Abstract
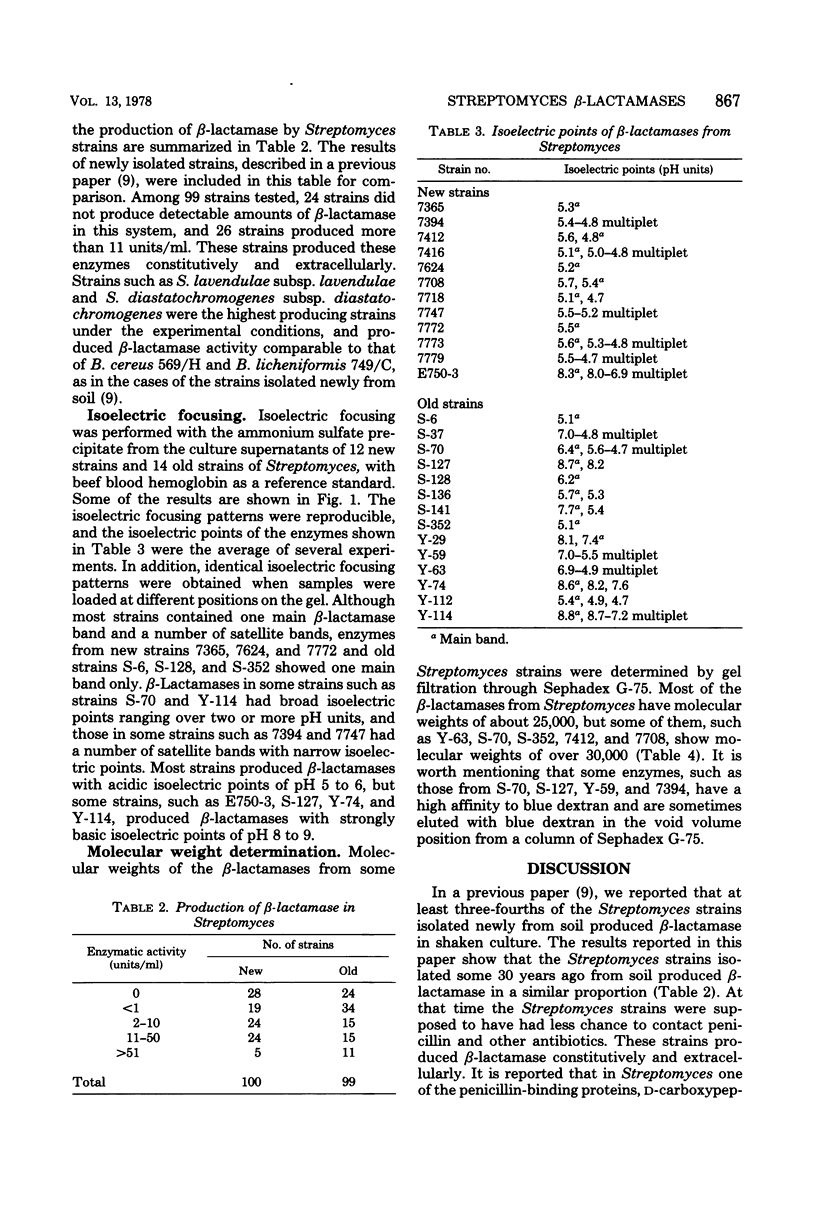
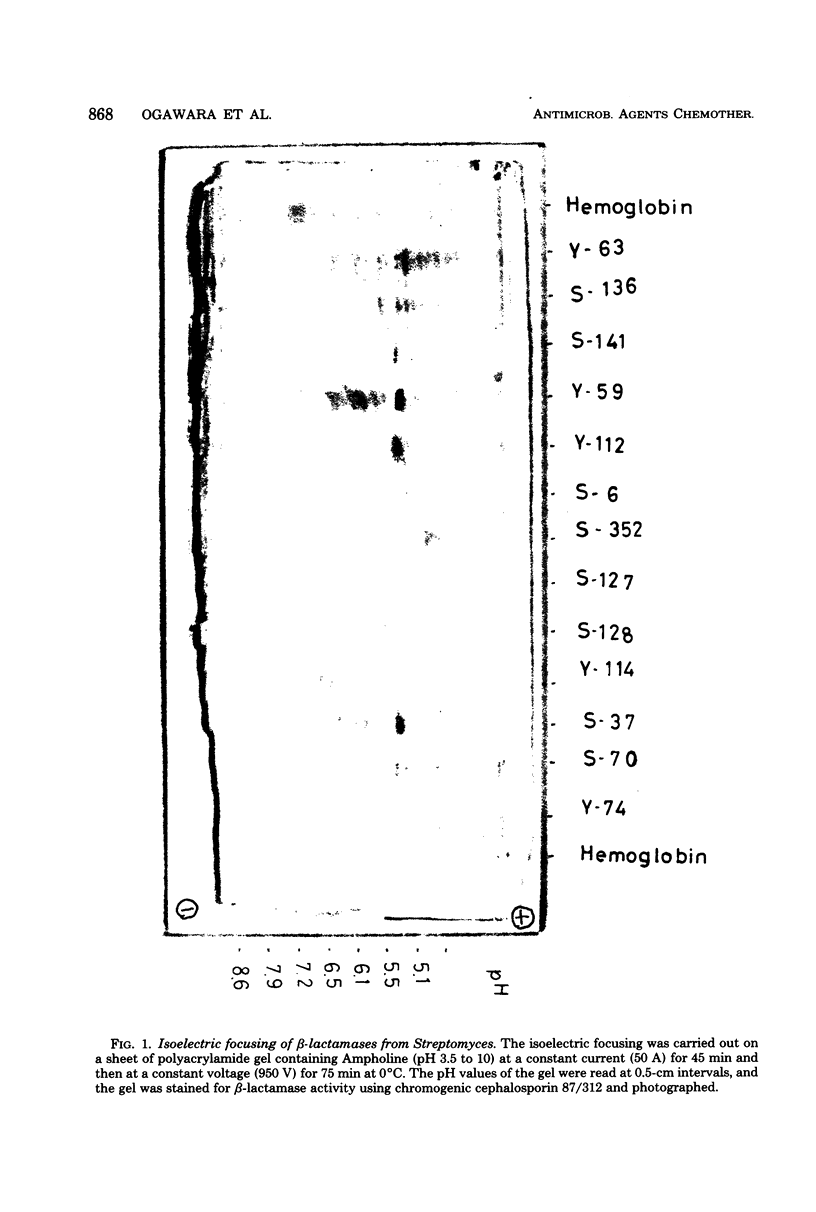
Productivity and property of β-lactamases of Streptomyces strains isolated from soil some 30 years ago were studied in comparison with those of the newly isolated strains. At least three-quarters of the Streptomyces strains produced β-lactamase constitutively and extracellularly, mainly as penicillinases, as in the cases of those from the newly isolated strains. Strains such as S. albus, S. diastatochromogenes, S. fradiae, and S. lavendulae were the highest producing strains, and the amounts of β-lactamase activity they produced were comparable to those produced by Bacillus cereus 569/H and B. licheniformis 749/C. In isoelectric focusing, most strains contained one main β-lactamase band with a number of satellite bands, but some strains contained one band only. Although β-lactamases from most strains showed isoelectric points of pH 5 to 6, some strains produced β-lactamases with strongly basic isoelectric points of pH 8 to 9. Molecular weights were between 20,000 and 30,000. From these results, it is suggested that the proportion of the producing strains of Streptomyces and the properties of the β-lactamases have not been affected significantly by the introduction of penicillin into the natural environment, in contrast to the cases of other microorganisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Vanderhaeghe H., Adriaens P., Degelaen J., De Graeve J. Fate of thiazolidine ring during fragmentation of penicillin by exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Nature. 1976 Apr 1;260(5550):451–454. doi: 10.1038/260451a0. [DOI] [PubMed] [Google Scholar]
- Kushner D. J., Breuil C. Penicillinase (beta-lactamase) formation by blue-green algae. Arch Microbiol. 1977 Mar 1;112(2):219–223. doi: 10.1007/BF00429338. [DOI] [PubMed] [Google Scholar]
- Matthew M., Harris A. M. Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976 May;94(1):55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- Ogawara H. A specific cephalosporin-binding protein of Citrobacter freundii. Biochim Biophys Acta. 1976 Jan 20;420(1):155–164. doi: 10.1016/0005-2795(76)90354-8. [DOI] [PubMed] [Google Scholar]
- Ogawara H., Nozaki S. Effect of acriflavine of the production of beta-lactamase in Streptomyces. J Antibiot (Tokyo) 1977 Apr;30(4):337–339. doi: 10.7164/antibiotics.30.337. [DOI] [PubMed] [Google Scholar]
- Ogawara H. Penicillin-binding proteins of Escherichia coli. Comparison of a strain carrying an R-factor and the parent strain. Biochim Biophys Acta. 1977 Mar 28;491(1):223–231. doi: 10.1016/0005-2795(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Ogawara H. Production and property of beta-lactamases in Streptomyces. Antimicrob Agents Chemother. 1975 Oct;8(4):402–408. doi: 10.1128/aac.8.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Umezawa H. Affinity labeling of an Escherichia coli beta-lactamase. Biochim Biophys Acta. 1973 Dec 19;327(2):481–489. doi: 10.1016/0005-2744(73)90431-2. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Saz A. K. An introspective view of penicillinase. J Cell Physiol. 1970 Dec;76(3):397–403. doi: 10.1002/jcp.1040760318. [DOI] [PubMed] [Google Scholar]




