Abstract
Background and Objectives:
This cytogenetic study detects a wide variety of common, rare and novel chromosomal abnormalities in patients with hematological disorders, providing valuable diagnostic and prognostic information.
Materials and Methods:
We addressed the utility of the cytogenetic technique in 50 patients of pediatric acute leukemia prospectively.
Results:
Successful cultures were found in 44 patients (88%) and abnormal karyotypes in 22 (44%). The common abnormalities like hyperdiploidy, del(6q), t(1;19)(q23;p13), t(4;11)(q22;q23), t(9;22)(q34;q11), rare t(2;7)(q23;p11) and t(4;12)(q21;p13) and a novel translocation t(7;9)(q22;q21) were observed in acute lymphoblastic leukemia. In acute myeloid leukemia, t(8;21)(q22;q22), del(16)(q22), t(15;17)(q22;q21) and t(9;11)(p22;q23) were commonly seen.
Conclusion:
Chromosomal abnormalities of this small group of patients are compared with the relevant literature with respect to the incidence rate and prognosis.
Keywords: Acute leukemia, cytogenetic, diagnosis, karyotype, pediatric
INTRODUCTION
Leukemia is the most common form of pediatric cancer, and accounts for about one-third of childhood malignancies. Acute leukemias represent clonal hematological disorders that arise from at least two or more genetic alterations in susceptible hematologic cells. The predominant variety of pediatric leukemia is acute lymphoblastic leukemia (ALL) with an annual incidence in different ountries varying from 0.9 to 4.7 per 100,000 children; less common is acute myeloid leukemia (AML), having an incidence of only one-quarter of that observed in ALL.[1] In India, the relative proportion varies between 25% and 40%. Sixty percent to 85% of all leukemia reported is ALL.[2] Conventional cytogenetics helps to classify the hematological malignancies that are associated with specific chromosomal abnormalities, and play an important role in diagnosis, allocation of treatment and prognosis. It has been reported that clonal chromosome abnormalities are present in the majority of successfully karyotyped patients, i.e. 79–85% in pediatric acute leukemia.[3] This is due to technical improvements and increased level of experience in the detection of subtle structural aberrations. On the basis of cytogenetic analysis, molecular methods have improved the pediatric hematologists’/oncologists’ ability to accurately and rapidly perform risk stratification.[4]
This is a prospective cytogenetic study of 50 patients of pediatric acute leukemia to know the clinical utility of cytogenetics among the south Indian population. Findings were compared and correlated with the relevant literature.
MATERIALS AND METHODS
Heparinised bone marrow aspirates were collected from 50 pediatric acute leukemic patients with age ranging between 6 months to 14 years, from the Pediatric Oncology Department, Kidwai Memorial Institute of Oncology, Bangalore, India and processed in cytogenetic laboratory for chromosomal analysis. Clinical and hematological findings were collected from files.
The bone marrow cells were cultured for direct, 24- and 48-h without mitogen in RPMI-1640 (Gibco, Invitrogen Corporation, Life Technologies, 3175 Staley Road, Grand Island, NY 14072, USA Cat: 23400-021) medium supplemented with 15% fetal bovine serum (Gibco) at 37°C. After incubation, the cells were exposed to colcemid (Gibco, 0.10 μg/ml) for 30 min, followed by hypotonic treatment (0.075 M KCl) for 20 min, and fixed with Carnoy's fixative (methanol:glacial acetic acid 3:1) and kept overnight in a refrigerator. The next day, air dry slides were made. The chromosomes were G-banded with the trypsin digestion method and stained with Giemsa. All the slides were screened and available metaphases were analyzed visually. Metaphases of good morphology were captured and analyzed using the Applied Spectral Imaging (ASI) software. The karyotypes were interpreted according to the International System for Human Cytogenetic Nomenclature.[5]
RESULTS
Of 50 cytogenetically analyzed patients, 30 (60%) were male and 20 (40%) were female, with a ratio of 3:2 and age range between 6 months and 14 years, with a mean of 7.11. Successful cultures were found in 44 patients (88%). Among the successful cultures, abnormal karyotypes (Ab.K) were seen in 22 (44%) patients, which includes 14 (63.6%) ALL and eight (36.4%) AML. Normal karyotype (NK) in 16 (32%) and poor morphology (PM) of not analyzable quality was seen in six (12%). The remaining six (12%) were not informative (NI) for the present study due to a lack of metaphase. Among ALLs, hyperdiploidy mn=50 and 49-54 was found in two patients showing an incidence of 14.2%, and translocations were detected in six patients (42.8%). Five patients revealed del(6)(q) (35.7%). Among them, it was a solitary abnormality in three, associated with del(9)(q21) in one and t(1;19)(q23;p13) in yet another (20%). The common abnormalities like t(9;22)(q34;q11) and t(4;11)(q22;q23) in patient nos. 9 and 11 and the novel and rare abnormalities like t(7;9)(q22;q21), t(2;7)(q23;p11) and t(4;12)(q21;p13) [Figure 1] were solely observed in patients nos. 10, 13 and 14, respectively [Table 1].
Figure 1.
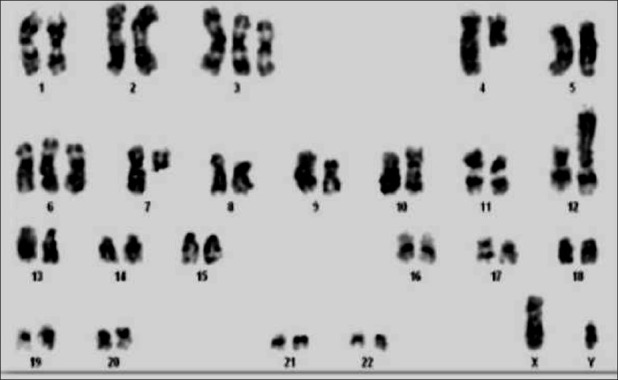
A rare karyotype of acute lymphoblastic leukemia showing 48, XY, +3, t(4;12)(q21;p13), +6, del(7)(q11)
Table 1.
Abnormal karyotypes in ALL and AML patients of present study
In AMLs, the numerical abnormalities like trisomy 6 and 13 were found in two patients, whereas trisomy 8 along with structural abnormality was seen in one (12.5%). The translocation was detected in six patients (75%). The recurrent abnormality, viz. t(8;21)(q22;q22), was seen in four patients (50%); two of them were associated with del(16)(q22) (25%), one with trisomy 8 and another with loss of sex chromosome [Figure 2]. t(15;17)(q22;q21) was found in one patient (12.5%) and t(9;11)(p22;q23) along with del(9)(q22) in another (12.5%). The del(16) along with NK was observed in one patient (12.5%) [Table 1].
Figure 2.
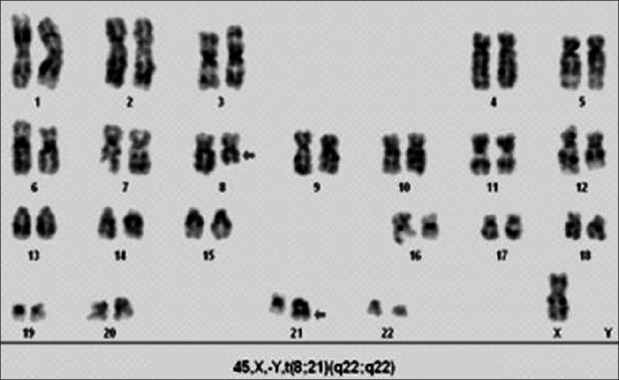
A karyotype of acute myeloid leukemia showing 45, X, -Y, t(8; 21) (q22; q22)
DISCUSSION
Karyotyping is a technique that provides critical diagnostic and prognostic information that allows the delivery of appropriate treatment. This technique solely depends on tissue culture success and availability of metaphases. The success rate depends on the sample condition, type of the disease and culture conditions. The success rate of culture varies from lab to lab; 77.6–90% and 87% was reported by Heng et al.[3] and Christine et al.,[6] respectively. Our study demonstrates that the practice of the direct culture method maximizes the culture success rate up to 86%.
Cytogenetic classification in ALL is based on numerical and structural chromosomal abnormalities. Hyperdiploidy was found to have a good prognosis[4,6] unless associated with structural aberrations, making it less favorable.[4,7] In the current study, hyperdiploidy was seen in two cases (14.2%), which is higher than a study from Egypt showing 3-9%.[4] Among the structural abnormalities, del(6)(q) is a frequent and well-known chromosomal abnormality, either solely in approximately 30% or with other additional abnormalities.[8] We found del(6)(q21-25) in three patients (60%) as the sole cause and in two patients along with other abnormalities (40%).
Chromosomal translocations have been identified in approximately 75% of pediatric ALL.[11] Among translocations, t(9;22)(q34:q11), t(4;11)(q22;q23) and t(1;19) (q23;p13) were reported in approximately 2-5%, 4% and 6%, respectively.[9] The former two types are known to be associated with a poor prognosis. The later one with unbalanced translocation is associated with good prognosis. In our study, we found an incidence of 7.1% of the above translocations. This difference could represent a truly geographic genetic heterogeneity, although bearing in mind the sample size. Herada et al.[10] have reported three cases of ALL with t(4;12)(q11-13;p12-13) associated with unique characteristics of B lymphoid phenotype. We had a similar karyotype of this type with a different break point of (4q). An interesting and important finding of the present study is t(2;7)(q23;p11) and t(7;9)(q22;q21) in two patients. To the best of our knowledge, these two abnormalities have not been reported elsewhere in pediatric ALL. The prognostic significance of these two translocations is yet to be evaluated in long-term follow-up.
Cytogenetic analysis is one of the most powerful and independent prognostic indicator in AML. The recurrent abnormalities, viz. t(8;21)(q22;q22), inv(16)(p13;q22)/t(16;16)(p13;q22), del(16)(q22) and t(15;17)(q22;q21) are associated with a good prognosis. The NKs of AML considered as intermediate prognosis and with numerical and structural abnormalities were considered to be poor prognosis.[2,11] In the present study, the most common abnormality t(8;21)(q22;q22) was detected in 50% of AML patients, and as a sole abnormality in 25%. The incidence rate is higher than in the literature.[11,12] Patients with t(8;21)(q22;q22), del(16)(q22) and t(15;17)(q22;q21) have good prognosis. The treatment outcome of trisomy 6 and 13 and t(9;11)(p22;q23) was found to be poor.[12] Two patients of our study belong to the category of poor prognosis.
Ongoing cytogenetic studies are warranted in larger groups of hematologic malignancies to identify newly acquired chromosomal abnormalities that may contribute to diagnosis and prognosis and increase the utility of cytogenetics in the development of targeted therapeutic drugs. Conventional cytogenetic is cost-effective, simple and allows an overall view of the genome. This can be effectively combined with other molecular methods like florescent in situ hybridization (FISH), which is essential to enable the detection of cryptic rearrangements that lead to the quantification of the NK, poor morphology and cultures showing no metaphases.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Manola KN. Cytogenetics of pediatric acute myeloid leukemia. Eur J Haematol. 2009;83:391–405. doi: 10.1111/j.1600-0609.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 2.Arora RS, Eden TO, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer. 2009;46:264–73. doi: 10.4103/0019-509X.55546. [DOI] [PubMed] [Google Scholar]
- 3.Heng JL, Chen YC, Quah TC, Liu TC, Yeoh AE. Dedicated cytogenetics factor is critical for improving karyotyping results for childhood leukaemias - experience in the National University Hospital, Singapore 1989-2006. Ann Acad Med Singapore. 2010;39:102–6. [PubMed] [Google Scholar]
- 4.Settin A, Al Haggar M, Al Dosoky T, Al Baz R, Abdelrazik N, Fouda M, et al. Prognostic cytogenetic markers in childhood acute lymphoblastic leukemia. Indian J Pediatr. 2007;74:255–63. doi: 10.1007/s12098-007-0040-z. [DOI] [PubMed] [Google Scholar]
- 5.Brothman AR, Persons DL, Shaffer LG. Nomenclature evolu tion: Changes in the ISCN from the 2005 to the 2009 edition. Cytogenet Genome Res. 2009;127:1–4. doi: 10.1159/000279442. [DOI] [PubMed] [Google Scholar]
- 6.Ito C, Kumagai M, Manabe A, Coustan-Smith E, Raimondi SC, Behm FG, et al. Hyperdiploid acute lymphoblastic leukemia with 51 to 65 chromosomes: A distinct biological entity with a marked propensity to undergo apoptosis. Blood. 1999;93:315–20. [PubMed] [Google Scholar]
- 7.Zemanova Z, Michalova K, Sindelarova L, Smisek P, Brezinova J, Ransdorfova S, et al. Prognostic value of structural chromosomal rearrangements and small cell clones with high hyperdiploidy in children with acute lymphoblastic leukemia. Leuk Res. 2005;29:273–81. doi: 10.1016/j.leukres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Amare P, Gladstone B, Varghese C, Pai S, Advani S. Clinical significance of cytogenetic findings at diagnosis and in remission in childhood and adult acute lymphoblastic leukemia: Experience from India. Cancer Genet Cytogenet. 1999;110:44–53. doi: 10.1016/s0165-4608(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH. Childhood leukemias. N Engl J Med. 1995;332:1618–30. doi: 10.1056/NEJM199506153322407. [DOI] [PubMed] [Google Scholar]
- 10.Harada H, Harada Y, Eguchi M, Dohy H, Kamada N. Characterization of acute leukemia with t(4;12) Leuk Lymphoma. 1997;25:47–53. doi: 10.3109/10428199709042495. [DOI] [PubMed] [Google Scholar]
- 11.Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: Clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999;94:3707–16. [PubMed] [Google Scholar]
- 12.Moorman AV, Ensor HM, Richards SM, Chilton L, Schwab C, Kinsey SE, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: Results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010;11:429–38. doi: 10.1016/S1470-2045(10)70066-8. [DOI] [PubMed] [Google Scholar]


