Abstract
BACKGROUND:
Erythropoietin (EPO) as a major stimulator of red blood cell (RBC) production play a key role on brain protection and have a caring effect on neurons from hypoxic or traumatic injury. The objective of this trial was to study the safety and efficacy of recombinant human EPO (rhEPO) on level of consciousness and other outcomes in patient with post traumatic diffuse axonal injury (PTDAI).
METHODS:
In a controlled double-blind randomized clinical trial, 54 patients aged 20-47 years were randomly allocated to 2 groups. Subjects in intervention group (n = 27) received 2000U open-label rhEPO (Erythropoietin-ß; Roche, Gren-zach-Wyhlen, Germany) subcutaneously for six doses in two weeks (on days: 2, 4, 6, 8 and 10). The efficacies of the intervention were evaluated by GCS (Glasgow Coma Scale) and GOS (Glasgow Outcome Scale).
RESULTS:
The patients that were treated by rhEPO improved earlier with the difference between the treatment groups occurring on the day 10 (score differences of 9.6 for GCS and 1.9 for GOS). The better course of the rhEPO-treated patients continued throughout the remaining study period. The hematocrit and red blood cell counts did not increase to levels exceeding the normal range in rhEPO patients.
CONCLUSIONS:
Intravenous EPO was well tolerated in diffuse axonal injury and was associated with an improvement in patients’ outcome in 2 weeks.
KEYWORDS: Erythropoietin, Outcome, Diffuse Axonal Injury
Traumatic injury of the nervous system initiates a composite cascade of proinflammatory mediators leading to apop-tosis and necrosis of neurons, especially oligo-dendrocytes as well as endothelial cells.1 Diffuse axonal injury (DAI), one of the most common and debilitating forms of traumatic brain injury, is caused by sudden acceleration- deceleration injuries that induce turning forces in the brain parenchyma. DAI is characterized by paralysis and loss of consciousness. Initially, it was thought that axonal disruption was responsible for the observed clinical manifesta-tions in DAI.2 Wolf and colleagues revealed that traumatic axonal deformation induces abnormal sodium influx through mechanically sensitive sodium channels, which subsequently triggers an increase in intra-axonal calcium by opening the voltage-gated calcium channel with reversal of the sodium–calcium exchange.3 Currently, increased trans-membrane calcium influx is considered a critical and in-itiating event in the pathogenesis of DAI. This has led to the emergence of calcium channel blockers such as nimodipine as potential the-rapeutic options for patients with DAI.2 Eryt-hropoietin (EPO) is a member of the cytokine type I super-family, which exerts anti-apoptotic and anti-inflammatory properties and enhances mobilization and proliferation of neuronal stem cells.1 Recent studies have demonstrated an increased local production of EPO and its receptor following brain injury.4 In ex-perimental studies, administration of recombi-nant human EPO (rhEPO) improved neurolog-ical recovery, decreased lipid peroxidation and minimized ultra structural changes following spinal cord injury.1 All these investigations implied a potent neuroprotection by EPO and some have used this agent for neuroprotective therapy after traumatic brain injuries. Yamamoto et al. showed that EPO activated neural voltage-gated calcium channels, by which it increased nitric oxide synthesis in the hippo-campus.5 EPO may also interact with neural membranes by increasing calcium influx through T-type voltage-dependent calcium channels.6 If true, one may hypothesize that EPO could actually enhance the progression of DAI early after traumatic brain injury when voltage-gated calcium channels become activated. Hence, in this study, we used erythropoietin in patients with DAI to see if it could change the prognosis of the victims.
Methods
The study protocol was approved by the Ethical Committee of Isfahan University of Medical Sciences. A total of 54 patients (All of them men between 20-47 years old) were recruited to evaluate the use of rhEPO in outcome of DAI. The study was a controlled double-blind randomized clinical trial. For blinding, a pharmacist prepared and numbered identical vials containing either saline (0.9% NaCl) or rhEPO reconstituted in saline. The vials were randomly assigned to patients upon enrollment and the contents of each vial were known only by the pharmacist. None of the clinicians who performed neurologic or imaging analyses had access to any of the serum laboratory data (platelet or reticulocyte counts) during the study. Serum EPO levels were analyzed only after unblinding. Study groups turned out to be highly comparable with respect to baseline characteristics. Twenty seven patients comprised intervention group that received 2000U open-label rhEPO erythropoietin (erythropoie-tin-ß; Roche, Grenzach-Wyhlen, Germany) subcutaneously for six doses in two weeks (on days 2, 4, 6, 8 and 10). The study's endpoints were GCS (Glasgow Coma Scale) during study and GOS (Glasgow Outcome Scale) at the end. Additional clinical variables were collected on days 2, 4, 6, 8 and 10 including serum EPO, hematocrit, hemoglobin, leukocyte and throm-bocyte counts, partial prothrombin time (PTT), C-reactive protein (CRP), ferritin, transferrin, iron, electrolytes, glucose, blood urea nitrogen (BUN) and creatinine.
Only patients with DAI and GCS between 4 until 8 were accepted (Inclusion Criteria). A cranial computerized tomography (CT) scan was performed to exclude any kind of he-morrhage at first. Exclusion criteria were de-fined in part to facilitate evaluation of the study by homogenizing the sample popula-tion, as well as in consideration of the litera-ture on rhEPO application in other indications. They included any contraindication to CT scan, unclear onset of clinical signs, quickly resolv-ing neurologic symptoms after 24 hours, GCS < 4 and > 8, surgery within the last 4 weeks. All those patients suffered from subarachnoid hemorrhage (SAH) or intracerebral hemorr-hage (ICH) as well as patients with any kind of brain neoplasm, septic embolism, endocarditis, abnormal hemodynamic abnormalities and any severe internal medicine diseases were ex-cluded. Statistical analyses were done using unpaired student's t-test. Statistical signific-ance was accepted for p < 0.05.
Results
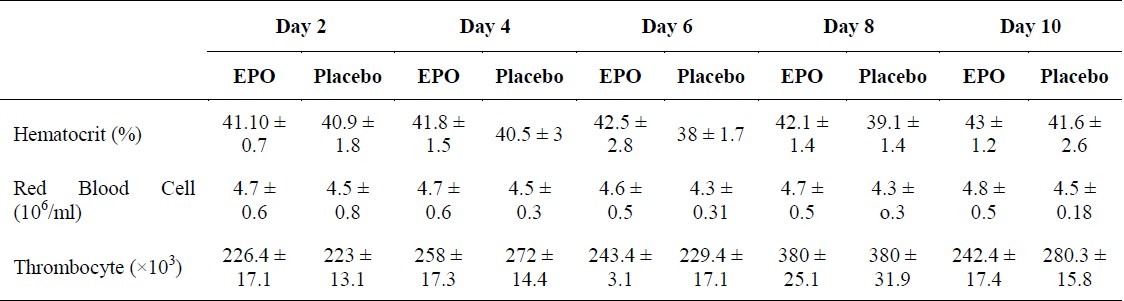
Fifty four DAI patients screened for participation in the trial. Most common reasons for exclusion were contraindications of EPO, age older than 60 years and quickly resolving neurologic symptoms. Baseline characteristics did not show any difference between two groups. In the efficacy study, four deaths occurred; two within the placebo arm (day 6 and 10, due to malignant brain edema and sepsis, respectively) and two within the rhEPO arm (day 18 and 28 due to pneumonia, sepsis, and multi-organ failure). Average time to drug infusion was 5 hours for rhEPO (ranged from 2 hours and 40 minutes to 7 hours and 55 minutes) and 4 hours and 45 minutes for placebo (ranged from 3 hours and 20 minutes to 7 hours 45 minutes). The use of intravenous heparin during the course of the study was not different between the two groups. Follow-up neurologic outcome scorings were obtained on days 2, 4, 6, 8 and 10, using the GCS. Table 1 shows differences of GCS from admission till the final step as well as GOS at the end of study when the patients were discharge from hospital. Table 2 shows red blood cells (RBC) count, hematocrit and thrombocyte counts of several measurements. In fact, the rhEPO-treated patients improved earlier in which the difference between two groups occurred on day 10 (score differences of 9.6 in the GCS and 1.9 in the GOS). The better course of the rhEPO-treated patients continued throughout the rest of study period. The hematocrit and RBC counts did not increase to the levels exceeding the normal range in rhEPO group, but there was only a tendency toward higher levels of these parameters in the this group as compared to controls. In contrast, the hematocrit of placebo-treated patients fell slightly. Thrombocyte counts showed comparable increases by day 10 in both treatment groups.
Table 1.
The comparison of patients outcome between erythropoietin and placebo group
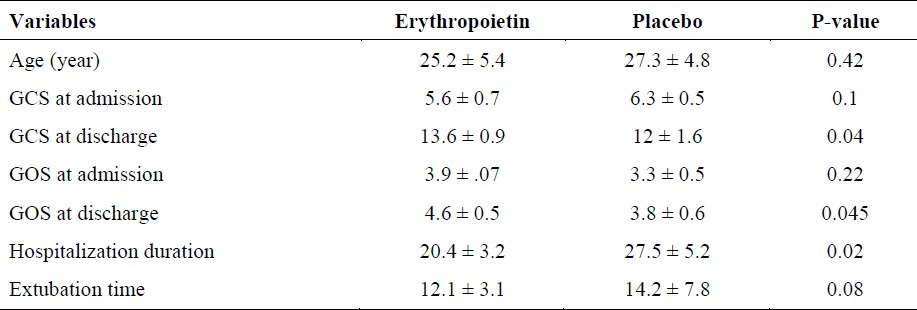
Table 2.
Hematocrit, red blood cell counts and thrombocyte of patients in consecutive measurements
Discussion
Low level expression of EPO and its receptors has long been reported in the brain especially after brain injury.7,8 One study showed that even though traumatic brain derived EPO is doubtful to donate to serum EPO concentration, cerebral EPO could face is up regulated by harmful condition such as hypoxia.9 It was also shown that in vitro rhEPO could protect neuronal cells against hypoxic injury.9 Consequently, the possibility arise that brain's EPO may provide a paracrine protective role and therefore administration of EPO could be useful to reduce consequences of brain injury. Initial experiments confirmed this concept using intrathecal administration of rhEPO.10,11 By intraventricular injection of rhEPO, it is theatrically possible to reduce potential peripheral absorption but this approach is not easy in clinical medicine especially for edematous brain which does not let to have a suitable access to ventricles. Therefore, this problem caused that recent studies use high dose of rhEPO for sufficient concentration of drug and that higher systemic administration doses of rhEPO was found to be necessary to achieve neuroprotective effect in animal models of brain injury.1,6,9 According to these studies and a number of therapeutic trials for head trauma and DAI in animals as well as studies about stroke of human, no broadly applicable, safe and efficacious treatment has been identified.12,13
In this study, GCS and GOS after administration of EPO were better than baseline and patients in EPO group significantly had shorter hospitalization time; but no difference between two groups in extubation time was found. In our study all the cases were selected among men because of two reasons; first, after DAI occurs on male gender and second, to eliminate the role of ovarian hormones which may play a endogenous neuroprotectants role as in many studies. Accumulating epidemiological data have suggested that young female patients have improved clinical prognoses after traumatic brain injury.14–17 Although peripherally administered rhEPO was shown to penetrate the blood brain barrier (BBB) and reduce brain injury following a variety of insults,18–20 its potential neuroprotective efficacy in an in vivo model of experimental TBI has been scarcely investigated.18,21–25 On the other hand, the ways by which EPO may have its influences and beneficial effects are not clear. Available evidence point that EPO acts in a synchronized fashion at multiple levels to limit the production of tissue-injuring molecules such as glutamate,26 reverse vasospasm,27 attenuate apoptosis,28 modulate inflammation,18 and recruit stem cells.29 The present study demonstrated that EPO confers neuroprotection in patient with DAI and emphasized its beneficial effect on neurological outcome showing that GCS and GOS improved following brain trauma. Till now, many evidences have shown that EPO is a neuroprotective agent and many in vitro and in vivo studies on traumatic brain injury confirmed it but till now there are only hardly any studies showing the effect of ruEPO after clinical traumatic brain injury.18,21–25,30 Although the mechanisms by which EPO acts as neuroprotective are still a matter of controversy, an increasing number of evidence suggests that EPOR activation following EPO binding inhibits neuronal apoptosis28,19 Prevention of neuronal apoptosis involves the activation of JAK-2 and nuclear factor (NF)-kB signaling pathways.19 In addition, EPO also appears to prevent apoptotic injury through an Akt dependent mechanism.31 one mechanism explaining the neuroprotective effect of ruEPO was shown to depend on inhibition of nitric oxide production.30 Accordingly, it is absolutely necessary to consider further studies to find additional information about the possibilities of clinical use of EPO after DAI.
Authors’ Contributions
SA planned the study and finalized it, prepared the first version of manuscript and revised final version for publish. MRS and AH did the statistical analysis of manuscript and revised final version. All authors read and approved the final manuscript.
Acknowledgments
The authors wish to sincerely thank the support of all the colleagues in Kashani Hospital Medical Center affiliated to Isfahan University of Medical Sciences in Isfahan, Iran. Furthermore, our special thanks go to the patients, who wholeheartedly and actively assisted us to carry out this research. No conflict of interest existed. This prospective randomized study was approved by the Ethics Committee of our university, (Isfahan University of Medical Sciences) and all patients gave written, informed consent.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002;99(14):9450–5. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng D, Ma Y, Zhang Y, Plets C, Goffin J, Chen J. Controlled study of nimodipine in treatment of patients with diffuse axonal injury. Chin J Traumatol. 2000;3(2):85–8. [PubMed] [Google Scholar]
- 3.Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21(6):1923–30. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101(3):271–6. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Koshimura K, Sohmiya M, Murakami Y, Kato Y. Effect of erythropoietin on nitric oxide production in the rat hippocampus using in vivo brain microdialysis. Neuroscience. 2004;128(1):163–8. doi: 10.1016/j.neuroscience.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Assandri R, Egger M, Gassmann M, Niggli E, Bauer C, Forster I, et al. Erythropoietin modulates intracellular calcium in a human neuroblastoma cell line. J Physiol. 1999;516(Pt 2):343–52. doi: 10.1111/j.1469-7793.1999.0343v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan CC, Eckardt KU, Ratcliffe PJ. Organ distribution of erythropoietin messenger RNA in normal and uremic rats. Kidney Int. 1991;40(1):69–76. doi: 10.1038/ki.1991.181. [DOI] [PubMed] [Google Scholar]
- 8.Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8(4):666–76. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 9.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76(1):105–16. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 10.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95(8):4635–40. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19(6):643–51. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M, Schaebitz W. An overview of acute stroke therapy: past, present, and future. Arch Intern Med. 2000;160(21):3196–206. doi: 10.1001/archinte.160.21.3196. [DOI] [PubMed] [Google Scholar]
- 13.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 14.Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12(9):805–8. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- 15.Kirkness CJ, Burr RL, Mitchell PH, Newell DW. Is there a sex difference in the course following traumatic brain injury? Biol Res Nurs. 2004;5(4):299–310. doi: 10.1177/1099800404263050. [DOI] [PubMed] [Google Scholar]
- 16.Mostafa G, Huynh T, Sing RF, Miles WS, Norton HJ, Thomason MH. Gender-related outcomes in trauma. J Trau-ma. 2002;53(3):430–4. doi: 10.1097/00005373-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol Behav. 1999;68(1-2):81–6. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- 18.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412(6847):641–7. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 20.Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10(2):93–8. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- 21.Ozturk E, Demirbilek S, Kadir BA, Saricicek V, Gulec M, Akyol O, et al. Antioxidant properties of propofol and erythropoietin after closed head injury in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(6):922–7. doi: 10.1016/j.pnpbp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. Heat acclimation increases hypoxia-inducible factor 1alpha and erythropoietin receptor expression: implication for neuroprotection after closed head injury in mice. J Cereb Blood Flow Metab. 2005;25(11):1456–65. doi: 10.1038/sj.jcbfm.9600142. [DOI] [PubMed] [Google Scholar]
- 23.Siren AL, Radyushkin K, Boretius S, Kammer D, Riechers CC, Natt O, et al. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129(Pt 2):480–9. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- 24.Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de Looij Y, et al. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab. 2007;27(7):1369–76. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- 25.Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, et al. Erythropoietin is neuro-protective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of ex-perimental closed head injury. FASEB J. 2005;19(12):1701–3. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276(42):39469–75. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 27.Grasso G. Neuroprotective effect of recombinant human erythropoietin in experimental subarachnoid hemorrhage. J Neurosurg Sci. 2001;45(1):7–14. [PubMed] [Google Scholar]
- 28.Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neu-ron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99(4):2258–63. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733–43. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, et al. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401(3):349–56. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 31.Bao H, Jacobs-Helber SM, Lawson AE, Penta K, Wickrema A, Sawyer ST. Protein kinase B (c-Akt), phosphatidyli-nositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells) Blood. 1999;93(11):3757–73. [PubMed] [Google Scholar]


