Abstract
Background: Hemorrhagic shock and trauma are associated with acidosis and altered coagulation. A fall in pH has been reported to attenuate the activity of recombinant activated Factor VII (rFVIIa) in vitro. However, it is not known if acidosis induced by hemorrhagic shock or infusion of HCl attenuates FVIIa activity in vivo. The purpose of this study was to determine if acidosis, induced by two methods, affects recombinant FVIIa (rFVIIa) activity in swine, and if correction of the pH restores rFVIIa activity to normal. Methods: Acidosis was induce in anesthetized swine in two separate models: 1) HCl infusion (n=10) and 2) hemorrhage/hypoventilation (n=8). Three groups per model were used: Control (pH7.4), Acidosis (arterial pH7.1) and Acidosis-Corrected (bicarbonate infusion to return pH from 7.1 to 7.4). Pigs were then injected with rFVIIa (90 μg/kg) or vehicle (saline) at target pH and arterial blood samples were taken for measurement of coagulation function, including Thromboelastography -TEG, Thrombin Generation, Activated Clotting Time, Prothrombin Time, activated Partial Thromboplastin Time, Fibrinogen Concentration and Platelet count before and 5min after injection of rFVIIa. Results: Acidosis led to a hypocoagulation as measured by almost all coagulation parameters in both models. Furthermore, the change in coagulation function produced after infusion of rFVIIa was not different between control, acidosis and acidosis-corrected groups for all coagulation parameters measured. Conclusion: Acidosis associated with hemorrhagic shock or HCl infusion led to a hypocoagulation that was not corrected with bicarbonate infusion. Furthermore, acidosis did not affect rFVIIa function, and correction of the acidosis with bicarbonate had no effect on rFVIIa function in these models. This suggests that in vivo acidosis did not diminish rFVIIa function.
Keywords: Hemorrhage, acidosis, hypoventilation, hypocoagulation, thromboelastography, thrombin generation, rotational thromboelastogram, fibrinogen, platelets, coagulation parameters, recombinant factor VIIa
Introduction
Hemorrhage and trauma are the leading cause of death in young adults, and is the principal cause of death on the battlefield [1-6]. This is partially due to the difficulty in controlling and managing trauma patients who are cold, acidotic and coagulopathic, and require continuous resuscitation because of persistent bleeding [7,8]. Hypocoagulation can occur during hemorrhagic shock and trauma, and has been partially attributed to metabolic acidosis that develops as a result of reduced blood perfusion of tissue beds and organs [9-12]. Acidosis has been shown to impair coagulation as measured by thrombelastography in whole blood [13], decrease the activity of Factors V and IX, reduce platelet aggregation [14], increase fibrinogen consumption [15], reduce platelet count, thrombin generation, maximum clot strength, and prolong PT, aPTT, and Activated Clotting Time [16-19]. Acidosis has been reported to significantly reduce activated Factor VII (FVIIa) activity and function in vitro [20] and its correction (or normalization of pH) has been suggested before clinical use of rFVIIa [21,22].
FVII is one of the many coagulation factors that are important for normal hemostasis. Humans with FVII deficiency experience intra-arterial and mucocutaneous bleeding analogous to hemophilia A or B (deficient in Factor VIII and Factor IX). Mice lacking FVII die in-utero or soon after birth due to vascular and hemostatic defects [23]. Recombinant FVIIa (rFVIIa) has been used clinically off label to control and decrease excessive bleeding in trauma patients. The use of rFVIIa was shown to significantly reduce the amount of red blood cells used in 20% of trauma patients requiring massive transfusion [24]. Furthermore, rFVIIa reduced the amount of diffuse bleeding, the need for blood products, and improved prothrombin time (PT) and activated partial thromboplastin time (aPTT) [25]. Case studies report beneficial effects of rFVIIa including diminished bleeding, improved visibility in the surgical field, and a decreased need for blood products [26,27]. However the use of rFVIIa in these situation is not without controversy [28].
Hypocoagulation caused by acidosis may benefit from rFVIIa therapy. However, it is not known if acidosis attenuates rFVIIa activity in vivo as has been suggested in vitro [20]. Furthermore, if acidosis does affect rFVIIa activity, it is not known if correction of acidosis will return FVIIa activity to normal levels. The purpose of this study was to determine if acidosis affects rFVIIa activity in vivo and if so, whether correction of the pH returns rFVIIa activity to normal.
Materials and methods
This study was approved by the Animal Care and Use Committee of the US Army Institute of Surgical Research. All animals received care and were used in strict compliance with the Guide for the Care and Use of Laboratory Animals [29].
Yorkshire cross-bred female pigs weighing 40.3±0.3 kg were purchased from Midwest Research Swine (Gibbon, MN). Before surgery, venous blood samples were collected from pigs and complete blood count (CBC) and coagulation parameters (PT, aPTT and fibrinogen concentration) were measured to ensure that these values were within the normal range before proceeding with experimentation. On the day of surgery, pigs were premedicated with buprenorphine (0.01 mg/kg, intramuscular [i.m.]) for analgesia and glycopyrrolate (0.01 mg/ kg, i.m.) to reduce saliva secretion and block vagally mediated bradycardia during the surgical procedure. Animals were then induced with Telazol (4-6 mg/kg, i.m.) and isoflurane, intubated and mechanically ventilated. These concentrations were dictated by our veterinary staff for each study.
Prior to surgery, tidal volume and ventilation rate were adjusted to maintain an end-tidal PCO2 of 40 ± 5 mmHg. Maintenance fluid, lactated Ringer’s (LR), was administered at 5 mL/kg/hr through a venous line placed in an ear vein.
The right carotid artery was cannulated for the direct measurements of blood pressure (systolic, diastolic, and mean) and heart rate throughout the experiment. The right jugular vein and right femoral vein were catheterized for administering drugs or fluids. The right femoral artery was cannulated for hemorrhage, and re-infusion of shed blood. Arterial blood samples were taken from the carotid artery. Venous blood samples were taken from the jugular vein. At the end of the experiment, all pigs were euthanized with 1cc/10 lbs of Fatal-Plus®, IV.
Two separate experimental methods were used to induce acidosis (pH7.1) in this study: 1) by IV infusion of 0.2M HCl solution into the pigs (n=10) and 2) by a combination of controlled hemorrhage and hypoventilation (n=8). Anesthesia was maintained between 0.8 and 1.5% isoflurane added to air/oxygen with 31% oxygen in the hemorrhage/hypoventilation model and 100% oxygen for the HCl-infusion model as per veterinary recommendations.
In order to determine if acidosis affects rFVIIa function, we measured various coagulation parameters (as described below) from blood samples of control and acidotic pigs before and after rFVIIa injection. To determine if correction of the acidosis with bicarbonate returns rFVIIa function to normal in both models, we infused acidotic pigs with bicarbonate until arterial pH was 7.4 (acidosis-corrected) and then injected rFVIIa. Three groups of pigs were used per method; control (pH7.4), acidosis (pH7.1) and acidosis-corrected (pH7.1 to 7.4). A timeline of this experiment is graphically represented in Figure 1 for each group in both methods.
Figure 1.
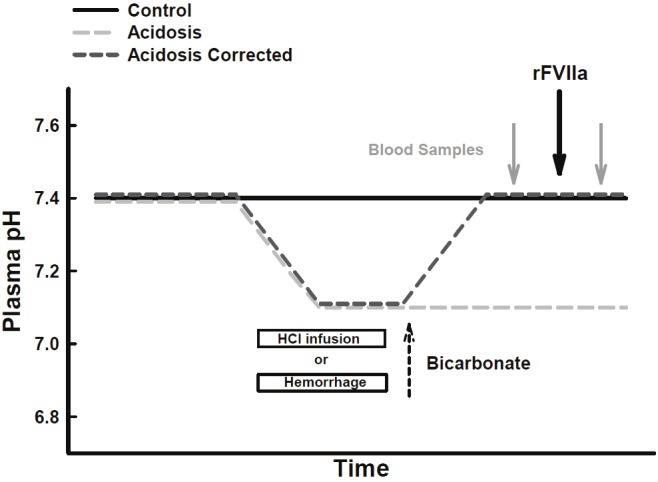
Description of experimental models. Time line showing the relationship between injection of rFVIIa, blood sampling, induction of acidosis by either hemorrhage or HCl-infusion, and correction of acidosis with bicarbonate. Total experimental time varied from pig to pig.
HCl-induced acidosis
After surgery was completed, inhalation anesthesia was reduced and maintained at 0.8-1.5% isoflurane. Two of the three groups of pigs were made acidotic by infusion of HCl (0.2M, IV) at 2-10 ml/min until a pH of arterial blood reached 7.1. This model has been previously described by our laboratory [17,18]. The amount of HCl needed to lower pH was 2594±119 ml (64±3 ml/kg) and was infused over 253±21 minutes. One of the acidotic groups was infused IV (266±23 ml at 100 ml/min) with sodium bicarbonate (8.4% injectable solution) until pH stabilized at 7.4 (acidosis-corrected group). The other acidosis group (acidosis) received the same volume of saline instead of bicarbonate. A control group (control) was infused with saline (instead of HCl and bicarbonate). The volume of saline, rate of infusion and time given were matched to the acidotic groups, and the pH in the control group remained at 7.4. Recombinant FVIIa (90 mg/kg, NovoSeven, Novo Nordisk) was injected iv into each group after pH had stabilized. Blood samples were taken before and 5 min after injection of rFVIIa for determination of coagulation parameters (described below).
Hemorrhage/hypoventilation-induced acidosis
After surgery was completed, 100-500 mg/kg*min of ketamine was administered via the femoral vein and the isoflurane reduced to 1%. This plane of anesthesia allowed breathing to be entirely controlled by mechanical ventilation.
A baseline pH of 7.42 was achieved by slight adjustment of the ventilator’s tidal volume and/or rate. To induce acidosis, two of the three groups of pigs (Acidosis and acidosis-corrected) were subjected to a controlled hemorrhage (50 ml/min) and bled to a mean arterial pressure of 30 mmHg by additional bleeding or reinfusion of shed blood. During hemorrhage, blood was collected in CPD blood bags and stored at 37°C. If arterial pressure fell below 30 mmHg, whole blood was reinfused from these bags to maintain mean arterial pressure at 30 mmHg. CaCl2 (100 mg/ml) was injected (1 ml/100ml blood reinfused) to maintain ionized Ca++ levels at normal levels. The control group (control) was not hemorrhaged. Blood samples (1.5 ml over lithium heparin) were taken from the carotid artery every 15 min to monitor arterial blood gases, ionized Ca++ and pH using an IRMA TruPoint Blood Analysis System (International Technidyne Corporation, Edison, NJ). The volume of controlled hemorrhage in the acidosis and acidosis-corrected groups (combined) was 1464±39 ml (36±1.0 ml/kg) and was removed to maintain mean arterial pressure at 30mmHg. The amount of blood reinfused was 801±74 ml (16.0±1.6 ml/kg). The total amount of blood removed after hemorrhage and reinfusion was 663±69 ml (16±1.5 ml/kg).
Arterial pH was measured every 15 min. Hemorrhage led to an initial fall in arterial pH, which plateaued at pH 7.33±0.01 at 116±6 min post hemorrhage. At this point, ventilation was decreased (tidal volume and/or ventilation rate were adjusted) until arterial pH fell to 7.1. In the acidosis-corrected group, 100 ml/40 kg body weight of sodium bicarbonate (8.4% injectable solution) was injected IV and ventilation (tidal volume/ rate) was adjusted to pre-hemorrhage levels until pH stabilized at normal levels (7.4). The control and acidosis groups were given an infusion of 100 ml saline/kg body weight as a volume control for the bicarbonate that was infused into the acidosis-corrected group.
Recombinant FVIIa (90 μg/kg, NovoSeven, Novo Nordisk) was injected IV into each group when the desired pH was reached. Blood samples were taken before and 5 min after injection of rFVIIa for determination of coagulation parameters (Figure 1).
Plasma fibrinogen concentration, PT and aPTT were measured using the BCS Coagulation System (Dade Behring, Deerfield, IL). Platelet counts were measured using an ABX Pentra 120 Hematology Analyzer (ABX Diagnostics, Irvine, CA).
Activated Clotting Time (ACT) was measured in fresh whole blood (Hemochron, International Technidyne Corporation, Edison, NJ) based on the manufacturer’s instructions.
Thrombelastography (TEG) was done immediately after the blood samples were collected. TEG was run on a TEG Hemostasis Analyzer 5000 (Haemonetics, Braintree, MA) using native whole arterial blood (no anticoagulants). The accuracy of the TEG machines was checked daily using quality control standards obtained from Hemoscope. For this assay, clotting was initiated by adding 10 μL of human recombinant tissue factor (Innovin, diluted 1:200 with saline) to 340 μL of fresh blood samples and the clotting profile traced. Samples were tested in triplicate and tracing continued until 30 minutes after the clot reached maximum strength. The following variables were measured for each sample at the pig’s normal body temperature (39°C): R-time (min, the time that the initial fibrin formation is detected); K-time (min, the time to clot formation); α angle (degrees, the speed of clot development); and MA (mm, the maximum amplitude or strength of the developed clot) as described previously [30].
Thrombin generation was analyzed by a fluorogenic method using a specialized plate reader and software (Fluoroskan Ascent, Calibrated Automated Throbinoscope, ThermoScientific, Waltham, MA). Thrombin generation was measured in platelet-poor plasma (80 μL) mixed with a reagent containing phospholipids and tissue factor in 96-well plates. Added to this mixture was a buffer containing a substrate that is enzymatically cleaved by thrombin to produce a fluorescent product. Calcium chloride was added to neutralize anticoagulant. Thrombograms were analyzed to determine endogenous thrombin potential (ETP), maximum thrombin concentration (peak), time to peak (ttPeak), and Lagtime. All assays were performed in triplicate.
Data were analyzed by Two-Way ANOVA Corrected for Repeated Measure followed by multiple comparison analysis using Holm-Sidak Method. One-Way ANOVA was used to analyze the blood gases and chemistry, and changes in coagulation parameters followed by multiple comparison analysis using Holm-Sidak Method. If the normality test failed, then log10 transform of data was performed or Kruskal Wallis One-Way ANOVA on Ranks was performed followed by multiple comparisons by Dunn’s Method. Statistics were performed using SigmaStat ® solfware. Data were expressed as the mean ± standard error of the mean (SEM). P<0.05 was considered significant.
Results
HCl-induced acidosis
Infusion of a solution of 0.2N HCl lowered arterial pH from 7.4 to 7.1, and reduced HCO3 - and Base Excess (BE) (Table 1). An infusion of 8% bicarbonate solution was also successful in returning arterial pH to 7.4, and restoring HCO3 - and BE to near control levels.
Table 1.
Blood gases measured prior to injection of rFVIIa in the HCl model
| Control | Acidosis | Acidosis corrected | |
|---|---|---|---|
| n=10 | |||
| pH | 7.38±0.01 | 7.14±0.01 | 7.43±0.01 |
| PaCO2 (mmHg) | 47.3±1.8 | 43.0±1.8 | 55.0±3.1 |
| PaO2 (mmHg) | 433.6±26.4 | 400.9±33.1 | 455.4±16.6 |
| HCO3 - (mM) | 32.4±1.0 | 14.5±0.7* | 31.4±1.5 |
| BE (mM) | 7.3±0.9 | -13.9±0.7* | 5.2±1.2 |
Values represent Mean±Standard Error of the Mean.
= Significant difference (P<0.05) from Control by 1Way ANOVA followed by pair-wise Post Hoc test (Holm-Sidac method).
Hemorrhage/hypoventilation
In all animals, severe hemorrhagic shock, by itself, failed to lower arterial pH below 7.3 even though HCO3 - and BE fell significantly (Table 2). Combining hemorrhage (metabolic acidosis) with a decrease in respiration (respiratory acidosis) successfully lowered arterial pH to 7.1 (Table 2). Bicarbonate infusion with normalization of respiration returned arterial pH to 7.4 and restored PaCO2, PaO2 and BE to baseline (Table 3). Ionized calcium was maintained near control in the hemorrhage model by injecting CaCl2 (1mg/ml of reinfused blood) to prevent plasma calcium from falling below 1mM and hinder the ability of the blood to clot. Table 4 shows that ionized Calcium remained above 1.2 mM. Hemorrhage/hypoventilation also led to a significant rise in plasma lactate and potassium (Table 4) which is an indication of the severity of this shock model.
Table 2.
Blood gases measured before and after hemorrhage and hypoventilation
| Before Hemorrhage | Hemorrhage to 30mmHg | Hemorrhage/Hypoventilation | |
|---|---|---|---|
| n=16 | |||
| pH | 7.42±0.01 | 7.33±0.01* | 7.12±0.02* # |
| PaCO2 (mmHg) | 49.5±1.3 | 48.2±1.8 | 94.0±5.2* # |
| PaO2 (mmHg) | 142.8±3.3 | 133.5±8.6 | 99.6±9.6* # |
| HCO3 - (mM) | 31.2±0.7 | 24.3±1.1* | 29.5±1.2# |
| BE (mM) | 5.8±0.6 | -1.5±1.0* | -2.1±1.2* |
Values represent Mean±Standard Error of the Mean from combined hemorrhage groups (Acidosis and Acidosis-corrected).
= Significant difference (P<0.05) from Before Hemorrhage,
= Significant difference (P<0.05) from Hemorrhage to 30mmHg, by 1Way ANOVA CRM with pair-wise multiple comparison Post Hoc test (Holm-Sidac method).
Table 3.
Blood gases measured prior to injection of rFVIIa in Hemorrhage/Hypoventilation model
| Control | Acidosis | Acidosis corrected | |
|---|---|---|---|
| n=8 | n=8 | n=8 | |
| pH | 7.42±0.01 | 7.10±0.01 | 7.42±0.01 |
| PaCO2 (mmHg) | 51.3±1.3 | 104.6±4.7* | 50.5±2.4 |
| PaO2 (mmHg) | 146.6±6.0 | 97.6±9.3* | 160.1±7.8 |
| HCO3 - (mM) | 32.2±0.7 | 31.5±1.4 | 32.2±1.5 |
| BE (mM) | 6.7±0.6 | -0.9±1.2* | 6.6±1.2 |
Values represent Mean±Standard Error of the Mean.
= Significant difference (P<0.05) from Control by 1Way ANOVA with pairwise multiple comparison Post Hoc test (Holm-Sidac method).
Table 4.
Plasma Chemistry in Hemorrhage/Hypovolemia Model prior to rFVIIa injection
| Control | Acidosis | Acidosis-corrected | |
|---|---|---|---|
| n=8 | n=8 | n=8 | |
| iCa (mM) | 1.37±0.02 | 1.31±0.02 | 1.24±0.03* |
| Na (mM) | 138.9±0.7 | 139.1±0.8 | 140.0±0.4 |
| K (mM) | 4.6±0.1 | 5.9±0.3* | 6.7±0.4* |
| Cl (mM) | 98.3±0.6 | 99.1±0.5 | 96.8±0.6 |
| Glucose (mM) | 3.77±0.73 | 3.35±1.01 | 1.58±0.34 |
| Lactate (mM) | 0.85±0.13 | 4.87±0.99* | 7.15±1.54* |
Values represent Mean±Standard Error of the Mean.
= Significant difference (P<0.05) from Control by 1Way ANOVA with pairwise multiple comparison Post Hoc test (Holm-Sidac method).
Effect of rFVIIa on coagulation in normal, acidotic, and acidosis-corrected swine
Generally, acidosis caused a hypocoagulation in the swine that was not corrected with bicarbonate injection. Irrespective of this hypocoagulation, infusions of rFVIIa improved coagulation by most parameters measured (specifics described below). Furthermore, the increase, or change in coagulation after rFVIIa infusions was not different between the control and acidosis groups, or between the acidosis and acidosis-corrected groups in either acidosis-induced model. This suggests that acidosis, or correction of acidosis did not affect the function of rFVIIa. The following is a detailed analysis of these findings.
PT, aPTT and ACT
Acidosis induced by HCl infusion significantly prolonged PT, aPTT and ACT (Figure 2). In contrast, PT, aPTT and ACT were not significantly affected by the acidosis caused by hemorrhage/hypoventilation.
Figure 2.
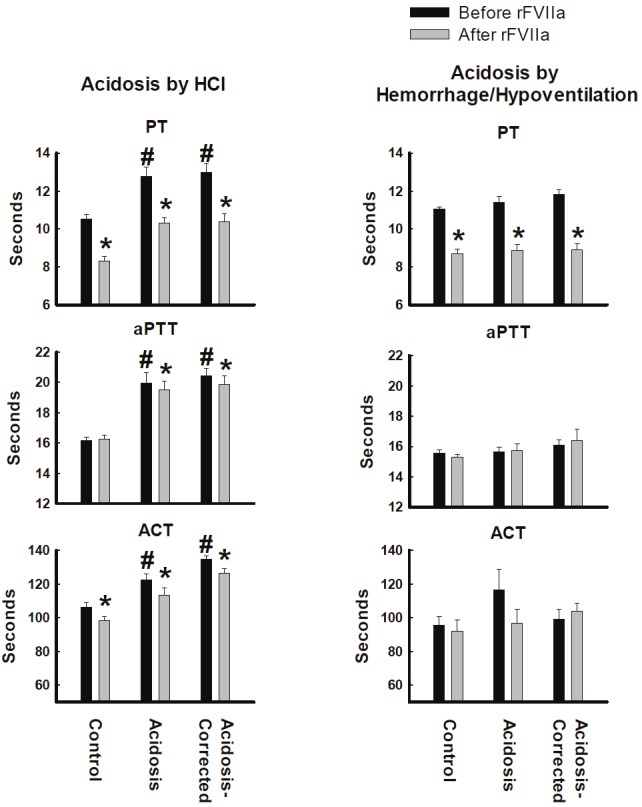
Clotting values from whole blood or plasma collected from pigs made acidotic by either infusion of 0.2M HCl (n=10) or hemorrhage/hypoventilation (n=8). PT = Prothrombin Time, aPTT = activated Partial Thromboplastin Time, ACT- Activate Clotting Time. *P<0.05 comparing before and after rFVIIa injection. #P<0.05 compared to Control group. Values = Mean±Standard Error of the Mean.
Recombinant FVIIa reduced PT in normal, acidosis and acidosis-corrected swine for both HCl- and hemorrhage/respiratory-induced acidosis (Figure 2). Infusions of rFVIIa led to a small, but significant decrease in aPTT in the acidosis and acidosis-corrected groups in the HCl Model (Figure 2). Infusion of FVIIa caused no change in aPTT in the Hemorrhage/Respiratory Model, but deceased ACT in the control, acidosis and acidosis-corrected groups in the HCl Model (Figure 2). Recombinant rFVIIa had no significant effect on ACT in the hemorrhage/hypoventilation acidosis model.
TEG
In the HCl model, acidosis significantly prolonged K-time, and reduced both MA and α angle (Figure 3). Although the hemorrhage/hypoventilation model showed similar trends, the changes in K time, MA and α angle did not achieve statistical significant.
Figure 3.
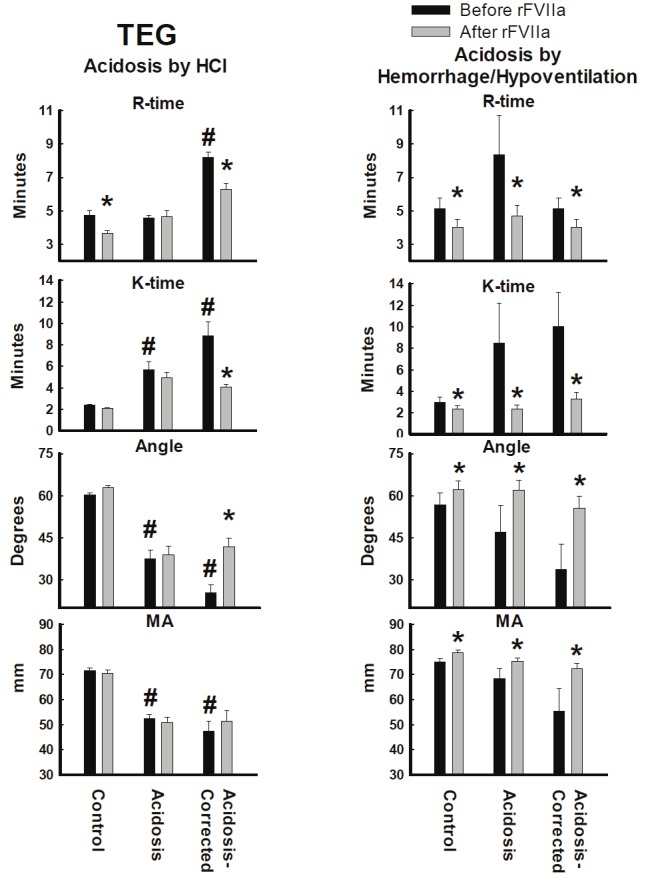
TEG parameters from whole blood collected from pigs made acidotic by either infusion of 0.2M HCl (n=10) or hemorrhage/hypoventilation (n=8). R= reaction time, K=clotting time, α angle= rate of clot formation, MA=maximum clot strength. *P<0.05 comparing before and after rFVIIa injection. #P<0.05 compared to Control group. Values = Mean±Standard Error of the Mean.
Infusion of rFVIIa in the hemorrhage/hypoventilation model significantly reduced R-time and K-time, and increased in α-angle and MA in control, acidosis and acidosis-corrected swine (Figure 3). In the HCl model, infusion of rFVIIa led to a significant decrease in R-time in the control group, and a significant decrease in R-time and K-time, and an increase in the α-angle of the acidosis-corrected group (Figure 3). MA did not significantly change after rFVIIa injection in HCl-induce acidosis for any of the three groups.
Thrombin generation
Induction of acidosis significantly decreased both Peak and ETP in both acidosis models (Figure 4). Lagtime and ttPeak did not significantly change in either model after induction of acidosis.
Figure 4.
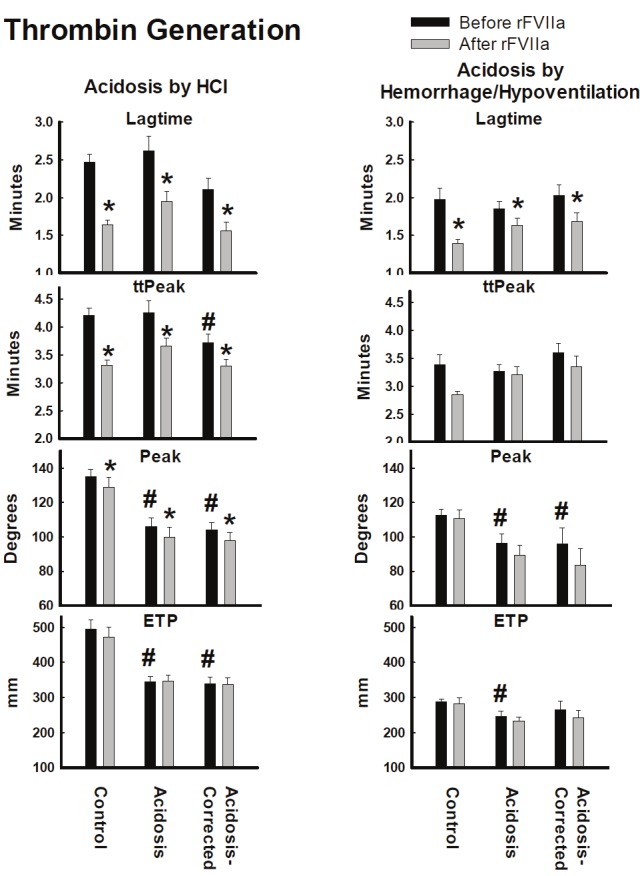
Thrombin Generation assay from plasma collected from pigs made acidotic by either infusion of 0.2M HCl (n=10) or hemorrhage/hypoventilation (n=8). ETP = Endogenous Thrombin Potential, Peak = Maximum Thrombin Concentration, Lag Time = time to start of thrombus formation, ttPeak = time to Peak. *=P<0.05 comparing before and after rFVIIa injection. #=P<0.05 compared to Control group. Values = Mean±Standard Error of the Mean.
In the HCl model, infusion of rFVIIa, significantly reduced the Lagtime, ttPeak and Peak in all three groups (Figure 4). However, there was no significant effect on ETP. In the hemorrhage/Hypoventilation model, infusion of rFVIIa only significantly reduced Lagtime (Figure 4).
Fibrinogen and platelets
The induction of acidosis by HCl infusion led to a significant fall in fibrinogen concentration and platelet count (Figure 5). In contrast, induction of acidosis had no significant affect on fibrinogen concentration or platelet count in the hemorrhage/hypoventilation model. Furthermore, infusion of rFVIIa had no affect on either parameter in either model.
Figure 5.
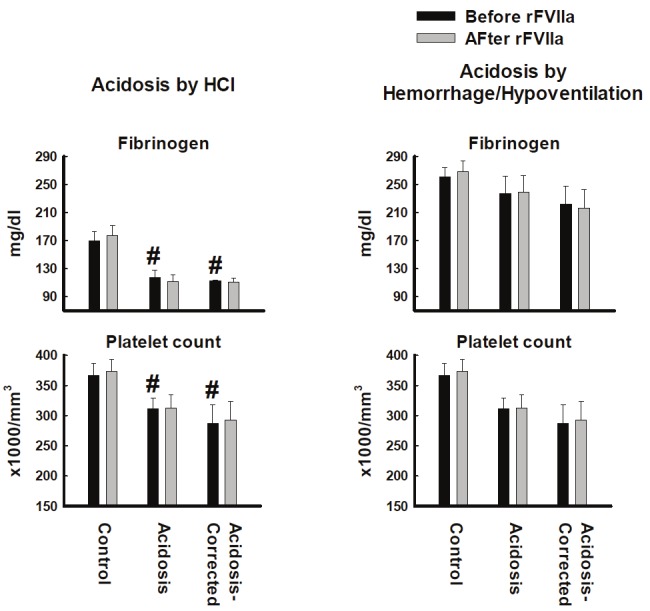
Fibrinogen concentration and platelet count from blood collected from pigs made acidotic by either infusion of 0.2M HCl (n=10) or hemorrhage/hypoventilation (n=8). #P<0.05 compared to Control group. Values = Mean±Standard Error of the Mean.
Discussion
These data confirm that acidosis induced a hypocoagulation in swine that could not be corrected by correcting pH. These data also show that infusion of rFVIIa improved coagulation by most of the parameters that were measured in both acidosis models. These findings suggest two important observations; 1) that acidosis did not suppress rFVIIa function in vivo and 2) correction of the acidosis was not necessary for rFVIIa to improve coagulation.
HCl infusion or hemorrhage coupled with decreased respiration was successful in inducing acidosis in swine. The acidosis led to a hypocoagulation as measured by almost all coagulation parameters and is in agreement with our previous report [31]. In the HCl model, the hypocoagulation may be partially explained by the decrease in the fibrinogen concentration and platelet count. This suggests that HCl partially destroyed fibrinogen and platelets and that normal levels could not be recoved by correction of pH. Fibrinogen is the major substrate of clotting that is converted to fibrin by thrombin, then undergoes covalent cross-linking catalyzed by factor XIIIa to form a stable clot. Platelets adhere and aggregate to the site of injury and provide a surface for generation of thrombin thereby potentiating clot formation. A decrease in fibrinogen concentration and platelet count would lead to a decreased rate of clot formation and clot strength. Since ACT and TEG use whole blood for their analysis, the resulting hypocoagulation as measured by these assays may partially be explained by a depletion of fibrinogen and platelets in the HCl Model. A fall in fibrinogen concentration and platelet count after HCl infusion in swine has been reported previously [17,18]. In these studies, the fall in fibrinogen concentration was probably due to an increase in consumption and not a fall in synthesis of fibrinogen [15]. HCl-induced acidosis has been shown to decrease platelet aggregation [14] which can lead to hypocoagulation. In the present study, HCl-induced acidosis led to a significant fall in fibrinogen concentration and platelet count. However, acidosis induced by hemorrhage/hypoventilation did not lead to a significant fall in fibrinogen concentration or platelet count suggesting that this hypocoagulation is probably due to other mechanisms.
There were some differences in the rFVIIa-induced changes in coagulation between the HCl-induced and the hemorrhage/hypoventilation induced acidosis models. Although rFVIIa significantly shortened PT in all groups in both experimental models, rFVIIa had no significant effect on aPTT in the hemorrhage/hypoventilation model, and a small, but probably not clinically significant effect on the acidosis group in the HCl model (Figure 2). The biggest difference between the two experimental models was the effect of rFVIIa on TEG and Thrombin Generation. In the hemorrhage/hypoventilation model, all TEG parameters changed significantly after rFVIIa infusion in the control, acidosis and acidosis-correction groups (Figure 3). In the HCl model, TEG parameters significantly changed only in the acidosis-corrected group, suggesting that the changes made by HCl infusion could not be reversed by rFVIIa injection. The reverse was true for thrombin generation (Figure 4). Recombinant FVIIa significantly decreased lagtime, ttPeak and Peak in the HCl model. Peak and ttPeak were unaffected by rFVIIa in the hemorrhage/hypoventilation model (Figure 5).
Overall, acidosis (by either model) did not affect the coagulation responses to rFVIIa. The differences in the responses between models tended to be in the degree of change seen after rFVIIa infusion. These differences are most likely explained by the difference in the cause of acidosis. HCl infusion significantly affected Fibrinogen and platelet levels as compared to Hemorrhage/Hypoventilaton. Lesperance et al [32] recently demonstrated that lactic acidosis in swine led to an coagulopathy that was not corrected with bicarbonate. However, rFVIIa decreased clotting time which is consistent with our observations.
Recombinant FVIIa increases the ability of the blood to coagulate by activating the extrinsic and common pathway that ends in fibrin polymerization [33,34]. The ability to form a clot is critically important in trauma patients. However, trauma is often associated with acidosis, and acidosis has been shown in this and other studies [13,17,18] to decrease the ability of the blood to clot and decrease rFVIIa activity [20,21]. This situation has led Hall et al [22] and others to question if arterial blood should be pH corrected before administering rFVIIa clinically. This report shows that rFVIIa improves coagulation in acidotic blood. Furthermore, correction of pH did not affect the increase in coagulation after rFVIIa injection in either the HCl or Hemorrhage/hypoventilation Models. This is especially evident in Prothombin Time, the classic measure of the extrinsic pathway function and a parameter that is directly affected by rFVIIa.
In conclusion, these data showed that acidosis caused a hypocoagulation in swine that was not reversed by correction of pH with bicarbonate. These data also showed that HCl caused irreversible damage to figrinogen and plateles and that rFVIIa function was not affected by acidosis. As the effects of rFVIIa were similar between the acidosis and pH corrected groups, these data suggest that pH correction is not required before giving rFVIIa.
Acknowledgements
Supported by the US Army Medical Research and Medical Command.
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Conflict of interest statement
The authors declare that they have no competing financial interests.
References
- 1.Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma Mortality in Mature Trauma Systems: Are We Doing Better? An Analysis of Trauma Mortality Patterns, 1997-2008. J Trauma. 2010;69:620–6. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, Butler FK. Causes of death in U. S. Special Operations Forces in the global war on terrorism: 2001-2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, Holcomb JB. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003-2004 versus 2006. J Trauma. 2008;64:S21–26. doi: 10.1097/TA.0b013e318160b9fb. discussion S26-27. [DOI] [PubMed] [Google Scholar]
- 4.Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health. 2000;90:523–526. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Tchaikovski SN, VAN Vlijmen BJ, Rosing J, Tans G. Development of a calibrated automated thrombography based thrombin generation test in mouse plasma. J Thromb Haemost. 2007;5:2079–2086. doi: 10.1111/j.1538-7836.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 7.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 8.Mikhail J. The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues. 1999;10:85–94. [PubMed] [Google Scholar]
- 9.Bruegger D, Kemming GI, Jacob M, Meisner FG, Wojtczyk CJ, Packert KB, Keipert PE, Faithfull NS, Habler OP, Becker BF, Rehm M. Causes of metabolic acidosis in canine hemorrhagic shock: role of unmeasured ions. Crit Care. 2007;11:R130. doi: 10.1186/cc6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darlington DN, Jones RO, Marzella L, Gann DS. Changes in regional vascular resistance and blood volume after hemorrhage in fed and fasted awake rats. J Appl Physiol. 1995;78:2025–2032. doi: 10.1152/jappl.1995.78.6.2025. [DOI] [PubMed] [Google Scholar]
- 11.Darlington DN, Tehrani MJ. Blood flow, vascular resistance, and blood volume after hemorrhage in conscious adrenalectomized rat. J Appl Physiol. 1997;83:1648–1653. doi: 10.1152/jappl.1997.83.5.1648. [DOI] [PubMed] [Google Scholar]
- 12.Gann DS, Carlson DE, Byrnes GJ, Pirkle JC Jr, Allen-Rowlands CF. Impaired restitution of blood volume after large hemorrhage. J Trauma. 1981;21:598–603. doi: 10.1097/00005373-198108000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom M, Schott U, Romner B, Reinstrup P. Acidosis impairs the coagulation: A thromboelastographic study. J Trauma. 2006;61:624–628. doi: 10.1097/01.ta.0000226739.30655.75. [DOI] [PubMed] [Google Scholar]
- 14.Marumo M, Suehiro A, Kakishita E, Groschner K, Wakabayashi I. Extracellular pH affects platelet aggregation associated with modulation of store-operated Ca(2+) entry. Thromb Res. 2001;104:353–360. doi: 10.1016/s0049-3848(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 15.Martini WZ, Holcomb JB. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann Surg. 2007;246:831–835. doi: 10.1097/SLA.0b013e3180cc2e94. [DOI] [PubMed] [Google Scholar]
- 16.Martini WZ, Dubick MA, Wade CE, Holcomb JB. Evaluation of tris-hydroxymethylaminomethane on reversing coagulation abnormalities caused by acidosis in pigs. Crit Care Med. 2007;35:1568–1574. doi: 10.1097/01.CCM.0000266682.74602.6A. [DOI] [PubMed] [Google Scholar]
- 17.Martini WZ, Pusateri AE, Uscilowicz JM, Delgado AV, Holcomb JB. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58:1002–1009. doi: 10.1097/01.ta.0000156246.53383.9f. discussion 1009-1010. [DOI] [PubMed] [Google Scholar]
- 18.Martini WZ, Dubick MA, Pusateri AE, Park MS, Ryan KL, Holcomb JB. Does bicarbonate correct coagulation function impaired by acidosis in swine? J Trauma. 2006;61:99–106. doi: 10.1097/01.ta.0000215574.99093.22. [DOI] [PubMed] [Google Scholar]
- 19.Viuff D, Lauritzen B, Pusateri AE, Andersen S, Rojkjaer R, Johansson PI. Effect of haemodilution, acidosis, and hypothermia on the activity of recombinant factor VIIa (NovoSeven) Br J Anaesth. 2008;101:324–331. doi: 10.1093/bja/aen175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng ZH, Wolberg AS, Monroe DM 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–891. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- 21.Dutton RP, Parr M, Tortella BJ, Champion HR, Bernard GR, Boffard K, Bouillon B, Croce MA, Dimsits J, Holcomb JB, Leppaniemi A, Vincent JL, Hauser CJ. Recombinant activated factor VII safety in trauma patients: results from the CONTROL trial. J Trauma. 71:12–19. doi: 10.1097/TA.0b013e31821a42cf. [DOI] [PubMed] [Google Scholar]
- 22.Hall K, Forrest P, Sawyer C. The effects of acidosis and hypothermia on blood transfusion requirements following factor VII administration. Anaesth Intensive Care. 2007;35:494–497. doi: 10.1177/0310057X0703500405. [DOI] [PubMed] [Google Scholar]
- 23.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108:1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins JG, Schreiber MA, Wade CE, Holcomb JB. Early versus late recombinant factor VIIa in combat trauma patients requiring massive transfusion. J Trauma. 2007;62:1095–1099. doi: 10.1097/TA.0b013e31804798a4. discussion 1099-1101. [DOI] [PubMed] [Google Scholar]
- 25.Martinowitz U, Kenet G, Segal E, Luboshitz J, Lubetsky A, Ingerslev J, Lynn M. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001;51:431–438. doi: 10.1097/00005373-200109000-00002. discussion 438-439. [DOI] [PubMed] [Google Scholar]
- 26.Korte WC, Moor S. Near fatal hemorrhage in traumatic bilateral leg amputation with coagulopathy, acidosis, and hypothermia and salvage therapy with recombinant factor VIIa. J Trauma. 2007;63:E1–4. doi: 10.1097/01.ta.0000246956.72328.6c. [DOI] [PubMed] [Google Scholar]
- 27.Dutton RP, Hess JR, Scalea TM. Recombinant factor VIIa for control of hemorrhage: early experience in critically ill trauma patients. J Clin Anesth. 2003;15:184–188. doi: 10.1016/s0952-8180(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 28.Wade CE, Eastridge BJ, Jones JA, West SA, Spinella PC, Perkins JG, Dubick MA, Blackbourne LH, Holcomb JB. Use of recombinant factor VIIa in US military casualties for a five-year period. J Trauma. 69:353–359. doi: 10.1097/TA.0b013e3181e49059. [DOI] [PubMed] [Google Scholar]
- 29.Council NR. guide for the care and use of laboratory animals. Washington D.C.: National Academy Press; 1996. [Google Scholar]
- 30.Pusateri AE, Ryan KL, Delgado AV, Martinez RS, Uscilowicz JM, Cortez DS, Martinowitz U. Effects of increasing doses of activated recombinant factor VII on haemostatic parameters in swine. Thromb Haemost. 2005;93:275–283. doi: 10.1160/TH04-03-0200. [DOI] [PubMed] [Google Scholar]
- 31.Darlington DN, Kheirabadi BS, Delgado AV, Scherer MR, Martini WZ, Dubick MA. Coagulation changes to systemic acidosis and bicarbonate correction in swine. J Trauma. 2011;71:1271–1277. doi: 10.1097/TA.0b013e318214f522. [DOI] [PubMed] [Google Scholar]
- 32.Lesperance RN, Lehmann RK, Harold DM, Beekley AC, Sebesta JA, Martin MJ. Recombinant factor VIIa is effective at reversing coagulopathy in a lactic acidosis model. J Trauma Acute Care Surg. 72:123–129. doi: 10.1097/TA.0b013e318224e24a. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009;108:1433–1446. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 34.Mackman N. Tissue-specific hemostasis: role of tissue factor. J Thromb Haemost. 2008;6:303–305. doi: 10.1111/j.1538-7836.2008.02873.x. [DOI] [PubMed] [Google Scholar]


