because the actual effectiveness of a polypill has not been rigorously tested, we also conducted a sensitivity analysis to evaluate the impact of its assumed effectiveness on our study results.
Mortality
We obtained age-specific mortality rates for post-MI events from the Agency for Healthcare Research and Quality's nationwide inpatient sample (Choudhry et al. 2008b) and age-specific total mortality rates from the 2006 U.S. Life Table (Arias 2010). Long-term excess mortality after an MI event was modeled based on a cohort study of post-MI patients (Brønnum-Hansen et al. 2001).
Quality of Life
We assigned a utility to each health state that reflected the preference for, or desirability of, that health state. Utilities at baseline and post-MI events were taken from studies that used standardized methods (the time trade-off or standard gamble technique) (Gore et al. 1995; Gunz et al. 2000; Nichol et al. 2004; Shrive et al. 2005; Hanmer et al. 2006; Xie et al. 2006). For patients with multiple conditions, the utilities for the associated conditions were multiplied together. We assumed that a stroke, for example, reduced a patient's quality of life by same percentage regardless of whether CHF also is present.
Costs
Drug costs were obtained from a major online pharmacy (http://www.drugstore.com) and calculated on the basis of the distribution of drug components of combination pharmacotherapy in a managed care population (Choudhry et al. 2011a). To consider the impact of patent expiration of atorvastatin in 2011 and rosuvastatin in 2016 on the drug price, we assumed that the generic price of each statin would become comparable with that of generic simvastatin 2 years after patent expiration, with halfway between the prices of each statin and generic simvastatin 1 year after patent expiration (Choudhry et al. 2011b). We used the price of the most costly brand-name drugs that may be contained in the polypill (i.e., atorvastatin 80 mg) as its base-case price. We used published sources to estimate the annual intervention costs of mailed education and disease management (Murray et al. 2007; Smith et al. 2008). The direct medical and nonmedical costs of post-MI events were taken from a previous cost-effectiveness analysis based on a weighted average of Medicare diagnosis-related group hospital payments for these events and other published literature (Choudhry et al. 2008b). All costs are presented in 2010 U.S. dollars and were inflated using the medical care component of the U.S. Consumer Price Index (U.S. Department of Labor 2012).
Outcomes and Analysis
We measured health benefits in quality-adjusted life years (QALYs) gained and sought to identify the single intervention that would provide the greatest improvement in health outcomes at a socially acceptable cost per QALY gained. First, we calculated the difference in costs and effectiveness of each intervention compared with usual care. Second, we performed an incremental cost-effectiveness analysis by ranking the interventions in order of increasing cost. If an intervention was more or equally costly and less effective than a competing intervention, it was ruled out by “simple dominance.” After the exclusion of interventions exhibiting simple dominance from the rank-order list, we calculated the incremental cost-effectiveness ratio (ICER) of each intervention as the additional cost of that strategy divided by its additional health benefit compared with the next most costly intervention. If an intervention was less effective and less efficient (i.e., had a higher ICER) than another intervention, it was ruled out by “extended dominance.” After the exclusion of interventions exhibiting extended dominance from the rank-ordered list, we recalculated ICERs of the remaining interventions. After these standard methods, each nondominated intervention was compared with the next most costly intervention.
Model Validation
The median survival in the simulated cohort was 9.4 years. The proportions of patients who experienced recurrent MI, CHF, and stroke in 5 years after a first MI event were 23, 17, and 4 percent, respectively. All of these estimates correlated well with available observational data (Roger et al. 2011).
Results
Cost-Effectiveness Compared with Usual Care
We estimated that the absolute increase in adherence from each of the interventions was 2 percent for mailed education, 5 percent for mailed education plus disease management, 6 percent for disease management, 10 percent for polypill use, 12 percent for polypill use plus mailed education, and 14 percent for polypill use plus disease management (Appendix Table 1). All of the evaluated strategies improved health outcomes compared with usual care. Only mailed education both improved health outcomes and reduced health spending (i.e., $315 per patient). Mailed education plus disease management, disease management, polypill use, polypill use plus mailed education, and polypill use plus disease management cost $74,600, $69,200, $133,000, $113,000, and $142,900 per QALY gained, respectively, versus usual care (Table 2).
Table 2.
Base-Case Results
| Cost ($) | Difference | |||||||
|---|---|---|---|---|---|---|---|---|
| Strategy | Intervention | Drug | Health Care | Total | Total QALY | Cost ($) | QALYs | C/E Ratio, $/QALY |
| Cost-effectiveness compared with usual care | ||||||||
| Usual care | 0 | 2,839 | 99,928 | 102,767 | 4.4756 | Reference | Reference | Reference |
| Mailed education | 77 | 3,009 | 99,365 | 102,451 | 4.4848 | −315 | 0.0092 | −34,300 |
| Mailed education plus disease management | 2,459 | 3,231 | 98,644 | 104,334 | 4.4966 | 1,567 | 0.0210 | 74,600 |
| Disease management | 2,750 | 3,300 | 98,421 | 104,471 | 4.5003 | 1,704 | 0.0246 | 69,200 |
| Polypill use | 0 | 9,010 | 98,067 | 107,077 | 4.5080 | 4,311 | 0.0324 | 133,000 |
| Polypill use plus mailed education | 77 | 9,279 | 97,719 | 107,075 | 4.5138 | 4,307 | 0.0381 | 113,000 |
| Polypill use plus disease management | 2,751 | 9,739 | 97,123 | 109,613 | 4.5235 | 6,846 | 0.0479 | 142,900 |
| Incremental cost-effectiveness* | ||||||||
| Mailed education | 77 | 3,009 | 99,365 | 102,451 | 4.4848 | Reference | Reference | Reference |
| Usual care | 0 | 2,839 | 99,928 | 102,767 | 4.4756 | 315 | −0.0092 | Dominated† |
| Mailed education plus disease management | 2,459 | 3,231 | 98,644 | 104,334 | 4.4966 | 1,882 | 0.0118 | Dominated‡ |
| Disease management | 2,751 | 3,300 | 98,421 | 104,471 | 4.5003 | 138 | 0.0036 | 130,800 |
| Polypill use plus mailed education | 77 | 9,279 | 98,067 | 107,077 | 4.5080 | 2,604 | 0.0135 | 193,000 |
| Polypill use | 0 | 9,010 | 97,719 | 107,075 | 4.5138 | 3 | −0.0057 | Dominated† |
| Polypill use plus disease management | 2,751 | 9,739 | 97,123 | 109,613 | 4.5235 | 2,538 | 0.0098 | 259,500 |
Incremental cost-effectiveness ratios were calculated by dividing the difference in QALY by the difference in cost between an intervention of interest and the next most costly, nondominated intervention.
Ruled out by simple dominance because a strategy was less effective and more costly than a competing strategy.
Ruled out by extended dominance because a strategy was less effective and less efficient (i.e., had a higher ICER) than a competing strategy.
C/E, cost-effectiveness; QALY, quality-adjusted life year.
Incremental Cost-Effectiveness
When rank ordered by costs, usual care was eliminated by simple dominance because it was more costly and less effective than mailed education (Table 2). Mailed education plus disease management and polypill use were eliminated by extended dominance because they were less effective and less efficient (i.e., had a higher ICER) than disease management and polypill use plus mailed education, respectively. Among the remaining strategies, mailed education was the reference strategy because it was the least costly, nondominated option. In a secondary analysis excluding the strategies that involved polypill use, usual care remained dominated by mailed education. Mailed education plus disease management was dominated by extended dominance because it was less effective and less efficient (i.e., had a higher ICER) than disease management. Compared with mailed education, disease management had an ICER of $130,800 per QALY gained.
Sensitivity Analyses
Our results were robust to a wide range of plausible estimates of the effectiveness of combination pharmacotherapy, the impact of each intervention on medication adherence, and the cost of each intervention. At a conventional cost-effectiveness threshold of $100,000 per QALY gained, mailed education would be preferred over usual care as long as the effectiveness of combination pharmacotherapy was at least 18 percent of that assumed by our base-case estimates (i.e., that combination pharmacotherapy reduced the risk of reinfarction, stroke, and CHF by at least 11, 9, and 6 percent, respectively). If the relative increase in adherence from mailed education was at least 1.2 percent, mailed education would be cost saving compared with usual care. Under our base-case assumption about the relative increase in adherence from mailed education, mailed education would be preferred as long as the cost of mailed education was below $70 per year. Under the assumption that the relative increase in adherence from mailed education was 3 percent (i.e., our worst-case assumption), disease management would be preferred regardless of the cost of mailed education (Figure 2). If the relative increase in adherence from disease management was above 18 percent, disease management would be preferred over mailed education. Under our base-case assumption about the relative increase in adherence from disease management, disease management would be preferred if the cost of disease management was below $295 per year. Even under the assumption that the relative increase in adherence from disease management was 25 percent (i.e., our best-case assumption), disease management would not be preferred if the cost of disease management was above $536 per year (Figure 2). Mailed education plus disease management would be preferred if the effectiveness of disease management for patients who did not respond to mailed education was greater than 71 percent of that of disease management for all medication takers.
Figure 2.

Multiway Sensitivity Analyses of the Relative Increases in Adherence and Annual Costs Associated with Mailed Education and Disease Management
Note. The figures show regions of optimal strategy under the assumption that maximum cost per quality-adjusted life year (QALY) of $100,000 is acceptable. D, disease management; M, mailed education.
Under the base-case assumption about the relative increase in adherence from polypill use, polypill use plus mailed education would be preferred over mailed education if the monthly cost of the polypill decreased to $163, and become potentially cost saving if its monthly cost decreased to $100 or less. However, even under the assumption that the relative increase in adherence from polypill use was 60 percent (i.e., our best-case assumption), polypill use plus mailed education would not be preferred if the polypill had a submultiplicative effect of less than 91 percent effectiveness compared with those of its components administered separately (Figure 3). The rank order of strategies did not change and the ICER for each strategy remained relatively stable across a wide range of assumptions evaluated in other sensitivity analyses (Appendix Table 1).
Figure 3.
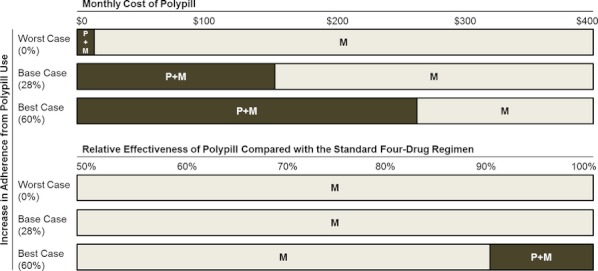
Multiway Sensitivity Analyses of the Price of the Polypill, the Relative Increase in Adherence from Polypill Use, and Relative Effectiveness of the Polypill Compared with those of Its Components Administered Separately
Note. The figures show regions of optimal strategy under the assumption that maximum cost per quality-adjusted life year (QALY) of $100,000 is acceptable.
Discussion
Nonadherence to post-MI medications has a substantial effect on cardiovascular morbidity and mortality (Rasmussen, Chong, and Alter 2007; Ho et al. 2008; Jakevicius, Li, and Tu 2008). Because there are many contributors to nonadherence, a variety of strategies may be used to address this problem (Peterson, Takiya, and Finley 2003; Kripalani, Yao, and Haynes 2007; Haynes et al. 2008). We conducted a comparative cost-effectiveness analysis of interventions designed to improve medication adherence among patients prescribed secondary prevention medications following MI. We found that a program involving the mailing of educational materials would both improve health outcomes and reduce costs from a societal perspective. In contrast, disease management, polypill use, and the combined strategies of mailed education, disease management, and polypill use appear less cost effective under conservative literature-based assumptions.
Gaps in knowledge about a patient's condition and why treatments were prescribed are believed to be central contributors of nonadherence. Smith and colleagues evaluated the impact of an intervention involving two educational mailings 2 months apart on post-MI β-blockers and found that the use of β-blockers increased by approximately 6 percent (Smith et al. 2008). Based on this, we estimated that mailed education will improve post-MI health outcomes and reduce lifetime spending by $315 per patient. Applied to 600,000 Medicare beneficiaries who have an acute MI every year, this would translate into almost $0.2 billion in lifetime savings (Roger et al. 2011). Our results were robust to many of the model assumptions. In particular, mailed education would continue to dominate usual care (i.e., be more effective and less costly) even if the absolute increase in adherence from the intervention was substantially smaller than our base-case estimate. These results are consistent with a recent systematic review of patient education in the management of coronary heart disease, which showed its potential to be cost saving by reducing subsequent health care utilization (Brown et al. 2011). Furthermore, in the current policy climate where efforts to improve health care quality must be coupled with efforts to contain costs, our analysis highlights the value of a low-cost strategy that could be easily scaled to large populations.
Several disease management programs have demonstrated favorable results using multidisciplinary approaches—targeting more than one reason for nonadherence (Peterson, Takiya, and Finley 2003; Kripalani, Yao, and Haynes 2007; Haynes et al. 2008). While there is substantial diversity across programs, they are, in general, resource intensive to develop, implement, and maintain (McCall and Cromwell 2011). Although we found that disease management was more effective than mailed education, it was less efficient and its ICER was higher than generally accepted cost-effectiveness thresholds in the United States (Gold et al. 1996). Disease management would become reasonably cost effective if the cost of disease management was lower than $295 per patient per year. Telephone-based care management may be a less costly alternative to the intervention we evaluated, although the impact of this strategy on improving adherence in post-MI patients remains unclear (Wennberg et al. 2010). The results of our analysis suggest that disease management that targeted patients who did not respond to mailed education does not appear reasonably cost effective. However, whether interventions should be targeted to patients who were nonadherent only or all medication takers has yet to be determined (Cutrona et al. 2012).
The complexity of prescribed medications has been identified as another important cause of medication underuse, particularly because patients are required to incorporate their medication taking into their daily schedules and lifestyles (Choudhry et al. 2011c). Interventions, such as polypill use, could make medication-taking behavior simpler, could be scaled to a larger number of patients, and has been particularly advocated in the developing world context (Wise 2005; Gaziano, Opie, and Weinstein 2006). The results of our analysis suggest the widespread use of a secondary prevention polypill, particularly when combined with mailed education, could be cost effective, and potentially cost saving, if its price were less than $100 per month. Our base-case analysis was based on a monthly cost of $200, based upon the price of the current prices of the constituent drugs, which include atorvastatin 80 mg per day. While a polypill is not commercially available in North America or Europe, several combination pills are already available in India. The existing data suggest that a primary prevention polypill (i.e., aspirin, simvastatin, atenolol, ramipril, and thiazide) improves individuals' cardiovascular risk profile to a similar extent as those of its components administered separately (Yusuf et al. 2009; Patel et al. 2010). Of course, the expected benefits of a polypill must be balanced by the fact that even minor side effects to one component of a polypill may lead to its total discontinuation and hence the loss of benefit from all of the component drugs. Even under the most optimistic assumption about the increase in adherence, polypill use was no more reasonably cost effective if it had submultiplicative effect of less than 91 percent effectiveness compared with those of its components administered separately. Ongoing randomized controlled trials are expected to provide further insight into the efficacy as well as the improvement in adherence achieved by the use of a polypill for secondary prevention (http://ClinicalTrials.gov 2011; Sanz et al. 2011).
Our analysis is subject to several limitations. We restricted the set of interventions to those that have specifically been evaluated among patients with the established diagnosis of cardiovascular disease. There are numerous adherence interventions that could possibly be applied but which have not been tested (Peterson, Takiya, and Finley 2003; Kripalani, Yao, and Haynes 2007; Haynes et al. 2008). We focused on patient- or provider-focused interventions to improve medication adherence and did not include policy interventions, such as the elimination of out-of-pocket costs for secondary prevention medications (Choudhry et al. 2011a), as a comparative arm. We assumed that adherence interventions were applied to all medication takers. We assumed that adherence interventions had an equal impact on adherence to each drug component of combination pharmacotherapy. With the exception of a polypill-based intervention, it is not precisely known whether this assumption will hold. We assumed that the marginal impact of each intervention remained constant over time. There is a paucity of long-term data to establish the proportion of patients who will remain adherent once interventions are performed.
Poor adherence to secondary prevention medications remains a significant barrier to the prevention of recurrent morbidity and mortality among post-MI patients. To date, the use of interventions to improve adherence is rare in routine clinical practice. Our comparative effectiveness research suggests that health outcomes coupled with medication adherence can be improved by implementing a mail education program within the resources potentially acceptable in the U.S. health care system. The use of a polypill would have the potential to become cost effective as the medication cost drops, although several key data are currently lacking.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This work was supported by an unrestricted research grant from CVS Caremark to Brigham and Women's Hospital.
Disclosures: None.
Disclaimers: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Table S1: Base-Case Proportion of Patients Adherent to Different Number of Drug Components.
Table S2: Additional Sensitivity Analyses.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agency for Healthcare Research and Quality. 2011. “Closing the Quality Gap Series: Comparative Effectiveness of Medication Adherence Interventions” [accessed on March 31, 2012]. Available at http://www.effectivehealthcare.ahrq.gov/ehc/products/296/764/Medication-Adherence_Protocol_20110818.pdf. [DOI] [PubMed]
- Arias E. 2010. “United States Life Tables, 2006. National Vital Statistics Report. Volume 58, Number 21. Hyattsville, MD. National Center for Health Statistics; 2010” [accessed March 31, 2012]. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_21.pdf.
- Bangalore S, Kamalakkannan G, Parker S, Messerli FH. “Fixed-Dose Combinations Improve Medication Compliance: A Meta-Analysis”. American Journal of Medicine. 2007;120(8):713–9. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Benner JS, Glynn RJ, Mogun H, Weinstein MC, Avorn J. “Long-Term Persistence in Use of Statin Therapy in Elderly Patients”. Journal of the American Medical Association. 2002;288(4):455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- Brønnum-Hansen H, Jørgensen T, Davidsen M, Madsen M, Osler M, Gerdes LU, Schroll M. “Survival and Cause of Death after Myocardial Infarction: The Danish MONICA Study”. Journal of Clinical Epidemiology. 2001;54(12):1244–50. doi: 10.1016/s0895-4356(01)00405-x. [DOI] [PubMed] [Google Scholar]
- Brown JP, Clark AM, Dalal H, Welch K, Taylor RS. “Patient Education in the Management of Coronary Heart Disease”. Cochrane Database of Systematic Review. 2011;12(23):CD008895. doi: 10.1002/14651858.CD008895.pub2. [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. “Trend in Adherence to Secondary Prevention Medications in Elderly Post-Myocardial Infarction Patients”. Pharmacoepidemiology and Drug Safety. 2008a;17(12):1189–96. doi: 10.1002/pds.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry NK, Patrick AR, Antman EM, Avorn J, Shrank WH. “Cost-Effectiveness of Providing Full Drug Coverage to Increase Medication Adherence in Post-Myocardial Infarction Medicare Beneficiaries”. Circulation. 2008b;117(10):1261–8. doi: 10.1161/CIRCULATIONAHA.107.735605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH, Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial “Full Coverage for Preventive Medications for Myocardial Infarction”. New England Journal of Medicine. 2011a;365(12):2088–97. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Patrick AR, Glynn RJ, Avorn J. “The Cost-Effectiveness of C-Reactive Protein Testing and Rosuvastain Treatment for Patients with Normal Cholesterol Levels”. Journal of the American College of Cardiology. 2011b;57(7):784–91. doi: 10.1016/j.jacc.2010.07.059. [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, Pakes J, Brennan TA, Shrank WH. “The Implication of Therapeutic Complexity on Adherence to Cardiovascular Medications”. Archives of Internal Medicine. 2011c;171(9):814–22. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. 2011. “UMPIRE-Use of a Multidrug Pill in Reducing Cardiovascular Events” [accessed on March 31, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT01057537.
- Connor J, Rafter N, Rodgers A. “Do Fixed-Dose Combination Pills or Unit-of-Dose Packaging Improve Adherence? A Systematic Review”. Bulletin of the World Health Organization. 2004;82(12):935–9. [PMC free article] [PubMed] [Google Scholar]
- Cutrona SL, Choudhry NK, Stedman M, Servi A, Liberman JN, Brennan T, Fischer MA, Brookhart MA, Shrank WH. “Physician Effectiveness in Interventions to Improve Cardiovascular Medication Adherence: A Systematic Review”. Journal of General Internal Medicine. 2010;25(10):1090–6. doi: 10.1007/s11606-010-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona SL, Choudhry NK, Fischer MA, Servi AD, Stedman M, Liberman JN, Brennan TA, Shrank WH. “Targeting Cardiovascular Medication Adherence Interventions”. Journal of the American Pharmacists Association. 2012;52(3):381–97. doi: 10.1331/JAPhA.2012.10211. [DOI] [PubMed] [Google Scholar]
- Danchin N, Cucherat M, Thillez C, Durand E, Kadri Z, Steq PG. “Angiotensin-Converting Enzyme Inhibitors in Patients with Coronary Artery Disease and Absence of Heart Failure or Left Ventricular Systolic Dysfunction: An Overview of Long-Term Randomized Controlled Trials”. Archives of Internal Medicine. 2006;166(7):787–96. doi: 10.1001/archinte.166.7.787. [DOI] [PubMed] [Google Scholar]
- Dargie HJ. “Effect of Carvedilol on Outcome after Myocardial Infarction in Patients with Left-Ventricular Dysfunction: The CAPRICORN Randomised Trial”. Lancet. 2001;357(9266):1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- Gaziano TA, Opie LH, Weinstein MC. “Cardiovascular Disease Prevention with a Multidrug Regimen in the Developing World: A Cost-Effectiveness Analysis”. Lancet. 2006;368(9536):679–86. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Seigel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- Gore JM, Granger CB, Simoons ML, Sloan MA, Weaver WD, White HD, Barbash GI, Van de Werf F, Aylward PE, Topol EJ, Califf RM, the GUSTO-I Investigators “Stroke after Thrombolysis. Mortality and Functional Outcomes in the Gusto-I Trial. Global Strategies to Open Occluded Coronary Arteries”. Circulation. 1995;92(10):2811–8. doi: 10.1161/01.cir.92.10.2811. [DOI] [PubMed] [Google Scholar]
- Gunz DA, Kuntz KM, Jacobson GA, Avorn J. “Cost-Effectiveness of 3-Hydroxy-3-Methilglutaryl Coenzyme A Reductase Inhibitor Therapy in Older Patients with Myocardial Infarction”. Annals of Internal Medicine. 2000;132(10):780–7. doi: 10.7326/0003-4819-132-10-200005160-00003. [DOI] [PubMed] [Google Scholar]
- Hanmer JW, Lawrence F, Anderson JP, Kaplan RM, Fryback DG. “Report of Nationally Representative Values for the Noninstitutionalized US Adult Population for 7 Health-Related Quality-of-Life Scores”. Medical Decision Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- Haynes RB, Yao X, Kripalani S, Garg A, McDonald HP. “Interventions for Enhancing Medication Adherence”. Cochrane Database of Systematic Review. 2008;2:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. “Medication Nonadherence Is Associated with a Broad Range of Adverse Outcomes in Patients with Coronary Artery Disease”. American Heart Journal. 2008;155(4):772–9. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Jakevicius CA, Li P, Tu JV. “Prevalence, Predictor, and Outcomes of Primary Nonadherence after Acute Myocardial Infarction”. Circulation. 2008;117(8):1028–36. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- Kramer JM, Hammill B, Anstrom KJ, Fetterolf D, Snyder R, Charde JP, Hoffman BS, Allen LaPointe N, Peterson E. “National Evaluation of Adherence to β-Blocker Therapy for 1 year after Acute Myocardial Infarction in Patients with Commercial Health Insurance”. American Heart Journal. 2006;152(3):454. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Kripalani S, Yao X, Haynes RB. “Interventions to Enhance Medication Adherence in Chronic Medical Conditions. A Systematic Review”. Archives of Internal Medicine. 2007;167(6):540–50. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- Kushner FG, Hand M, Anderson SC, Jr, Smith SB, 3rd, King JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Green DE, Jr, Casey LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines “2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines”. Circulation. 2009;120(22):2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. “The Polypill in the Prevention of Cardiovascular Diseases: Key Concepts, Current Status, Challenges, and Future Directions”. Circulation. 2010;122(11):2078–88. doi: 10.1161/CIRCULATIONAHA.109.873232. [DOI] [PubMed] [Google Scholar]
- McCall N, Cromwell J. “Results of the Medicare Health Support Disease-Management Pilot Program”. New England Journal of Medicine. 2011;365(18):1704–12. doi: 10.1056/NEJMsa1011785. [DOI] [PubMed] [Google Scholar]
- Muntner P, Mann D, Wildman RP, Shimbo D, Fuster V, Woodward M. “Projected Impact of Polypill Use among US Adults: Medication Use, Cardiovascular Risk Reduction, and Side Effects”. American Heart Journal. 2011;161(4):719–25. doi: 10.1016/j.ahj.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, Stroupe KT, Wu J, Clark D, Smith F, Gradus-Pizlo I, Weinberger M, Brater DC. “Pharmacist Intervention to Improve Medication Adherence in Heart Failure: A Randomized Trial”. Annals of Internal Medicine. 2007;146(10):714–25. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- Newby LK, Allen LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. “Long-Term Adherence to Evidence-Based Secondary Prevention Therapies in Coronary Artery Disease”. Circulation. 2006;113(2):203–12. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- Nichol G, Kaul P, Huszti E, Bridges JF. “Cost-Effectiveness of Cardiac Resynchronization Therapy in Patients with Symptomatic Heart Failure”. Annals of Internal Medicine. 2004;141(5):343–51. doi: 10.7326/0003-4819-141-5-200409070-00102. [DOI] [PubMed] [Google Scholar]
- Patel A, Shah T, Shah G, Jha V, Ghosh C, Desai J, Khamar B, Chackraborty BS. “Preservation of Bioavailability of Ingredients and Lack of Drug-Drug Interactions in a Novel Five-Ingredient Polypill (Polycap): A Five-Arm Phase I Crossover Trial in Healthy Volunteers”. American Journal of Cardiovascular Drugs. 2010;10(2):95–103. doi: 10.2165/11532170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Peterson AM, Takiya L, Finley R. “Meta-Analysis of Trials of Interventions to Improve Medication Adherence”. American Journal of Health-System Pharmacy. 2003;60(7):657–65. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- Rasmussen JN, Chong A, Alter DA. “Relationship between Adherence to Evidence-Based Pharmacotherapy and Long-Term Mortality after Acute Myocardial Infarction”. Journal of the American Medical Association. 2007;297(2):177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, Ford G, de Simone ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee “Heart Disease and Stroke Statistics-2011 Update: A Report from the American Heart Association”. Circulation. 2011;123(4):e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz G, Fuster V, Guzmán L, Guglietta A, Arnáiz JA, Martízez F, Sarria A, Roncaglioni MC, Taubert K. “The Fixed-Dose Combination Drug for Secondary Cardiovascular Prevention Project: Improving Equitable Access and Adherence to Secondary Cardiovascular Prevention with a Fixed-Dose Combination Drug. Study Design and Objectives”. American Heart Journal. 2011;162(5):811–7. doi: 10.1016/j.ahj.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Shrive FM, Manns BJ, Galbraith PD, Knudtson ML, Ghali WA, APPROACH Investigators “Economic Evaluation of Sirolimus-Eluting Stents”. Canadian Medical Association Journal. 2005;172(3):345–51. doi: 10.1503/cmaj.1041062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Kramer JM, Perrin N, Platt R, Roblin DW, Lane K, Goodman M, Nelson WW, Yang X, Soumerai SB. “A Randomized Trial of Direct-to-Patient Communication to Enhance Adherence to β-Blocker Therapy Following Myocardial Infarction”. Archives of Internal Medicine. 2008;168(5):477–83. doi: 10.1001/archinternmed.2007.132. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Labor. 2012. “U.S. Consumer Price Index for Medical Care for All Urban Consumers” [accessed on March 31, 2012]. Available at http://www.bls.gov/cpi/
- Wennberg DE, Marr A, Lang L, O'Malley S, Bennett G. “A Randomized Trial of a Telephone Case-Management Strategy”. New England Journal of Medicine. 2010;363(13):1245–55. doi: 10.1056/NEJMsa0902321. [DOI] [PubMed] [Google Scholar]
- Wise J. “Polypill Holds Promise for People with Chronic Disease”. Bulletin of the World Health Organization. 2005;83(12):885–7. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2003. “Adherence to Long-Term Therapies: Evidence for Action.” [accessed on March 31, 2012]. Available at http://www.who.int/chp/knowledge/publications/adherence_report/en/
- Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, Labarthe DR. “Impact of Stroke on Health-Related Quality of Life in the Noninstitutionalized Population in the United States”. Stroke. 2006;37(10):2567–72. doi: 10.1161/01.STR.0000240506.34616.10. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Pais P, Afzal R, Xavier D, Teo K, Eilkelboom J, Sigamani A, Mohan V, Guputa R, Thomas N, Indian Polycap Study (TIPS) “Effects of a Polypill (Polycap) on Risk Factors in Middle-Aged Individuals without Cardiovascular Disease (TIPS): A Phase II, Double-Blind, Randomized Trial”. Lancet. 2009;373(9672):1341–51. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


