Abstract
Background:
Glioblastoma multiforme (GBM) is the most common astrocytoma in adults and has a poor prognosis, with a median survival of about 12 months. But, it is rare in children. We report our experience on the pediatric population (20 years or younger) with GBM.
Patients and Methods:
Twenty-three patients with GBM who were treated at our hospital during 1990–2008 were evaluated.
Results:
The mean age was 15.2 years, and the majority of them (14/23) were male. All had received radiotherapy and some had also received chemotherapy. The mean survival was 16.0 months. Two cases survived more than 5 years. Age, radiation dose and performance status were significantly related to survival.
Conclusion:
GBM in pediatric patients were not very common in our center, and prognosis was unfavorable.
Keywords: Glioblastoma multiforme, pediatrics, survival
INTRODUCTION
Glioblastoma multiforme (GBM), which consists of 17% of adult intracranial neoplasm, is a frightening disease.[1] No risk factor except ionizing radiation is known to be associated with GBM.[2] GBM is one of the most aggressive cancers in humans. Despite advances in surgery and adjuvant treatment, the average survival is about 1 year, which has not been improved significantly during the last three decades.[3,4] After temozolomide was introduced as an effective chemotherapy, both concurrent with radiotherapy and as an adjuvant chemotherapy, survival only improved for 2 months.[5] GBM is rare among children.[6] In this retrospective study, we describe the demography, treatment and results for our hospital pediatric (20 years or younger) GBM patients.
PATIENTS AND METHODS
In this study, we describe our GBM patients and their treatment outcome for those who were 20 years old or younger and were treated in an academic tertiary referral center. All the patients had pathologic diagnosis of GBM. The patients’ demographic characteristics, disease factors (size, location, etc.), treatment (operation, radiotherapy/chemotherapy technique and dose) and outcome were obtained from the patients’ records and by phone, if needed.
Patients
Twenty-three cases with 20 years or less at the time of diagnosis were treated at our center during an 18-year period from January 1990 to December 2008. Performance status (PS) was defined according to the Eastern Cooperative Oncology Group (ECOG) scoring system.[7]
Treatment
Although the aim of surgery was maximal safe resection of the tumor, most (12) cases had undergone only stereotactic biopsy. In six patients, it was due to poor PS and in the other six cases, it was due to deep-seated or eloquent location of the tumor. Most patients received postoperative radiotherapy, except those with poor PS. The patients received radiotherapy with cobalt-60 units or 9 MV X-ray photons from a linear accelerator. In these series, 10 cases who were treated before 2000 initially received 40 Gy as whole brain radiotherapy followed by 14 Gy as involved field with a 2–2.5 cm margin and, since 2000, the remaining 13 cases were treated with involved field radiotherapy with a 2–2.5–cm margin. Some cases could not complete their radiotherapy course or only received short-term palliative radiotherapy (30 Gy in 10 fractions). The patients with significant tumor-related or postsurgical complications such as severe anorexia, nausea, vomiting (due to persistent increased intracranial pressure), weight loss, dysphagia and respiratory distress (due to midbrain and/or brain stem involvement) and decreased level of consciousness could not complete their radiotherapy plan.
After curative or palliative radiotherapy, chemotherapy was considered for some patients, but those with poor PS did not receive chemotherapy. Four cases of these series were treated in the recent 5 years; however, despite the availability of temozolomide in recent years, these cases did not receive temozolomide due to lack of medical insurance coverage for this drug and low socioeconomic status.
Statistical analysis
Disease or progression-free survival was defined as the duration from the date of histologic diagnosis to the date of relapse or disease progression or censored at the date of last follow-up. Overall survival was defined as the duration from the date of histologic diagnosis to the date of death resulting from any cause or censored at the date of last follow-up. Multivariate survival analysis was performed using the stepwise Cox's proportional hazards regression model. All the variables with a statistically significant P-value in the univariate analysis were included in a stepwise multivariate logistic regression analysis to identify the independent predictors for overall survival. The hazard ratio (HR) for death, with the 95% confidence interval (CI), was calculated for the treatment groups. The significance of differences in survival was evaluated using the log-rank test. P-values less than 0.05 were considered as significant.
RESULTS
Patients and tumor characteristics
Twenty-three cases were eligible to enter the study [Table 1]. They were between 10 and 20 years old. Their mean age was 15.2 years and the median age 16 years. Fourteen patients were male and the other nine patients were female (male/ female ratio: 1.55). Eleven cases had right-sided and 12 cases had left-sided lesions. Twelve (52%) tumors were located in the occipital lobe. Six (26%) tumors were in the temporoparietal lobe and five (22%) in the frontal lobe.
Table 1.
Patients’ characteristics and treatment outcome
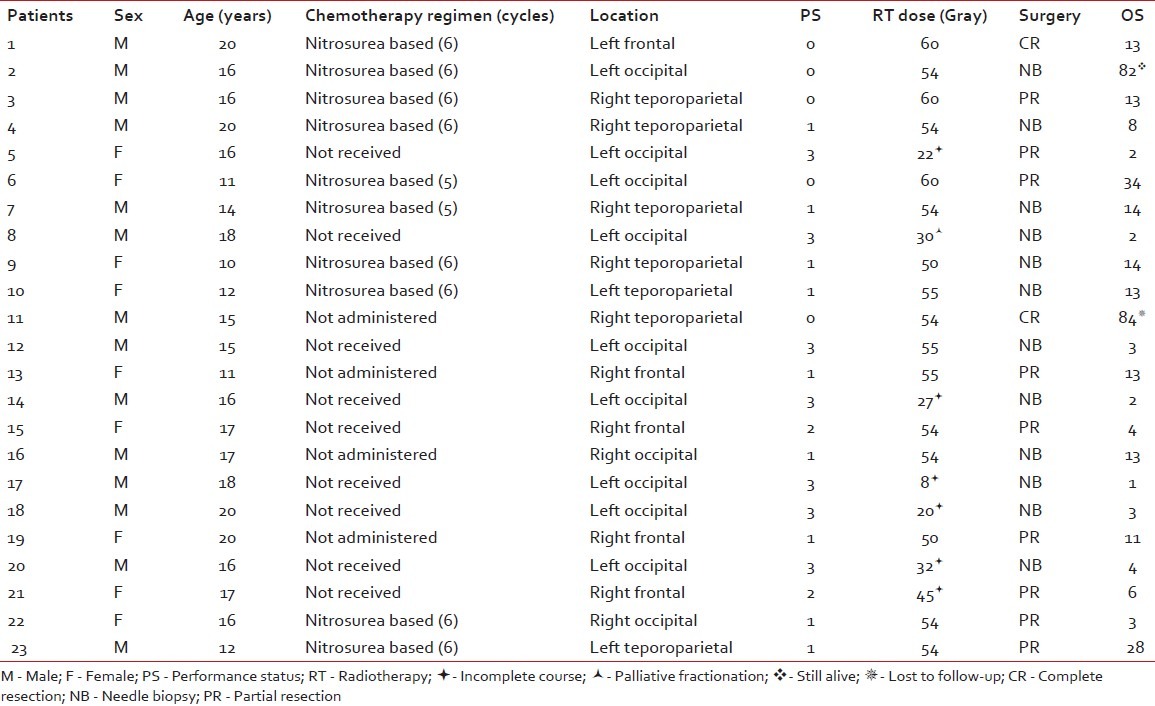
Surgery
According to the imaging reports, the mean tumor size was 6.6 cm (range 3–12 cm). Twelve patients underwent only stereotactic needle biopsy. In nine cases, partial tumor resection was performed. Complete resection was performed for two patients.
Treatment
For one patient, due to a poor general condition, palliative radiotherapy with 30 Gy in 10 fractions was administered. Six cases did not finish their treatment course and died during the radiotherapy course. From those who successfully finished their radiotherapy course, 10 cases received 54 Gy or less and six cases received greater than 54 Gy.
After radiotherapy, some patients received chemotherapy (mean 2.47 cycles). Ten patients received nitrosurea-based chemotherapy. Thirteen patients did not receive any chemotherapy (nine of them due to poor general condition). No patient received temozolomide.
Survival
The median progression-free and overall survival rates were 9 and 11 months, respectively. Four (17%) patients lived for more than 2 years. Two of them died due to tumor recurrence in the thirs year (one had 34 and the other had 28 months survival). Another one is still alive after 82 months and has regular follow-up. The other one missed his follow-up after 84 months. The 1-, 2-, 3- and 5-year progression-free survival rates were 17.4%, 17.4%, 8.7% and 8.7%, respectively. The 1-, 2-, 3- and 5-year overall survival were 47.8%, 17.4%, 8.7% and 8.7%, respectively. The pathologies of those with more than 5 years of survival were reviewed to confirm GBM diagnosis.
Prognostic factors
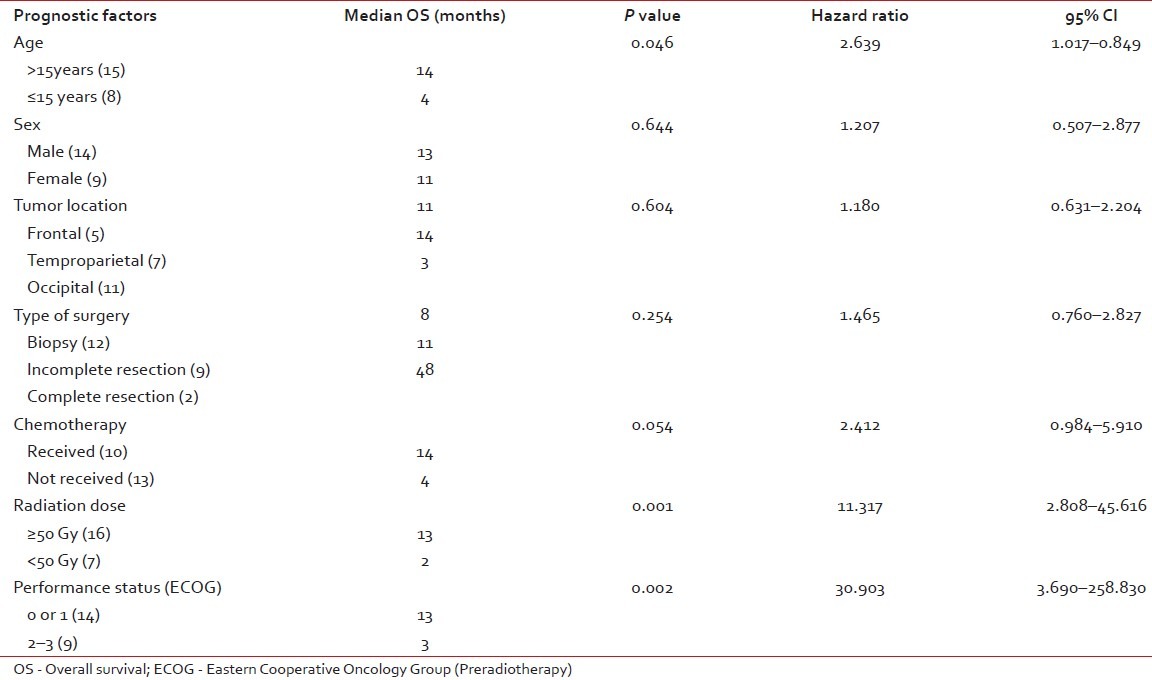
All potential prognostic factors including age, sex, tumor location, type of surgery, chemotherapy, radiation dose and preradiotherpay ECOG PS were analyzed to find out their impact on median survival of the patients with GBM. In univariate analysis, the patients who had received a radiation dose of less than 50 Gy had a significantly poorer outcome compared with those who had received more than 50 Gy (P=0.001). In addition, good ECOG PS (P=0.002) was found to have a favorable effect on median survival [Table 2]. In multivariate analysis using the stepwise logistic regression model, only ECOG PS of 0 or 1 (P<0.001) retained statistical significance.
Table 2.
Univariate analysis of prognostic factors for overall survival
DISCUSSION
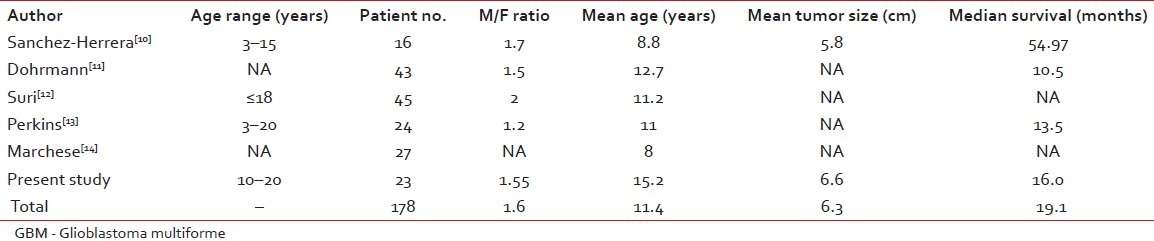
GBM is the most common adult brain tumor, and has poor prognosis. It is more common in men, and the male/female ratio is 1.3:1.45. Median survival is about 1 year and, even in some studies, less than 12 months (4, 8 and 9 months). Although it is not so usual, a patient being alive for more than 3 years is considered as long-term survival. GBM is associated with some hereditary diseases such as Li- Fraumeni syndrome and Turcot's syndrome, but it is usually sporadic.[8] GBM has no precursor lesion when occurring primarily, and is usually an adult population disease.[9] However, secondary GBMs arise from other astrocytomas after years.[10] There are reports from other centers on pediatric GBM [Table 3]. We studied GBM in young age (20 years or less) during 18 years from 1990 to 2008.
Table 3.
Major reported studies of pediatric GBM
The most effective treatment in achieving long-term survival is surgical excision of the tumor, removal and then chemoradiotherapy followed by adjuvant chemotherapy by temozolomide. This schedule is now the standard treatment[2,15–17] Nitrosurea-based chemotherapy was previously used, but with small benefits.[15]
Some prognostic factors have been proposed and have been tested in studies. Among them, younger age (<40 years), extent of surgery (completeness of removal) and good PS are the strongest factors.[8,18,19] Because of infiltrative margin of the tumor, complete resection is usually not so applicable.[15,19,20] Some other factors such as hemoglobin level and midline shift are also suggested.[19] Postoperative radiotherapy also has a definite role in survival prolongation[9,19] But, dose escalation by hyperfractionation and newer devices such as cyberknife did not lead to survival benefit.[15,21]
In our patients, only two cases had complete tumor resection. Their survivals were 13 and 84 months. Three patients, with more than 2 years of survival (82, 34 and 28 months), had undergone incomplete tumor resection.
Nitrosurea-based chemotherapy in an adjuvant setting produced a small survival benefit.[15] Chemotherapy was not so effective in GBM when temozolomide emerged as an effective treatment.[22] Because of borderline results in the pretemozolomide era, some of our patients did not receive any chemotherapy. For four cases who did not receive adjuvant chemotherapy, the survival rates were 84, 13, 13 and 11 months.
Sánchez-Herrera et al. in a retrospective study at the Hospital Infantil de México Federico Gómez (HIMFG) in Mexico City reported pediatric GBM (<18 years) in their center.[10] There were 348 pediatric brain tumors during a 10-year period. Among them, 16 (4.5%) cases were GBM. In their study, tumor size (mean size 5.79 cm) had no relation to survival and outcome. But, the extent of resection correlated with survival. The survival in this study was much longer than ours and also other similar studies, as shown in Table 3. The mean age in their study was 8.8, which was lower than that of our patients’ mean age. The youngest patient in our study was 10 years old.
Dohrmann et al. in a study on 488 children with central nervous system neoplasm found 43 (8.8%) cases who were GBM affected.[11] This study was conducted in 1976 and, at that time, the longest overall survival was 22 months. They concluded a similar outcome when comparing with adult GBM. In their study, the frontal lobe was the most common primary site. In our series, more than half of the patients had occipital primary location.
Grovas et al., in a phase II study, used carmustine, thiotepa and etoposide followed by autologous stem cell rescue. From 11 cases, three patients had prolonged survival of 2.9, 3.9 and 5.1 years. They concluded that high-dose chemotherapy with stem cell transplant is feasible, but lung toxicity due to BCNU was prohibitive.[23]
Perkins et al. performed a retrospective study at the Washington University on 24 cases during 1970–2008. The patients’ mean age was 11 years (range 3–20 years), and 54% were male. Whole brain radiotherapy was used in seven cases and, comparing with newer techniques such as three-dimensional conformal radiotherapy (3DCRT), survival was shorter. But, attractively, 3DC-radiotherapy was not statistically superior to the conventional techniques. Nowadays, whole brain radiotherapy is not a part of GBM radiotherapy. Among their practice, only two cases were in the posterior fossa, but more than half of our patients had occipital tumors. The median survival was 13.5 months. In their study, age, race, gender or tumor location was not a prognostic factor. But, the extent of resection was associated with prolonged survival time. Among them, three cases had more than 5 years of survival.[13]
As shown in Table 3, our results were similar to other studies. Accordingly, we found five previously reported series including 155 cases on pediatric GBM. Overall survival in our patients was 3 months less than the mean, and the male to female ratio was as in other studies.
Genetic aberration and immunology in GBM is under investigation and their evaluation on treatment outcome has entered clinical practice. For example, bevacizumab and temozolomide are recently introduced to be effective in survival prolongation.[2] Suri et al. showed differences in genetics in pediatric and adult GBM.[12] This may reveal differences in treatment outcome or may lead to different treatments. Temozolomide is associated with survival improvement in adult GBM patients, but it is not so effective in pediatric cases. This may be related to O6-methylguanine-DNA methyltransferase (MGMT) gene expression.[23] Temozolomide administration in pediatric GBM requires further studies. Mutations of tumor suppressor genes and protooncogenes play important roles in the pathogenesis and progression of GBM. These molecular genetic alterations are thus important targets for use in the early detection of these neoplasms. Therefore, the molecular analysis and profiling approaches using immunohistochemistry would provide novel diagnostic and prognostic perceptions into the biology of GBM. In this series, there were no data regarding molecular markers and, currently, these markers are not routinely checked in patients with GBM in our institution.[14,19]
CONCLUSION
Pediatric GBM is not very common as adult; however, prognosis remains poor as seen in adult population. Although a small category may have prolonged survival, further investigations are necessary to disclose this group's characteristics, especially genetic and molecular markers.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Nasrin Shokrpour at the Center for Development of Clinical Research of Nemazee Hospital for editorial assistance.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Katz DS, Poe LB, Winfield JA, Corona RJ., Jr A rare case of cerebellar glioblastoma multiforme in childhood: MR imaging. Clin Imaging. 1995;19:162–4. doi: 10.1016/0899-7071(94)00042-b. [DOI] [PubMed] [Google Scholar]
- 2.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–83. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 3.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 4.Noda SE, El-Jawahri A, Patel D, Lautenschlaeger T, Siedow M, Chakravarti A. Molecular advances of brain tumors in radiation oncology. Semin Radiat Oncol. 2009;19:171–8. doi: 10.1016/j.semradonc.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Robins HI, Chang S, Butowski N, Mehta M. Therapeutic advances for glioblastoma multiforme: current status and future prospects. Curr Oncol Rep. 2007;9:66–70. doi: 10.1007/BF02951428. [DOI] [PubMed] [Google Scholar]
- 6.Borgo MC, Pereira JL, Lima FB, Brandão RA, Carvalho GT, Costa BS. Glioblastoma multiforme in childhood: a case report. Clinics (Sao Paulo) 2010;65:923–5. doi: 10.1590/S1807-59322010000900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 8.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 9.Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D, et al. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79–87. doi: 10.1215/15228517-2007-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez-Herrera F, Castro-Sierra E, Gordillo-Domínguez LF, Vaca-Ruiz MA, Santana-Montero B, Perezpeña-Diazconti M, et al. Glioblastoma multiforme in children: experience at Hospital Infantil de Mexico Federico Gomez. Childs Nerv Syst. 2009;25:551–7. doi: 10.1007/s00381-008-0780-8. [DOI] [PubMed] [Google Scholar]
- 11.Dohrmann GJ, Farwell JR, Flannery JT. Glioblastoma multiforme in children. J Neurosurg. 1976;44:442–8. doi: 10.3171/jns.1976.44.4.0442. [DOI] [PubMed] [Google Scholar]
- 12.Suri V, Das P, Pathak P, Jain A, Sharma MC, Borkar SA, et al. Pediatric glioblastomas: A histopathological and molecular genetics study. Neuro Oncol. 2009;11:274–80. doi: 10.1215/15228517-2008-092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins Sm, Rubin JB, Leonard JR, Smyth MD, El Naqa I, Michalski JM, et al. Glioblastoma in children: A single institution experience. Int J Radiat Oncol Biol Phys. 2011;80:1117–21. doi: 10.1016/j.ijrobp.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Marchese MJ, Chang CH. Malignant astrocytic gliomas in children. Cancer. 1990;65:2771–8. doi: 10.1002/1097-0142(19900615)65:12<2771::aid-cncr2820651227>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Piroth MD, Gagel B, Pinkawa M, Stanzel S, Asadpour B, Eble MJ. Postoperative radiotherapy of glioblastoma multiforme: analysis and critical assessment of different treatment strategies and predictive factors. Strahlenther Onkol. 2007;183:695–702. doi: 10.1007/s00066-007-1739-5. [DOI] [PubMed] [Google Scholar]
- 16.Robins HI, Chang S, Butowski N, Mehta M. Therapeutic advances for glioblastoma multiforme: current status and future prospects. Curr Oncol Rep. 2007;9:66–70. doi: 10.1007/BF02951428. [DOI] [PubMed] [Google Scholar]
- 17.Buckner JC, Brown PD, O’Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82:1271–86. doi: 10.4065/82.10.1271. [DOI] [PubMed] [Google Scholar]
- 18.Shinoda J, Sakai N, Murase S, Yano H, Matsuhisa T, Funakoshi T. Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection. J Neurooncol. 2001;52:161–71. doi: 10.1023/a:1010624504311. [DOI] [PubMed] [Google Scholar]
- 19.Odrazka K, Petera J, Kohlova T, Dolezel M, Vaculikova M, Zouhar M, et al. Prognostic impact of hemoglobin level prior to radiotherapy on survival in patients with glioblastoma. Strahlenther Onkol. 2003;179:615–9. doi: 10.1007/s00066-003-1097-x. [DOI] [PubMed] [Google Scholar]
- 20.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–9. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 21.Villavicencio AT, Burneikiene S, Romanelli P, Fariselli L, McNeely L, Lipani JD. Survival following stereotactic radiosurgery for newly diagnosed and recurrent glioblastoma multiforme: A multicenter experience. Neurosurg Rev. 1999;32:417–24. doi: 10.1007/s10143-009-0212-6. [DOI] [PubMed] [Google Scholar]
- 22.Li SW, Qiu XG, Chen BS, Zhang W, Ren H, Wang ZC, et al. Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin Med J (Engl) 2009;122:1245–9. [PubMed] [Google Scholar]
- 23.Grovas AC, Boyett JM, Lindsley K, Rosenblum M, Yates AJ, Finlay JL. Regimen-related toxicity of myeloablative chemotherapy with BCNU, thiotepa, and etoposide followed by autologous stem cell rescue for children with newly diagnosed glioblastoma multiforme: Report from the Children's Cancer Group. Neuro Oncol. 1999;33:83–7. doi: 10.1002/(sici)1096-911x(199908)33:2<83::aid-mpo4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


