Abstract
Prostaglandin E2 (PGE2), the most relevant eicosanoid promoting inflammation and tumorigenesis, is formed by cyclooxygenases (COXs) and PGE2 synthases from free arachidonic acid. Preparations of the leaves of Salvia officinalis are commonly used in folk medicine as an effective antiseptic and anti-inflammatory remedy and possess anticancer activity. Here, we demonstrate that a standard ethyl acetate extract of S. officinalis efficiently suppresses the formation of PGE2 in a cell-free assay by direct interference with microsomal PGE2 synthase (mPGES)-1. Bioactivity-guided fractionation of the extract yielded closely related fractions that potently suppressed mPGES-1 with IC50 values between 1.9 and 3.5 μg/ml. Component analysis of these fractions revealed the diterpenes carnosol and carnosic acid as potential bioactive principles inhibiting mPGES-1 activity with IC50 values of 5.0 μM. Using a human whole-blood assay as a robust cell-based model, carnosic acid, but not carnosol, blocked PGE2 generation upon stimulation with lipopolysaccharide (IC50 = 9.3 μM). Carnosic acid neither inhibited the concomitant biosynthesis of other prostanoids [6-keto PGF1α, 12(S)-hydroxy-5-cis-8,10-trans-heptadecatrienoic acid, and thromboxane B2] in human whole blood nor affected the activities of COX-1/2 in a cell-free assay. Together, S. officinalis extracts and its ingredients carnosol and carnosic acid inhibit PGE2 formation by selectively targeting mPGES-1. We conclude that the inhibitory effect of carnosic acid on PGE2 formation, observed in the physiologically relevant whole-blood model, may critically contribute to the anti-inflammatory and anticarcinogenic properties of S. officinalis.
Introduction
Leaves of Salvia officinalis (sage) are used as culinary herb and in folk medicine to treat sore throats, dyspepsia, and diverse inflammatory diseases in the Western world (Johnson, 2011). Among the multiple polyphenols identified in sage, the o-diphenolic diterpenes carnosol and carnosic acid are most abundant (Johnson, 2011). In addition to their strong antioxidant character, carnosol and carnosic acid exert potent anti-inflammatory and anticarcinogenic properties (Johnson, 2011). They impair the proliferation of several cancer cell lines and induce apoptosis (Dörrie et al., 2001; Steiner et al., 2001; Visanji et al., 2006; Hussein et al., 2007; Khan et al., 2007; Johnson et al., 2008, 2010; Yesil-Celiktas et al., 2010; Tsai et al., 2011), reduce tumor growth in athymic nude mice implanted with prostate carcinoma cells (Johnson et al., 2010), interfere with carcinogen-induced mammary tumorigenesis in rats (Singletary et al., 1996), inhibit phorbol-12-myristate-13-acetate-induced ear inflammation and tumor promotion in mice (Huang et al., 1994), and decrease adenoma formation in APC(Min/+) mice (Moran et al., 2005). On the molecular level, carnosol and carnosic acid may interfere with multiple signaling pathways that are deregulated during inflammation and cancer, including nuclear factor κB (Lo et al., 2002; Huang et al., 2005), p38 mitogen-activated protein kinase (Lo et al., 2002), extracellular signal-regulated kinase (Lo et al., 2002), phosphatidylinositol-3-kinase (Martin et al., 2004), protein kinase C (Subbaramaiah et al., 2002), cyclooxygenase (COX) (Laughton et al., 1991; Subbaramaiah et al., 2002), androgen and estrogen receptors (Johnson et al., 2010), the B-cell lymphoma-2 protein family (Dörrie et al., 2001), β-catenin (Moran et al., 2005), and intracellular Ca2+ (Lee et al., 2006; Poeckel et al., 2008). However, only a few direct targets of carnosol and carnosic acid have been described so far. Rau et al. (2006) ascribed the long-term anti-inflammatory properties of carnosol and carnosic acid to the activation of peroxisome proliferator-activated receptor γ. Moreover, we proposed that the inhibition of human 5-lipoxygenase (Laughton et al., 1991; Poeckel et al., 2008) contributes to their immediate anti-inflammatory effects. Direct molecular targets explaining the anticarcinogenic effects of carnosol and carnosic acid are not described.
Exaggerative prostaglandin (PG) E2 formation links carcinogenesis to inflammation (Rådmark and Samuelsson, 2010). During biosynthesis of PGE2, arachidonic acid is transformed to PGH2 by cyclooxygenases and further converted to PGE2 by PGE2 synthases (Koeberle and Werz, 2009). The isoenzymes COX-2 and microsomal PGE2 synthase (mPGES)-1 are functionally coupled and responsible for the inducible PGE2 formation under pathophysiological conditions (Koeberle and Werz, 2009). Both isoenzymes are induced by proinflammatory stimuli and overexpressed in various cancers (Koeberle and Werz, 2009). Accordingly, genetic or pharmacological inhibition of COX-2 or mPGES-1 reduces inflammation, fever, and pain in numerous cellular and animal studies as well as tumorigenesis, angiogenesis, and metastasis (Smith et al., 2000; Koeberle and Werz, 2009; Rådmark and Samuelsson, 2010).
Here, we identified an ethyl acetate extract of S. officinalis and its active principles carnosol and carnosic acid as direct inhibitors of mPGES-1. Carnosic acid, but not carnosol, inhibited PGE2 biosynthesis in a physiologically relevant human whole-blood assay at low micromolar concentrations that can be achieved in vivo after oral administration. Neither the biosynthesis of other prostanoids than PGE2 nor the activity of cell-free COX-1 or COX-2 was markedly affected, suggesting a preferred interference with mPGES-1 underlying the suppression of cellular PGE2 biosynthesis.
Materials and Methods
Solvents and Reagents
All solvents used for phytochemical work were obtained from VWR International (Darmstadt, Germany). Solvents for HPLC were provided by Merck (Darmstadt, Germany). Ultrapure water was produced by a Sartorius Arium 611 UV water purification system (Sartorius AG, Göttingen, Germany). Carnosol, carnosic acid, ursolic acid (Baricevic et al., 2001), oleanolic acid (Werz, 2007), and rosmarinic acid (Werz, 2007) (Sigma-Aldrich, Deisenhofen, Germany) were dissolved in dimethyl sulfoxide (DMSO) and stored in the dark at −20°C, and freezing/thawing cycles were kept to a minimum. The thromboxane synthase inhibitor (E)-7-phenyl-7-(3-pyridyl)-6-heptenoic acid (CV4151) (Kato et al., 1985) and the mPGES-1 inhibitor 2-(2-chlorophenyl)-1H-phenanthro[9,10-d]imidazole (MD52) (Côté et al., 2007) were kindly provided by Dr. Stefan Laufer (University of Tuebingen, Tuebingen, Germany) and Dr. Manfred Schubert-Zsilavecz (University of Frankfurt, Frankfurt, Germany), respectively. Materials used were: DMEM/high-glucose (4.5 g/l) medium, penicillin, streptomycin, trypsin/EDTA solution (PAA, Coelbe, Germany); PGH2 (Larodan, Malmö, Sweden); 11β-PGE2, PGB1, 3-[1-(4-chlorobenzyl)-3-t-butylthio-5-isopropylindol-2-yl]-2,2-dimethylpropanoic acid (MK-886), human recombinant COX-2, and ovine COX-1 (Cayman Chemical, Ann Arbor, MI). All other chemicals were obtained from Sigma-Aldrich (Deisenhofen, Germany) unless otherwise stated.
Plant Material
Dried, cut leaves of S. officinalis (2 kg) were purchased from Kottas Pharma GmbH (Vienna, Austria). A voucher specimen (JR-20100802-A1) is deposited at the Institute of Pharmacy/Pharmacognosy, University of Innsbruck (Innsbruck, Austria).
Extraction and Fractionation of S. officinalis and Sample Preparation
Ground leaves (94 g) of S. officinalis were extracted five times for 30 min with 600 ml of ethyl acetate in an ultrasonic bath at room temperature. The combined extracts were evaporated to dryness, yielding 14.62 g, and 13.9 g of the extract were separated by flash silica gel 60 (40–63 μm; 202 g; Merck) column chromatography (3.8 × 47.5 cm) using petroleum ether as eluent with stepwise (200 ml each) increasing amounts of dichloromethane, followed by ethyl acetate and finally acetone, yielding 11 fractions.
The ethyl acetate extract of S. officinalis and its fractions were solubilized in DMSO, stored at −20°C in the dark, and analyzed within 4 weeks. Fraction 1 was not completely soluble in DMSO at 0.3 μg/ml, even after extensive sonification. The insoluble pellet was removed by centrifugation.
HPLC Quantification
To determine the content of carnosol and carnosic acid, standard solutions were diluted to appropriate concentrations. The ethyl acetate extract of S. officinalis and its 11 fractions were dissolved in acetonitrile and, when necessary, in a mixture of acetonitrile and tetrahydrofuran (fractions 9 and 10). Every sample was analyzed by HPLC three times. The amount of carnosol and carnosic acid found in the samples was calculated as the percentage of the weight of the dry extract and fractions. Limits of quantification were determined as the signal-to-noise ratio of 10. HPLC-diode-array detection analyses were performed on a Shimadzu (Kyoto, Japan) UFLC-XR instrument, equipped with auto sampler, photo diode array, and on-line degasser and column thermostat. Stationary phase was Max RP 80A column (150 × 4.6 mm, 3.5-μm particle size; Phenomenex, Torrance, CA). Mobile phase was double-distilled water (A) and methanol containing 1% acetic acid (B). Flow rate was 1.0 ml/min with detection wavelength at 284 nm and solvent gradient at start 35% A, 65% B; 20 min 2% A, 98% B; stop 30 min; post time 10 min.
Cells
Human platelets were freshly isolated from leukocyte concentrates obtained at the Blood Center of the University Hospital Tuebingen (Tuebingen, Germany) according to Albert et al. (2002). In brief, leukocyte concentrates were prepared by centrifugation (4000g, 20 min, 20°C) from venous blood from healthy adult donors who did not take any medication for at least 7 days. Blood cells were immediately separated by dextran sedimentation and centrifugation on Nycoprep cushions (PAA). The supernatant was mixed with phosphate-buffered saline (PBS), pH 5.9 (3:2 v/v) and centrifuged (2100g, 15 min, room temperature). The pellet was washed with PBS, pH 5.9/0.9% NaCl (1:1, v/v) and resuspended in PBS, pH 7.4 and 1 mM CaCl2 to obtain isolated human platelets.
Human lung adenocarcinoma epithelial A549 cells were cultured in DMEM/high-glucose (4.5 g/l) medium supplemented with heat-inactivated fetal calf serum (10%, v/v), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C and 5% CO2, detached by using 1× trypsin/EDTA when confluent, and reseeded at 2 × 106 cells in 20 ml of medium in 175-cm2 flasks.
Determination of mPGES-1 Activity in Microsomes of A549 Cells
Microsomes of A549 cells were prepared, and mPGES-1 activity was determined according to Koeberle et al. (2008). In brief, cells were treated with interleukin-1β (2 ng/ml) at 37°C and 5% CO2, harvested after 48 h, and frozen in liquid nitrogen. After reuptake of the cells in ice-cold homogenization buffer (0.1 M potassium phosphate buffer, pH 7.4, 1 mM phenylmethanesulphonyl fluoride, 60 μg/ml soybean trypsin inhibitor, 1 μg/ml leupeptin, 2.5 mM glutathione, and 250 mM sucrose) and incubation for 15 min, cells were sonicated on ice (3 × 20 s) and subjected to differential centrifugation at 10,000g for 10 min and 174,000g for 1 h at 4°C. The microsomal fraction (pellet) was resuspended in homogenization buffer, analyzed for its protein content by using a protein assay kit (Bio-Rad Laboratories GmbH, Munich, Germany), and diluted in potassium phosphate buffer (0.1 M, pH 7.4) containing 2.5 mM glutathione to a final concentration of 25 to 50 μg protein/ml. After preincubation with the test compounds (dissolved in DMSO) for 15 min at 4°C, the reaction (100 μl of total volume, 4°C) was initiated by the addition of PGH2 (20 μM, final concentration) and terminated after 1 min by the addition of 100 μl of stop solution (40 mM FeCl2, 80 mM citric acid, and 10 μM 11β-PGE2 as internal standard). PGE2 was extracted and analyzed by reversed phase-HPLC as described previously (Koeberle et al., 2008). The final concentration of DMSO in the reaction volume was always adjusted to 1%. Addition of PGH2 to a reaction mix already containing stop solution and a reaction mix containing heat-inactivated microsomes yielded relative amounts of PGE2 of 8.9 ± 0.8 and 11.0 ± 1.4%, respectively. Results are expressed as percentage of the vehicle (DMSO)-treated control.
Determination of PGE2, 6-Keto PGF1α, 12(S)-Hydroxy-5-cis-8,10-trans-Heptadecatrienoic Acid, and Thromboxane B2 Formation in Human Whole Blood
Blood from healthy adult volunteers was obtained by venipuncture and collected in monovettes containing heparin (20 U/ml), and prostanoid formation was analyzed according to Koeberle et al. (2009b). In brief, for the determination of PGE2 and 6-keto PGF1α, aliquots of whole blood (0.8 ml) were mixed with thromboxane synthase inhibitor CV4151 (1 μM) and aspirin (50 μM) and adjusted to a total volume of 1 ml with 10 mM potassium phosphate buffer, pH 7.4, 3 mM KCl, 140 mM NaCl, and 6 mM D-glucose. CV4151 and aspirin were omitted for the determination of 12-HHT and thromboxane B2 formation. Samples were preincubated with the test compounds for 5 min at room temperature and stimulated with lipopolysaccharide (LPS; 10 μg/ml) for 5 h at 37°C. After prostanoid formation was stopped on ice, the samples were centrifuged (2300g, 10 min, 4°C) and analyzed for 6-keto PGF1α and thromboxane B2 by ELISA (Enzo Life Sciences Inc., Lausen, Switzerland) and for 12-HHT by HPLC. For the determination of PGE2, the supernatant was acidified with citric acid (30 μl, 2 M) and centrifuged (2300g, 10 min, 4°C). Then, PGE2 was separated by solid-phase extraction and reversed phase-HPLC and quantified by using a PGE2 enzyme immunoassay kit (Enzo Life Sciences).
Determination of COX-2 Product Formation in Intact A549 Cells
A549 cells (1 × 106), which do not express COX-1 (Asano et al., 1996), were resuspended in 1 ml of PBS containing CaCl2 (1 mM) and preincubated with the test compounds for 15 min at 37°C, and arachidonic acid (30 mM) was added. After 15 min at 37°C, the samples were put on ice and centrifuged (300g, 5 min, 4°C), and supernatants were analyzed for the amount of COX-2-derived 6-keto PGF1α by using a 6-keto PGF1α High Sensitivity enzyme immunoassay Kit (Enzo Life Sciences).
Determination of COX-1 Product Formation in Washed Platelets
Freshly isolated platelets (108/ml PBS containing 1 mM CaCl2) were preincubated with the test compounds for 5 min at room temperature. Addition of 5 μM arachidonic acid for 5 min at 37°C evoked COX-1-derived 12-HHT (which is nonenzymatically formed from PGH2) and thromboxane B2 formation. 12-HHT was extracted and analyzed by HPLC as described previously (Albert et al., 2002). Thromboxane B2 was quantified by ELISA (Enzo Life Sciences Inc.).
Activity Assays of Isolated COX-1 and COX-2
Effects on ovine COX-1 and human COX-2 enzyme activity were determined as described previously (Koeberle et al., 2008). In brief, purified ovine COX-1 (50 units) or human recombinant COX-2 (20 units) were preincubated in 1 ml of reaction mixture containing 100 mM Tris buffer, pH 8, 5 mM glutathione, 5 μM hemoglobin, and 100 μM EDTA with the test compounds for 5 min at 4°C. After prewarming for 60 s at 37°C, the samples were incubated with arachidonic acid (COX-1, 5 μM; COX-2, 2 μM) for 5 min. COX-derived 12-HHT was extracted and then analyzed by HPLC as described previously (Albert et al., 2002).
Statistics
Data are expressed as mean ± S.E. IC50 values were determined by graphical analysis using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA). The program GraphPad Instat (GraphPad Software Inc., San Diego, CA) was used for statistical comparisons. Statistical evaluation of the data was performed by one-way ANOVAs for independent or correlated samples followed by Tukey HSD post hoc tests. A p value < 0.05 was considered significant.
Results
An Ethyl Acetate Extract of Sage Inhibits mPGES-1
Sage extracts have anti-inflammatory and anticarcinogenic properties (Johnson, 2011). Because PGE2 formation through the inducible PGE2 synthase mPGES-1 plays a critical role in the progression of both inflammation and cancer (Koeberle and Werz, 2009), we investigated whether an ethyl acetate extract of the leaves of S. officinalis may inhibit mPGES-1. The contents of carnosol and carnosic acid in the extract were determined to be 4.3 and 20.1%, respectively. The ethyl acetate extract was tested in a cell-free mPGES-1 activity assay. The direct substrate of mPGES-1, PGH2, is enzymatically converted in this assay to PGE2 by mPGES-1 from microsomal preparations of interleukin-1β-stimulated A549 cells. MK-886 (10 μM), used as reference, suppressed mPGES-1 activity by 74.5 ± 1.5% as expected (Koeberle et al., 2008). The ethyl acetate extract of S. officinalis concentration-dependently inhibited mPGES-1 with an IC50 of 5.0 μg/ml (Fig. 1).
Fig. 1.
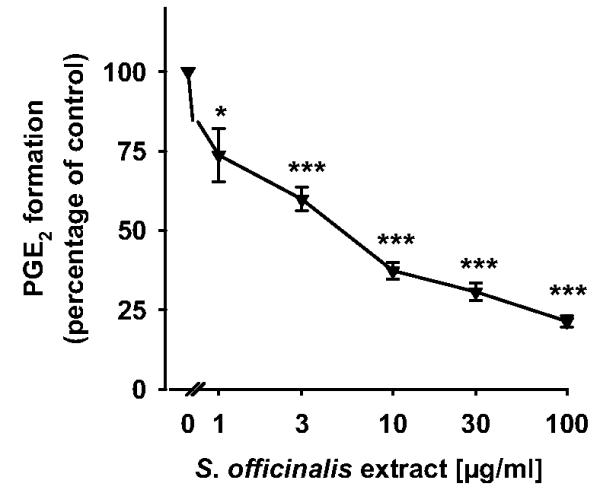
An ethyl acetate extract of S. officinalis inhibits mPGES-1 activity. Microsomal preparations of interleukin-1β-stimulated A549 cells were preincubated with an ethyl acetate extract of S. officinalis for 15 min at 4°C, and the reaction was started with 20 μM PGH2. After 1 min at 4°C, the reaction was terminated by using a stop solution containing FeCl2 and 11β-PGE2 (1 nmol) as internal standard. The concentration-response curve for the ethyl acetate extract of S. officinalis is shown. The 100% value corresponds to 246 ± 4 ng of PGE2. Data are given as mean + S.E. (n = 3-5). *, p < 0.05 or ***, p < 0.001 versus vehicle (DMSO) control, ANOVA + Tukey HSD post hoc tests.
Bioactivity-Guided Fractionation and Characterization of the S. officinalis Extract
To identify the active constituents of the ethyl acetate extract of S. officinalis responsible for the inhibition of mPGES-1, the extract was fractionated by means of silica gel column chromatography. The 11 fractions obtained were then tested for their inhibitory effects against mPGES-1 in our in vitro mPGES-1 activity assay. Fractions 2 and 4 to 8 showed pronounced mPGES-1 inhibitory effects (IC50 between 1.9 and 3.5 μg/ml), whereas the inhibitory activity of the other fractions was moderate or absent (Table 1). HPLC analysis indicated the presence of carnosol and carnosic acid (between 1.3 and 46.6%) in all active fractions with the exception of fraction 2, which, however, was a minor fraction representing only approximately 1% of the fractionated ethyl acetate extract. Even though there was not a clear correlation between the content of the two diterpenes in the single fractions and their magnitude of mPGES-1 inhibitory effects (Table 1), carnosol and carnosic acid were regarded as primarily responsible for the mPGES-1-inhibiting activity of the crude ethyl acetate extract of S. officinalis.
TABLE 1.
IC50 values for inhibition of mPGES-1 (n = 3) and content of carnosol and carnosic acid of fractions 1 to 11
The content of carnosol and carnosic acid was determined by HPLC and is given as percentage of the fraction’s dry weight.
| Fraction | IC50 | Carnosol | Carnosic acid |
|---|---|---|---|
| μg/ml | % | ||
| 1a | N.I. (at 3 μg/ml) | < LOQ | < LOQ |
| 2 | 2.1 | < LOQ | < LOQ |
| 3 | 15.3 | < LOQ | < LOQ |
| 4 | 1.9 | 3.4 | 1.3 |
| 5 | 2.0 | 25.2 | 27.3 |
| 6 | 3.1 | 12.6 | 46.6 |
| 7 | 2.4 | 9.3 | 30.4 |
| 8 | 3.5 | 6.2 | 17.5 |
| 9 | 8.5 | 2.0 | 5.6 |
| 10 | 17.2 | 0.9 | 0.9 |
| 11 | 17.5 | < LOQ | < LOQ |
N.I., no inhibition; LOQ, limit of quantification.
Incompletely dissolved.
Inhibition of mPGES-1 by Carnosol and Carnosic Acid
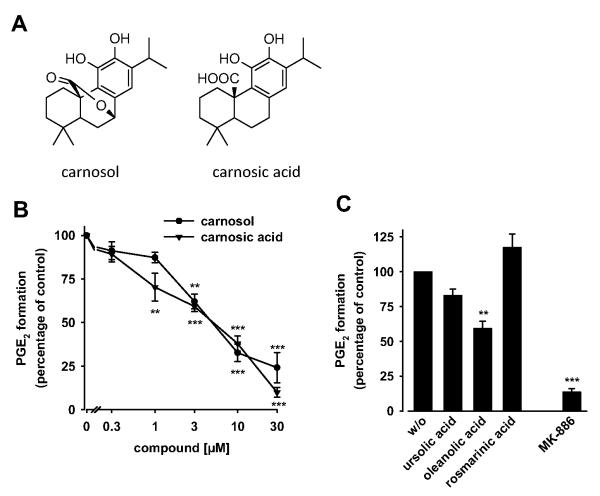
Because carnosol or carnosic acid were enriched in the active fractions of the sage ethyl acetate extract (with the exception of fraction 2), we investigated the effect of these diterpenes on mPGES-1 activity in the microsomes of A549 cells. In fact, both compounds concentration-dependently inhibited mPGES-1 activity with IC50 values of 5 μM each (Fig. 2B). It is noteworthy that the inhibition of mPGES-1 by the ethyl acetate extract of Salvia officinalis perfectly matches with the content and IC50 values of carnosol and carnosic acid, suggesting a major role of carnosol and carnosic acid for the inhibition of mPGES-1 by the extract. Recently, we identified boswellic acid triterpenes as inhibitors of mPGES-1 (Siemoneit et al., 2011). However, triterpenes found in sage extracts, like ursolic acid or oleanolic acid (Baricevic et al., 2001; Ninomiya et al., 2004), hardily (ursolic acid) or at least less efficiently (oleanolic acid) suppressed mPGES-1 activity at a concentration of 10 μM (Fig. 2C). In addition, the polyphenol rosmarinic acid (10 μM) failed to inhibit mPGES-1 activity.
Fig. 2.
Carnosol and carnosic acid inhibit mPGES-1 activity. A, structures of carnosol (left) and carnosic acid (right). B, concentration-response curves for carnosol and carnosic acid. The effect of carnosol and carnosic acid on mPGES-1 activity was determined under the same conditions as described in the legend to Fig. 1. C, effect of vehicle (DMSO, w/o), ursolic acid, oleanolic acid, rosmarinic acid, and the mPGES-1 control inhibitor MK-886 (10 μM, each) on mPGES-1 activity. Values of 100% correspond to 306 ± 63 ng of PGE2. Data are given as mean + S.E. (n = 3-5). **, p < 0.01 or ***, p < 0.001 versus vehicle (DMSO) control, ANOVA + Tukey HSD post hoc tests.
Carnosic Acid Suppresses PGE2 Formation in LPS-Stimulated Whole Blood
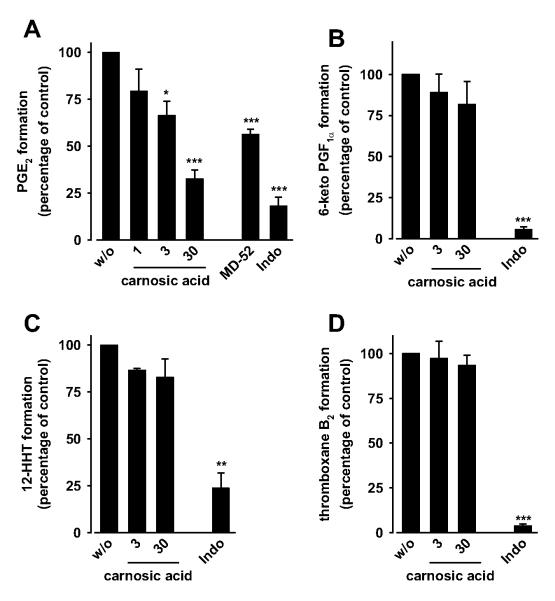
Potent inhibition of PGE2 bio-synthesis in a cell-free assay is not necessarily preserved under more physiological conditions such as in whole blood. Therefore, we investigated whether carnosol and carnosic acid suppress PGE2 formation in LPS-stimulated human whole blood, which emerged as a routine assay for selecting candidate COX-2 and mPGES-1 inhibitors for preclinical investigation (Hawkey, 1999; Koeberle and Werz, 2009). Carnosic acid concentration-dependently inhibited PGE2 formation in human whole blood with an IC50 value of 9.3 μM (Fig. 3A), whereas carnosol surprisingly did not show an effect up to 100 μM (data not shown). MD52 (2 μM) and indomethacin (10 μM), used as mPGES-1 and COX reference inhibitors, respectively, suppressed PGE2 formation as expected (Fig. 3A) and described previously (Koeberle et al., 2009a).
Fig. 3.
Effect of carnosol and carnosic acid on prostanoid formation in human whole blood. Aliquots of heparinized human whole blood (treated with 1 μM thromboxane synthase inhibitor and 50 μM aspirin for A and B) were preincubated with vehicle (DMSO, w/o) or carnosic acid for 5 min at room temperature, and prostanoid formation was induced by addition of 10 μg/ml LPS. Indomethacin (Indo, 10 μM) and MD52 (2 μM) were used as controls. A and C, PGE2 (A) and 12-HHT (C) were extracted from blood plasma by reversed-phase-18 solid-phase extraction, separated by reversed phase-HPLC, and quantified by ELISA (A) or UV detection (C). B and D, 6-keto PGF1α (B) and thromboxane B2 (D) were directly determined in the blood plasma by ELISA. Values of 100% correspond to 45.8 ± 5.2 ng/ml PGE2, 12.4 ± 1.6 ng/ml 6-keto PGF1α, 23.9 ± 3.2 ng/ml 12-HHT, and 67.8 ± 4.8 ng/ml thromboxane B2. Data are given as mean + S.E. (n = 1-4). *, p < 0.05; **, p < 0.01; or ***, p < 0.001 versus vehicle (DMSO) control, ANOVA + Tukey HSD post hoc tests.
Carnosic Acid Is a Selective Inhibitor of mPGES-1 within Prostanoid Biosynthesis
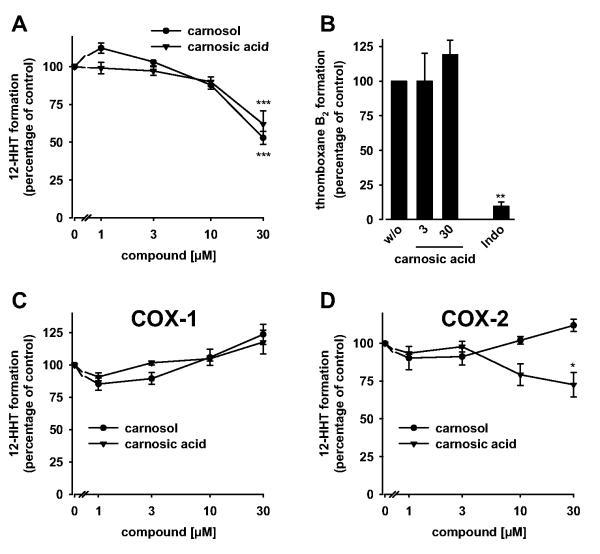
If mPGES-1 would be the only target within prostanoid biosynthesis, carnosic acid should not interfere with the biosynthesis and release of other prostanoids than PGE2. In fact, carnosic acid neither significantly suppressed the formation of 6-keto PGF1α (a stable metabolite of PGI2; Fig. 3B), 12-HHT (which can be nonenzymatically formed from the COX product PGH2; Fig. 3C) nor thromboxane B2 (Fig. 3D) in LPS-stimulated whole blood. Accordingly, carnosic acid failed to suppress COX-2-derived 6-keto PGF1α formation from exogenous arachidonic acid in human lung epithelial A549 cells (up to 100 μM; data not shown). COX-1 activity, measured as 12-HHT formation in arachidonic acid-treated platelets, was inhibited (Fig. 4A) but only at high concentrations of carnosic acid (≥ 30 μM). Inhibition of COX-1 could not be confirmed in platelets for thromboxane B2 as biomarker (Fig. 4B) or for the isolated enzyme (Fig. 4C). In addition, the activity of isolated COX-2 was only slightly suppressed by 30 μM carnosic acid (Fig. 4D), suggesting mPGES-1 as a preferential functional target of carnosic acid within prostanoid biosynthesis. The reference inhibitors indomethacin (10 μM; COX-1) and celecoxib (5 μM; COX-2) suppressed prostanoid formation (6-keto PGF1α, 12-HHT, and thromboxane B2) under the respective assay conditions as expected (Figs. 3 and 4B and data not shown) and described previously (Koeberle et al., 2008; Bauer et al., 2011).
Fig. 4.
Effects of carnosol and carnosic acid on COX activity. Effects on 12-HHT (A) and thromboxane B2 formation (B) in intact platelets and on the activity of isolated bovine COX-1 (C) and human recombinant COX-2 (D). Platelets (108/ml) or COX isoenzymes were preincubated with carnosol or carnosic acid for 5 min, and 12-HHT (A, C, and D) or thromboxane B2 formation (B) was initiated with arachidonic acid. After 5 min at 37°C, 12-HHT was determined by reversed-phase-HPLC as described (A, C, and D); and thromboxane B2 was quantified by ELISA (B). Prostanoids formed in the absence of test compounds (100%, control) were: 92.2 ± 23.1 ng/ml 12-HHT (A) and 0.58 ± 0.03 μg/ml thromboxane B2 (B) for intact platelets, 201.7 ± 54.0 μg/ml 12-HHT for COX-1 (C), and 32.9 ± 5.7 μg/ml 12-HHT for COX-2 (D). Data are given as mean + S.E. (n = 3-5). *, p < 0.05; **, p < 0.01; or ***, p < 0.001 versus vehicle (DMSO) control, ANOVA + Tukey HSD post hoc tests.
Discussion
Anticarcinogenic and anti-inflammatory properties of sage extracts and its constituents carnosol and carnosic acid have been described for numerous cellular and animal models (Johnson, 2011). Although diverse mechanisms have been discussed, only a few direct molecular targets have been identified (Johnson, 2011). In particular, targets responsible for the anticancer effects of carnosol and carnosic acid are still elusive. Research over the last decade revealed a prominent role of mPGES-1-derived PGE2 not only in inflammation but also for tumor progression, vascularization, and metastasis (Koeberle and Werz, 2009; Rådmark and Samuelsson, 2010). Accordingly, inhibitors of mPGES-1 (or the upstream enzyme COX-2) possess prominent anti-inflammatory and antitumorigenic activities (Koeberle and Werz, 2009). Previous studies have shown that carnosol and carnosic acid impair the proliferation of cancer cells, particularly from prostate, breast, and lung (Singletary et al., 1996; Hussein et al., 2007; Johnson et al., 2008, 2010; Yesil-Celiktas et al., 2010), prevent tumorigenesis in APC(Min/+) mice (Moran et al., 2005), and suppress phorbol ester-induced ear inflammation (Huang et al., 1994). Inducible PGE2 formation occurs under all these experimental conditions. Prostate, breast, and lung cancer cells overexpress mPGES-1 (Koeberle and Werz, 2009), knockout of the mPGES-1 gene reduces intestinal cancer growth in APC-mutant mice (Nakanishi et al., 2008), and treatment with phorbol esters results in induction of mPGES-1 protein (Pham et al., 2006). From this, we speculated that an interference with mPGES-1 might contribute to the pleiotropic anticarcinogenic and anti-inflammatory activities of carnosol and carnosic acid. In fact, our study confirms carnosol and carnosic acid as active principles of sage selectively suppressing PGE2 synthesis by the inhibition of mPGES-1. Neither the moderate inhibition of COX isoenzymes as described here and by others (Laughton et al., 1991; Subbaramaiah et al., 2002; Mengoni et al., 2011; Barni et al., 2012) nor a potential interference with other prostanoid synthases might explain the preferred inhibition of PGE2 formation. However, whether other PGE2 synthase isoenzymes than mPGES-1 are additionally inhibited by carnosol and carnosic acid remains elusive.
Extracts of S. officinalis contain a variety of secondary metabolites, including diterpenoids, triterpenoids, flavonoids, and phenolic glycosides (Gonzalez et al., 1989; Lu and Foo, 2000; Jäger et al., 2009). Among the most abundant metabolites are the phenolic diterpenes carnosol and carnosic acid, which reach concentrations up to 8.1% in acetone extracts of sage (Okamura et al., 1994) and even 24.4% in the ethyl acetate extract as described here. Comparing the IC50 values of the ethyl acetate extract and carnosol and carnosic acid, these pure compounds are mainly responsible for the observed suppression of mPGES-1. The inhibitory potential of fraction 2, however, which does not contain carnosol and carnosic acid, leads to the conclusion that there must be further compounds in the ethyl acetate extract contributing to the inhibitory effect on mPGES-1. This is also underlined by the fact that the IC50 values do not correspond exactly with the amount of carnosol and carnosic in fractions 4 to 8. Therefore, other substances contribute to an unknown extend to mPGES-1 inhibition and may act synergistically as well. For example, S. officinalis contains a variety of cyclic polyphenols, a structural class that is well represented among mPGES-1 inhibitors (Koeberle and Werz, 2009).
In addition to inhibition of mPGES-1, carnosol and carnosic acid might affect prostanoid biosynthesis by direct inhibition of COX or suppression of COX-2 protein induction as described previously (Laughton et al., 1991; Subbaramaiah et al., 2002; Mengoni et al., 2011; Barni et al., 2012). We could confirm an inhibition of COX product formation (i.e., 12-HHT) by carnosol and carnosic acid for human platelets stimulated with arachidonic acid. Substantially higher concentrations of carnosol or carnosic acid than for inhibition of mPGES-1 activity were required, however. Because arachidonic acid is directly converted to PGH2 by COX, and PGH2 can react nonenzymatically to 12-HHT, we were surprised that neither carnosol nor carnosic acid inhibited isolated COX isoenzymes. The discrepancy might be explained by an inhibition of thromboxane synthase that is abundantly expressed in platelets and can catalyze the conversion of PGH2 to 12-HHT (Shen and Tai, 1986). Thus, 12-HHT is formed both nonenzymatically and through thromboxane synthase. However, the failure of carnosic acid to inhibit the formation of thromboxane B2 (which is exclusively formed from PGH2 by thromboxane synthase) precludes thromboxane synthase as a major target of carnosic acid. Together, these observations question a direct interaction of carnosol and carnosic acid with COX and suggest other intracellular points of attack. Note that interference of carnosol and carnosic acid with COX-2 and PGE2 synthase induction is unlikely under our experimental settings but might be of importance in vivo or for prolonged incubation times that allow an efficient up-regulation of inducible enzymes. With an incubation time of only 5 h, our LPS-stimulated whole-blood assay was optimized to minimize effects on protein expression, particularly of mPGES-1 whose expression is delayed relative to COX-2 (Koeberle and Werz, 2009).
The potency of carnosol and carnosic acid to interfere with COX-product formation (i.e., 12-HHT and 6-keto PGF1α synthesis) was strongly reduced in the physiologically relevant whole-blood assay compared with isolated platelets. Such a shift in potency is often observed for amphiphilic compounds like carnosol and carnosic acid and ascribed to plasma protein binding (Koeberle and Werz, 2009). The IC50 for the inhibition of PGE2 formation by carnosic acid is also shifted by a factor of 2 but still remains at low micromolar concentrations (IC50 = 9.3 μM) in contrast to the IC50 value of carnosol. Whether differences in plasma protein binding, cellular uptake, or metabolism underlie the different potencies of carnosol and carnosic acid in inhibiting PGE2 formation in LPS-stimulated human whole blood has yet to be answered. Oral administration of carnosic acid to mice resulted in peak plasma levels up to 44 μM (Satoh et al., 2008), implying that carnosic acid inhibits PGE2 formation in whole blood at pharmacologically highly relevant concentrations. Several studies described a redirection of PGH2 toward the biosynthesis of other prostaglandins upon inhibition of mPGES-1, which might detrimentally compensate for PGE2 (Koeberle and Werz, 2009). For carnosol and carnosic acid, a redirection was not observed to 6-keto PGF1α, thromboxane B2, or 12-HHT, eventually because of the overlapping cellular inhibition of mPGES-1 and COX isoenzymes.
As dual inhibitors of mPGES-1 (as shown here) and 5-lipoxygenase [as reported previously (Poeckel et al., 2008)], carnosol and carnosic acid possess a beneficial pharmacological profile. Dual inhibition of inducible PGE2 and leukotriene biosynthesis is considered advantageous compared with interference with prostanoid formation alone. Combined inhibitors might not only exhibit an increased anti-inflammatory and anticarcinogenic potential but also seem to be associated with reduced side effects (Celotti and Laufer, 2001; Rådmark and Samuelsson, 2010). Particularly, gastrointestinal, renal, and cardiovascular intolerance have been reported for COX-1/2 inhibitors (Koeberle and Werz, 2009). Such side effects were not observed for the treatment with traditional remedies containing S. officinalis or reported within the limited number of preclinical studies using carnosol, carnosic acid, or plant extracts enriched in either of them.
Taken together, we 1) identified an ethyl acetate extract of S. officinalis to potently inhibit mPGES-1, 2) ascribed this inhibition to carnosol and carnosic acid through bioactivity-guided fractionation, 3) confirmed the inhibition of mPGES-1 by carnosol and carnosic acid in cell-free and cellular assays, and 4) characterized the specificity of carnosol and carnosic acid for mPGES-1 within prostanoid biosynthesis. Our data suggest that the selective inhibition of mPGES-1-derived PGE2 formation may contribute to the anticarcinogenic and anti-inflammatory properties of carnosol and carnosic acid, which are major ingredients of the traditional herbal remedy S. officinalis.
Acknowledgments
We thank Gertrud Kleefeld and Katrin Fischer for expert technical assistance.
This work was supported by the Standortagentur Tirol and the Austrian Science Fund [Grant DNTI S10703].
ABBREVIATIONS
- COX
cyclooxygenase
- 12-HHT
12(S)-hydroxy-5-cis-8,10-trans-heptadecatrienoic acid
- LPS
lipopolysaccharide
- PG
prostaglandin
- mPGES
microsomal PGE2 synthase
- PBS
phosphate-buffered saline
- HPLC
high-performance liquid chromatography
- DMSO
dimethyl sulfoxide
- ANOVA
analysis of variance
- ELISA
enzyme-linked immunosorbent assay
- HSD
honestly significant difference
- LOQ
limit of quantification
- CV4151
(E)-7-phenyl-7-(3-pyridyl)-6-heptenoic acid
- MD52
2-(2-chlorophenyl)-1H-phenanthro[9,10-d]-imidazole
- MK-886
3-[1-(4-chlorobenzyl)-3-t-butylthio-5-isopropylindol-2-yl]-2,2-dimethylpropanoic acid
Footnotes
Authorship Contributions
Participated in research design: Rollinger, Stuppner, Werz, and Koeberle.
Conducted experiments: Bauer, Kuehnl, Scherer, and Koeberle.
Contributed new reagents or analytic tools: Rollinger, Northoff, Stuppner, and Werz.
Performed data analysis: Bauer, Kuehnl, and Koeberle.
Wrote or contributed to the writing of the manuscript: Rollinger, Stuppner, Werz, and Koeberle.
Parts of this work were described previously: Koeberle A (2009) Identification and characterization of microsomal prostaglandin E2 synthase-1 inhibitors. Ph.D. thesis, Pharmaceutical Institute, University of Tuebingen, Tuebingen, Germany.
Contributor Information
Julia Bauer, Department for Pharmaceutical Analytics, Pharmaceutical Institute, University of Tuebingen, Tuebingen, Germany.
Susanne Kuehnl, Institute of Pharmacy/Pharmacognosy and Center for Molecular Biosciences Innsbruck, University of Innsbruck, Innsbruck, Austria.
Judith M. Rollinger, Institute of Pharmacy/Pharmacognosy and Center for Molecular Biosciences Innsbruck, University of Innsbruck, Innsbruck, Austria
Olga Scherer, Chair of Pharmaceutical/Medicinal Chemistry, Institute of Pharmacy, University of Jena, Jena, Germany.
Hinnak Northoff, Institute for Clinical and Experimental Transfusion Medicine, University Medical Center, Tuebingen, Germany.
Hermann Stuppner, Institute of Pharmacy/Pharmacognosy and Center for Molecular Biosciences Innsbruck, University of Innsbruck, Innsbruck, Austria.
Oliver Werz, Chair of Pharmaceutical/Medicinal Chemistry, Institute of Pharmacy, University of Jena, Jena, Germany.
Andreas Koeberle, Chair of Pharmaceutical/Medicinal Chemistry, Institute of Pharmacy, University of Jena, Jena, Germany.
References
- Albert D, Zündorf I, Dingermann T, Müller WE, Steinhilber D, Werz O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem Pharmacol. 2002;64:1767–1775. doi: 10.1016/s0006-2952(02)01387-4. [DOI] [PubMed] [Google Scholar]
- Asano K, Lilly CM, Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1996;271:L126–L131. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, Zupancic A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol. 2001;75:125–132. doi: 10.1016/s0378-8741(00)00396-2. [DOI] [PubMed] [Google Scholar]
- Barni MV, Carlini MJ, Cafferata EG, Puricelli L, Moreno S. Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells. Oncol Rep. 2012;27:1041–1048. doi: 10.3892/or.2012.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Koeberle A, Dehm F, Pollastro F, Appendino G, Northoff H, Rossi A, Sautebin L, Werz O. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem Pharmacol. 2011;81:259–268. doi: 10.1016/j.bcp.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Celotti F, Laufer S. Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res. 2001;43:429–436. doi: 10.1006/phrs.2000.0784. [DOI] [PubMed] [Google Scholar]
- Côté B, Boulet L, Brideau C, Claveau D, Ethier D, Frenette R, Gagnon M, Giroux A, Guay J, Guiral S, et al. Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors. Bioorg Med Chem Lett. 2007;17:6816–6820. doi: 10.1016/j.bmcl.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Dörrie J, Sapala K, Zunino SJ. Carnosol-induced apoptosis and down-regulation of Bcl-2 in B-lineage leukemia cells. Cancer Lett. 2001;170:33–39. doi: 10.1016/s0304-3835(01)00549-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez AG, Abad T, Jimenez IA, Ravelo AG, Luis JG, Aguiar Z, Andres LS, Plasencia M, Herrera JR, Moujir L. A first study of antibacterial activity of diterpenes isolated from some salvia species (Lamiaceae) Biochem Sys Ecol. 1989;17:293–296. [Google Scholar]
- Hawkey CJ. COX-2 inhibitors. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-κB and c-Jun. Biochem Pharmacol. 2005;69:221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Hussein AA, Meyer JJ, Jimeno ML, Rodríguez B. Bioactive diterpenes from Orthosiphon labiatus and Salvia africana-lutea. J Nat Prod. 2007;70:293–295. doi: 10.1021/np0680376. [DOI] [PubMed] [Google Scholar]
- Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14:2016–2031. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011;305:1–7. doi: 10.1016/j.canlet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5′-AMP-activated protein kinase (AMPK) pathway. Pharm Res. 2008;25:2125–2134. doi: 10.1007/s11095-008-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ, Syed DN, Suh Y, Heren CR, Saleem M, Siddiqui IA, Mukhtar H. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev Res (Phila) 2010;3:1112–1123. doi: 10.1158/1940-6207.CAPR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Ohkawa S, Terao S, Terashita Z, Nishikawa K. Thromboxane synthetase inhibitors (TXSI). Design, synthesis, and evaluation of a novel series of omega-pyridylalkenoic acids. J Med Chem. 1985;28:287–294. doi: 10.1021/jm00381a005. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- Koeberle A, Bauer J, Verhoff M, Hoffmann M, Northoff H, Werz O. Green tea epigallocatechin-3-gallate inhibits microsomal prostaglandin E2 synthase-1. Biochem Biophys Res Commun. 2009a;388:350–354. doi: 10.1016/j.bbrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Koeberle A, Northoff H, Werz O. Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem Pharmacol. 2009b;77:1513–1521. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Koeberle A, Siemoneit U, Bühring U, Northoff H, Laufer S, Albrecht W, Werz O. Licofelone suppresses prostaglandin E2 formation by interference with the inducible microsomal prostaglandin E2 synthase-1. J Pharmacol Exp Ther. 2008;326:975–982. doi: 10.1124/jpet.108.139444. [DOI] [PubMed] [Google Scholar]
- Koeberle A, Werz O. Inhibitors of the microsomal prostaglandin E2 synthase-1 as alternative to non steroidal anti-inflammatory drugs (NSAIDs)-a critical review. Curr Med Chem. 2009;16:4274–4296. doi: 10.2174/092986709789578178. [DOI] [PubMed] [Google Scholar]
- Laughton MJ, Evans PJ, Moroney MA, Hoult JR, Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem Pharmacol. 1991;42:1673–1681. doi: 10.1016/0006-2952(91)90501-u. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Jin YR, Lim Y, Hong JT, Kim TJ, Chung JH, Yun YP. Antiplatelet activity of carnosol is mediated by the inhibition of TXA2 receptor and cytosolic calcium mobilization. Vascul Pharmacol. 2006;45:148–153. doi: 10.1016/j.vph.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry. 2000;55:263–267. doi: 10.1016/s0031-9422(00)00309-5. [DOI] [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Mengoni ES, Vichera G, Rigano LA, Rodriguez-Puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno S, Vojnov AA. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia. 2011;82:414–421. doi: 10.1016/j.fitote.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Moran AE, Carothers AM, Weyant MJ, Redston M, Bertagnolli MM. Carnosol inhibits β-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res. 2005;65:1097–1104. [PubMed] [Google Scholar]
- Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, Xu D, Rosenberg DW. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- Ninomiya K, Matsuda H, Shimoda H, Nishida N, Kasajima N, Yoshino T, Morikawa T, Yoshikawa M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg Med Chem Lett. 2004;14:1943–1946. doi: 10.1016/j.bmcl.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Okamura N, Fujimoto Y, Kuwabara S, Yagi A. High-performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus oficinalis and Salvia oficinalis. J Chromatogr A. 1994;679:381–386. [Google Scholar]
- Pham H, Shafer LM, Slice LW. CREB-dependent cyclooxygenase-2 and microsomal prostaglandin E synthase-1 expression is mediated by protein kinase C and calcium. J Cell Biochem. 2006;98:1653–1666. doi: 10.1002/jcb.20899. [DOI] [PubMed] [Google Scholar]
- Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, Hörnig C, Steinhilber D, Schubert-Zsilavecz M, Werz O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91–97. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Rådmark O, Samuelsson B. Microsomal prostaglandin E synthase-1 and 5-lipoxygenase: potential drug targets in cancer. J Intern Med. 2010;268:5–14. doi: 10.1111/j.1365-2796.2010.02246.x. [DOI] [PubMed] [Google Scholar]
- Rau O, Wurglics M, Paulke A, Zitzkowski J, Meindl N, Bock A, Dingermann T, Abdel-Tawab M, Schubert-Zsilavecz M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor γ. Planta Med. 2006;72:881–887. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RF, Tai HH. Immunoaffinity purification and characterization of thromboxane synthase from porcine lung. J Biol Chem. 1986;261:11592–11599. [PubMed] [Google Scholar]
- Siemoneit U, Koeberle A, Rossi A, Dehm F, Verhoff M, Reckel S, Maier TJ, Jauch J, Northoff H, Bernhard F, et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br J Pharmacol. 2011;162:147–162. doi: 10.1111/j.1476-5381.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Steiner M, Priel I, Giat J, Levy J, Sharoni Y, Danilenko M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer. 2001;41:135–144. doi: 10.1080/01635581.2001.9680624. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Cole PA, Dannenberg AJ. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 2002;62:2522–2530. [PubMed] [Google Scholar]
- Tsai CW, Lin CY, Lin HH, Chen JH. Carnosic acid, a rosemary phenolic compound, induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neurochem Res. 2011;36:2442–2451. doi: 10.1007/s11064-011-0573-4. [DOI] [PubMed] [Google Scholar]
- Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–136. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Werz O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007;73:1331–1357. doi: 10.1055/s-2007-990242. [DOI] [PubMed] [Google Scholar]
- Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr. 2010;65:158–163. doi: 10.1007/s11130-010-0166-4. [DOI] [PubMed] [Google Scholar]