Abstract
Autoimmune disease (AD) is one of the emerging noncommunicable diseases. Remission is a possibility in AD, but current treatment strategies are not able to achieve this. We have well-established protocols for infections, oncology, metabolic diseases, and transplantation which are often used as models for the management of AD. Studies and observations suggest that in contrast to diseases used as a role model, AD has wide variability, different causative and pathogenic process, which is highly dynamic, making the current treatment strategies to fall short of expected complete remission. In this brief review, it is attempted to highlight the current understanding of AD and the probable gaps in the treatment strategies. Few hypothetical suggestions to modify the treatment protocols are presented.
KEY WORDS: Autoimmune disease, homeostasis, immune-modulation, immunosuppressive drugs, strtegies
Introduction
Epidemiological studies have indicated autoimmune diseases (ADs) to be the 10th most common cause of mortality in developing countries. The incidences of AD, including minor AD, such as thyroiditis, iridocyclitis, etc., are estimated to be around 10%.[1,2] The treatment strategies and outcome in the majority of ADs have significantly improved in the past two decades. The disease like systemic lupus erythematosus (SLE), which has aggressive course, had a mortality of more than 50% by the end of 5 years two decades ago. At present SLE survival has significantly improved with reported 90% survival beyond 5 years.[3] But, morbidity and mortality in substantial percentage of these treated AD remain unchanged, which suggest that there is a gap in the treatment protocols used currently in the management of ADs.[4] The current therapeutic strategies in AD, though aim at long-lasting remission, are achieved in only few ADs. Even these ADs which go for remission by their natural history have a tendency to go for spontaneous remission. Thus, in majority of ADs the targets achieved are reduction in symptoms and improving the quality of life. The current strategies are successful in preventing specific organ damage where possible or at least delaying the organ damage. The current understanding of immunobiology of AD has brought forward a large amount of information on disease mechanism and has led to the development of newer drugs and biologicals. In spite of all these advances cure or long-lasting remission still remains elusive in majority of systemic Ads, i.e., rheumatoid arthritis (RA), SLE, vasculitis, etc. In this article we have attempted to review the different models of pharmacological therapy, which are often considered as role models and the relevance of these approaches in the management of ADs as well as in designing the protocols of therapy for ADs. We have attempted to suggest few changes based on current understanding of the management of AD.
The Concept of Autoimmunity
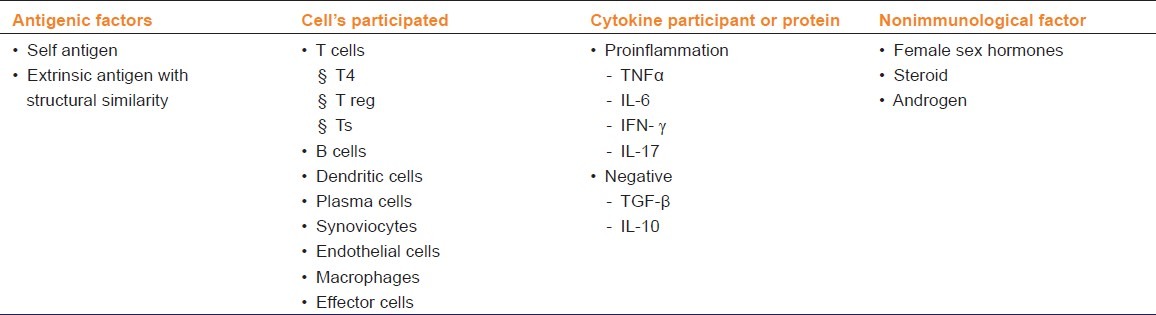
The concept of AD started as a rare possibility, but now AD, has been established to be one of the leading noncommunicable diseases across the globe. Understanding of autoimmunity has progressed significantly. The mechanisms of autoimmunity are now well understood from gross structural level to micromolecular levels. Initially, the theories of causation were more focused on the development of bad clones and have now extended to the bad clone selection, and breakdown in tolerance at different sites.[5] The current understanding of immunology in AD has significantly changed. However, the etiology and the causative mechanisms of AD are not completely understood.[6–8] The basic pathological processes mediating AD are now largely delineated. Even the pooled clinical observations on ADs have supplemented them. Few of these observations are worth noting and they are presented in Table 1. The most interesting inference which emerges from these observations is that autoimmunity and immune response (IR) involved in AD are not unidirectional and one-step response. The autoimmune process is a dynamic process, progressing from one end of lower affinity to higher affinity response and changes over a period of time.[9–11] The changes occurring in AD can promote the disease process either toward resolution or toward worsening. The studies previously performed exploring a single possible error, in a single cell line, a predefined antibody, a specific protein molecule like cytokine, and chemokine are inconclusive. In vitro experiments as well as animal models have demonstrated that some of the cells described and a few of these cytokines or ligands are critical in the development of AD.[12] But the studies conducted in human beings demonstrate that the observations of the animal experiments concurs with only 60–70% of studied population.[13] Even the targeted therapy on a single component of the immune system such as B cell targeting, anti-TNF strategies, anti-T cell strategies have provided better response in selected fraction of patients of autoimmune rheumatic disease explaining the possible role of multiple factors both at cellular level as well at the level of mediators [Table 2].[14] The clonal hypothesis, though appeared logical, has not been supported by many studies. All studies have failed to identify to date a single specific clonality of B cell or T cell in any of the autoimmune-mediated rheumatic disease.[15] Though studies had identified the increase in secretion of some specific cytokine mediators, these changes are not uniform in all the patients with the same AD.[13,16] In addition, the dynamics of cytokine production changes over a period of time, sometime day-to-day depending upon the environmental stimuli, hormones, nutritional status, infection load, exercise, physical activity, etc.[17,18] This dynamism has been appreciated by the studies from basic science. But, their clinical applications need further exploration,[19] which is the basic focus of this review.
Table 1.
Observations unique to autoimmune disease (AD)

Table 2.
Factors involved in the development and maintenance of autoimmune disease
Current Approach in AD
The current approach in AD can be broadly categorized into two categories: (1) symptomatic or replacement therapy (a conservative approach) and (2) the immunosuppressive or immune-modulation therapy (aggressive therapy). Autoimmune thyroid disease is the best example, which is predominantly managed either by reducing the thyroxin production at the time of hyperfunctioning or by replacing the hormone once the gland is damaged,[20] whereas in systemic diseases like SLE which targets vital organs such as kidney, etc. the primary treatment is to use immunosuppressive to prevent further organ damage. The response to immunosuppression in AD is seen in 60–70% initially, and subsequently the disease may progress or may stop responding to the drug used. Some of the ADs may go for clinical remission (no demonstrable clinical activity) to relapse after sometime.[21] In a small percentage of patients, the AD does go for long-lasting remission. Significant changes have occurred in the approach to AD with immunosuppressive therapy in last few decades. In the initial years, the immunosuppressive drugs used were nonspecific and were interfering in larger pathways and cells. Currently more target-specific drugs are available which have reduced the toxicity of immunosuppressant drugs on other collateral systems and produces a more profound immunosuppression effect.[22] Even these targeted immunosuppressive therapies have not increased the remission rate significantly including the diseases like RA.[23] In earlier years, the fear of serious infections as a result of immunosuppression were holding back the prescription of immunosuppression to less severe AD and were introduced in later part of AD. Improved intensive care and the management of infectious diseases have encouraged using higher immunosuppression and the combinations of these drugs. Combined with the observation of improved outcome in AD with early introduction of immunosuppression has encouraged their early use.[24] These changes have significantly improved the overall outcome of ADs and have paved the way to rethink on the need to achieve long-lasting remission. The fact that a significant proportion of patients with AD go for spontaneous remission and the same can be induced with immune modulators suggests remission to be a possible target. To achieve this target what is the best model of treatment in current practice which can serve as an ideal model or should AD be the approached model of its own?
Treatment Models in Current Medical Practice
Infectious Disease
The goal in the management of an infectious disease is to eliminate the infective organism from the host and to achieve a pathogen-free state. Thus, the recommendations in the management of infectious diseases are simple, such as identifying that the condition is caused by infection, isolating the appropriate microorganism, and administering the antimicrobial depending upon the sensitivity, with adequate symptom control and other supportive measures.[25–27] Recommendations broadly suggest to initiate an antibiotic after the isolation and establishing the infective microorganism. But, with reference to bacteria and fungi, as broad-spectrum antimicrobials are available in majority of the clinical setting, often antibiotics are started empirically; waiting to be replaced with specific antimicrobials.[28] In viral disease, since available antivirals are specific to virus and have narrow spectrum of activity, they are chosen after the confirmation of the presence of virus. In addition, in some of the viral infections, current guidelines suggest to introduce antivirals only in specific clinical and virological setting, for example, in chronic persisting viral infections like HIV and hepatitis virus. The recommendation depends upon both the host and viral factors, such as amount of pathology developed in the host and viral load.[29–31] In a nutshell, the treatment in an infectious disease focuses on the microbe responsible for the disease and its susceptibility to the drug. Though some of the host factors are considered in planning the therapy, the ultimate target is to eliminate the microbe close to zero wherever possible.
Oncology Disease
The goal in the management of a tumor or malignancy is to reduce the tumor burden and preferably completely.[32] Thus the steps in management of oncology include identification of the tumor and approximating its biological and pathological characterization, estimation of tumor burden, and associated host factors, if any. The treatment strategies are chosen from the available modalities to eliminate the malignant cells, keeping a close watch on toxicity to benefit ratio. This depends on type of malignancy, stage of disease, and functional status of the patients.[33]
Metabolic Diseases
The Metabolic Diseases (MDs) are the complex group of diseases, which may occur as a consequence of a single-step defect or a series of alteration in the metabolic process. The defect in few of the inherited or acquired metabolic disease could be deficiency of a single molecule, enzyme or hormone, like thyroxin in hypothyroidism.[34] In the circumstances of deficit prompt replacement of the deficient hormone or possible substrate would improve the patient. In some circumstances though the defect appears to be centering on a single defect (either excess or deficiency), the metabolic process may have wider repercussion. Simple substituting or altering that single defect in these circumstances may not offer a satisfactory result. The replacement of the deficient factor (hormone) and circumventing the interfered metabolic pathway or reducing or regulating the excess, offer a solution in MDs to a great extent. In a nutshell, the principle and the goal of the therapy in MDs is restoration of altered metabolic defect close to normal.[35]
The above described are the three fundamental groups of naturally occurring disease processes other than immune mediated disease (IMD). These three models are often considered as role models in the management of disease and have found tremendous success over a period of time.
Transplant Rejection Protocol
The management of transplant rejection by using immunosuppression is another modality which is used as a role model in the management of AD. But these are iatrogenic disease models and are approached with different perspectives. Reduction in immune activation and thereby rejection is the basic principle in transplant rejection management. Various combinations of immunosuppression for different length of time are used. Transplanting from adequately compatible donors and aggressive immunosuppression has changed the scenario of the transplant.
What are the Lessons Learnt from the Current Approach and What are the Changes Needed?
There are adequate reviews suggesting that current treatment strategies with reference to AD are not satisfactory.[21,36] The major focus of management is antiinflammatory and immunosuppressive therapy to reduce the immune activation and thereby to reduce the inflammatory damage. Various cytokines and cells which participate in the ongoing inflammatory process are targeted. Single or combination immunosuppressive are used based on the evidence gathered from several controlled clinical trials.[37,38] Thus at present the basic principle in the current approach to AD is immunosuppression.
Immunosuppression and AD
The current approach of immunosuppression resembles the concept of transplant rejection, but the immunosuppression used is much lesser in AD than what is normally prescribed in transplant rejection management. The immune homeostasis principle suggests that AD can be caused by lymphopenia and resurgence of the autoreactive clones.[38–40] The possible role of the homeostatic failure in the development of autoreactive clone has been demonstrated in studies on primary immunodeficiency (PID) and in animal models. There are increased incidences of developing a second AD in patients with preexisting AD. Both PID and acquired deficiency state (HIV), immunosuppression by infection or by drugs have increased incidence of AD.[41,42] The continued use of antitumor necrosis factor (anti-TNF) therapy, anti-T cell strategies have led to the production of different autoantibodies, but only few patients present with clinical manifestations.[43] Thus immunosuppression has been demonstrated to cause AD in different circumstances.
In contrast the evidence-based clinical studies have demonstrated that immunosuppressive therapy in AD substantially reduces the inflammatory damage and disease manifestation. But the response is not consistent and remission is achieved in few. Recent experiments with NOD mice have demonstrated the potential of immunosuppression in inducing AD.[40] Even the total immune-ablative therapy with reconstitution of the immune system by autotransplant of hematopoietic stem cell is accompanied by significant relapse, though remission may be present for a longer period.[44] It has often been stated that to cure an AD one must replace the “sick,” autoaggressive immune system with a genetically “healthy” one. The concept of clonal elimination is probably true for malignant clonal disease, is over simplistic for AD.[44] Autoimmune reactions being networks of autoreactivity (cells, cytokines, and growth factors) [Table 2], there are concrete clinical examples suggesting that the issue is not so simple and the concept of “resetting” the immune system should be the goal rather than ablating, which is practiced in the past few years.
Multiple Players, Simplified Targets
More than one group of cells may be actively involved in AD and targeting them together can be useful, such as, macrophage and T cells.[45] The multiple players both cellular elements, cytokine, and other factors actively influencing AD are depicted in Table 2. Immunological reaction is a self-regulated and self-perpetuating, time bound, and sequential process. Both proactive and counter regulatory processes get activated during the disease process. The development of autoimmune disease is not a one-step error. The process of both rectifier (counter regulatory mechanism) as well as promoter of the disease may be acting simultaneously. The hypothetical model is depicted in Figure 1. The immunosuppressive drugs may often inhibit or disturb the cells which could be inhibitory to AD, such as reduction in T regulatory cells, which could have otherwise antagonized the proinflammatory status.[46] The lower response rate to single-targeted immunosuppressive approach may be explained by failure to interfere in multilevel pathways, which are contributing to the disease process. Thus, a multipronged approach probably in a time-sequenced manner may be a more appropriate approach to AD.
Figure 1.
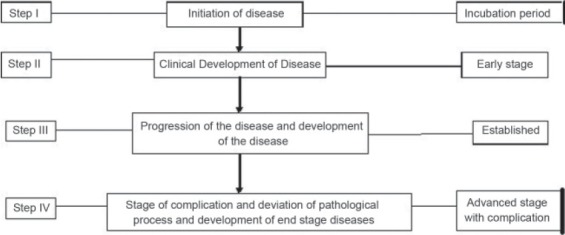
Autoimmune disease is not a one-step process. It moves through stage of preclinical evolution to fully established disease. The cells, cytokines, and other pathological process differ through these stages. Reversibility is best seen in first two steps
Syndromic Approach
Another major drawback in the current approach of AD is the arbitrary classification; often the diagnosis and the pathological processes are not appropriately connected. ADs are classified as the connective tissue disease, vasculitis, etc. predominantly based on clinical features and few antibody markers.[12] The drug therapy used despite different definition of autoimmune syndromes in the end drugs used are combinations of different immunosuppressive drugs or modulators. We find few of the drugs being effective in majority of ADs, such as steroids, and some of the immunosuppressive, while only few of them are disease specific. For example, anti-TNF drugs work in diseases like RA, ankylosing spondylitis and related arthritis, psoriatic arthritis, and inflammatory bowel disease. In the same group of anti-TNF strategies, anti-TNF receptor do not help in Crohn's disease while Infliximab works well.[47] Some of the drugs like methotrexate, azathioprine, and mycophenolate have been found to be useful in much wider AD. The responses do vary between syndromes, but this variation occurs even within the same syndrome. For instance in RA only 70% of patients showed significant response to methotrexate. Thus, the disease classification based on immune pathogenesis may help more to device the treatment protocols in contrast to the syndromic approach
Current Clinical Trials Status
One needs to have a relook at the areas focused in the current research work in AD. To recapitulate the quote that “events of the past 2 years have, in sometimes disconcerting ways, demonstrated that ‘evidence-based’ medicine has been looking in wrong places to meet many of the most important needs of the patients it serve.”[48] The current studies in AD are looking at reducing the symptoms, signs, and the impact of the disease on different organs. For instance in SLE with Lupus Nephritis (LN) the target is to salvage kidney and thereby improving the quality of life.[49] Similarly, in RA the end points evaluated are the disease activity and the reduced erosions of the joints and deformities. Current evidence and studies favor to manage the AD as a chronic disease, rather than looking at a disease which can have remission.
In addition there is a significant variability among patients suffering from AD. The genetic factors, the stage of the disease, the immunological response, and the other metabolic factors are responsible for variation in response. The current approach and recommendations in most of the ADs thus appear to be based on a reductionist approach ignoring this variability. Recently, many reviews have focused on this issue.[50–52] There are proposals to personalize the medical therapy to an individual patient utilizing the biomarkers.[50,51] The failure to achieve long-lasting remission or sustained low-disease activity in majority of the patients followed for longer period of time clearly suggests that continued usage of the same immunosuppressive regimen have certain limitations. Studies have shown that failure rates with conventional disease modifying antirheumatic drug (DMARD) therapy in RA can reach 75% over a follow-up period of 5 years.[21,53,54] The reasons for failure of immunosuppressive are often attributed to the metabolic or pharmacokinetic property of the drugs. But most often ignored is the dynamic variation occurring in the IR, for example, reduced B cell wherein anti-B cell drug may not work any longer, or development of different clones. Theoretically, it looks simpler to propose that silencing the targeted IR by promoting tolerance and increasing T regulatory cells should give a safe and a long-lasting response in AD. The sequencing of immune modulators with specific and guided biomarker of immune activation may provide a step closer to remission. This approach of sequencing immune modulator needs further clinical trial.
Dosing Protocols
Selection of dose of immunosuppressive and immune modulators needs a relook. There is no clarity on dosing protocols currently in AD. With an exception of few drugs, the dose of an immunosuppressive is considered empirically. For example, methotrexate dose has a range from 7.5 mg to 25 mg per week while treating RA. Typically a low-to-moderate dose of methotrexate is initiated and if no response and without toxicity dose is escalated to best tolerable dose. In some of the immunosuppressive drugs like azathioprine and cyclophosphamide, dosing is often guided by weight of the patient and the best-tolerated dose. Nearly, 90 years ago there was a suggestion that for the great majority of the drugs, the starting dose as so much per kilogram should be abandoned.[55] The relationship of dosage of a drug to the size of an animal treated, especially in regard to the failures to cure in trypanosomiasis, and other protozoan diseases in man and in other large animals, is the simple example of failure of such dosing protocols. The critical issue in AD, which is an IMD, is the quantity and quality of IR rather than the body weight of the individual. IR does not depend on body mass or surface area. The factors determining the quantity and quality of immunological activity responsible for the pathological process largely remain unclear. However, the intensity of the immunosuppression regimen and usage of different immunosuppressive drug is guided in some of the diseases such as SLE by the extent of organ involved and the severity of disease.[50] In RA treatment recommended preference DMARD to be used is based on the algorithm synthesized based on therapeutic toxicity ratio: such as to start with methotrexate and later with other drugs. However recent recommendation has suggested to stratify the disease as mild, moderate, and severe based on clinical and serological markers; and to use either combination or single DMARD.[56] The aggressive therapies in the majority of ADs are the combinations of the drugs used as monotherapy or newer biological with higher immunosuppressive effect. Dosage of immunosuppressive drug in aggressive disease is based on the patient's body weight or body surface area and not on immunological parameters. Thus, currently the dosing principle in AD is more on the aggressive nature of the disease, organ involved and the body weight of the patient. With an exception of few drugs, the dosage is often titrated against the control of disease activity and the best-tolerated dose by the patients. Keeping the safety concern of the patient, dosing principles based on the body weight and best-tolerated dose may be appropriate. But the efficacy parameters need to be ascertained. The issue we need to currently address is – Should the dosing be best guided by the quantity of immune activation and the pattern of IR? But we lack the biomarkers to assist us and unfortunately clinical features are not completely congruent with IR.
Comparison of Other Protocols with AD Protocols
The infectious disease model and oncology models are often used in designing the protocol in AD. This may not be suitable in AD, since AD is not caused by an extrinsic agent or a specific cell which can be successfully eliminated to contain the disease. Though earlier hypothesis believes AD to be caused by a bad clone, the current literature does not support the hypothesis. However, the combination protocols were introduced in AD, encouraged by the improved outcome in tuberculosis and malignancy by the use of combination chemotherapy and antimicrobials (infectious and oncology model). But the remission with combination therapy in AD could not be achieved. The combinations have significantly improved the clinical outcome. Unlike metabolic defects the error in AD are multiple level and dynamic; these characteristic features of AD make the metabolic models to fail. The current therapy of AD is comparable to the way the disease diabetes is managed.[57] The immune activation or aberration is compared to disregulated glucose metabolism. These models justify continued use of immunosuppression like oral hypoglycemic in diabetes and keep the inflammation under a tight control. Immunosuppression is the current standard method in management of AD. It is time to review the utility and limitation of these protocols and to work toward regimen which can induce remission restoring normal homeostasis. In LN the oncology protocol of remission induction followed by maintenance is utilized. Thus in LN high dose of immune suppressive therapy is used to induce remission (reduction in disease activity) and then it is maintained with lower immune suppression. Evidence from clinical trials suggests that the protocol of induction and remission gives a better outcome. But in theory the mechanism of success of therapy is not comparable to explanation in oncology. In contrast to cancer chemotherapy where remission is achieved, in LN the treatment protocol of induction and remission gives a better clinical response, but not remission. Though the models used are not explainable by the disease process they have improved the outcome. Rationalizing the approach in building a better model for AD based on pathogenesis may improve the outcome and achieve the most ideal goal, i.e., remission.
Conclusion
The current approach to AD has significantly improved the outcome and has raised a new hope for a possible remission. Immunosuppression is the mainstay of therapy currently. The current strategy of immunosuppression though helps in reducing symptoms but the overall impact on the disease is not effective, especially in inducing remission or a long-lasting cure. The commonly used role model of therapies of infectious disease, oncology, and metabolic disease are not applicable in toto in the management of AD. AD being an immune dysregulation, with multistep aberration and being a dynamic disease the current approach of managing AD as a chronic disease have not achieved the much-desired remission. Thus, a treatment protocol should be based on biomarkers and other immunological markers in a stepwise manner to restore a homeostatic nonaggressive immune system. This is a possible target since spontaneous remission is well documented in AD. A systematic approach to design a personalized treatment to AD should help to achieve this goal.
Footnotes
Source of Support: Nil.
Conflicts of interest: No.
References
- 1.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29:1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: Improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urowitz MB, Gladman DD. How to improve morbidity and mortality in systemic lupus erythematosus. Rheumatology (Oxford) 2000;39:238–44. doi: 10.1093/rheumatology/39.3.238. [DOI] [PubMed] [Google Scholar]
- 4.McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3:e297. doi: 10.1371/journal.pmed.0030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 6.Adams DD, Knight JG, Ebringer A. Autoimmune diseases: Solution of the environmental, immunological and genetic components with principles for immunotherapy and transplantation. Autoimmun Rev. 2010;9:525–30. doi: 10.1016/j.autrev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Doria A, Sarzi- Puttini P, Shoenfeld Y. Infections, rheumatism and autoimmunity: The conflicting relationship between humans and their environment. Autoimmun Rev. 2008;8:1–4. doi: 10.1016/j.autrev.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9:A271–6. doi: 10.1016/j.autrev.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Christen U, von Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–67. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest. 2007;117:871–3. doi: 10.1172/JCI31860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci U S A. 1995;92:875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 13.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–71. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Robinson DB, El-Gabalawy HS. Emerging biologic therapies in rheumatoid arthritis: Cell targets and cytokines. Curr Opin Rheumatol. 2005;17:274–9. doi: 10.1097/01.bor.0000160778.05389.dc. [DOI] [PubMed] [Google Scholar]
- 15.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren's syndrome. J Clin Invest. 1998;102:938–46. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manz RA, Moser K, Burmester GR, Radbruch A, Hiepe F. Immunological memory stabilizing autoreactivity. Curr Top Microbiol Immunol. 2006;305:241–57. doi: 10.1007/3-540-29714-6_12. [DOI] [PubMed] [Google Scholar]
- 17.Domínguez Rodríguez A, Abreu González P, García MJ, de la Rosa A, Vargas M, Marrero F. Circadian variations in proinflammatory cytokine concentrations in acute myocardial infarction. Rev Esp Cardiol. 2003;56:555–60. doi: 10.1016/s0300-8932(03)76916-4. [DOI] [PubMed] [Google Scholar]
- 18.Gokhale R, Chandrashekara S, Vasanthakumar KC. Cytokine response to strenuous exercise in athletes and non-athletes-an adaptive response. Cytokine. 2007;40:123–7. doi: 10.1016/j.cyto.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Moudgil KD, Choubey D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J Interferon Cytokine Res. 2011;31:695–703. doi: 10.1089/jir.2011.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer PA, Cooper DS, Levy EG, Ladenson PW, Braverman LE, Daniels G, et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. Standards of Care Committee, American Thyroid Association. JAMA. 1995;273:808–12. [PubMed] [Google Scholar]
- 21.van der Kooij SM, de Vries- Bouwstra JK, Goekoop- Ruiterman YP, van Zeben D, Kerstens PJ, Gerards AH, et al. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis. 2007;66:1356–62. doi: 10.1136/ard.2006.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–9. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 23.Böhm M, Luger TA, Schneider M, Schwarz T, Kuhn A. New insight into immunosuppression and treatment of autoimmune diseases. Clin Exp Rheumatol. 2006;24(1 Suppl 40):S67–71. [PubMed] [Google Scholar]
- 24.Verstappen SM, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, Borg EJ, et al. Five-year followup of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheum. 2003;48:1797–807. doi: 10.1002/art.11170. [DOI] [PubMed] [Google Scholar]
- 25.Bisno AL, Gerber MA, Gwaltney JM, Jr, Kaplan EL, Schwartz RH. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of Group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis. 2002;35:113–25. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 26.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 27.Deresinski S. Principles of antibiotic therapy in severe infections: Optimizing the therapeutic approach by use of laboratory and clinical data. Clin Infect Dis. 2007;45(Suppl 3):S177–83. doi: 10.1086/519472. [DOI] [PubMed] [Google Scholar]
- 28.Casadevall A. Crisis in infectious diseases: Time for a new paradigm? Clin Infect Dis. 1996;23:790–4. doi: 10.1093/clinids/23.4.790. [DOI] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services [Internet] [Last accessed on 2011 Oct 14]. pp. 1–167. Available from: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL002534.pdf .
- 30.Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 31.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55:245–64. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Simon C, Watson M, Drake A, Fenton A, McLoughlin C. Principles of cancer treatment. [Last accessed in 2008];InnovAiT [Internet] 1:107–18. Available from: http://rcgp-innovait.oxfordjournals.org/content/1/2/107.full.pdf+html . [Google Scholar]
- 33.Perry MC. Principles of cancer therapy. In: Goldman L, Ausiello D, editors. Cecil Medicine. Philadelphia: Saunders Elsevier; 2007. p. 192. [Google Scholar]
- 34.Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281–8. doi: 10.7326/0003-4819-121-4-199408150-00010. [DOI] [PubMed] [Google Scholar]
- 35.Chakrapani A, Wraith JE. Principles of management of the more common metabolic disorders. Curr Paediatrics. 2002;12:117–24. [Google Scholar]
- 36.Cohen IR. Treatment of autoimmune disease: To activate or to deactivate? Chem Immunol. 1995;60:150–60. [PubMed] [Google Scholar]
- 37.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–82. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 38.Dalakas MC. Therapeutic targets in patients with inflammatory myopathies: Present approaches and a look to the future. Neuromuscul Disord. 2006;16:223–36. doi: 10.1016/j.nmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: A two-hit model. Clin Immunol. 2006;120:121–8. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 40.Kaminitz A, Mizrahi K, Yaniv I, Stein J, Askenasy N. Immunosuppressive therapy exacerbates autoimmunity in NOD mice and diminishes the protective activity of regulatory T cells. J Autoimmun. 2010;35:145–52. doi: 10.1016/j.jaut.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Schuetz C, Niehues T, Friedrich W, Schwarz K. Autoimmunity, autoinflammation and lymphoma in combined immunodeficiency (CID) Autoimmun Rev. 2010;9:477–82. doi: 10.1016/j.autrev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Dhasmana DJ, Dheda K, Ravn P, Wilkinson RJ, Meintjes G. Immune reconstitution inflammatory syndrome in HIV- infected patients receiving antiretroviral therapy: Pathogenesis, clinical manifestations and management. Drugs. 2008;68:191–208. doi: 10.2165/00003495-200868020-00004. [DOI] [PubMed] [Google Scholar]
- 43.Singh JA, Christensen R, Wells GA, Suarez- Almazor ME, Buchbinder R, Lopez- Olivo MA, et al. Biologics for rheumatoid arthritis: An overview of Cochrane reviews. Cochrane Database Syst Rev. 2009:CD007848. doi: 10.1002/14651858.CD007848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyndall A, Gratwohl A. The use of high dose immunoablative therapy with hematopoietic stem cell support therapy in the treatment of severe autoimmune diseases. Int J Hematol. 2002;76:218–22. doi: 10.1007/BF03165249. [DOI] [PubMed] [Google Scholar]
- 45.Sibilia J. Novel concepts and treatments for autoimmune disease: Ten focal points. Joint Bone Spine. 2004;71:511–7. doi: 10.1016/j.jbspin.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyrin- Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: Meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–53. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: Prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553–60. doi: 10.18553/jmcp.2008.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195–205. doi: 10.1136/ard.2007.070367. [DOI] [PubMed] [Google Scholar]
- 50.Scherer HU, Dörner T, Burmester GR. Patient-tailored therapy in rheumatoid arthritis: An editorial review. Curr Opin Rheumatol. 2010;22:237–45. doi: 10.1097/BOR.0b013e328337b832. [DOI] [PubMed] [Google Scholar]
- 51.Isaacs JD, Ferraccioli G. The need for personalised medicine for rheumatoid arthritis. Ann Rheum Dis. 2011;70:4–7. doi: 10.1136/ard.2010.135376. [DOI] [PubMed] [Google Scholar]
- 52.Carlson RJ. The disruptive nature of personalized medicine technologies: Implications for the health care system. Public Health Genomics. 2009;12:180–4. doi: 10.1159/000189631. [DOI] [PubMed] [Google Scholar]
- 53.Maetzel A, Wong A, Strand V, Tugwell P, Wells G, Bombardier C. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2000;39:975–81. doi: 10.1093/rheumatology/39.9.975. [DOI] [PubMed] [Google Scholar]
- 54.Aletaha D, Smolen JS. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. J Rheumatol. 2002;29:1631–8. [PubMed] [Google Scholar]
- 55.Moore B. The relationship of dosage of a drug to the size of the animal treated, especially in regard to the cause of the failures to cure trypanosomiasis, and other protozoan diseases in man and in large animal. Biochem J. 1909;4:323–30. doi: 10.1042/bj0040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 57.Emery P. Treatment of rheumatoid arthritis. BMJ. 2006;332:152–5. doi: 10.1136/bmj.332.7534.152. [DOI] [PMC free article] [PubMed] [Google Scholar]


