Abstract
The increasing incidence of Multi Drug Resistance Tuberculosis (MDR-TB) and Extensively Drug Resistance TB (XDR-TB) worldwide highlight the urgent need to search for newer anti-tuberculosis compounds. It has been determined that pharmaceutical plant, hops (Humulus lupulus), possesses some antibacterial effect. In this study, the antimycobacterial effect of this plant on rifampin sensitive and resistant strains of Mycobacterium tuberculosis were examined. Sensitivity and resistance of 37 Iranian isolates of M. tuberculosis to rifampin was determined by proportion method. Ethanolic extract of hops was prepared using maceration method. PCR-SSCP and direct sequencing were used for confirming existence of mutations in 193-bp rpoB amplicons related to the rifampin resistance in Mycobacterium tuberculosis isolates. Two different concentrations of hops alcoholic extract (4 and 8 mg/ml) were prepared and its effects against 21 resistant and 15 sensitive isolates was determinate using proportion method. Six different mutations in the 193-bp amplified rpoB gene fragments and seven distinguishable PCR-SSCP patterns in 21 Iranian rifampin resistant isolates were recognized. This study showed that the percentage of resistance and the type of mutations were correlated with the PCR-SSCP patterns and the type of mutations in rpoB gene (P<0.05). The results of hops antimycobacterial effect showed that different concentrations of hops ethanolic extract (4 and 8 mg/ml) had a remarkable inhibitory effect on rifampin sensitive and resistant isolates of Mycobacterium tuberculosis. Identification of the effective fraction of hops against Mycobacterium tuberculosis is a further step to be studied.
Keywords: Mycobacterium tuberculosis, Humulus lupulus, Ethanolic extract, Antimycobacterial effect
INTRODUCTION
Currently, one third of the world's population is infected with M. tuberculosis and each year there are 2-3 million deaths worldwide caused by tuberculosis (TB)(1). TB is also a leading cause of death among people with human immunodeficiency virus (HIV). Individuals infected with HIV are very susceptible to TB and often develop this disease before other manifestations of AIDS become apparent(2,3). Today, strains of TB that are resistant to all major anti-TB drugs have emerged. The emergence of resistance to antimicrobials, though is a natural biological occurrence, has become an important public health issue in many developing countries as the treatment of TB requires the use of more expensive drugs for a longer treatment period. The increasing incidence of Multi Drug Resistance Tuberculosis (MDR-TB) and Extensively Drug Resistance TB (XDR-TB) worldwide highlight the urgent need to search for newer anti-tuberculosis compounds. There is, therefore, an urgent need for new, inexpensive TB drugs which are more effective and with fewer side effects. Medicinal plants offer a great hope to fulfill these needs and have been used for curing diseases for many centuries. So far, few plants have been tested against bacteria and mycobacteria and a few plant species have been thoroughly investigated for their medicinal properties(4). Iran is one of the few countries in the world which has unique wealth of medicinal plants and vast traditional knowledge of using of herbal medicine for curing of various diseases(5). Humulus lupulus (hops) have been used with some success in the treatment of premature ejaculation and priapism. The characteristic bitterness imparted by the addition of hops to the brewing process is mainly due to the presence of the bitter acids, which are prenylated acylphloroglucinol derivatives(6). Bitter acids are divided into the alpha-acids, with humulone the major compound, and the beta-acids, with lupulone the major compound. Alpha-acids isomerize during the brewing process to form iso-alpha acids, which themselves have a bitter taste(7). Hops also contain xanthohumol, a prenylated chalcone compound that shows cytoprotective and other health-promoting activities(8). Hops resins are subdivided into hard and soft based on their solubility. Hard resins are of little significance while soft resins contribute to the flavoring and preservative properties. Alpha acids have a mild antibiotic effect against Gram-positive bacteria(9). Alcoholic extracts of hops show a strong spasmolytic property on smooth muscle and are effective on visceral tension such as in nervous colitis, nervous dyspepsia, palpitations, nervous or irritable coughs, and asthma(10,11).
Recent scientific studies support the claims for its use in dysmenorrheal and amenorrhea as an antioxytocic property(7). Hops may also be used as a poultice. Its antibacterial qualities also stimulate gastric juice production. Based on the ethno medical information on these plants, they were screened against two Gram-positive bacteria, B. subtilis and S. aureus(12). A study undertaken by Stavri and coworkers showed that components of hops have an antimycobacterial effect against M. fortuitum(13). The study undertaken by Shapouri and coworkers clearly showed that the aqueous, acetonic and ethanolic extracts of hops were effective on Brucella at laboratory and intramacrophage bacteria(14). However, it has not well reported the antibacterial effects of ethanolic extracts of hops toward Mycobacterium tuberculosis that has been recognized as the major organism involved in deaths worldwide. In this study, the antibacterial effect of hops extracts against Mycobacterium tuberculosis and some of the other typical species of bacteria including Bacillus subtilis (PTCC 1023), E. coli (PTCC 1276), Pseudomonas aeruginosa (PTCC 1430) and Staphylococcus aureus (PTCC 1112) was investigated.
MATERIALS AND METHODS
Preparation of hops ethanolic extract
Whole hops major plants (stems, leaves and roots) were collected from the Herbal Department of Agriculture Research Center in Isfahan, identified and authenticated by a plant taxonomist at the Department of Pharmacognosy. The specimen was washed and cut into small pieces and dried in shade (25°C) by the air drying method for 7 days and then were grinded with electrical grinder. The extracts were filtered, and then the filtrate was collected and concentrated up to 10 ml in vacuum pump at 40°C. The concentrated extracts sterilized by 0.22 μm filters. The alcohol extract, was collected into a clean sterile bottles, labeled and stored at -20°C until further use(12,15).
Polymerase chain reaction-single-strand conformation polymorphism (PCR-SSCP) method
PCR-SSCP was used as a rapid screening test for the detection of mutations in the rpoB gene and so rifampin sensitivity phenotype. Chromosomal DNA of clinical isolates was extracted by “DNA mini-prep” procedure using hexadecyl trimethyl ammonium bromide (CTAB). About 10 ng of extracted DNA was used for the PCR amplification. The DNA of drug-susceptible standard strain, H37Rv (ATCC 27294) was used as the control sample. PCR was performed as following: PCR reactions (50 μl) contained 10 ng target DNA, 15 pmol primers, 2 mM dNTP (Pharmacia Biotech), 2.5 U Taq polymerase (MBI Fermentas), 1.5 mM MgCl2, and 5 μl 10 × buffer. The reaction was performed in a thermal cycler from PCR Hybid (Omnigene). The sequence of primers for the rpoB locus were rpo 105 Forward (5’-CGTGGAGGCGATCACACCGCAGACGT-3’) and primer rpo 273 Reverse (5’-GACCTCCAGCCCGGCACGCTCACG-3’), which produced a 193-bp amplicons. Samples were then subjected to one cycle of 96°C for 5 min, followed by 40 cycles of 96°C for 1 min, 55°C for 1 min, 72°C for 30 S, and one final cycle of 72°C for 10 min(28,30). PCR products were run on 2% agarose gel and examined for the presence of the 193-bp band after ethidium bromide staining. The PCR products were SSCP analyzed by electrophoresis on 10% (w/v) acrylamide gels. In brief, the SSCP gel was made by mixing 10 ml 40% (w/v) acrylamide solution, 25.6 ml H2O, 4 ml 10 × TBE, 30 ml TEMED, and 300 ml ammonium persulfate. About 6 ml of amplified product was mixed with 4 ml of loading buffer (95% formamide, 20 mM EDTA, and 0.05% bromophenol blue) and the mixtures was boiled for 4 min, cooled on ice for 5 min, and then loaded on the acrylamide gel at 2 W for 14-16 h in cold room. The gel was silver stained by using 10% (v/v) glacial acetic acid as fix/stop solution; silver nitrate solution (contained 2 g AgNO3, 2 l ultrapure water and 3 ml 37% formaldyde) as stain solution and solution contained 60 g sodium carbonate, 2 l ultrapure water, 3 ml 37% formaldehyde and 400 ml sodium thiosulfate as developing solution(28,30). SSCP assays were repeated at least two times with all isolates.
Bacterial isolates
Thirty seven M. tuberculosis isolates from different region of Iran were characterized by conventional and molecular methods by specific amplification of the Direct Repeat (DR) gene region that is only present in M.tuberculosis complex(16,17). Sensitivity and resistance of M. tuberculosis isolates at concentrations of 0.1 μg/ml rifampin was determined by proportion method(18,19). All isolates were cultured in 7H9 broth medium for ten days. Subsequently, LJ medium without and with rifampin were inoculated with 0.1 ml of 10-2 and 10-4 dilutions of a McFarland 1.0 standard of each isolate of M. tuberculosis. The inoculated plates were then incubated at 37°C. The percentage inhibition of CFU was determined after 3 weeks of inoculation(20,21). M. tuberculosis H37Rv (ATCC 25177, standard strain for susceptibility testing) strain was used as the control in this study.
The study of hops antimycobacterial effect
The antimycobacterial effect of hops alcoholic extract on M. tuberculosis was determined by proportion method. Antibacterial effects of hops on the resistant and sensitive isolates of M. tuberculosis exposed to different concentrations of the hops alcoholic extract (4 mg/ml and 8 mg/ml) were determined according to the standard procedures(19). M. tuberculosis H37Rv (ATCC 25177) was used as the control in this experiment. The subcultures prepared in Middlebrook 7H9 broth enriched with oleic acid-albumin-dextrose- catalase (OADC) supplements incubated (5-10 % CO2) for10 days at 37°C. A standard suspension of 107 CFU/ml (equivalent to # 1 McFarland standard) M. tuberculosis was used in the inoculation process. Media were prepared as 10 ml slopes in glass bottles inoculated with 0.1 ml of suspension and incubated at 37°C. The percentage inhibition of cfu was determined after 3 weeks of inoculation.
In addition to this study, the antibacterial effect of hops was examined on some Gram-positive and Gram-negative bacteria including Bacillus subtilis (PTCC 1023), E. coli (PTCC 1276), Pseudomonas aeruginosa (PTCC 1430) and Staphylococcus aureus (PTCC 1112). On Mueller-Hinton (Merck, Germany), 5-mm diameter wells were prepared and then from 1.5 × 108 cfu/ml of bacterial suspension cultivated on plates. Wells were loaded with hops extracts dilutions (70 μl of 4 mg/ml and 8 mg/ml dilutions) and plates were incubated for 48 h at 37°C and the zone of growth inhibition was measured (12,15,21).
Nucleotide sequence accession number
Partial sequences of mycobacterial rpoB genes were deposited in GenBank under accession numbers JQ314433.1 to JQ314437.1
RESULTS
The results of susceptibility testing indicated that percentage of resistance were from less than 1 to 31 on LJ medium contained 4 mg rifamoin /ml. This was less than 1 for all sensitive isolates. Based on PCR-SSCP results, the rifampin-resistance isolates were classified into seven groups including patterns 1, 4 and 5 with 4 bands, 2 and 3 with 5 bands, 7 with 3 bands and 6 with 3 bands similar to H37Rv (Fig. 1). Specific mutations identified in the rifampin resistance M. tuberculosis isolates were in 81-bp region of the rpo gene, codons: 531 (TCG/TTG), 523 (GGG/GGT), 516 (GAC/GTC), 526 (CAC/TAC), 511 (CTG/TTG), and 512 (AGC/TCG).
Fig. 1.
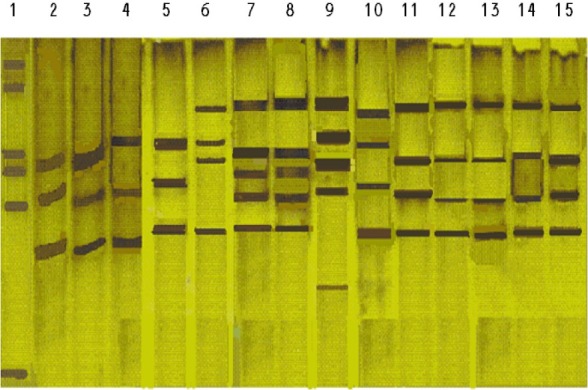
PCR-SSCP patterns of 3 sensitive and 10 rifampin-resistant M. tuberculosis isolates. From left, Lane: 1: ladder 50, lane 2 is H37Rv, lanes 3, 4, and 5 are sensitive strains and their patterns are indistinguishable from that of M. tuberculosis H37Rv (lane 2). Lanes 6 to 15 are rifampin-resistant strains and their patterns are distinguishable from that of M. tuberculosis H37Rv. Lane 6 is pattern 1 rifampin-resistant strains with 4 bands, Lanes 7 and 8, pattern 2 rifampinresistant with 5 bands, Lane 9, pattern 3 rifampin-resistant with 5 bands different from 7 and 8, Lane 10, pattern 4 rifampin-resistant with 4 bands different from Lane 6 and 11 to 15, pattern 5 rifampin resistant with 4 similar bands. All strains show distinguishable patterns from that of M. tuberculosis H37Rv pattern.
Ethanolic extract of hops was prepared using maceration. Antimycobacterial effect of 4 and 8 mg/ml alcoholic extract against rifampin-resistant and sensitive M. tuberculosis isolates were determined using proportion method. Two groups of sensitive and resistant to rifampin M. tuberculosis isolates were detected. In this study hops extract (4 mg/ml and 8 mg/ml) had an inhibitory effect against M. tuberculosis strains. The ethanolic extract from female flowers of hops, showed the strong inhibitory against M. tuberculosis strains (Table1).
Table 1.
The effect of different concentration of Humulus lupulus (hops) alcoholic extracts on rifampin sensitive and resistant M. tuberculosis.

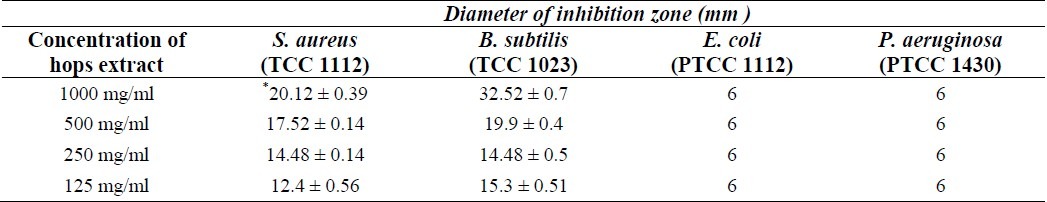
Antimicrobial effect of hops against indicator bacteria was investigated by using well plate agar. The extract of hops displayed strong activity against Gram-positive bacteria including Staphylococcus aureus (20.12 ± 0.39), (X ± SD) and Bacillus subtilis (22.52 ± 0.7) (Table 2). However, this plant had a weak effect against some Gram-negative bacteria such as E. coli and Pseudomonas aeruginosa. Hops extract show the best MIC value for the ethanolic extracts against the Gram-positive bacteria. The ethanolic extract was exceptionally active against M. tuberculosis.
Table 2.
The effect of different concentration of Humulus lupulus (hops) alcoholic extracts on indicator bacteria.
DISCUSSION
In the last decade, TB has reemerged as one of the leading causes of death with nearly 3 million deaths annually(21). The emergence of AIDS and decline of socio-economic standards contribute to the disease's resurgence in industrialized countries(22,23). The prevalence of MDR strains of M. tuberculosis is an important reason for the resurgence of TB as a major disease in many parts of the world. In many countries, medicinal plants are used by traditional medical practitioners to combat TB. The increasing incidence of MDR-TB and XDR-TB worldwide highlight the urgent need to search for newer anti-tuberculosis compounds drugs(22,24,26). There is, therefore, an urgent need for new, inexpensive TB drugs which are effective and with fewer side effects. Medicinal plants offer a great hope to fulfill these needs and have been used for curing diseases for many centuries. A number of studies have revealed that the essential oil of female flowers of hops contains α-bisabolene, caryophyllene, cuminyl aldehyde, and carvone oxide, which are known to exhibit antimicrobial properties(27). Extract of hops has been reported to exhibit significant antimicrobial activity against certain bacteria. The ethanolic extract of hops may be responsible for some of the activities of this plant. The plants screened in this study are used in traditional medicinal practices for the treatment of various diseases including TB. PCR-SSCP analysis, involves amplification of a segment of the gene encoding for the specific drug target and comparison of PCR products of drug-sensitive and drug-resistant strains by non-denaturing electrophoresis, in which mutations usually result in an altered pattern (24,25,28). However recent studies have questioned its sensitivity and specificity(29,30). Most of resistant strains (95.2%) showed PCR-SSCP patterns different from that of H37Rv; 28.6% had four bands and 23.8% had three bands. These findings demonstrate that frequencies of particular PCR-SSCP patterns in RMP-resistant M. tuberculosis isolates from Iran have some differences from those that have been reported from other geographic areas(28–34). Nonetheless of local differences, our data also demonstrated that SSCP analysis is highly specific and sensitive for detecting mutations in the rpoB gene in rifampin-resistant M. tuberculosis. The sensitivity of this method was 95.2% with the specificity of 93.8%. Thus this method can be considered as a convenient method for detection of mutations responsible for conferring rifampin resistance. The results of rpoB sequencing of rifampin resistance isolates showed that 85.7% have a single mutation in 81-bp region of rpoB gene. Mutations associated with nucleotide replacements in codons 513 and 526 were associated with high percentage resistance to rifampin whereas mutations in codons 523 and 512 were observed in low-percentage rifampin resistance. Other authors have reported high and low levels of resistance associated with specific nucleotide replacements(35).
In this study, whole hops plants were collected, identified and authenticated and the alcohol extract was prepared into clean sterile bottles. The antimycobacterial effect of different concentrations of hops alcoholic extract (4 mg/ml and 8 mg/ml) on rifampin- resistant and sensitive isolates of M. tuberculosis was determined by proportion method according to standard procedures(16,19). Antimicrobial effect of hops against indicator bacteria was investigated using well plate agar. The ethanolic extract showed a strong inhibitory activity against M. tuberculosis strains (Table1). All of the Mycobacteria isolates inhibited by the hops completely. The results showed antimycobacterial activity with MIC values ranging from 400 to 800 μg/ml and results clearly indicated the effectiveness of this plant to control tuberculosis in vitro. The results were comparable to those of the standard drug (rifampin) in sensitive M. tuberculosis strains. This may be due to the bioactive constituents, such as alkaloids, tannins and flavonoids, present in the extracts(36,37). On the other hand, the ethanolic extract of hops displayed strong activity against Gram-positive bacteria including Staphylococcus aureus (20.12 ± 0.39, (X ± SD) and Bacillus subtilis (22.52 ± 0.7) Table 2. A previous study has shown that the aqueous and methanol extracts of S. persica inhibited most bacteria, including Staphylococcus aureus, Streptococcus mutans, Streptococcus faecalis, Streptococcus pyogenes, Lactobacillus acidophilus, Pseudomonas aeruginosa, and the fungi Candida albicans though with varying effectiveness(37,38). Cladwell and coworkers reported that 3-epioleanolic acid and oleanolic acid isolated from Junellia tridens had shown antimyco-bacterial activity with MIC values ranging from 16 to 128 μg/ml(39). Grange and Snell tested the activity of bromhexine and ambroxol, semisynthetic derivatives of vasicine from A. vasica against M. tuberculosis and found that the MIC for ambroxol was 50 to 100 mg/L and for bromhexine, the MIC was 6 to 12 mg/L at pH 6.5 after dissolving the compounds in DMSO(40). Gordien and coworkers found MIC values ranging from 21.1 to 92.4 μg/ml for terpenoids isolated from Juniperous communis(41). Murillo and coworkers reported MIC values of 100 μg/ml for the flavones isolated from Haplopappus sonorensis(42).
Our results are similar to the activity of these natural compounds. Okunade and coworkers described 88 naturally occurring compounds, and in some cases synthetic analogues, largely from plants, fungi and marine organisms that demonstrated significant activity in the in vitro bioassays against M. tuberculosis and other Mycobacterial species(43). We believe that the ethanolic extract of hops can play an important role in the management of tuberculosis.
Gao and Newton and coworkes reported that there were a number of plants, supposedly used in the traditional medicine to treat TB, which did not demonstrate any antimyco-bacterial activity against M. aurum and M. smegmatis(44,45). The author suggested that the plants may be used to treat the symptoms of the disease rather than actually cure the disease itself. A number of studies have revealed that the essential oil of female flowers of hops contain α-bisabolene, caryophyllene, cuminyl aldehyde, and carvone oxide, which are known to exhibit antimicrobial properties. In a study done by Vasconcelos, the alcoholic extract of hops exhibited growth of Helicobacter pylori(27). Flythe et al. showed that rumen bacteria were sensitive to antimicrobial components in hops(46). Stavri and coworkers have shown that hexane extract of strobile hops (Humulus lupulus) have an antimycobacterial effect on the fast-growing mycobacterial species, Mycobacterium fortuitum(13). The antimycobacterial property observed in the oil and extract is probably due to the presence of furanocoumarin in dill(47). In a study by Lo Cantore and coworkers, however, essential oil extracted from fennel showed a lesser antibacterial effect compared to coriander oil in inhibition of Escherichia coli and Bacillus megaterium(48). Yamaguchi N. and coworkers have shown that components from hops extract, indicated strong inhibitory activities against Propioni bacterium acnes, and Staphylococcus pyogenes(9).
Our results correlate with the antimicrobial effects observed with the traditional uses of hops reported by Stavri and coworkers and suggest that this plant may serve as a source of new antimicrobial agents that are effective against problematic drug-resistant infection(13).
CONCLUSION
The results of the study of hops antimycobacterial effect showed that different concentrations of hops ethanolic extract (4 and 8 mg/ml) had a remarkable inhibitory effect on sensitive and resistant phenotype of M. tuberculosis. Identification of the effective fraction of hops against M. tuberculosis is a further step to be studied.
ACKNOWLEDGMENT
This assay was supported by grant No.83179 from Isfahan university of medical sciences and grant 444584123 from Islamic Azad University of Qom.
REFERENCES
- 1.Zumla A, Mwaba P, Squire SB, Grange JM. The tuberculosis pandemic- which way now? J Infection. 1999;38:74–79. doi: 10.1016/s0163-4453(99)90072-5. [DOI] [PubMed] [Google Scholar]
- 2.Grange JM, Davey RW. Detection of antituberculosis activity in plant extracts. J Appl Bacteriol. 1990;68:587–591. doi: 10.1111/j.1365-2672.1990.tb05224.x. [DOI] [PubMed] [Google Scholar]
- 3.Lall N, Meyer JJM. In vitro inhibition of drug-resistant and drug sensitive strains of Mycobacterium tuberculosis by ethnobotanically selected South African plants. J Ethnopharmacol. 1999;66:347–354. doi: 10.1016/s0378-8741(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 4.Verzele M, De Keukeleire D. Chemistry and analysis of hops and beer bitter acids. New York: Elsevier; 1991. [Google Scholar]
- 5.Jaskula B, Kafarski P, Aerts G, De Cooman L. A kinetic study on the isomerization of hop α-acids. J Agric Food Chem. 2008;56:6408–6415. doi: 10.1021/jf8004965. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Shidi B. Evalution of antimicrobial properties of some medical plants used in Iran. J Ethnopharm. 2004;11:42–48. [Google Scholar]
- 9.Yamaguchi N, Yamaguchi KS, Ono M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine. 2009;16:369–376. doi: 10.1016/j.phymed.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Zanoli P, Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J Ethnopharmacol. 2008;116:383–396. doi: 10.1016/j.jep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Kac J, Plazar J, Mlinaric A, Zegura B, Lah TT, Filipic M. Antimutagenicity of hops (Humulus lupulus L.): Bioassay-directed fractionation and isolation of xanthohumol. Phytomedicine. 2008;15:216–220. doi: 10.1016/j.phymed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Kermanshahi R, Nasr B, Esmi J, Asghari GH. The study of antibacterial effect of Humulus lupulus on some of Gram-positive and Gram-negative bacteria. J Medicinal plants. 2009;30:92–97. [Google Scholar]
- 13.Stavri M, Schneider R, O’Donnell G, Lechner D, Bucar F, Gibbons S. The antimycobacterial components of hops (Humulus lupulus) and their dereplication. Phytother Res. 2004;18:774–776. doi: 10.1002/ptr.1527. [DOI] [PubMed] [Google Scholar]
- 14.Shapouri R, Rahnema M. Evaluation of antimicrobial effect of hops extracts on intramacrophages Brucella abortus and B. melitensis. Jundishapur J Microbiol. 2011;4:51–58. [Google Scholar]
- 15.Eloff JN. Which extracting should be used for the screening and isolation of antimicrobial component from plants? J of Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 16.Bahremand A. Laboratory Procedures for the Isolation and Identification of Mycobacteria. Iran: Pastor Institute of Iran Press; 1992. [Google Scholar]
- 17.Tavakoli A, Radi E, Tamizifar H, Pishva E, Salehi R. Molecular fingerprinting of Mycobacterium tuberculosis isolates from patients in Isfahan province using spoligotyping method. J Sci I.R.I. 2005;23:108–114. [Google Scholar]
- 18.Williams DL, Spring L, Collins L, Miller LP, Heifets LB, Gangadharam PRJ, et al. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:1853–1857. doi: 10.1128/aac.42.7.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charley W, Mitchell M, Kidd K. The Antimycobacterial Susceptibility Tests. New York: Williams & Wilkins Press; 1999. Antibiotics laboratory medicine; pp. 127–175. [Google Scholar]
- 20.Nasr Isfahani B, Tavakoli A, Salehi M, Tazhibi M. Detection of rifampin resistance patterns in Mycobacterium tuberculosis strains isolated in Iran by polymerase chain reaction-single-strand conformation polymorphism and direct sequencing methods. Mem Inst Oswaldo Cruz. 2006;101:597–602. doi: 10.1590/s0074-02762006000600004. [DOI] [PubMed] [Google Scholar]
- 21.Bloom BR, Murray C. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 22.Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen JO, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 23.Ashok R, Awdhesh K, Nishat A. Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives. Emerg Infect Dis. 1998;4:195–210. doi: 10.3201/eid0402.980207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snider D, Roper WL. The new tuberculosis. N Engl J Med. 1992;326:703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- 25.Iseman M. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 26.Kato-Maeda M, Sifuentes-Osornio J, Bobadilla-del-Valle M, Ruiz-Palacios GM, Ponce-de-Leon A. Drug resistance among acid-fast bacilli. Lancet. 1999;353:1709–1715. doi: 10.1016/s0140-6736(05)77019-7. [DOI] [PubMed] [Google Scholar]
- 27.Rezaie M. Study of Humulus lupulus components. Invest. Med. Frag. Plants. 3:1–13. 1378. [Google Scholar]
- 28.Telenti A, Imboden P, Marchesi F, Schmidheini T, Bodmer T. Direct, automated detection of rifampin-resistant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob Agents Chemother. 1993b;37:2054–2058. doi: 10.1128/aac.37.10.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Cho S, Bang H, Lee J, Bae G, Kim S. Molecular analysis of rifampin-resistant Mycobacterium tuberculosis isolated from Korea by polymerase chain reaction-single strand conformation polymorphism sequence analysis. Int J Tuberc Lung Dis. 1998;2:585–589. [PubMed] [Google Scholar]
- 30.Miriam BV, Alfredo PL, Catalina AH, Gilberto VA, Midori KM, Peter MS, et al. rpoB gene mutations in rifampin-resistant Mycobacterium tuberculosis identified by polymerase chain reaction single-stranded conformational polymorphism. Emerg Infec Dis. 2001;7:1010–1013. doi: 10.3201/eid0706.010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochi A, Vareldzis B, Styblo K. Multi-drug resistant tuberculosis and control. Res Microbiol. 1993;144:104–110. doi: 10.1016/0923-2508(93)90023-u. [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, Kim SY, Park BH, Lyu MA, Park IK, Bai GH, et al. Mutations in the rpoB gene of Mycobacterium tuberculosis that interfere with PCR-SSCP analysis for rifampin susceptibility testing. J Clin Microbiol. 1997;35:492–494. doi: 10.1128/jcm.35.2.492-494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Kim SJ, et al. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB) J Clin Microbiol. 1999;37:1714–1720. doi: 10.1128/jcm.37.6.1714-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M. Detection of rifampin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993a;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 35.Kapur V, Li L L, Iordanescu S, Hamrick M, Wanger BN, Mooser JM. Characterization by automated DNA sequencing of mutations in the gene rpoB encoding the RNA polymerase B subunit in rifampin resistant Mycobacterium tuberculosis strains from New York City and Texas. J Clin Microbiol. 1994;32:1095–1098. doi: 10.1128/jcm.32.4.1095-1098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–688. [Google Scholar]
- 37.Sathiya M, Muthuchelian K. Phytochemical Investigation and Antibacterial Screening of Ethanolic Leaf Extract of Sapindus emarginatus Vahl. Ethnobotanical Leaflets. 2008;12:891–895. [Google Scholar]
- 38.Al-bayati FA, Sulaiman KD. In Vitro Antimicrobial Activity of Salvadora persica L.Extracts against Some Isolated Oral Pathogens in Iraq. Turk J Biol. 2008;32:57–62. [Google Scholar]
- 39.Caldwell S, Franzblau E, Suarez B. Timmermann, Oleanane triterpenes from Junellia tridens. J Nat Prod. 2000;63:1611–1614. doi: 10.1021/np0002233. [DOI] [PubMed] [Google Scholar]
- 40.Grange J M, Snell N J C. Activity of bromohexine and ambroxol, semi synthetic dvt. Of vacisine from the Indian shrub Adhatoda vasica against Mycobacterium tuberculosis in vitro. J. Ethnopharmacol. 1996;50:49–53. doi: 10.1016/0378-8741(95)01331-8. [DOI] [PubMed] [Google Scholar]
- 41.Gordien A Y, Gray A I, Franzblau S G, Seidel V. Antimycobacterial terpenoids from Juniperus communis. (Cuppressaceae) J Ethnopharmacol. 2009;126:500–505. doi: 10.1016/j.jep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Murillo J I, Dimayuga R E, Malmstrom J, Christophersen C, Franzblau SG. Antimycobacterial flavones from Haplopappus sonorensis. Fitoterapia. 2003;74:226–230. doi: 10.1016/s0367-326x(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 43.Okunade A L, Elvin-Lewis M P, Lewis W H. Natural antimycobacterial metabolites: current status. Phytochemistry. 2004;65:1017–1032. doi: 10.1016/j.phytochem.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Al-bayati FA, Sulaiman KD. In vitro Antimicrobial Activity of Salvadora persica extracts against Some Isolated Oral Pathogens in Iraq. Turk J Biol. 2008;32:57–62. [Google Scholar]
- 45.Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria Canadensis. J Ethnopharmacol. 2002;79:57–67. doi: 10.1016/s0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 46.Flythe MD. The antimicrobial effects of hops (Humulus lupulus L.) on ruminal hyper ammonia-producing bacteria. Lett Appl Microbiol. 2009;48:712–717. doi: 10.1111/j.1472-765X.2009.02600.x. [DOI] [PubMed] [Google Scholar]
- 47.Stavri M, Gibbons S. The antimycobacterial constituents of Dill (Anethum graveolens) Phytother. 2005;11:938–941. doi: 10.1002/ptr.1758. [DOI] [PubMed] [Google Scholar]
- 48.Lo Cantore P, Lacobellis NS, DeMarco A, Capasso F, Senatore F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller var vulgare (Miller) essential oils. Agric Food Chem. 2004;52:7862–7866. doi: 10.1021/jf0493122. [DOI] [PubMed] [Google Scholar]


