Abstract
Oxidative stress has been implicated as a prominent determinant in the development of several diseases such as atherosclerosis. Anti atherosclerotic effects of L-serine have been shown previously but its responsible mechanisms remained unidentified. This study aimed to investigate the antioxidant and cytoprotecrtive effects of L-serine and its possible mechanisms. For this purpose, cell viability analysis and nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2) activity, heme oxygenase-1 (HO-1) concentration, total Nitric Oxide (NOx) production were evaluated in oxidative stress-induced Human Umbilical Vein Endothelial Cells (HUVECs) pretreated by L-serine. Cytoprotective effects of L-serine was measured through 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Nrf2 activity and HO-1 concentration were determined in the cell lysate by commercial immunoassay methods. NOx was assayed in the supernatant of culture medium through colorimetric Griess method. Pretreatment with L-serine (0.1-3.2 mM) protected endothelial cells from hydrogen peroxide-mediated cell cytotoxicity (H2O2, 0.5 mM) and lead to significant induction of Nrf2 activity, HO-1 expresssion and NOx production. These findings demonstrated that L-serine has antioxidant and cytoprotective effects through the elevation of some crucial antioxidant factors such as Nrf2, HO-1 and NO.
Keywords: Nrf2, Heme oxygenase-1, Nitric oxide, MTT, L-serine, Antioxidant
INTRODUCTION
Oxidative stress causes endothelial dysfunction and has a prominent role in the pathogenesis of atherosclerosis and cardiovascular diseases(1,2). Antioxidant small molecules such as some amino acids protect endothelium against oxidative injuries through several mechanisms(3). Some amino acids have been proposed to greatly decline endothelial cell injury caused by reactive oxygen species (ROS)(4). Antiatherogenic properties of the amino acid L-serine in hypercholesterolemic rabbits has been already reported by our group(5). The exact underlying mechanism for the antiatherogenic effects of L-serine is not yet fully understood. A possible mechanism might be the induction of antioxidant mediators and protein genes which protect cells from oxidative stress injury. In recent years, extensive studies have fulfilled the pivotal role of nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2) as an antioxidant and anti inflammatory mediator(6). Nrf2 is a redox-sensitive transcription factor, which promotes the production of many phase 2 antioxidant enzymes such as heme oxygenase-1 (HO-1) through regulating of antioxidant response elements (ARE) that encodes phase 2 detoxification enzymes, thereby protects the endothelium against cytotoxic electrophiles or ROS(7). Also it has been demonstrated that Nrf2 protects cells/tissues from inflammatory injuries(6,8). Increased HO-1 activity has been reported as a possible mechanism through which the antiatrogenic properties of some antioxidant amino acids such as L-alanine and L-methionine have been explained(9,10). HO-1 is an inducible enzyme that degrades heme. Degradation of heme leads to the elevation of bilirubin which has strong antioxidant effects at physiological levels. Bilirubin is inversely related to atherogenic risk factors and protects endothelium against oxidative stress(11–13). Carbon monoxide (CO) is another product of heme degradation that exerts a vasoerelaxant effect through regulation of K+ channels in smooth muscle cells as well as lead to vasodilation by guanylate cyclase induction(11). Nitric oxide (NO) is an important agent through which some antioxidant molecules prevent endothelial cells from pro-inflammatory responses that lead to the endothelial cells death(14). NO has a crucial role in regulation of vascular function as a potent vasorelaxant, and vascular muscle cell migration and proliferation inhibitor. Furthermore, there is a significant relationship between Nrf2, HO-1, heme degradation products and NO bioavailability(15). One possible mechanism through which L-serine exerts its antiatherogenic effects might be through the elevation of Nrf2, HO-1 and NO. In the present study, we aimed to evaluate the possible antioxidant effects of L-serine through increasing of Nrf2 activity, HO-1 concentration, and total nitric oxide (NOx) levels in human umbilical vein endothelial cells (HUVECs) induced oxidative stress mediated by H2O2.
MATERIALS AND METHODS
Cell culture
HUVEC line was purchased from National Cell Bank of Iran (Pasteur Institute, Iran). The cells were cultured in 25 and/or 75 cm2 culture flasks in Dulbecco's Modified Eagle's Medium (DMEM; Gibco) containing 10% fetal bovine serum (Gibco-Invitrigen), penicillin-streptomycin mixture (100 U/ml Penicillin and 100 μg/ml streptomycin,Gibco) and incubated in circumstance of 5% CO2 and 95% air at 37°C incubator. The medium of cultured cells renewed every 48 h.
Evaluation of cell viability
In order to determine the protective effect of L-serine on H2O2-oxidative cell injury, cytotoxicity assay was performed using 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay(16). Briefly cell suspension (2 × 104 cells/180 μl medium) was transferred to 96-well microtiter plates. The medium also contained 10% fetal bovine serum. After 24 h incubation at 37°C, the cells were treated by L-serine (Sigma) in the concentration range of 0.1 to 3.2 and 10 mM (final concentration in wells) for an additional 24 h. Then, the cells were washed out with phosphate buffered saline (PBS, PH 7.4), new medium and hydrogen peroxide (Merck KGaA Co.) at the concentration of 0.5 mM was added to the wells and incubated for 2 h. Afterward, catalase (0.1 mg/ml) was added as an oxidative stress stopper. The cells were then washed out with PBS and renewed by medium. 20 μl MTT (0.5 mg/ml; Sigma) was later added per well and incubated for 3 h in 37°C. Finally MTT reacted with living cells and converted by mitochondrial enzyme to foramazan crystals with dark purple color which is an insoluble component that dissolved by dimethyl sulfoxide (DMSO) and its optical density (OD) measured in 550 nm wavelength by microplate reader (BioTek Instruments, EL × 800 TM, USA). The wells just containing cell suspension without any treatment with L-serine or exposing through H2O2 were assumed as a negative control. DMEM was used as the blank. The percentage of cell viability in the negative control was considered as 100. Cell viability determined through a relevant known formula(17):
Cell viability (%)=(OD test-OD blank/ OD negative control-OD blank)*100
For obtaining appropriate concentration and time for inducing oxidative stress through H2O2, half maximal inhibitory concentration (IC50) experiments were performed. The cytotoxicity effect of H2O2 was stopped by the addition of catalase (0.1 mg/ml, Sigma) for removing excess hydrogen peroxide in wells as described previously(18).
Nrf2 activity assay
To determine Nrf2 activity, the HUVECs was pretreated with L-serine (0.1 to 3.2 and 10 mM) and exposed to the oxidative stress by 0.5 mM H2O2. At first, the media was removed from dish and the cells were washed out three times with a mixture of PBS and phosphatase inhibitor which then gently scraped from dish by cell lifter/scraper without trypsynizing or enzymatic scraping. The nuclear extract was prepared using the nuclear extract kit (Trans AM™ Nrf2, Active Motif, Carlsbad, USA, Cat No:40010). The activity of Nrf2 in the nuclear extract was assayed using a commercial Nrf2 activity kit (Active Motif, Carlsbad, USA, Cat No:50296)(19) which is based on the DNA-binding Enzyme-Linked Immunosorbent Assay (ELISA). According to the manual of the kit, for providing quantitative results, the detection range of this kit was 0.15 to 2.5 μg/ml of nuclear extract per well. Equal and distinguished concentrations of nuclear extracts that were diluted in lysis buffer (1.25 μg/ml) added per well. For this, nuclear extract protein concentrations were evaluated through Bradford based protein quantification assay kit (Bioo Scientific, Max Discovery, USA, Cat No: 3440-01) according to the instruction provided by the manufacturer.
HO-1 concentration assay
The amount of HO-1 in cell lysates was measured using a human HO-1 ELISA Kit (Assay Designs, Stressgen, Enzo Life Sciences, UK, Cat No: ADI-EKS-800). For this purpose, the cultured cells that pretreated with L-serine (0.1 to 3.2 and 10 mM) and exposed to the oxidative stress through H2O2 (0.5 mM) were scraped by cell lifter and then centrifuged. The cell pellet was washed three times with cold PBS. For every 1 × 106 cells, 1 ml of lysis buffer of the kit was applied to prepare cell lysate. HO-1 in cell lysate was measured according to the instruction provided by the manufacturer. The intra-assay and inter-assay precision of this kit were <10%(20).
Total NO assay
The amount of NOx production in the cultured cells that pretreated with L-serine (0.1 to 3.2 and 10 mM) and then exposed to oxidative stress by 0.5 mM H2O2, was assessed through determination of NOx level in medium or supernatant of harvested cells using a commercial kit (Cayman Chemicals Company, Ann Arbor, MI,USA, Cat No:780001) based on the Griss reaction(21,22) according to the instructions of its manufacturer. The intra-assay and inter-assay coefficient of variations for the assay were 2.7% and 3.4%, respectively.
Sample's OD in all experiments was detected using an absorbance microplate reader and all of the cells used in this study had been passaged 3 to 5 times.
Statistical analysis
All data were analyzed by SPSS software version 16.0. Statistical analysis were accomplished through One-way ANOVA for determining differences between multiple experimental groups (different concentration of L-serine treatment), followed by LSD or bonferoni post hoc test for comparing two groups. In this study, the results were determined and expressed as mean ± standard deviation (SD) in triplicate independent, experiments. Statistical significance was considered P<0.05.
RESULTS
Cytoprotective effects of L-serine against oxidative stress induced cell injury on HUVECs
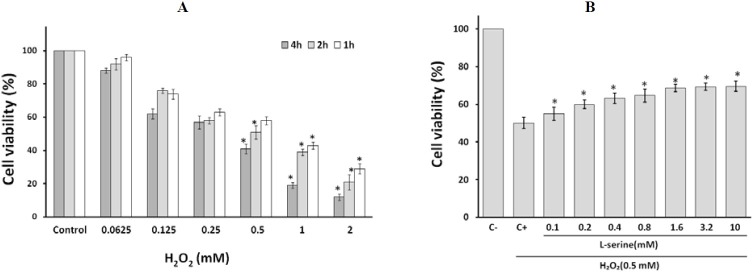
HUVECs exposed to various concentration of H2O2 (0.0625-2mM) for three different time periods (1,2 and 4 h) remarkably reduced cell viability (51%) at 0.5 mM concentration for 2 h (IC50). Through this experiment, appropriate time and concentration of H2O2 for inducing oxidative stress on HUVECs was achieved. (Fig. 1A). The addition of L-serine (0.1 to 10 mM) to the cultured HUVECs markedly decreased cell death caused by the exposure to hydrogen peroxide and 10 mM concentration of L-serine did not have any meaningful result rather than 3.2 mM (Fig. 1B). Half maximal effective concentration (EC50) of L-serine for this experiment was 0.45 mM. Furthermore, gallic acid, an organic and phenolic acid, which is a known high potent antioxidant and its cytoprotective and antioxidant effects on HUVECs described previously(23), was applied for controlling the cell viability assessment in this study. Gallic acid significantly increased cell viability (Data not shown).
Fig. 1.
Effect of L-serine on hydrogen peroxide mediated cytotoxicity in HUVECs. A) The viability of HUVECs against various concentration of H2O2 (0.0625-2 mM) for 1, 2 and 4 h. * indicates significant differences compared with negative control for expressing half maximal cytoxicity of H2O2 at P<0.05 B) The protective effects of L-serine evaluated at 7 different concentrations. C- and C+ are negative and positive controls, respectively. Values are expressed as mean ± SD. * indicates significant differences as compared with positive control at P<0.05.
L-serine pretreatment effect on Nrf2 activity in HUVECs under oxidative stress condition
L-serine (0.1 to 10 mM) caused an elevation in Nrf2 activity in HUVECs as determined by measuring the activity of Nrf2 in the nuclear extract of the cultured cells (Fig. 2). Nrf2 activity of the nuclear extract samples are expressed as the amount of their ODs at 450 nm. EC50 of L-serine for induction Nrf2 activity was 0.2 mM.
Fig. 2.
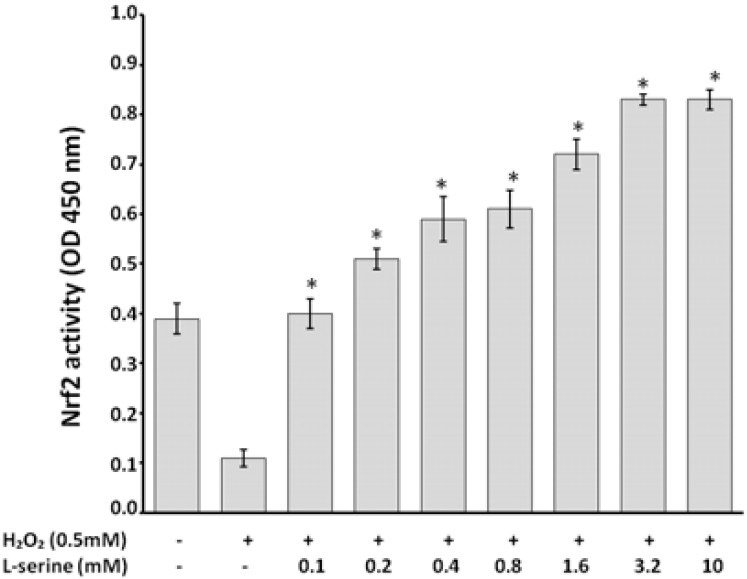
L-serine pretreatment increased Nrf2 activation in HUVECs that exposed to oxidative stress through H2O2 as determined in the nuclear extract of the cultured cells. * indicates significant differences as compared with positive control at P<0.05.
HO-1 concentration influenced by L-serine on HUVECs under oxidative stress circumstances
HO-1 induction by L-serine occurred at various protein levels determined by ELISA (Fig. 3). As illustrated in the figure, incubation of cells with L-serine (0.1 to 10 mmol/L) followed by exposure to the H2O2 (0.5 mM, 2 h) lead to a significant elevation of HO-1 protein expression (Fig. 3). EC50 of L-serine for HO-1 expression was found to be 0.55 mM. The effect after 3.2 mM of L-serine became constant.
Fig. 3.
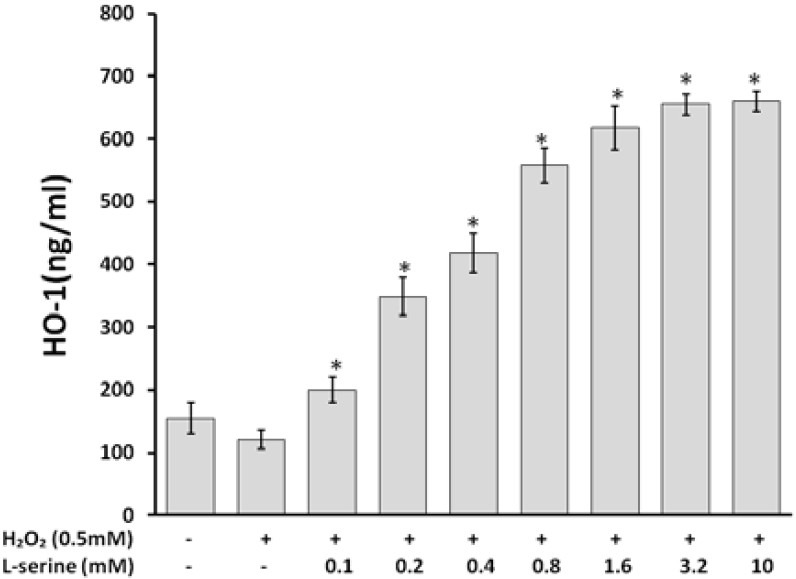
Effect of L-serine on HO-1 concentration in HUVECs induced cell injury by H2O2. L-serine, in the range of 0.1 to 3.2 mM had an increased effect on HO-1 induction in the cells. * indicates significant differences as compared with positive control at P<0.05.
Evaluation of NOx production on HUVECs pretreated with L-serine under oxidative stress condition
The levels of nitrate and nitrite ( the end products of NO) in the culture medium of cells pretreated with various concentrations of L-serine (0.1 to 10 mM) for 24 h under oxidative stress for 2 h under H2O2 (0.5 mM) were measured (Fig. 4). L-serine at concentration of 3.2 mM exerted the greatest effects on NO production in the cultured cells and EC50 of L-serine for NOx production was found to be 0.25 mM.
Fig. 4.

NOx production in the medium of HUVECs pretreated with different levels of L-serine. * indicates significant differences as compared with positive control at P<0.05
DISCUSSION
The results of the present study showed that the amino acid L-serine has cytoprotective and antioxidant effects against oxidative stress induced in HUVECs with H2O2, especially at concentration range of 0.1 to 3.2 mM with no more activity at concentration above 3.2 mM (10 mM). The cytoprotective and antioxidant effects may be brought about through the elevation of antioxidant agents such as Nrf2, HO-1and NO.
The antioxidant properties of some other amino acids such as L-alanine(10), L-arginine(24), L-tryptophan(25), L-methionine(9) and L-cystiene(26) have been evaluated and the underlying antioxidant mechanisms are addressed. Antinerotoxic effects of L-serine, in vitro and in vivo, have been previously demonstrated(27). Antiatherogenic feature of L-serine has been demonstrated in hypercholestrelemic rabbits in our previous study but its responsible mechanisms and mediators have not been determined(5). However, we hypothesized that the antioxidant and anti-inflammatory features of L-serine may, in part, account for this property. L-serine is a simple and neutral amino acid with no active metabolites such as 3-hydroxy antranilic acid or NO and agmatine as seen with L-tryptophan or L-arginine, respectively. Especial group like thiol group as present in L-cystiene is absent in the chemical structure of L-serine. We, therefore, decided to conduct more experiments to disclose the possible underlying antioxidant mechanisms of L-serine.
It has been demonstrated that Nrf2 has antioxidant activity as a transcriptional factor which induces HO-1 expression and NO production. Althogh there is a causal link between Nrf2 activation and NO levels and bioavailibility(28,29), it is well established that induction of HO-1 leads to anti-inflammatory, anti-atherogenic and cytoprotective effects. HO-1 through the degradation of the pro-oxidant heme, produces bilirubin and CO that are important antioxidants(15,30–32). The importance of NO in endothelium and cardiovascular system integrity has been emphasized in many studies(14,33). The effects of L-serine on the elevation of Nrf2 activity, HO-1 expression and NO production observed in the present study, may be responsible for the antioxidant effects of the amino acid as determined through its cytoprotective effect. On the other hand, the cytoprotective effects of some amino acids such as L-alanine and L-glycine and structurally similar amino acids against free radical-induced injury that has been reported by some studies(4,34–35) are in consistence with our results.
CONCLUSION
In summary, our study for the first time demonstrated that L-serine exerts antioxidant and cytoprotective effects on HUVECs through induction of other antioxidant factors such as Nrf2, HO-1 and NO. Therefore, L-serine may have beneficial effects on endothelium homeostasis.
ACKNOWLEDGMENT
This study was financially supported by the research council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Antoniades C, Tousoulis D, Tentolouris C, Toutouzas P, Stefanadis C. Oxidative stress, antioxidant vitamins, and atherosclerosis. Herz. 2003;28:628–638. doi: 10.1007/s00059-003-2417-8. [DOI] [PubMed] [Google Scholar]
- 2.Tawfik HE, Cena J, Schulz R, Kaufman S. Role of oxidative stress in multiparity-induced endothelial dysfunction. Am J Physiol-Heart C. 2008;295:H1736–H1742. doi: 10.1152/ajpheart.87.2008. [DOI] [PubMed] [Google Scholar]
- 3.Klouche K, Morena M, Canaud B, Descomps B, Beraud J, Cristol J. Mechanism of in vitro heme-induced LDL oxidation: effects of antioxidants. Eur J Clin Invest. 2004;34:619–625. doi: 10.1111/j.1365-2362.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 4.Estacion M, Weinberg JS, Sinkins WG, Schilling WP. Blockade of maitotoxin-induced endothelial cell lysis by glycine and L-alanine. American Journal of Physiology-Cell Physiology. 2003;284:C1006–C1020. doi: 10.1152/ajpcell.00258.2002. [DOI] [PubMed] [Google Scholar]
- 5.Movahedian A, Naderi GA, Dashti GR, Asgary S, Zadhoosh F. Antioxidant effects of L-Serine against fatty streak formation in hypercholesterolemic animals. ARYA Atherosclerosis. 2010;2:126–129. [Google Scholar]
- 6.Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation Research/ Fundamental and Molecular Mechanisms of Mutagenesis. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Element AR. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 8.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol-Heart C. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 9.Erdmann K, Grosser N, Schröder H. L-methionine reduces oxidant stress in endothelial cells: role of heme oxygenase-1, ferritin, and nitric oxide. AAPS J. 2005;7:195–200. doi: 10.1208/aapsj070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schröder H. Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Bioph Res Co. 2004;314:351–355. doi: 10.1016/j.bbrc.2003.12.089. [DOI] [PubMed] [Google Scholar]
- 11.Wang CY, Chau LY. Heme oxygenase-1 in cardiovascular diseases: molecular mechanisms and clinical perspectives. Chang Gung Med J. 2010;33:13–24. [PubMed] [Google Scholar]
- 12.Idriss NK, Blann AD, Lip GYH. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008;52:971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Otterbein LE, Choi AMK. Heme oxygenase: colors of defense against cellular stress. Am J Physiol-Lung C. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 14.Foreman KE, Tang J. Molecular mechanisms of replicative senescence in endothelial cells. Exp Gerontol. 2003;38:1251–1257. doi: 10.1016/j.exger.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Pae HO, Son Y, Kim NH, Jeong HJ, Chang KC, Chung HT. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide. 2010;23:251–257. doi: 10.1016/j.niox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Ma ZC, Hong Q, Wang YG, Tan HL, Xiao CR, Liang QD, et al. Ferulic acid protects lymphocytes from radiation-predisposed oxidative stress through extracellular regulated kinase. Int J Radiat Biol. 2011;87:130–140. doi: 10.3109/09553002.2011.523510. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi-Aliabadi H, Minaiyan M, Dabestan A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res Pharm Sci. 2010;5:127–133. [PMC free article] [PubMed] [Google Scholar]
- 18.Kostyuk VA, Potapovich AI, Suhan TO, de Luca C, Korkina LG. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. European Journal of Pharmacology. 2011;658:248–256. doi: 10.1016/j.ejphar.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, et al. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. JBiol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Kim HA, Choi JS, Lee M. Delivery of hypoxia inducible heme oxygenase-1 gene using dexamethasone conjugated polyethylenimine for protection of cardiomyocytes under hypoxia. Bull Korean Chem Soc. 2009;30:897–901. [Google Scholar]
- 21.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Analytical biochemistry. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Shen L, She H, Yue S, Feng D, Luo Z. Nitric oxide-induced activation of NF–κB-mediated NMDA-induced CTP: phosphocholine cytidylyl transferase alpha expression inhibition in A549 cells. Cell Biol Toxicol. 2011;27:41–47. doi: 10.1007/s10565-010-9168-0. [DOI] [PubMed] [Google Scholar]
- 23.Whang WK, Park HS, Ham IH, Oh M, Namkoong H, Kim HK, et al. Methyl gallate and chemicals structurally related to methyl gallate protect human umbilical vein endothelial cells from oxidative stress. Exp Mol Med. 2005;37:343–352. doi: 10.1038/emm.2005.44. [DOI] [PubMed] [Google Scholar]
- 24.Raghavan SAV, Dikshit M. Vascular regulation by the-arginine metabolites, nitric oxide and agmatine. Pharmacol Res. 2004;49:397–414. doi: 10.1016/j.phrs.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Pae HO, Oh GS, Lee BS, Rim JS, Kim YM, Chung HT. 3-Hydroxyanthranilic acid, one of l-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis. 2006;187:274–284. doi: 10.1016/j.atherosclerosis.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RA, Lamb DJ, Leake DS. Mechanisms by which cysteine can inhibit or promote the oxidation of low density lipoprotein by copper. Atherosclerosis. 2003;169:87–94. doi: 10.1016/s0021-9150(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 27.Garofalo K, Penno A, Schmidt BP, Lee HJ, Frosch MP, von Eckardstein A, et al. Oral l-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type. JClin Invest. 2011;121:4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann GE, Rowlands DJ, Li FYL, De Winter P, Siow R. Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc Res. 2007;75:261–274. doi: 10.1016/j.cardiores.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related Factor (Nrf2) Contributes to Keep Endothelial NO Synthase (eNOS) in the Coupled State. Journal of Biological Chemistry. 2009;284:31579–31586. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Góra-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–39. [PubMed] [Google Scholar]
- 31.Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 32.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. JClin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Haro Miralles J, Martinez-Aguilar E, Florez A, Varela C, Bleda S, Acin F. Nitric oxide: link between endothelial dysfunction and inflammation in patients with peripheral arterial disease of the lower limbs. Interactive CardioVascular and Thoracic Surgery. 2009;9:107–112. doi: 10.1510/icvts.2008.196428. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg JM, Davis JA, Abarzua M, Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. JClin Invest. 1987;80:1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg J, Varani J, Johnson K, Roeser N, Dame M, Davis J, et al. Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol. 1992;140:457–471. [PMC free article] [PubMed] [Google Scholar]