Abstract
Aims: We tested whether an exposure to alcohol in late adolescence, an age of rapid increase in neuroactive steroid precursors, would increase voluntary alcohol consumption in adult rats and whether this effect would be modulated by finasteride, an inhibitor of neuroactive steroid synthesis. Methods: In Experiment 1, we exposed male Wistar rats to 8% alcohol during the dark cycle for 1 week during late adolescence [postnatal days (PNDs) 51–58], and then measured voluntary alcohol consumption 1 month later in adulthood (PNDs 91–104). In Experiment 2, finasteride was administered during the forced alcohol exposure in late adolescence and, in Experiment 3, during voluntary alcohol consumption in adulthood. Plasma was collected at the end of each finasteride treatment to confirm the reduction of plasma neuroactive steroid levels. Results: We found that a daily 12-h exposure to alcohol for 7 days in late adolescence significantly increased voluntary alcohol consumption (4-fold) a month later during adulthood. Finasteride administration in late adolescence increased group alcohol intake in late adolescence but did not block the effect of adolescent alcohol exposure on increasing alcohol preference in adulthood. There was no effect of finasteride treatment in adulthood on alcohol preference. Conclusions: A daily 12-h exposure to alcohol for 7 days in late adolescence was sufficient to induce chronically increased alcohol preference in adulthood, indicating that this age may be sensitive to the effects of alcohol.
INTRODUCTION
A national survey found that a majority of high school students had tried alcohol (Johnston et al., 2006). The survey also found that in addition to widespread alcohol use, many adolescents reported consuming five or more drinks in a single occasion. The prevalence of binge drinking in youth is of concern, as it has been found that even a short pattern of binge drinking in rats can lead to more prominent brain damage in adolescents than in adults (Crews et al., 2000). Overall, alcohol consumption in human adolescents can have negative effects on normal brain development, disrupting structure (De Bellis et al., 2000) as well as function (Squeglia et al., 2009).
Alcohol use at adolescence is also a strong predictor of later alcohol problems in humans. An epidemiologic survey revealed that individuals who started drinking before age 15 had a 40% rate of lifetime alcohol dependence, and that this rate dropped to 10% in individuals who started drinking at age 20 and older (Grant and Dawson, 1997). Little is known about the potential biological basis as to why the early onset of alcohol use is a predictor for the development of alcohol abuse and dependence, and why adolescence is a particularly sensitive age.
Given the high prevalence and potential impacts of alcohol consumption by youth, alcohol exposure paradigms using animal models that reliably increase later adult alcohol preference are needed to provide translational models of human adolescent alcohol exposure. Although the exact time frame of adolescence in the rat is still under debate, the age of adolescence has been broadly defined to extend from postnatal days (PNDs) 28–60 in male rats (Spear, 2000). Others have shown that in male rats, increases in sex steroid hormone levels are most rapid between PNDs 50 and 65 (Korenbrot et al., 1977). Most studies of the effects of early alcohol exposure on drinking preferences and other endophenotypes have focused on rodents in early adolescence (PNDs 21–34) and mid-adolescence (PNDs 34–46) (Spear, 2000; Slawecki and Betancourt, 2002; Varlinskaya and Spear, 2004; Siegmund et al., 2005; Vetter et al., 2007; Tambour et al., 2008; Garcia-Burgos et al., 2009; Maldonado-Devincci et al., 2010a, 2010b).
Studies that examine the long-term effects of adolescent alcohol exposure on subsequent alcohol preference have reported that binge alcohol exposure in early to mid-adolescence (PNDs 28–45) increases sweetened alcohol intake in young adulthood (PNDs 60–69) in male and female rats, but not if drinking was assessed slightly later in adulthood (PNDs 72–81) (Maldonado-Devincci et al., 2010a). Others reported that alcohol exposure in early to mid-adolescence (PNDs 28–40) did not have any significant effect on ethanol intake in adulthood (Slawecki and Betancourt, 2002; Vetter et al., 2007). Interestingly, we were unable to identify studies that employed alcohol exposure focused on late adolescence (e.g. PNDs 50–60). Examination of the effects of alcohol exposure during late adolescence when neuroactive steroid precursors are rapidly increasing may be of particular interest.
The adolescent period during which human alcohol exposure predicts increased risk of alcohol problems in adulthood is also the age when puberty occurs in humans (Tanner and Whitehouse, 1976). This is an age characterized by multiple hormonal changes, including marked increases in androgens and estrogens (Courant et al., 2010), accompanied by a significant increase in the levels of the GABAergic neuroactive steroid allopregnanolone in both males and females (Fadalti et al., 1999). Neuroactive steroids are endogenous, highly potent, positive allosteric modulators of GABAA receptor function (Paul and Purdy, 1992; Belelli and Lambert, 2005), which are synthesized endogenously in the brain as well as in peripheral tissues, such as the adrenals and liver, via the sequential action of the enzymes 5α-reductase (5α-R) and 3α-hydroxysteroid dehydrogenase (3α-HSD) (Mellon and Griffin, 2002).
Neuroactive steroids have anticonvulsant (Belelli et al., 1989), antinociceptive (Nadeson and Goodchild, 2001), antidepressant (Khisti et al., 2000; Uzunova et al., 2006) and anxiolytic (Bitran et al., 1991; Wieland et al., 1991; Akwa et al., 1999) properties. They have also been hypothesized to be involved in alcohol effects, tolerance and withdrawal (Morrow et al., 2001) as well as alcohol dependence (Milivojevic et al., 2011). Preclinical (Sanna et al., 2004) and clinical (Torres and Ortega, 2004) studies have shown that acute, high-dose alcohol exposure increases the production of neuroactive steroids, and that inhibition of 5α-reductase by finasteride can block this effect in rodents (VanDoren et al., 2000). Interestingly, more recent studies indicate that moderate doses of alcohol increase serum neuroactive steroids in rats, but not in mice, monkeys or humans (Porcu et al., 2010).
Pharmacologic manipulation of neuroactive steroid levels has been reported to moderate alcohol consumption in rodents. Administration of allopregnanolone, one of the most potent and widely studied neuroactive steroids, increases alcohol intake in male mice and rats (Janak et al., 1998; Sinnott et al., 2002; Ford et al., 2005b) but not female mice (Ford et al., 2008a) while blockade of neuroactive steroid synthesis with finasteride decreased alcohol consumption (Ford et al., 2005a, 2008a, 2008b). Others have found that administration of the neurosteroid precursor pregnenolone reduces alcohol consumption in alcohol-conditioned rats, implying that increased levels of neuroactive steroids may reduce alcohol consumption (Besheer et al., 2010).
We used a rat model to study the effects of a 1-week, 12 h per day forced alcohol exposure via drinking water in late adolescence (PNDs 51–58) on levels of voluntary alcohol consumption in adulthood (PNDs 91–104). As late adolescence is an age characterized by hormonal changes, we examined the potential role of the sex hormone-derived neuroactive steroid allopregnanolone in this process by blocking its synthesis with finasteride. We also examined the effects of finasteride treatment on the neuroactive steroid precursor pregnenolone. Based on existing literature, our hypothesis was that alcohol exposure in late adolescence would increase alcohol intake in adulthood and that blockade of neuroactive steroid synthesis during alcohol exposure in late adolescence would prevent this increased adult alcohol intake. Since many effects of alcohol are thought to be mediated through neuroactive steroid modulation of GABAA receptors, we hypothesized that a decreased neuroactive steroid response would reduce the effect of alcohol in adolescence and thereby blunt the reacquisition of alcohol consumption in adulthood. Administration of finasteride in adulthood after a week of voluntary drinking was conducted with the hypothesis that finasteride treatment would decrease the maintenance of elevated alcohol consumption that resulted from alcohol exposure in late adolescence.
MATERIALS AND METHODS
Ethics statement
All procedures were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The University of Connecticut Health Center (UCHC) is fully accredited by the American Association for Accreditation of Laboratory Care, Int'l (AAALAC), and holds an approved NIH Assurance and USDA License. All animal procedures were approved by the UCHC Animal Care Committee (ACC) permit # 100250-0614.
Animals
Time-pregnant Wistar rats (Harlan Laboratories, Indianapolis, IN, USA) were delivered to the University of Connecticut Health Center (UCHC) Animal Care Center at gestation day 16. Following birth, male rats from 8 to 9 litters for each experiment were weaned at PND 22 and divided into experimental groups that included balanced numbers of offspring from all litters to avoid litter effects on results. Rats were housed four to five per cage under a 12 h light, 12 h dark cycle (lights on from 0700 to 1900 hours) and at a constant temperature of 71°F and relative humidity of 50%. Rats had free access to standard laboratory food and water at all times, except during the forced alcohol exposure at which they had access to 8% alcohol instead of water during the dark cycle.
Procedure
All experiments consisted of three phases. Phase 1 consisted of PND 51–58 alcohol exposure ± finasteride. Phase 2 consisted of a 1-month alcohol abstinence which began on PND 58 and continued through PND 91. Phase 3 consisted of a voluntary alcohol assessment in adulthood which began on PND 91 and continued until PND 104 ± finasteride on PND 97–103.
Experiment 1: effect of alcohol exposure in late adolescence
Alcohol exposure
Ethanol (99.5%; Sigma- Aldrich) was diluted in tap water to an 8% concentration (v/v). In two separate runs of Experiment 1, animals were exposed to 8% alcohol (n = 24; six cages of four animals per cage) for 7 days beginning on PND 51. Due to the gregarious nature of rats and to avoid the confounding effects of isolation stress in late adolescence on alcohol intake and later preference, rats were housed in groups of four during this 7-day alcohol exposure period. The 8% alcohol solution was presented in the cage bottles as the only choice during the dark phase of the light–dark cycle. The amount consumed was measured at the end of the exposure. The control group (combined n = 23) was housed in the same manner, with access to regular drinking water. Throughout the entire experiment, animals had free access to standard laboratory food.
Voluntary drinking (alcohol preference) assessment
On PNDs 89–90, rats were isolated and allowed to habituate to single housing for 1–2 days. Beginning on PND 91, voluntary alcohol consumption was assessed for each animal in single housing by means of a continuous (i.e. 24 h/day), free choice between a bottle of water and a bottle containing 8% alcohol. The consumption was measured daily for 2 weeks. Bottle positions were switched daily in order to avoid any side preferences. Water and alcohol loss were measured in a separate cage without animals. The daily value of this loss was subtracted from the daily amounts of water and alcohol consumed by the animals. An alcohol preference was calculated by taking the amount of alcohol consumed and dividing it by the alcohol plus water consumed. Alcohol intake was calculated as grams of ethanol per kilogram of body weight.
Weights
Rats were weighed at each experimental phase in order to identify whether alcohol consumption impacted growth in the alcohol-exposed group.
Experiment 2: finasteride treatment paired with alcohol exposure in late adolescence
Rats were exposed to alcohol PNDs 51–58 as described above. Finasteride was commercially purchased from Sigma-Aldrich and administered intraperitonally (i.p.) at 50 mg/kg diluted in 1 ml of 20% w/v 2-hydroxypropyl-beta-cyclodextrin in saline. The dose of finasteride was chosen based on the previous studies in rodents (Lephart et al., 1996; VanDoren et al., 2000). A 2 × 2 design with drug treatment and alcohol exposure as between-subject factors resulted in four groups. One group received daily finasteride injections (n = 11) immediately prior to the overnight forced alcohol exposures. Another group received finasteride prior to water exposure (n = 10). A third group received vehicle injections prior to overnight alcohol exposures (n = 10) and a fourth received vehicle injections paired with water exposure (n = 10). Experimental injections were administered on PNDs 51–57 between 1500 and 1700 hours. Rats were injected with vehicle (20% w/v 2-hydroxypropyl-beta-cyclodextrin in saline) for 3 days prior to PND 51 finasteride or vehicle treatments to habituate to the injection procedure. Voluntary drinking was then measured a month later in adulthood (PNDs 91–104) as described for Experiment 1.
Experiment 3: finasteride treatment during voluntary drinking assessment in adulthood
Rats were exposed to alcohol or water in late adolescence as described in Experiment 1. A month later, alcohol preference was assessed as in Experiment 1, except that on the afternoon of PNDs 97–103, during the alcohol preference assessment phase rats were given daily i.p. injections of finasteride (50 mg/kg) or vehicle in a 2 × 2 design with adolescent alcohol exposure and adulthood drug treatment as between-subject factors, resulting in four groups. One group received finasteride (n = 7), having had forced alcohol exposure in late adolescence. Another group received finasteride, having had water exposure in late adolescence (n = 7). A third group received vehicle injections (n = 8), having had forced alcohol exposure in late adolescence and a fourth group received vehicle injections (n = 8), having had water exposure in late adolescence. Injections were administered daily on the last 7 days of the 14-day voluntary drinking assessment, between 1500 and 1700 hours. All animals received vehicle injections for 2 days prior to the finasteride/vehicle treatment to habituate to the injection procedure.
Plasma collection
Plasma was collected at the end of each finasteride treatment period (i.e. late adolescence or adulthood) to measure the effect of finasteride on peripheral neuroactive steroid levels as well as for analysis of blood ethanol concentrations using an Analox Alcohol Analyzer (Analox Instruments USA, Lunenburg, MA, USA). A subgroup of animals from each of the initial experimental groups was sacrificed by decapitation following deep isofluorane anesthesia. Trunk blood was collected into heparin tubes and placed immediately on ice. The animals were sacrificed in the morning (0900 to 1100 hours) to avoid circadian fluctuation in neuroactive steroid levels (Corpechot et al., 1997). The heparized blood samples were centrifuged at 3000 rpm (4°C) for 10 min, and the resulting plasma stored at −80°C. It is of note here that because the animals were sacrificed beginning at 0900 hours and the alcohol bottles were removed from cages at 0700 hours, we could not detect ethanol in the plasma samples above instrumentation background.
Neuroactive steroid analysis
Steroid extraction and separation
Plasma levels of allopregnanolone (5α-pregnan-3α-ol-20-one) and pregnenolone were measured using gas chromatography/mass spectrometry (GC/MS) with electron capture-negative chemical ionization. Steroids were extracted using the slightly modified methods described in Vallee et al. (2000). In brief, 0.3 mL of plasma was combined with 4 µl of an internal standard solution containing D4-5β-pregnane-3α-ol-20-one (24 pg/µl) and D4-pregnenolone (32 pg/µl) (Cambridge Isotope Laboratories, Andover, MA, USA) in order to control for variation in sample-to-sample extraction and derivatization efficiencies. An aliquot of 500 µl of methanol/water (v/v) was added to each sample, and vortex mixed. In parallel, a six-level, 2-fold dilution series of known concentrations of neuroactive steroid standards was prepared as above. The samples were then diluted with deionized water to a final concentration of 5% methanol. Samples were then extracted using C-18 solid-phase columns (Bond Elut 200 mg; Varian, Inc., Lake Forest, CA, USA), previously conditioned with 100% methanol (6 ml) followed by 5% methanol (6 ml). Following application of samples, C-18 cartridges were washed three times with 3 ml of 5% methanol (9 ml total) and neuroactive steroids eluted with 100% methanol (2 ml) into sialinized tubes, and then dried in a rotary evaporator for ∼2 h.
Derivatization
Neuroactive steroids were derivatized according to the method described in Ostlund et al. (1996), with slight modifications. Batches of eight samples were dissolved in 50 µl toluene, vortex mixed and allowed to sit at room temperature (RT) for 5–10 min in order to solubilize dried samples. An aliquot of 25 µl of 40% pyridine (pyridine/toluene; 40/60 v/v) was then added, vortex mixed and allowed to sit at RT for 3 min. Then, 25 µl of 10% pentafluorobenzoyl chloride (PFBC) (PFBC/toluene; 10/90 v/v) was added, vortex mixed and incubated for 10–12 min at RT. Samples were then mixed with 0.6 ml of deionized water and vortex mixed three times for 10 s each. The steroids were then extracted into an organic phase (1 ml of hexane applied twice) by vortex mixing for 20 s. The pooled hexane extracts were then dried in a rotary evaporator for ∼2 h.
Instrumentation
Derivatized samples were analyzed by gas chromatography (GC) coupled with electron capture-negative chemical ionization mass spectrometry (MS) according to the method described in Kim et al. (2000), with slight modifications. The analysis was performed using a Hewlett–Packard (HP) 5890 gas chromatograph coupled to an HP 5989B mass spectrometer using a select ion monitoring mode for each compound (512.5 m/z for allopregnanolone and 510.5 m/z for pregnenolone) to further increase sensitivity. A thin-film capillary column (30 m × 0.25 mm, 0.1 µm film thickness; Restek, Bellefonte, PA, USA) was used, which allowed the resolution of all four isoforms of tetrahydroprogesterone. The ratio of the molecular ion peak area for each steroid relative to the corresponding area of the density-labeled internal standard was calculated for each sample, and using a standard curve generated with each run, this ratio was converted to steroid concentrations. Each plasma sample was extracted and assayed in duplicate with the sample average used for data analysis.
Statistical analysis
Statistical analyses were performed using SPSS software (version 15, SPSS Inc., Chicago, IL, USA). Figures were created with Prism 5 (GraphPad Software Inc., San Diego, CA, USA). Total liquid intake during late adolescence for water vs. alcohol exposure was compared by the t-test. Change in weight as a function of time and group assignment was evaluated by linear mixed model analysis. Overnight alcohol intake during adolescent forced alcohol ± finasteride exposure (PNDs 51–58) was analyzed using repeated-measures ANOVA with day as within and finasteride treatment as between-subject factors. Alcohol preference and ethanol intake in adulthood were analyzed using repeated-measures ANOVA with day as within and alcohol treatment as between-subject factors. Analysis focused on voluntary drinking data for PNDs 95–104, as the drinking for PNDs 91–94 was variable and in general rapidly declining for all groups. Allopregnanolone and pregnenolone levels after late adolescent or adult finasteride treatment were analyzed using linear mixed models with the neuroactive steroids levels as the dependent variable, and alcohol and finasteride treatment as between-subject factors. A P-value of ≤0.05 was considered statistically significant.
RESULTS
Liquid intake and weights
The average amount of ethanol consumed per night during the late adolescence alcohol exposure was 3.2 ± 0.1 g/kg in Experiment 1, 3.3 ± 0.1 g/kg in the vehicle treated group in Experiment 2 and 3.6 ± 0.1 g/kg in Experiment 3. There were no differences in the total liquid consumed between the alcohol exposed and the control group during the PND 51–58 experimental late adolescence phase. Similarly, there were no differences in weights between groups (Fig. 1) at any phase of the experiments, although weights increased ∼2-fold with increasing age in all experiments [(F(1,92) = 595.65; P < 0.001) Experiment 1, Fig. 1A; (F(1,124) = 822.46; P < 0.001) Experiment 2, Fig. 1B; (F(1,104) = 555.97; P < 0.001) Experiment 3, Fig. 1C].
Fig. 1.
Weights at all phases of experiments. Weights were measured for water and alcohol exposed groups at all phases of Experiment 1 (A), Experiment 2 (B) and Experiment 3 (C). Data are displayed as mean ± SEM weight (in g).
Experiment 1: effects of alcohol exposure in late adolescence on adult alcohol preference
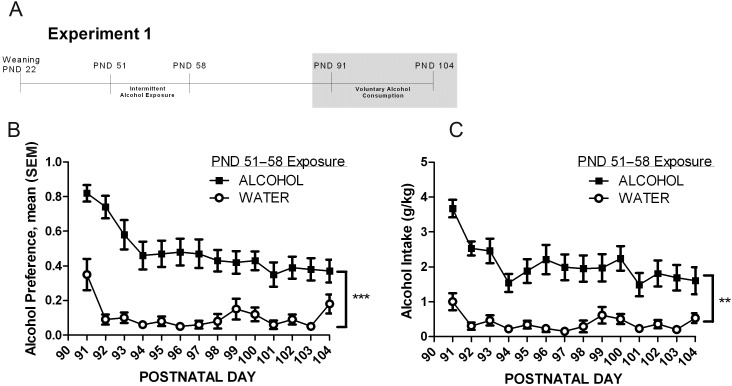
One month following a 7-day forced exposure to alcohol in late adolescence (PNDs 51–58), voluntary drinking was measured (PNDs 91–104) and daily alcohol preference and intake was calculated (see timeline, Fig. 2A). There were marked reductions in alcohol preference over the first 4 days in all groups (and in all experiments). After the first 4 days, alcohol preference stabilized at a mean of 42 ± 2% in the alcohol-exposed group compared with a mean of 9 ± 1% in the control group for the remaining 10 days of assessment (Fig. 2B). Alcohol preference was significantly higher (4-fold) during PNDs 95–104 in the group that experienced alcohol each night for 7 days in late adolescence (F(1,18) = 20; P < 0.001). Similarly, stable alcohol intake in the last 10 days of assessment in adulthood was 5-fold higher in the group that was exposed to alcohol in late adolescence (F(1,18) = 13.5; P = 0.002), where the alcohol exposed group had a mean daily alcohol intake of 1.9 ± 0.01 g/kg compared with a mean of 0.3 ± 0.05 g/kg in the control group (Fig. 2C).
Fig. 2.
Alcohol preference and alcohol intake in adulthood. (A) Experimental timeline with shaded bar indicating period of alcohol preference and alcohol intake measurement data. (B and C) Data are displayed as mean ± SEM of alcohol preference (B) and alcohol intake (g/kg) (C) measured daily over 14 days in adulthood, 1 month after an intermittent, 7-day exposure to 8% alcohol (ALCOHOL, n = 12) or water (CONTROL, n = 10). Main effects of alcohol exposure: **P < 0.01 and ***P < 0.001.
Experiment 2: effect of finasteride paired with alcohol exposure in late adolescence on alcohol intake
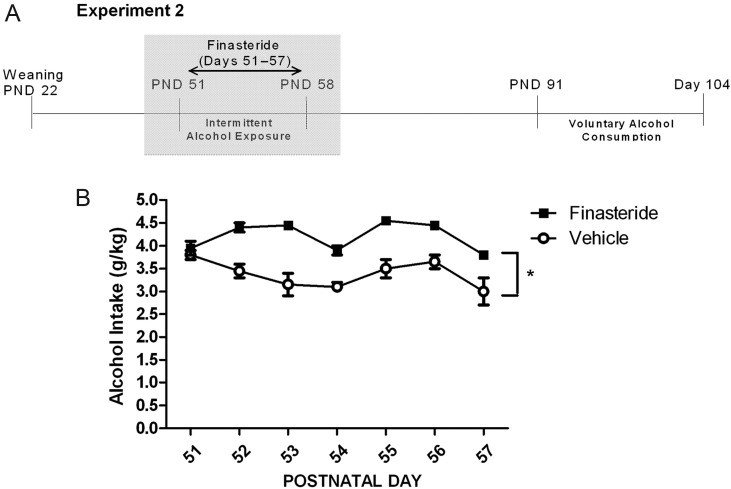
Alcohol intake was measured on mornings of PNDs 52–58 after the overnight availability of 8% alcohol as the only liquid choice in group housed rats receiving either vehicle or finasteride injections daily (see timeline, Fig. 3A). During this treatment, animals were in group-housing (five to six animals per cage, two cages per group) to avoid confounding interactive effects of social isolation stress and alcohol/finasteride treatment on subsequent voluntary drinking in adulthood. Alcohol intake per cage for each night was converted to average intake per rat expressed as grams per kilogram based on the average weight of animals at the end of the forced alcohol exposure period. Finasteride treatment during forced alcohol exposure on PNDs 51–58 increased alcohol intake 1.2-fold (F(1,2) = 21.9; P = 0.043), with a mean alcohol intake of 4.2 ± 0.1 g/kg in the finasteride group and 3.4 ± 0.1 g/kg in the vehicle group (Fig. 3B). Finasteride had no effect on volume of water consumed in the non-alcohol exposed groups.
Fig. 3.
Alcohol intake during finasteride treatment in late adolescence. (A) Experimental timeline with shaded bar indicating period of alcohol intake measurement data. (B) Alcohol intake was measured overnight as the only choice of liquid during the 7-day finasteride (n = 11) or vehicle (n = 10) treatment. Data are displayed as mean ± SEM of average intake per rat (in g/kg). Main effect of finasteride exposure: *P < 0.05.
Alcohol preference and alcohol intake were then measured a month later for 14 days in adulthood (PNDs 91–104) (see timeline, Fig. 4A). As in Experiment 1, there were marked reductions in alcohol consumption during the first 4 days, after which drinking stabilized. We, thus, focused our analysis on the stable alcohol preference and intake period, PND 95–104. Adolescent forced alcohol exposure increased alcohol preference 3.9-fold (F(1,17) = 20.6; P < 0.001), where the mean alcohol preferences in the alcohol-vehicle group was 33 ± 2 and 45 ± 2% in the alcohol–finasteride group (Fig. 4B) compared with 13 ± 2% in the water vehicle and 7 ± 1% in the water–finasteride group (Fig. 4C). Repeated-measures ANOVA revealed no statistically significant effects of finasteride or alcohol × finasteride interaction on alcohol preference for PNDs 95–104. Similarly, adolescent alcohol exposure increased adulthood alcohol intake 3.6-fold (F(1,17) = 29.3; P < 0.001), where the mean alcohol intake in the alcohol–vehicle group was 1.5 ± 0.11 and 2.1 ± 0.10 g/kg in the alcohol–finasteride group (Fig. 4D) compared with 0.7 ± 0.12 g/kg in the water–vehicle and 0.3 ± 0.09 g/kg in the water–finasteride group (Fig. 4E). Contrary to our hypothesis, finasteride did not block late adolescence alcohol exposure effects on alcohol preference or intake. When we analyzed all days of assessment, there was a trend toward a significant alcohol–finasteride interaction in alcohol preference (P = 0.065) and alcohol intake (P = 0.058). This trend level interaction was driven by the first 4 days of alcohol preference and intake assessment, where alcohol preference was elevated but rapidly declining in all conditions.
Fig. 4.
Adult alcohol preference and intake following paired alcohol exposure and finasteride treatment in late adolescence. (A) Experimental timeline with shaded bar indicating period of alcohol preference measurement data. (B, C, D and E) Voluntary alcohol consumption was measured as a choice between 8% alcohol and water in groups that were exposed in late adolescence to alcohol and finasteride (AlcFin, n = 7), or vehicle (AlcVeh, n = 6) (B, D), and groups that were exposed in late adolescence to water and finasteride (WatFin, n = 6) or vehicle (WatVeh, n = 6) (C, E). Data are displayed as mean ± SEM of alcohol preference score (B, C) and average alcohol intake per rat in g/kg (D, E).
Experiment 3: effect of adult finasteride treatment on alcohol preference following alcohol exposure in late adolescence
Rats were exposed to 8% alcohol or water on each night of PNDs 51–58 and then voluntary drinking was measured a month later for 14 days in adulthood (PNDs 91–104). During the last 7 days of the voluntary drinking measurement, finasteride was administered daily (see timeline, Fig. 5A) in order to test whether blockade of neuroactive steroid synthesis would reduce the maintenance of increased alcohol consumption resulting from forced alcohol exposure in late adolescence. Finasteride was given beginning at day 7 (rather than day 1) to allow rats to reach a stable drinking pattern before testing the effect of finasteride. Repeated-measures ANOVA was used to analyze alcohol preference separately for days 5–7, before finasteride was administered, and the days 8–14 during finasteride administration. During the 3-day period prior to finasteride administration (PNDs 95–97), forced alcohol exposure in late adolescence resulted in a 7-fold increase in alcohol preference (F(1,25) = 15.2; P = 0.001) (Fig. 5B), with a mean preference of 36 ± 3% for the alcohol group vs. a mean preference of 5 ± 1% in the control group. Similarly, adolescent alcohol exposure resulted in an 8-fold increase in alcohol intake (F(1,25) = 18.9; P < 0.001) (Fig. 5D), with a mean intake of 1.6 ± 0.1 g/kg for the alcohol group vs. a mean intake of 0.1 ± 0.01 g/kg for the control group. During the 7 days of finasteride or vehicle administration, forced alcohol exposure in late adolescence resulted in an average 4.2-fold increase in alcohol preference for the two groups (F(1,25) = 15.64; P = 0.001) (Fig. 5C) and a 4.4-fold increase in alcohol intake (F(1,25) = 15.28; P = 0.001) (Fig. 5E), with alcohol preferences of 34 ± 2% in the alcohol groups compared with 7 ± 7% in the control groups and alcohol intakes of 1.6 ± 0.2 g/kg in the alcohol groups and 0.3 ± 0.1 g/kg in the control groups. There was no effect of finasteride treatment or alcohol–finasteride interaction during the 7-day period of adulthood finasteride or vehicle administration on either alcohol preference or alcohol intake.
Fig. 5.
Alcohol preference and intake before and during finasteride treatment in adulthood. (A) Experimental timeline with shaded bar indicating period of alcohol preference measurement data. (B, C, D and E) Voluntary, free-choice alcohol consumption was measured for 7 days (B, D), after which finasteride or vehicle was administered daily (C, E) for groups exposed previously to alcohol or water in late adolescence (Alc_Fin, n = 7; Alc_Veh, n = 8; Wat_Fin, n = 7; Wat_Veh, n = 8). Data are displayed as mean ± SEM of alcohol preference score (B, C) and average intake per rat in g/kg (D, E). Main effects of alcohol: ***P ≤ 0.001.
Neuroactive steroid levels
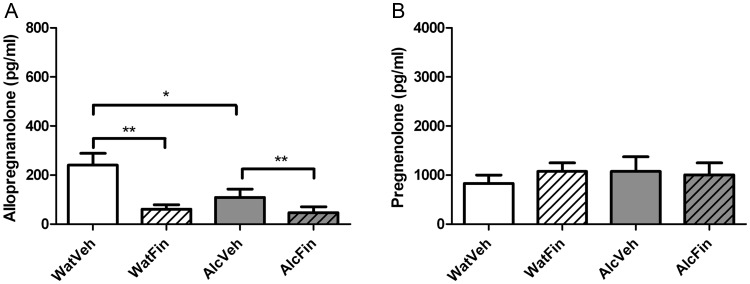
We measured levels of allopregnanolone (5α-pregnan-3α-ol-20-one) and pregnenolone in plasma using GC/MS after finasteride treatment in late adolescence and in adulthood. In adolescence (Fig. 6), both alcohol (F(1,12) = 4.76; P = 0.05) and finasteride (F(1,12) = 13.18; P = 0.003) treatment reduced allopregnanolone levels compared with the water vehicle group by 75 and 55%, respectively, but there was no significant alcohol–finasteride interaction (Fig. 6A). In contrast, the levels of pregnenolone, a precursor to progesterone and allopregnanolone, were unchanged by either PND 51–58 alcohol exposure or finasteride exposure, and there was no alcohol–finasteride interaction effect (Fig. 6B).
Fig. 6.
Neuroactive steroid levels after finasteride treatment in late adolescence. Levels of allopregnanolone (A) and pregnenolone (B) were measured using GC/MS after pubertal exposure to water and vehicle (WatVeh, n = 4), water and finasteride (WatFin, n = 4), alcohol and vehicle (AlcVeh, n = 4) and alcohol and finasteride (AlcFin, n = 4). Data are displayed as mean ± SEM (in pg/ml). Statistics of main effects of alcohol and finasteride for allopregnanolone levels are indicated as *P ≤ 0.05; **P < 0.01.
In adulthood (Fig. 7), adult PND 97–103 finasteride treatment reduced allopregnanolone levels by 83% (F(1,26) = 10.52; P = 0.003), but there was no significant effect of prior PND 51–58 alcohol treatment or alcohol–finasteride interaction effects (Fig. 7A). Levels of pregnenolone at PND 104 were unaffected by PND 51–58 alcohol treatment or PND 97–103 finasteride treatment, and there was no alcohol–finasteride interaction effect (Fig. 7B).
Fig. 7.
Neuroactive steroid levels after finasteride treatment in adulthood. Levels of allopregnanolone (A) and pregnenolone (B) were measured using GC/MS after finasteride treatment in the last week of the 2-week voluntary drinking assessment in a group exposed to water in late adolescence (WatFin, n = 7) and a group exposed to alcohol in late adolescence (AlcFin, n = 7). Control groups consisted of a group exposed to water in late adolescence and vehicle injections in adulthood (WatVeh, n = 8) and a group exposed to alcohol in late adolescence and vehicle in adulthood (AlcVeh, n = 8). Data are displayed as mean ± SEM (in pg/ml). Main effects of finasteride: **P < 0.01.
Finally, allopregnanolone and pregnenolone levels increased with age. There was a 1.8-fold increase in allopregnanolone from PND 58 to 104 and a 2-fold increase in pregnenolone during the same timeframe in the control groups. This finding demonstrates that male Wistar rats exhibit hormonal increases well beyond the adolescent age, i.e. PND 58, and that sex hormone precursors and sex hormone-derived neuroactive steroids are still increasing in early adulthood.
DISCUSSION
We found that a 1-week, 12-h per day forced exposure to alcohol in male Wistar rats during late adolescence significantly increased alcohol consumption in adulthood, despite a prolonged, 30-day period of abstinence. The alcohol used for the forced exposure, as well as the voluntary drinking assessment, was not paired with a sweetener or faded in, but was rather an 8% ethanol solution. This simple method proved to reproducibly increase adult alcohol consumption in all experiments, and we therefore believe it to be reliable. In addition, we believe that this model is clinically relevant as it involves late adolescent alcohol exposure and examines alcohol preference a substantial time later in adulthood when chronic alcohol use problems in humans often manifest. Our findings imply that in Wistar rats, late adolescence is an age sensitive to the effects of alcohol.
Alcohol intake in late adolescence was slightly increased when finasteride was paired with alcohol treatment during this time. In contrast to our hypothesis, this paired treatment in late adolescence did not moderate the effect of adolescent alcohol exposure on sustained alcohol consumption in adulthood. Similarly, finasteride treatment in adulthood did not have an effect on alcohol consumption in adult rats.
Studies of the long-term effects of alcohol exposure in adolescent rats on alcohol intake in adulthood have been limited in number and provide mixed results. For instance, adolescent binge-pattern alcohol exposure in female rats did not increase alcohol intake in adulthood (PNDs 72–81) following a period of abstinence (Maldonado-Devincci et al., 2010a). Similarly, intermittent adolescent exposure to alcohol vapors in male rats did not increase alcohol intake in adulthood after a prolonged period of abstinence (Slawecki and Betancourt, 2002). To our knowledge, there is only one report of adolescent alcohol exposure (via i.p. injection) that resulted in higher alcohol intake in the adult rat (Pascual et al., 2009). In this study, however, the increased alcohol intake in adulthood was only observed after a 1-week forced alcohol exposure in adulthood. Finally, all of these studies used alcohol exposure in early to mid-adolescence; the effect of alcohol in late adolescence has not been previously measured. The stage of adolescence may be an important factor regarding the prolonged effects of early alcohol exposure, as different stages of adolescence have been shown to predict a differential response to alcohol. For example, early adolescent rats have different responses to the hypnotic (Silveri and Spear, 1998) or anxiolytic (Varlinskaya and Spear, 2006) effects of alcohol compared with late adolescent rats.
Our original hypothesis was that blockade of neuroactive steroid synthesis either during adolescent or adult periods would decrease alcohol intake in rats. In contrast, we observed that finasteride treatment in late adolescence increased alcohol intake in adolescence (∼1.2 fold) but had no effect on the stable phase of enhanced alcohol preference and intake in adulthood resulting from adolescent alcohol exposure. Finasteride administration in adulthood similarly had no significant effect on alcohol preference in adulthood for rats exposed to alcohol in late adolescence. In contrast to our finding, previous studies reported that finasteride treatment reduced the acquisition of alcohol preference in young adult C57BL/6J male mice (Ford et al., 2008b). The opposing findings could potentially be a result of different methodologies in alcohol access and drinking pattern measurement. Whereas our rats had alcohol as the only fluid available in late adolescence during the dark phase, and for 24 h in adulthood, studies that observed decreased alcohol intake with finasteride treatment (Ford et al., 2005a, 2008a, 2008b) utilized a 2 h limited access paradigm to measure temporal patterns of alcohol intake. Additionally, genetic differences between genetically inbred C57BL/6J mice and the genetically heterogeneous Wistar rats may have contributed to this difference in findings. Furthermore, there are substantial species differences in neuroactive steroid levels and synthesis between male rats and mice. C57BL/6J male mice have substantially higher basal levels of allopregnanolone compared with male rats, and the same dose of ethanol increases allopregnanolone levels in rats and significantly decreases allopregnanolone levels in C57BL/6J mice (Porcu et al., 2010).
Allopregnanolone pretreatment has been shown to enhance alcohol intake patterns in male rats (Janak and Michael Gill, 2003) and male mice (Ford et al., 2005b), albeit only at low doses. High doses of allopregnanolone significantly suppressed alcohol intake in male mice (Ford et al., 2005b), consistent with our finding that decreased levels of allopregnanolone induced by finasteride treatment in late adolescence were associated with increased alcohol intake in adolescence. Also consistent with our findings is the report that administration of the neuroactive steroid precursor pregnenolone decreased ethanol self-administration in rats (Besheer et al., 2010).
Rats receiving finasteride injections into the amygdala to block local de novo neuroactive steroid synthesis exhibit higher anxiety-like behavior on the elevated plus maze than vehicle-treated rats (Walf et al., 2006). Another study observed that the anxiolytic effect of alcohol in rats is blocked by finasteride (Hirani et al., 2005). This increased anxiety with reduced levels of neuroactive steroids could be a potential mechanism contributing to the increased alcohol intake we observed in adolescent rats treated with finasteride together with forced alcohol exposure.
A second potential explanation for the increased alcohol consumption in adolescent rats that received alcohol paired with finasteride is that they may have experienced decreased sedative effects of alcohol. Prior studies have reported that the levels of brain neuroactive steroids correlate with the duration of loss of righting reflex (LORR) following i.p. alcohol administration in rats (Khisti et al., 2003). This study found that decreased levels of allopregnanolone due to adrenalectomy reduced the alcohol-induced duration of LORR, demonstrating that neuroactive steroids play a role in the sedative effects of alcohol. A human alcohol challenge study reported that finasteride pre-treatment reduced several subjective effects of alcohol in social drinkers (Pierucci-Lagha et al., 2005). A more recent study found that dutasteride, another inhibitor of 5α-reductase, reduced the sedative effects of alcohol (Covault et al., 2012). Low sensitivity to the effects of alcohol has been proposed as a risk for alcohol dependence (Schuckit, 1994). In our study, late adolescent alcohol exposure alone as well as alcohol exposure paired with finasteride decreased allopregnanolone levels, and were associated with an increased alcohol intake in late adolescence. It is, therefore, possible that neuroactive steroids mediate some effects of alcohol, and that decreased levels of neuroactive steroids blunt alcohol effects, leading to increased drinking during adolescent forced alcohol exposure. The lack of a similar effect of finasteride in adult rats may in part be related to the lower dose of alcohol consumed (1.9 ± 0.1 over 24 h in adult vs. 3.4 ± 0.1 g/kg over 12 h in adolescent rats).
In humans, alcohol is a potent activator of the hypothalamic–pituitary–adrenal (HPA) axis-mediated stress response when administered acutely, as indicated by rapid increases in glucocorticoids (Ellis, 1966) and chronic alcohol consumption in adults can disturb normal HPA axis functioning (Adinoff et al., 1998). HPA axis activation in turn stimulates synthesis and release of GABAergic neuroactive steroids (Morrow et al., 2006). Chronic alcohol exposure in adult rats has been shown to lead to a blunting of the stress response and blunted increases in neuroactive steroids in response to acute alcohol (Boyd et al., 2010). In this study, the acute alcohol exposure was after a 24-h withdrawal period from adult chronic alcohol exposure. Alcohol exposure in adolescence, when the HPA axis is maturing, may have different effects on the HPA axis compared with chronic alcohol exposure in adulthood.
In a study by Przybycien-Szymanska et al. (2011), male Wistar rats exposed to alcohol in adolescence followed by a prolonged abstinence displayed (a) lower baseline levels of corticosterone and (b) a heightened HPA axis response to acute doses of alcohol in adulthood without a habituation response to repeated doses of alcohol. These findings suggest that alcohol exposure during adolescence may interfere with normal development and reactivity of the HPA axis, and by extension, the HPA-mediated synthesis of neuroactive steroids.
In our study, adolescent alcohol exposure was associated with decreased levels of allopregnanolone in late adolescence (see Fig. 6, AlcVeh group). These results for adolescent rats are consistent with the findings of Przybycien-Szymanska et al. (2011), that is, adolescent alcohol exposure decreased the basal levels of corticosterone. In contrast, allopregnanolone levels in adulthood after 2 weeks of voluntary drinking were similar between the group of animals with low levels of alcohol consumption in adulthood (see Fig. 7, WatVeh group) and the group of animals that consumed high levels of alcohol in adulthood (see Fig. 7, AlcVeh group). Our results regarding alcohol effects in adulthood on allopregnanolone levels are harder to interpret as we did not measure the levels of allopregnanolone in adulthood before the voluntary drinking assessment. Based on the findings of Przybycien-Szymanska et al. (2011), i.e. adolescent alcohol exposure decreased the basal levels of corticosterone in adult rats after a long period of abstinence, we can speculate that adolescent alcohol exposure may have decreased allopregnanolone levels chronically, such that allopregnanolone levels were still reduced before voluntary drinking began, and that alcohol consumption in adulthood may have raised the levels of allopregnanolone similar to those in the control group as in the Przybycien-Szymanska et al. (2011) study, where adolescent alcohol-exposed rats had higher HPA axis activation to acute and chronic alcohol exposure.
Strengths of this study include that we used rats born and weaned in our facility in order to eliminate the confounding effects of transportation stress at an age sensitive to these effects. We also used a model of alcohol exposure that is relevant to the human experience, i.e. oral ingestion in a social setting. An additional strength of this study is that we assessed alcohol consumption in adulthood after a 30-day period of abstinence, and lastly, we confirmed the biochemical effect of the finasteride treatment by measuring the levels of the neuroactive steroid allopregnanolone.
Limitations of this study include that we examined only male Wistar rats; it would be important to examine drinking behavior in females and other rat strains using this paradigm. Our forced alcohol exposure in late adolescence was conducted with group-housed rats, which precluded correlations of PND 51–58 alcohol intake and PND 91–104 alcohol preference at an individual subject level. This choice was made in order to remove the possibly confounding effects of social isolation stress in order to isolate the effects of alcohol exposure. Lastly, since animals were sacrificed beginning at 0900 hours, and the availability of alcohol ceased at 0700 hours, there were no detectable levels of alcohol found in plasma samples collected in this study. While such data were not available, the adolescent alcohol intake of 3.4 ± 0.1 g/kg is a pharmacologically meaningful dose as acute doses of 3.5 g/kg lead to LORR (Khisti et al., 2003) and doses of 1.5 g/kg lead to changes in neuroactive steroid levels (Porcu et al., 2010).
In conclusion, our findings highlight that late adolescence is a vulnerable age for the effects of alcohol on later alcohol preference. This study offers a simple model of alcohol exposure in late adolescence which results in high alcohol preference in adulthood. This animal model may be useful in future research aimed at elucidating the mechanisms behind the increased risk for alcohol problems caused by adolescent alcohol exposure.
Funding
This work was supported by the UCHC Alcohol Research Center (NIH grant P60AA03510) and the Department of Psychiatry.
Acknowledgements
The authors would like to acknowledge the helpful suggestions of Dr. Lisa Conti in the design of this study and Ms. Linda Burian for technical assistance.
References
- Adinoff B, Iranmanesh A, Veldhuis J, et al. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Akwa Y, Purdy RH, Koob GF, et al. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–25. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol. 1989;166:325–9. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O'Buckley TK, et al. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring p rats. Alcohol Clin Exp Res. 2010;34:2044–52. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O'Buckley TK, et al. Chronic ethanol exposure produces tolerance to elevations in neuroactive steroids: mechanisms and reversal by exogenous ACTH. J Neurochem. 2010;115:142–52. doi: 10.1111/j.1471-4159.2010.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Collins BE, Carey MP, et al. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–80. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Courant F, Aksglaede L, Antignac JP, et al. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95:82–92. doi: 10.1210/jc.2009-1140. [DOI] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, et al. Effects of dutasteride on alcohol responses in a laboratory setting and on drinking in the natural environment. Alcohol Clin Exp Res. 2012;36:18a. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, et al. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23. [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–7. [PubMed] [Google Scholar]
- Fadalti M, Petraglia F, Luisi S, et al. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr Res. 1999;46:323–7. doi: 10.1203/00006450-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005a;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, et al. Neurosteroid modulators of GABA(A) receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005b;29:1630–40. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, et al. Ethanol intake patterns in female mice: influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depend. 2008a;97:73–85. doi: 10.1016/j.drugalcdep.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, et al. Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008b;32:1408–16. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Burgos D, Gonzalez F, Manrique T, et al. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33:722–8. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, et al. Evaluation of GABAergic neuroactive steroid 3alpha-hydroxy-5alpha-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology (Berl) 2005;180:267–78. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Janak PH, Michael Gill T. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–12. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, et al. Monitoring the Future: National Survey Results on Drug Use, 1975–2005, Vol 1: Secondary School Students. Bethesda, MD: National Institutes of Health; 2006. NIH Publication 06-5883. [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O'Buckley T, et al. Neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–65. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- Kim YS, Zhang H, Kim HY. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Anal Biochem. 2000;277:187–95. doi: 10.1006/abio.1999.4384. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Ladle DR, Jacobson NA, et al. Inhibition of brain 5 alpha-reductase in pregnant rats: effects on enzymatic and behavioral activity. Brain Res. 1996;739:356–60. doi: 10.1016/s0006-8993(96)01068-2. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, et al. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010a;96:476–87. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Badanich KA, Kirstein CL. Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol. 2010b;44:57–66. doi: 10.1016/j.alcohol.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. [Google Scholar]
- Milivojevic V, Kranzler HR, Gelernter J, et al. Variation in genes encoding the neuroactive steroid synthetic enzymes 5alpha-reductase type 1 and 3alpha-reductase type 2 is associated with alcohol dependence. Alcohol Clin Exp Res. 2011;35:946–52. doi: 10.1111/j.1530-0277.2010.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, et al. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, et al. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–77. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeson R, Goodchild CS. Antinociceptive properties of neurosteroids III: experiments with alphadolone given intravenously, intraperitoneally, and intragastrically. Br J Anaesth. 2001;86:704–8. doi: 10.1093/bja/86.5.704. [DOI] [PubMed] [Google Scholar]
- Ostlund RE, Jr, Hsu FF, Bosner MS, et al. Quantification of cholesterol tracers by gas chromatography–negative ion chemical ionization mass spectrometry. J Mass Spectrom. 1996;31:1291–6. doi: 10.1002/(SICI)1096-9888(199611)31:11<1291::AID-JMS424>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, et al. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, et al. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, et al. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34:432–42. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, et al. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–30. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–9. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, et al. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–45. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–47. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–8. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:2100–6. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–9. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 2004;172:352–5. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006;186:351–61. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Vallee M, Rivera JD, Koob GF, et al. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–66. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, et al. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–9. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague–Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5alpha-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology (Berl) 2006;186:302–11. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, et al. Anxiolytic activity of the progesterone metabolite 5 alpha-pregnan-3 alpha-o1-20-one. Brain Res. 1991;565:263–8. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]