Abstract
BACKGROUND:
The aim of the study was to assess the effects of combination of bortezomib moderate dose and continuous oral low dose melphalan and thalidomide and dexamethasone (BMTD regimen) in elderly patients aged ≥ 65 years with relapsed multiple myeloma (MM).
METHODS:
Twenty four patients with advanced MM were enrolled to receive eight 3-week treatment cycles with bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 followed by three 5-week cycles with bortezomib 1.3 mg/m2 on days 1, 8, 15, and 22. Within all cycles, dexamethasone 24 mg/d was given intravenously on the day of bortezomib injection and the day thereafter. In addition, patients received oral treatment of melphalan at a dose of 5 mg/d continuously for twenty days for every cycle.
RESULTS:
Mean age of study patients was 72.8 ± 6.4 years. All patients that completed at least one treatment cycle were evaluated for response. Complete, partial, and minor responses occurred in 19%, 65% and 6% of patients, respectively. Overall response rate was 90% (efficacy analysis).
CONCLUSIONS:
This study demonstrated potent in vivo activity of combination therapy with BMTD regimen in patients with relapsed MM, with an acceptable safety profile and high overall response rate.
KEYWORDS: Multiple Myeloma, Bortezomib, Dexamethasone, Melphalan, Thalidomide
Multiple myeloma (MM) is an aggressive and incurable hematological neoplasia, characterized by expansion of malignant plasma cells. For approximately 50 years, the combination of melphalan with prednisone was the reference therapy for elderly patients with multiple myeloma.1,2 Recently, however, the addition of thalidomide3 or bortezomib4 or lenalidomide5 to the standard melphalan plus prednisone combination has changed the treatment paradigm for elderly patients with multiple myeloma.4,6,7 For many years, the combination treatment with melphalan-prednisone has been its conventional chemotherapy, resulting in a median survival of about 3 years.2
The frequency of remission, the disease-free survival and overall survival have been improved in patients ≤ 65 years with the use of first-line high-dose chemotherapy, followed by autologous stem-cell transplantation.8 Indeed, two large randomized trials compared this procedure with standard chemotherapy and the overall 5-year survival improved from 12% to 52% in one trial, while the median survival increased from 42 to 54 months in the second Bortezomib in elderly patients with multiple myeloma trial.4,5 However, most patients’ relapse and further therapies were largely ineffective.6,7
In the past 10 years, new advances have been gained into the understanding of the biologic and molecular mechanisms of MM pathogenesis. Several studies have indeed shown a critical role of the bone marrow microenvironment in the development of this neoplastic disease.9 The interactions of MM cells with stromal cells and extracellular matrix trigger paracrine and autocrine loops of many cytokines involved in MM progression and activate intracellular signal pathways that promote bone destruction as well as survival, proliferation, drug resistance and genomic instability of myeloma cells.9,10 The identification of these mechanisms has led to the development of novel therapeutic options to target specific pathways involved in the pathogenesis of disease in order to disrupt functional interactions between MM cells with bone marrow microenvironment and to block autocrine self supporting circuits.11,12 These agents include the immunomodulatory drug thalidomide, the proteasome inhibitor bortezomib,9 and the thalidomide derivative lenalidomide.10 Clinical studies have shown encouraging results first in patients with relapsed/refractory MM, then in newly diagnosed patients. These drugs, alone and in combination, are now all approved treatment options for symptomatic MM.13–15
Present study was design to assess the effects of combination of bortezomib (B) moderate dose, continuous oral low-dose melphalan (M), thalidomide (T) and dexamethasone (D), [BMTD regimen] in elderly patients aged ≥ 65 years with relapsed MM.
Methods
Between April 2004 and January 2010, a total of 24 patients aged ≥ 65 years, with primary refractory or relapsed MM were enrolled in the study and followed up until June 2010. Patients with relapsed MM after ≥ 1 prior treatment and a minimal response of ≥ 2 months after their last line of therapy were eligible for enrollment. Additional eligibility criteria included measurable serum or urinary paraprotein, platelet count > 100 × 109/l, absolute neutrophil count > 1.5 ×109/l, and serum creatinine < 170 μmol/l. In addition, patients with peripheral neuropathy grade ≥ 2 or patients receiving any investigational drugs within 14 days of enrollment were excluded from the study. Ethics approval was obtained from the school of medicine in Isfahan University of Medical Sciences. All patients were informed about the purpose of the study and written informed consent was obtained from all of them.
Eligible patients received eight 3-week treatment cycles with bortezomib (VelCadeR JANSSEN-CILAG, Idron, France) 1.3 mg/m2 on days 1, 4, 8, and 11 and followed by three 5-week cycles with bortezomib 1.3 mg/m2 on days 1, 8, 16, and 22. Within all cycles, 24 mg/d dexamethasone (OSVAH pharmaceutical co. Tehran, Iran) was given intravenously on the day of bortezomib injection and the day thereafter. In addition, patients received continuous oral daily melphalan (AlkeranR 2 mg Comprese rivestite con film. Glaxo Smith Kline
S.P.A Agenzio Italiana del Farmaco: Maggio 2009) treatment at a dose of 5 mg/d for twenty days and 100 mg/day thalidomide (MyrinR Lipomed AG Fabrikmuttenwey 4. CH-4144 Arlesheim. Switzerland). Antiviral prophylaxis appears to be mandatory with Acyclovir 400 mg tablet every 6 h/day manufactured by Rous Darow Laboratories Tehran, Iran and or Amantidine cap 100 mg every 6 h/day made by Amin pharmatetical co. Isfahan, Iran.
Baseline evaluations included physical examination, blood counts, hepatic and renal function tests, bone marrow aspirate and biopsy, serum and urine protein electrophoreses for serum immunoglobulin and urinary light chains, β2-microglobulin and C-reactive protein. A chest X-ray and a complete radiological bone survey were also performed.
Safety was assessed throughout the study by physical examinations, recording of vital signs, general condition, oral mucosites, toxicity assessments and laboratory tests (hematology, clinical chemistry, complete blood count (CBC), platelet, WBC diff, alanine aminotransferase (ALT), aspartate aminotransferase, blood urea nitrogen (BUN), creatinine, urine analysis, chest X-Ray and abdominal-pelvic sonography). All patients that received at least one dose of the study's drugs were included in the toxicity evaluation. Two patients received additional erythropoietin 4000 iu subcutaneous injection every 3 days to control anemia.
Patients were evaluated before each treatment cycle to determine how their disease responded to the therapy. The preferred method was the measurement of monoclonal protein in the serum and urine. Bone marrow evaluation was incorporated to identify complete response.16 The free light chain assay allowed the determination of response in patients with immeasurable disease by serum and urine protein electrophoresis. Disease activity in all patients was measured with the serial of monoclonal protein in the serum or urine. Alternative methods included the evaluation of bone marrow plasma cell percentage and serum free light-chain measurements. Bone marrow plasma cell assessment and imaging studies, such as CT-scan and/or magnetic resonance imaging (MRI), were the only methods available for monitoring the small subset of myeloma patients that could not be measured by monoclonal protein or free light chain. The frequency of bone marrow assessment in these patients depended on other clinical parameters; patients who were felt to be responding well based on improvement in end-organ function needed bone marrow assessments less frequently compared with those in whom the response status was less clear.16
Applying the predefined dose modification criteria, there was no grade 4 anemia or leucopenia17 with this combination treatment. However, 23% of patients suffered from grade 2: Infection in one organ and 4% (one patient) grade 3 infections. Respiratory tract infection with septic course without underlying neutropenia was controlled with appropriate antibiotics (grade 4 Infection meaning widespread infection and septecemia).18 This 67-year-old patient had received four prior lines of treatment within 43 months since MM diagnosis.
Statistical analysis was performed by SPSS software version 17 (SPSS Inc, Chicago, IL, USA). All data was presented as means ± SD, number (%) or median [range] as appropriate. Statistical significance was accepted at p < 0.05 (two-tailed).
Results
Mean age of subjects was 72.8 ± 6.4 years [range 66-90 years]. Baseline demographic and clinical characteristics are summarized in table 1. Of 24 patients evaluated for response, 19% of patients achieved a complete response (CR), 65% a partial response (PR) and 6% a minor responses rate (MRR), resulting in an overall response rate (ORR) of 90%. Median event-free survival was 15 months, with a median overall survival of 25 months. The median number of treatment cycles with bortezomib/low-dose melphalan/dexamethasone and thalidomide was six cycles (median duration of treatment4.5 months). Eighteen of the 24 patients completed 11 cycles or achieved a confirmed complete response. The median time to first response was 45 days (range 1.4–6 months). In the responding patients, a partial response occurred within the first two cycles (69% after the first cycle and 22% after the second). The median time from diagnosis to study entry was 52 months. The median duration of follow-up from study entry was 26 months (range 20–32 months).
Table 1.
Patient demographic and baseline characteristics (n = 24)
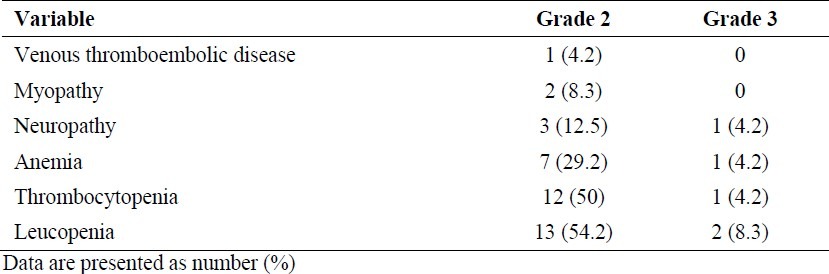
The most common grade 2 adverse events regardless of relation to study drug treatment are show in figure 1. The most commonly reported adverse events were thrombocytopenia, leukocytopenia, infection and fatigue. There was no apparent constipation or hyperglycemia with this drug combination. Grade 3 adverse events regardless of relation to study drug treatment were thrombocytopenia (one patient), infection (one patient), neuropathy (one patient), cardiovascular events (one patient), dyspnea (one patient) and edema (one patient). Table 2 shows the most common grade 2 and 3 adverse events in 24 relapsed elderly patients with MM.
Figure 1.
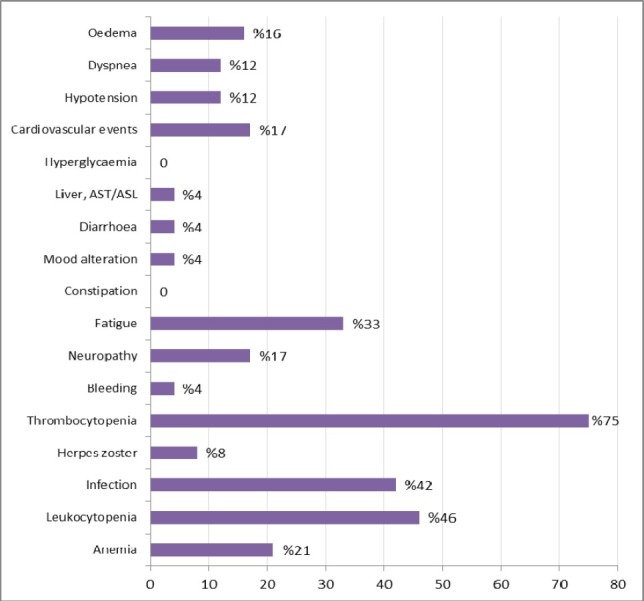
Grade 2 adverse events regardless of relation to study drug treatment (n= 24). Grade 3 adverse events were thrombocytopenia (one patient), infection (one patient), neuropathy (one patient), cardiovascular events (one patient), dyspnea (one patient) and edema (one patient).
Table 2.
Adverse events in 24 relapsed elderly patients with multiple myeloma
Discussion
The majority of MM patients will ultimately relapse after initial therapy. A mixture regimen using a novel agent backbone is an attractive therapeutic choice, potentially allowing longer duration of response compared with previous regimens.17,19–21 Bortezomib combination therapies are being increasingly explored, with a predictable rise in efficacy over monotherapy.
The rationale for dosing of melphalan 24 hour after bortezomib was based on in vitro studies demonstrating reserve of DNA repair pathways by bortezomib, therefore sensitizing both drug-sensitive and resistant myeloma cells to melphalan maximally after ≥10–16 hour.22 Treatment with single agent bortezomib at a dose of 1.3 mg/m2 effected at least partial responses in 27% of patients with relapsed and refractory MM, 38% of patients with a relapse after one to three previous therapies and 50% in patients relapsing during or after first-line therapy.15,20,23,24
In addition, it was indicated a dose response association with an inferior response rate of 33% with 1.0 mg/m2 of bortezomib. Median time to progression MM with single agent bortezomib was superior to high-dose dexamethasone (6.22 vs. 3.49 months).24 Overall survival in relapsed and refractory MM was 17 months.23 Combination with dexamethasone in patients with either stable or progressive disease on bortezomib alone resulted in 11% to 18% additional responses.14,20 However, it has not been established whether the addition of dexamethasone confers benefit in terms of TTP and overall survival compared to bortezomib alone.25
Conventional cytotoxic drugs may add to the efficacy of bortezomib.26 In MM, bortezomib has been shown to restore both cyclophosphamide- and doxorubicin- sensitivity to resistant cell lines and to synergize with melphalan in killing myeloma cells.21 In phase ½ trials assessing bortezomib and melphalan or doxorubicin combinations for relapsed or refractory MM, ORR of 68–73% were achieved.17,27 In elderly untreated myeloma patients, bortezomib plus melphalan and prednisone appeared significantly superior to historical melphalan and prednisone alone.28 In an attempt to further improve the efficacy of bortezomib/dexamethasone, the present phase 2 trials was initiated to study bortezomib/dexamethasone in combination with continuous low-dose melphalan administered orally.
This study demonstrated that bortezomib and melphalan therapy can be safely and effectively administered to patients with relapsed MM, with only one DLT (grade 4 neuropathy) observed during the phase I component.25 In response to the occurrence of myelosuppression in the first cohort, the criteria to define the MTD protocol were expanded to include the dose not leading to delays of more than 2 weeks to progress to the next cycle. This method for defining the MTD has been increasingly described for novel agent combination studies.17
The dose of oral melphalan (5.0 mg/d) used for this study was too lower than that used in other studies that combined intravenous melphalan doses of 20 mg/m2 with dexamethasone or 25 mg/m2 melphalan with thalidomide/prednisone.29 The lower-than-expected MTD protocol of melphalan in this study supports the hypothesis of synergistic cytotoxicity with bortezomib. Despite a lower melphalan dose, the responses observed were rapid (median time to response 45 days) and occurred at a high rate ORR 90% at the MTD protocol.
For patients with stable or progressive disease, the addition of dexamethasone had only a modest effect in improving responses. Importantly, lower doses of melphalan have been associated with lower incidence of toxicities, particularly in patients > 65 years.25 Although pharmacokinetics was not assessed in this study, the reduced pharmacokinetic variability of oral dosing of melphalan may account for the significant efficacy of this regimen and tolerable safety profile. In this study, the complication of treatment with combination therapy with bortezomib, low dose continuous oral melphalan, low-dose dexamethasone, thalidomide in patients with relapsed MM were very mild, controllable and with relative high result in responses.
In conclusion, this study demonstrated potent in vivo activity of combination therapy with bortezomib, low dose continuous oral melphalan, low-dose dexamethasone, thalidomide and melphalan in patients with relapsed MM, with an acceptable safety profile and high ORR. These findings indicated that further study in setting is warranted, comparing this regimen with the widely used bortezomib and dexamethasone combination.
Authors’ Contributions
TA Study design, Conduct of study, Manuscript preparation. MA Study design, Conduct of study, Data analysis, Manuscript preparation. AA Conduct of study, Manuscript preparation, Data Gathering. HM Conduct of study, Manuscript preparation, Data Gathering.
Acknowledgments
The authors thank clinicians who referred patients into the trial and the trial nurses at Sayed al Shohada Hospital. We thank all colleagues in research office of School of Medicine of Isfahan University of Medical sciences.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Cavo M, Benni M, Ronconi S, Fiacchini M, Gozzetti A, Zamagni E, et al. Melphalan-prednisone versus alternating combination VAD/MP or VND/MP as primary therapy for multiple myeloma: final analysis of a randomized clinical study. Haematologica. 2002;87(9):934–42. [PubMed] [Google Scholar]
- 3.Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thali domide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–14. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 4.Uldbrandsen N, Waage A, Gismin P, Turesson I, Juliusson G, Abildgaard N, et al. A randomized placebo controlled study with melphalan/prednisone vs Melphalan /prednisone / thalidomide: quality of life and toxicity. Haematologica. 2009;93(0209) [Google Scholar]
- 5.Waterman GN, Yellin O, Swift RA, Mapes R, Eades B, Ackerman E, et al. A modified regimen of pegylated liposomal doxorubicin, bortezomib, and dexamethasone is effective and well tolerated in the treatment of relapsed or refractory multiple myeloma. Ann Hematol. 2011;90(2):193–200. doi: 10.1007/s00277-010-1052-8. [DOI] [PubMed] [Google Scholar]
- 6.Myeloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16(12):3832–42. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22(2):231–9. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 8.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 9.Bonvini P, Zorzi E, Basso G, Rosolen A. Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma. Leukemia. 2007;21(4):838–42. doi: 10.1038/sj.leu.2404528. [DOI] [PubMed] [Google Scholar]
- 10.Richardson P, Jagannath S, Hussein M, Berenson J, Singhal S, Irwin D, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114(4):772–8. doi: 10.1182/blood-2008-12-196238. [DOI] [PubMed] [Google Scholar]
- 11.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87(3):1104–12. [PubMed] [Google Scholar]
- 12.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98(2):428–35. doi: 10.1182/blood.v98.2.428. [DOI] [PubMed] [Google Scholar]
- 13.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137(5):429–35. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 16.Chee CE, Kumar S, Larson DR, Kyle RA, Dispenzieri A, Gertz MA, et al. The importance of bone marrow examination in determining complete response to therapy in patients with multiple myeloma. Blood. 2009;114(13):2617–8. doi: 10.1182/blood-2009-01-198788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105(8):3058–65. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 18.Paradisi F, Corti G, Cinelli R. Infections in multiple myeloma. Infect Dis Clin North Am. 2001;15(2):373–viii. doi: 10.1016/s0891-5520(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 19.Popat R, Oakervee H, Williams C, Cook M, Craddock C, Basu S, et al. Bortezomib, low-dose intravenous melphalan, and dexamethasone for patients with relapsed multiple myeloma. Br J Haematol. 2009;144(6):887–94. doi: 10.1111/j.1365-2141.2008.07572.x. [DOI] [PubMed] [Google Scholar]
- 20.Kropff MH, Lang N, Bisping G, Domine N, Innig G, Hentrich M, et al. Hyperfractionated cyclophosphamide in combination with pulsed dexamethasone and thalidomide (HyperCDT) in primary refractory or relapsed multiple myeloma. Br J Haematol. 2003;122(4):607–16. doi: 10.1046/j.1365-2141.2003.04473.x. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin DH, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma:: final time-to-event results from the SUMMIT trial. Cancer. 2006;106(6):1316–9. doi: 10.1002/cncr.21740. [DOI] [PubMed] [Google Scholar]
- 22.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Invest. 2004;22(2):304–11. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. Clinical factors predictive of out-come with bortezomib in patients with relapsed, refractory multiple myeloma. Blood. 2005;106(9):2977–81. doi: 10.1182/blood-2005-02-0691. [DOI] [PubMed] [Google Scholar]
- 24.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–72. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 25.Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777–84. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kropff M, Bisping G, Wenning D, Berdel WE, Kienast J. Proteasome inhibition in multiple myeloma. Eur J Cancer. 2006;42(11):1623–39. doi: 10.1016/j.ejca.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Berenson JR, Yang HH, Sadler K, Jarutirasarn SG, Vescio RA, Mapes R, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24(6):937–44. doi: 10.1200/JCO.2005.03.2383. [DOI] [PubMed] [Google Scholar]
- 28.Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108(7):2165–72. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 29.Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825–31. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]


